Non-Covalent Interactions Between Quercetin and Rice Bran Protein: Mechanisms and Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RBP and RBP–Que Complexes
2.2.1. Extraction of RBP
2.2.2. Preparation of RBP–Que Complexes
2.3. Molecular Characteristics
2.3.1. Quercetin Binding Ratio
2.3.2. Particle Size, ζ-Potential and Polydispersity Index (PDI)
2.3.3. Turbidity
2.3.4. Molecular Flexibility
2.4. Structural Characteristics
2.4.1. Fourier Transform Infrared (FTIR) Spectrometry
2.4.2. Surface Hydrophobicity (H0)
2.4.3. Fluorescence Spectrometry
2.4.4. Fluorescence Quenching Mechanism
2.5. Functional Characteristics
2.5.1. Solubility
2.5.2. Antioxidant Activities
- DPPH radical scavenging assay
- 2.
- ABTS radical scavenging assay
2.5.3. Foaming Properties
2.5.4. Emulsifying Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Molecular Characteristics
3.1.1. Quercetin Binding Ratio
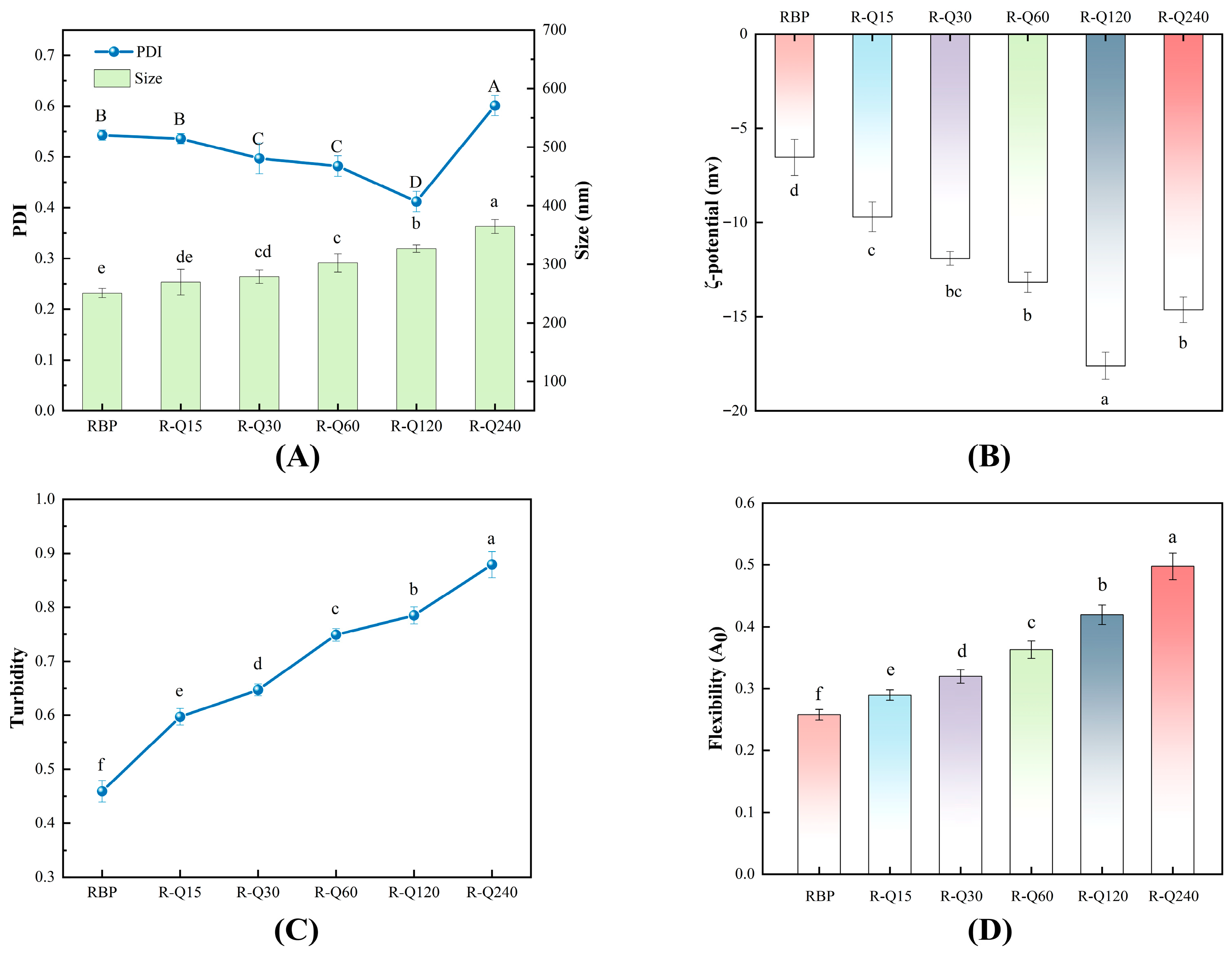
3.1.2. Particle Size and PDI
3.1.3. ζ-Potential
3.1.4. Turbidity
3.1.5. Flexibility
3.2. Structural Characteristics
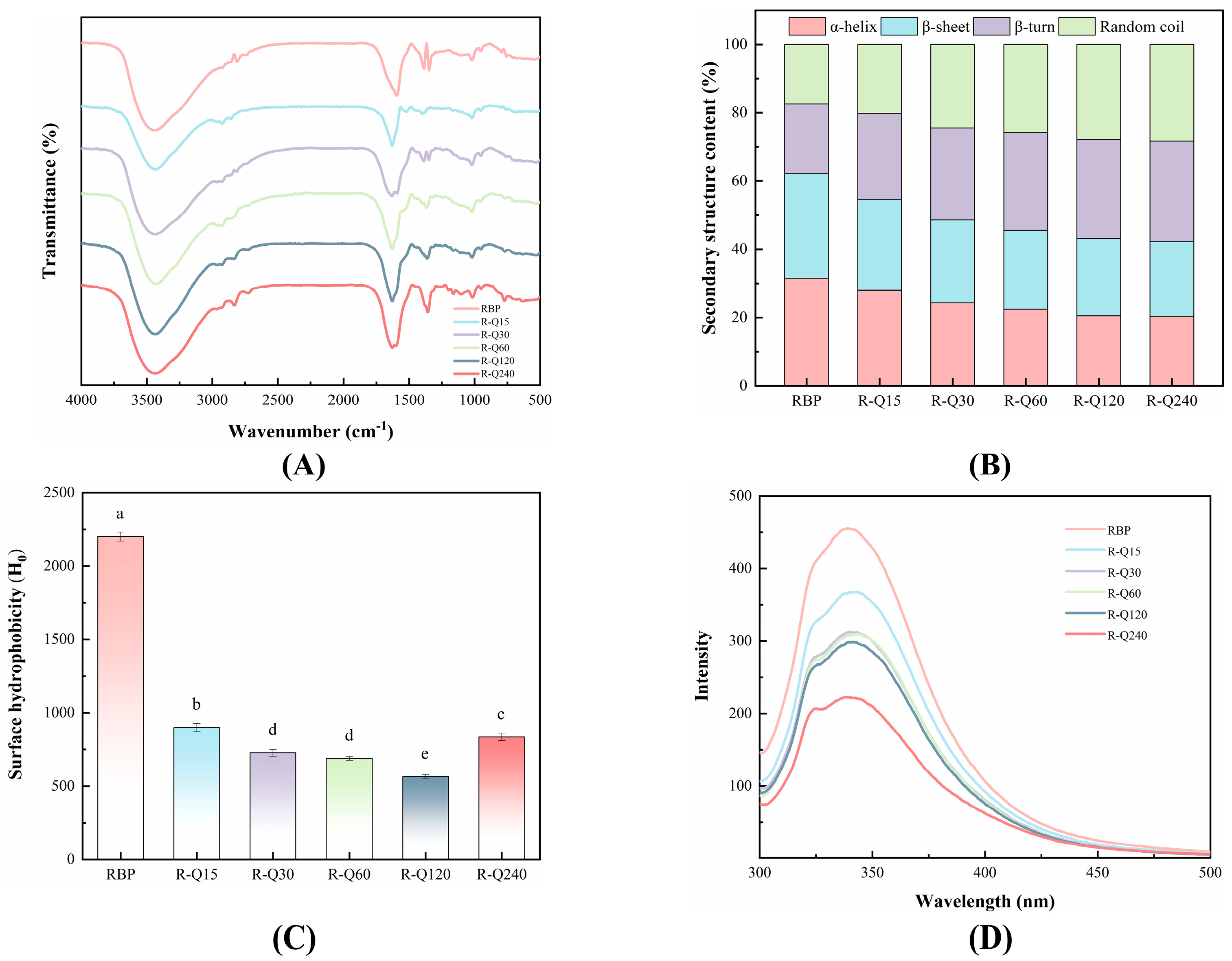
3.2.1. FTIR Spectra
3.2.2. Surface Hydrophobicity
3.2.3. Fluorescence Spectra
3.2.4. Fluorescence Quenching Mechanism
3.3. Functional Characteristics
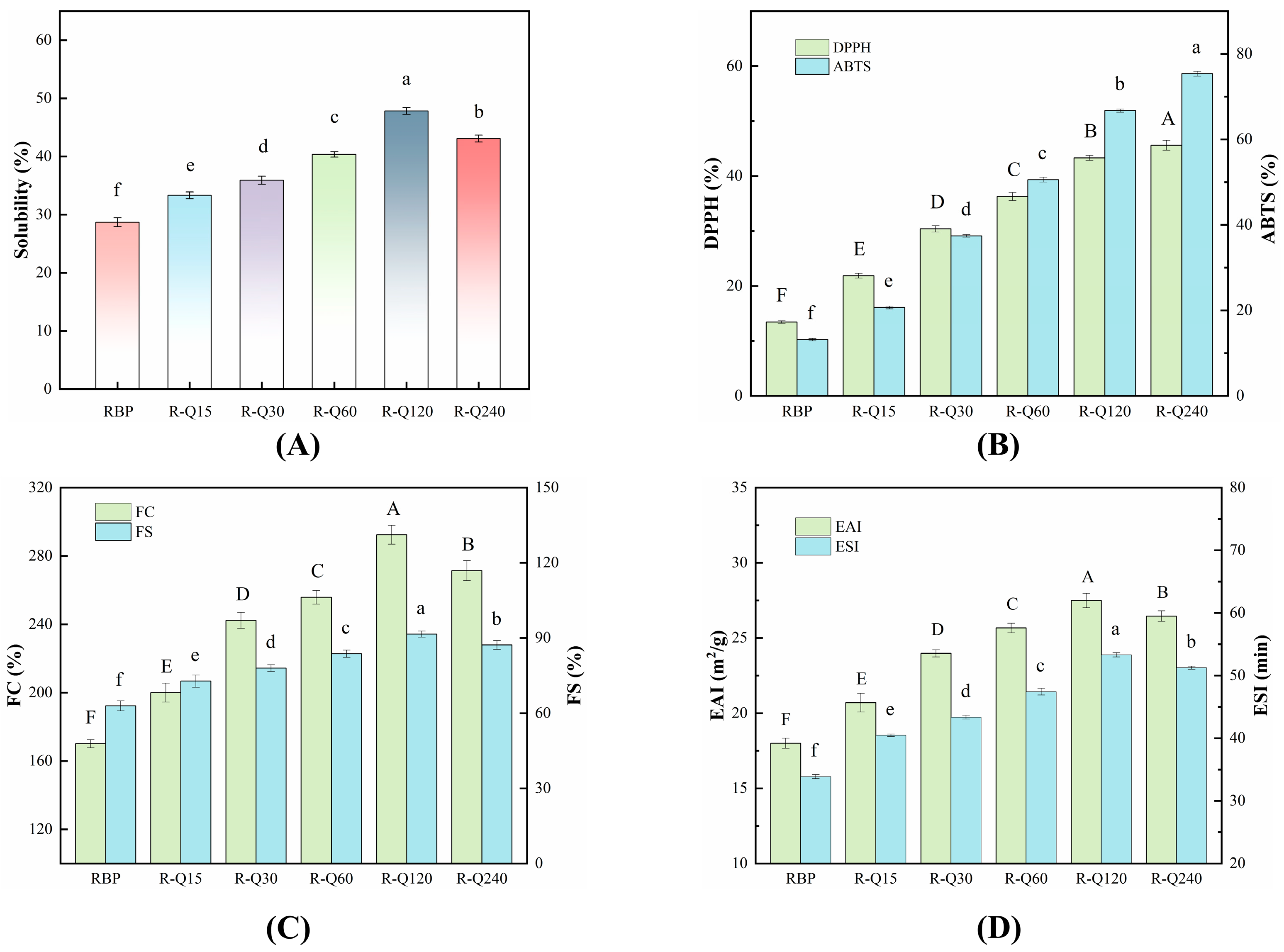
3.3.1. Solubility
3.3.2. Antioxidant Activities
3.3.3. Foaming Properties
3.3.4. Emulsifying Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RBP | Rice Bran Protein |
| Que | Quercetin |
| ANS | 8-Anilino-1-naphthalenesulfonic acid |
| SDS | sodium dodecyl sulfate |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| PDI | Polydispersity Index |
| FTIR | Fourier Transform Infrared |
| H0 | Surface Hydrophobicity |
| FC | Foaming Capacity |
| FS | Foaming Stability |
| EAI | Emulsifying Activity Index |
| ESI | Emulsifying Stability Index |
References
