Light-Emitting Diode [LED]-Driven Mechanisms for Postharvest Decay Control and Functional Quality Improvement in Fruits and Vegetables
Abstract
1. Introduction
2. LED Characteristics, Materials, and Wavelengths
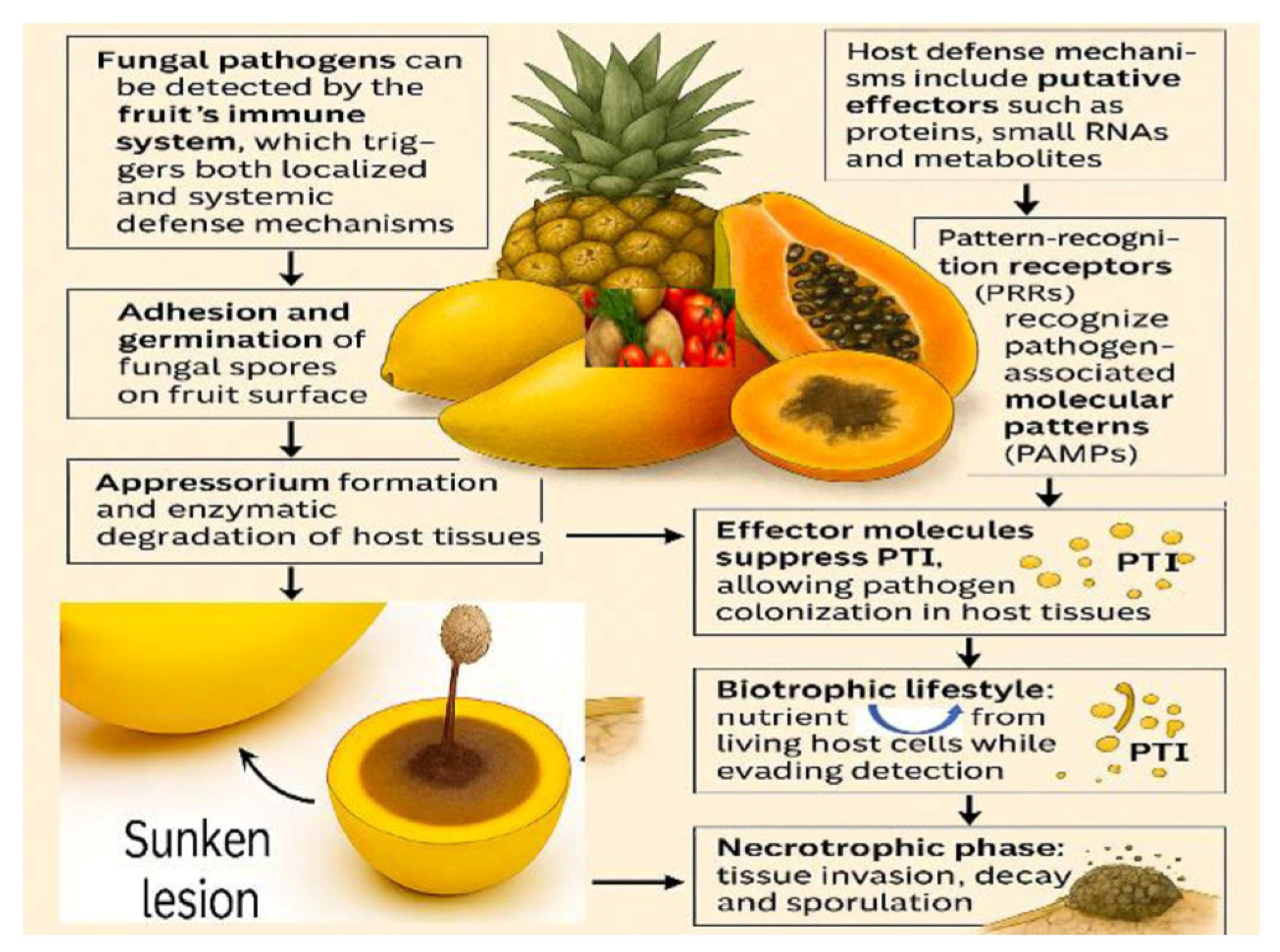
3. Rot Pathogenicity and Evasion of Defense Mechanisms in Fruits and Vegetables
4. LED Control of Fruit Rot-Related Pathogens in Fruits
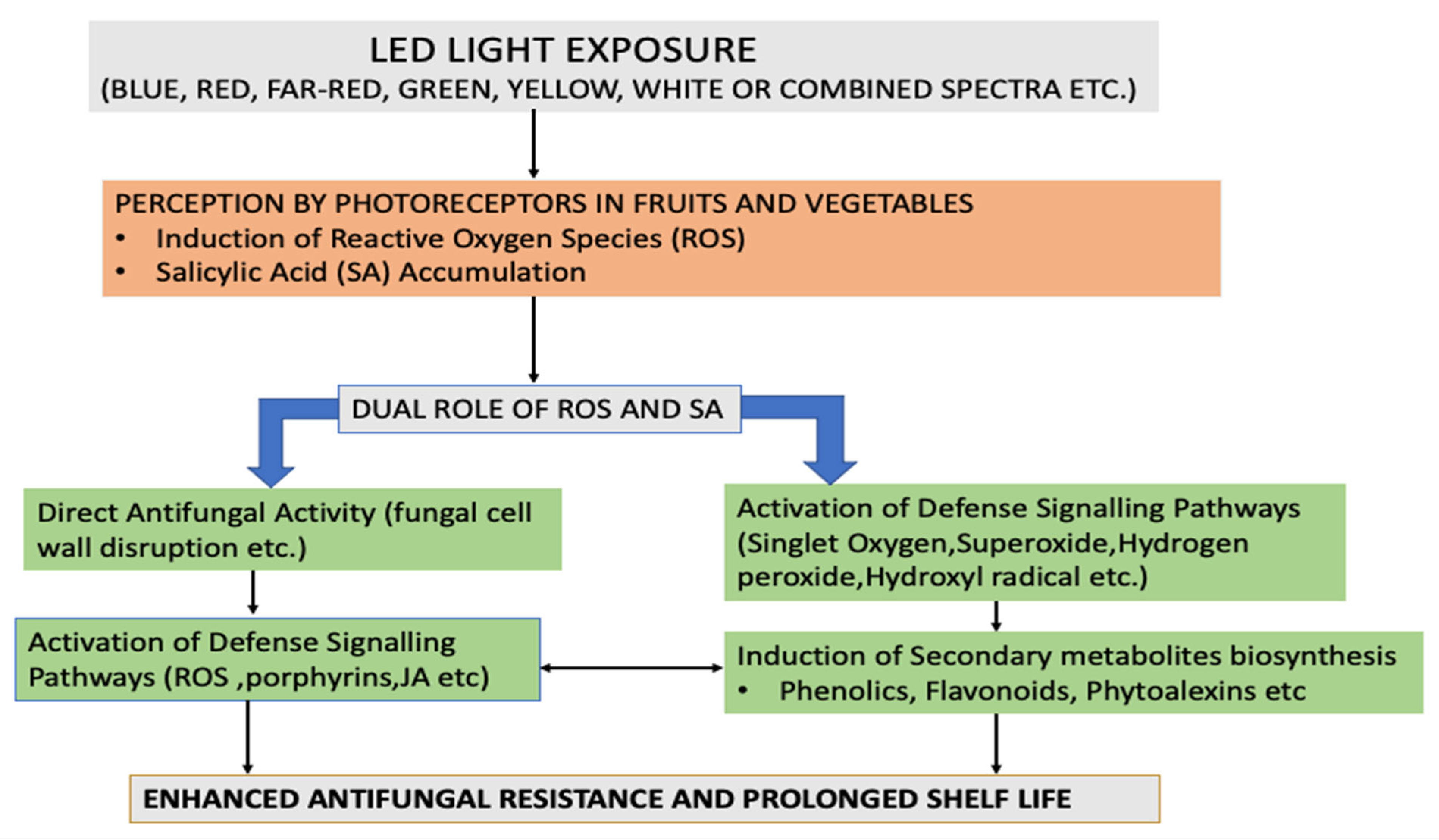
5. Mechanism of LED Light Action in Triggering Innate Biochemical Defense Response in Fruits and Vegetables
6. Gene Expression Pathways Triggered by LED Light Exposure
7. Phenolic Modulation for Improved Defense and Functional Value of Fruits and Vegetables with LED Light Treatments
8. Conclusions
9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devirgiliis, C.; Guberti, E.; Mistura, L.; Raffo, A. Effect of Fruit and Vegetable Consumption on Human Health: An Update of the Literature. Foods 2024, 13, 3149. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Nassarawa, S.S.; Abdelshafy, A.M.; Xu, Y.; Li, L.; Luo, Z. Effect of Light-Emitting Diodes (LEDs) on the Quality