- Perez-Ternero, C.; Alvarez de Sotomayor, M.; Herrera, M.D. Contribution of ferulic acid, γ-oryzanol and tocotrienols to the cardiometabolic protective effects of rice bran. J. Funct. Foods 2017, 32, 58–71. [Google Scholar] [CrossRef]
- Wang, S.R.; Wang, T.Y.; Sun, Y.; Cui, Y.J.; Yu, G.P.; Jiang, L.Z. Effects of high hydrostatic pressure pretreatment on the functional and structural properties of rice bran protein hydrolysates. Foods 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Ding, W.; Qu, W.; Oladejo, A.O.; Xiong, F.; Zhang, W.; He, R.; Ma, H. Alkali solution extraction of rice residue protein isolates: Influence of alkali concentration on protein functional, structural properties and lysinoalanine formation. Food Chem. 2017, 218, 207–215. [Google Scholar] [CrossRef]
- Ling, B.; Ouyang, S.; Wang, S. Effect of radio frequency treatment on functional, structural and thermal behaviors of protein isolates in rice bran. Food Chem. 2019, 289, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- Wu, W.; Kong, X.; Zhang, C.; Hua, Y.; Chen, Y. Improving the stability of wheat gliadin nanoparticles—Effect of gum arabic addition. Food Hydrocoll. 2018, 80, 78–87. [Google Scholar] [CrossRef]
- Luo, Y.; Pu, C.; Zhang, J.; Fu, Z.; Tang, W.; Sun, Q. Oil in water emulsion stabilized by glycated rice bran protein aggregates: Effect on interfacial behavior and in vitro digestion of emulsion. Food Hydrocoll. 2025, 158, 110530. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Qiu, L.; Sun, Y.; Xiong, H.; Ogra, Y. Improvement of the solubility and emulsifying properties of rice bran protein by phosphorylation with sodium trimetaphosphate. Food Hydrocoll. 2019, 96, 288–299. [Google Scholar] [CrossRef]
- Pereira, G.M.; Jun, S.; Li, Q.X.; Wall, M.M.; Ho, K.K.H.Y. Formation and physical characterization of soy protein-isoflavone dispersions and emulsions. LWT-Food Sci. Technol. 2023, 176, 114513. [Google Scholar] [CrossRef]
- Parolia, S.; Maley, J.; Sammynaiken, R.; Green, R.; Nickerson, M.; Ghosh, S. Structure-Functionality of lentil protein-polyphenol conjugates. Food Chem. 2022, 367, 130603. [Google Scholar] [CrossRef]
- Wang, S.R.; Li, X.Y.; Zhu, J.S.; Liu, H.C.; Liu, T.; Yu, G.P.; Shao, M.L. Covalent interaction between high hydrostatic pressure-pretreated rice bran protein hydrolysates and ferulic acid: Focus on antioxidant activities and emulsifying properties. J. Agric. Food Chem. 2021, 69, 7777–7785. [Google Scholar] [CrossRef]
- Wang, S.; Cui, Y.; Sun, Y.; Yu, G.; Wang, T. Insight into the effects of soybean isoflavone on the functional properties of rice bran protein: Focus on non-covalent interaction. LWT-Food Sci. Technol. 2025, 217, 117346. [Google Scholar] [CrossRef]
- Ma, N.; Duan, J.; Zhou, G.; Wang, X. Study of the mechanism of non-covalent interactions between chlorogenic acid and soy protein isolate: Multi-spectroscopic, in vitro, and computational docking analyses. Food Chem. 2024, 457, 140084. [Google Scholar] [CrossRef]
- Wang, N.; Liu, B.; Han, X.; Wang, L.; Yang, F.; Wang, T.; Yu, D. Tannic acid/non-covalent interaction-mediated modification of hemp seed proteins: Focused on binding mechanisms, oil-water interface behavior, and rheological properties. Food Hydrocoll. 2024, 157, 110483. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Del-Toro--Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz- Cruz, S.; López-Ahumada, G.A.; Carvajal-Millan, E.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Preparation and characterization of quercetin-loaded zein nanoparticles by electrospraying and study of in vitro bioavailability. J. Food Sci. 2019, 84, 2883–2897. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Ke, C.X.; Liu, B.S.; Dudu, O.E.; Zhang, S.Q.; Meng, L.; Wang, Y.; Wang, W.L.H.; Cheng, J.J.; Yan, T.S. Modification of structural and functional characteristics of casein treated with quercetin via two interaction modes: Covalent and non-covalent interactions. Food Hydrocoll. 2023, 137, 108394. [Google Scholar] [CrossRef]
- Cheng, J.J.; Dudu, O.E.; Zhang, J.J.Q.; Wang, Y.; Meng, L.; Wei, W.L.H.; Li, X.D.; Yan, T.S. Impact of binding interaction modes between whey protein concentrate and quercetin on protein structural and functional characteristics. Food Hydrocoll. 2023, 142, 108787. [Google Scholar] [CrossRef]
- Günal-Köroğlu, D.; Turan, S.; Capanoglu, E. Interaction of lentil protein and onion skin phenolics: Effects on functional properties of proteins and in vitro gastrointestinal digestibility. Food Chem. 2022, 372, 130892. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.M.; Liu, J.Y.; Zhang, H.J.; Gong, L.X.; Li, Z.F.; Liu, H.Z.; Wang, Z.Y. Effect of non-covalently bound polyphenols on the structural and functional properties of wheat germ protein. Food Hydrocoll. 2024, 149, 109534. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determination by use of stable free radicals. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Siddhuraju, P. The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (Jacq.) Marechal seed extracts. Food Chem. 2006, 99, 149–157. [Google Scholar] [CrossRef]
- Liu, F.; Sun, C.; Yang, W.; Yuan, F.; Gao, Y. Structural characterization and functional evaluation of lactoferrin–polyphenol conjugates formed by free-radical graft copolymerization. RSC Adv. 2015, 5, 15641–15651. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Hao, L.L.; Su, J.W.; Pei, M.Q.; Zhang, G.F.; Li, C.; Li, C.M.; Ma, X.K.; He, S.X.; Liu, L.B. Impact of non-covalent bound polyphenols on conformational, functional properties and in vitro digestibility of pea protein. Food Chem. 2022, 383, 132623. [Google Scholar] [CrossRef]
- Sui, X.N.; Sun, H.B.; Qi, B.K.; Zhang, M.; Li, Y.; Jiang, L.Z. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef]
- Chang, K.; Liu, J.; Jiang, W.; Zhang, R.; Zhang, T.; Liu, B. Ferulic acid-ovalbumin protein nanoparticles: Structure and foaming behavior. Food Res. Int. 2020, 136, 109311. [Google Scholar] [CrossRef]
- Liu, X.Z.; Chen, J.W.; Zhang, W.; Lin, X.; Fei, T.; Liu, Z.H.; Wang, L. Non-covalent interaction between lactoferrin and theaflavin: Focused on the structural changes, binding mechanism, and functional properties. Food Chem. 2024, 461, 140835. [Google Scholar] [CrossRef] [PubMed]
- Balange, A.K.; Benjakul, S. Effect of oxidized tannic acid on the gel properties of mackerel (rastrelliger kanagurta) mince and surimi prepared by different washing processes. Food Hydrocoll. 2009, 23, 1693–1701. [Google Scholar] [CrossRef]
- Dai, S.; Liao, P.; Wang, Y.; Tian, T.; Tong, X.; Lyu, B.; Miao, L.; Qi, W.; Jiang, L.; Wang, H. Soy protein isolate-catechin non-covalent and covalent complexes: Focus on structure, aggregation, stability and in vitro digestion characteristics. Food Hydrocoll. 2023, 135, 108108. [Google Scholar] [CrossRef]
- Poon, S.; Clarke, A.E.; Schultz, C.J. Effect of denaturants on the emulsifying activity of proteins. J. Agric. Food Chem. 2001, 49, 281–286. [Google Scholar] [CrossRef]
- Tang, C.H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- Wang, N.Z.; Ma, Z.H.; Ma, L.T.; Zhang, Y.; Zhang, K.D.; Ban, Q.F.; Wang, X.B. Synergistic modification of structural and functional characteristics of whey protein isolate by soybean isoflavones non-covalent binding and succinylation treatment: A focus on emulsion stability. Food Hydrocoll. 2023, 144, 108994. [Google Scholar] [CrossRef]
- Zhao, T.T.; Huang, L.; Luo, D.H.; Xie, Y.X.; Zhang, Y.H.; Zhang, Y.S.; Jiao, W.J.; Su, G.W.; Zhao, M.M. Fabrication and characterization of anchovy protein hydrolysates-polyphenol conjugates with stabilizing effects on fish oil emulsion. Food Chem. 2021, 351, 129324. [Google Scholar] [CrossRef]
- Xu, P.W.; Yue, X.J.; Yuan, X.F.; Zhao, B. Non-covalent interaction between hemp seed globulin and two hemp seed phenolic compounds: Mechanism and effects on protein structure, bioactivity, and in vitro simulated digestion. Int. J. Biol. Macromol. 2024, 255, 128077. [Google Scholar] [CrossRef]
- Yu, D.; Liu, B.; Wang, N.; Wang, T.; Wang, W.; Wang, L. Cavitation jet treatment on soybean protein isolate-arbutin non-covalent complexes: Multi-spectral analysis, molecular docking, interfacial properties. Food Hydrocoll. 2025, 164, 111190. [Google Scholar] [CrossRef]
- Huang, X.; Xia, B.; Liu, Y.; Wang, C. Non-covalent interactions between rice protein and three polyphenols and potential application in emulsions. Food Chem. X 2024, 22, 101459. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, X.; Zhang, S.X.; Wang, T.; Yu, D.Y. Non-covalent interaction between soybean protein isolate and naringenin: Focused on binding mechanism, interface behavior, and functional properties. Food Hydrocoll. 2024, 153, 109975. [Google Scholar] [CrossRef]
- Zhou, S.; Meng, L.; Lin, Y.; Dong, X.; Dong, M. Exploring the interactions of soybean 7S globulin with Gallic acid, Chlorogenic acid and (–)-epigallocatechin Gallate. Foods 2023, 12, 4013. [Google Scholar] [CrossRef]
- Chen, G.; Wang, S.T.; Feng, B.; Jiang, B.; Miao, M. Interaction between soybean protein and tea polyphenols under high pressure. Food Chem. 2019, 277, 632–638. [Google Scholar] [CrossRef]
- Li, L.; Yu, X.X.; Zhao, H.F.; Yu, B.K.; Zhang, Y.H.; Mu, Z.S. Study on molecular mechanism and functional properties of non-covalently binding between whey protein and chlorogenic acid. Int. J. Biol. Macromol. 2025, 316, 144768. [Google Scholar] [CrossRef]
- Liu, C.; Wang, N.; Li, L.; Wu, D.; Wang, L.; Zhang, N.; Yu, D. Effect of microwave plasma processing on the structure, physicochemical properties and functional properties of rice bran protein. Food Hydrocoll. 2025, 160, 110851. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Lan, Y.; Wang, X. Enhancing soy protein isolate flexibility through non-covalent ferulic acid modification: Implications for interfacial characteristics and protein-based emulsion performance. Food Struct. 2024, 42, 100400. [Google Scholar] [CrossRef]
- Yin, B.; Wu, Q.; Zheng, Z.; Wang, R.; Zhao, Y.; Zhao, W.; Wang, D.; Qin, P.; Zhao, S.; Kan, J.; et al. Effects of non-covalent binding of different proteins and apple polyphenols on structure and functional properties. Food Hydrocoll. 2025, 166, 111333. [Google Scholar] [CrossRef]
- Li, C.; Dai, T.; Chen, J.; Li, X.; Ti, L.; Liu, C.; McClements, D.J. Protein-polyphenol functional ingredients: The foaming properties of lactoferrin are enhanced by forming complexes with procyanidin. Food Chem. 2021, 339, 128145. [Google Scholar] [CrossRef]
- Yang, W.H.; Tu, Z.C.; Li, Q.H.; Kaltashov, I.A.; McClements, D.J. Utilization of sonication-glycation to improve the functional properties of ovalbumin: A high- resolution mass spectrometry study. Food Hydrocoll. 2021, 119, 106822. [Google Scholar] [CrossRef]
- He, W.Y.; Xu, H.X.; Lu, Y.Q.; Zhang, T.T.; Li, S.M.; Lin, X.; Xu, B.Q.; Wu, X.L. Function, digestibility and allergenicity assessment of ovalbumin–EGCG conjugates. J. Funct. Foods 2019, 61, 103490. [Google Scholar] [CrossRef]
- Yang, J.; Lamochi Roozalipour, S.P.; Berton-Carabin, C.C.; Nikiforidis, C.V.; van der Linden, E.; Sagis, L.M.C. Air-water interfacial and foaming properties of whey protein-sinapic acid mixtures. Food Hydrocoll. 2021, 112, 106467. [Google Scholar] [CrossRef]
- von Staszewski, M.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M.R. Green tea polyphenols-β-lactoglobulin nanocomplexes: Interfacial behavior, emulsification and oxidation stability of fish oil. Food Hydrocoll. 2014, 35, 505–511. [Google Scholar] [CrossRef]

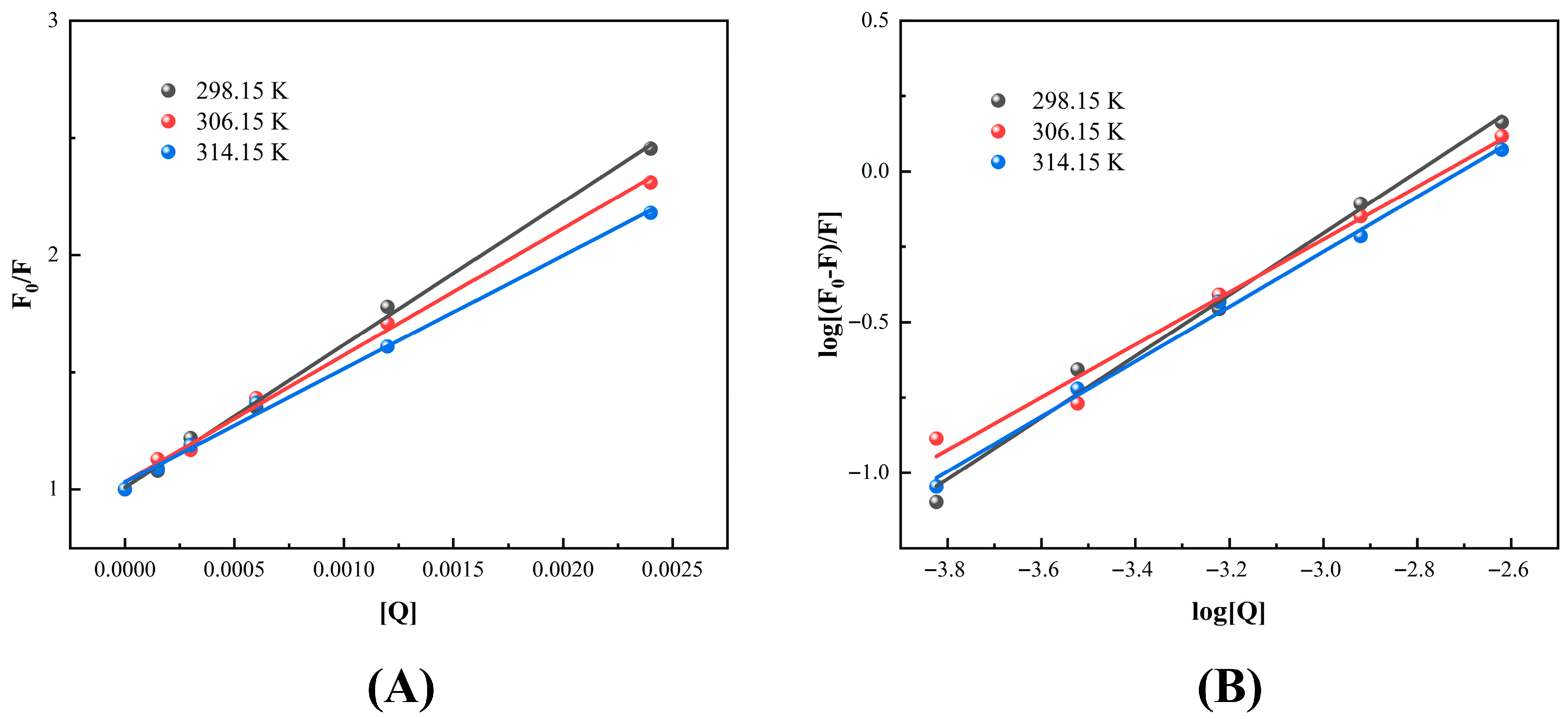
| T (K) | KSV (103 L mol−1) | Kq (1011 L mol−1 s−1) | R2 | Ka (102 L mol−1) | n | R2 |
|---|---|---|---|---|---|---|
| 298.15 | 0.61 ± 0.02 | 0.61 ± 0.02 | 0.9988 | 7.13 ± 0.02 | 1.02 ± 0.06 | 0.9945 |
| 306.15 | 0.54 ± 0.02 | 0.54 ± 0.02 | 0.9982 | 2.47 ± 0.02 | 0.87 ± 0.07 | 0.9919 |
| 314.15 | 0.48 ± 0.02 | 0.48 ± 0.02 | 0.9980 | 2.93 ± 0.01 | 0.91 ± 0.03 | 0.9979 |
| T (K) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | ΔG (kJ mol−1) |
|---|---|---|---|
| 298.15 | −43.88 | −11.33 | −16.28 |
| 306.15 | −14.02 | ||
| 314.15 | −14.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yu, D.; Wang, T.; Zhou, L.; Han, X. Non-Covalent Interactions Between Quercetin and Rice Bran Protein: Mechanisms and Functional Properties. Foods 2025, 14, 2923. https://doi.org/10.3390/foods14172923
Wang S, Yu D, Wang T, Zhou L, Han X. Non-Covalent Interactions Between Quercetin and Rice Bran Protein: Mechanisms and Functional Properties. Foods. 2025; 14(17):2923. https://doi.org/10.3390/foods14172923
Chicago/Turabian StyleWang, Shirang, Dianyu Yu, Tengyu Wang, Liping Zhou, and Xu Han. 2025. "Non-Covalent Interactions Between Quercetin and Rice Bran Protein: Mechanisms and Functional Properties" Foods 14, no. 17: 2923. https://doi.org/10.3390/foods14172923
APA StyleWang, S., Yu, D., Wang, T., Zhou, L., & Han, X. (2025). Non-Covalent Interactions Between Quercetin and Rice Bran Protein: Mechanisms and Functional Properties. Foods, 14(17), 2923. https://doi.org/10.3390/foods14172923






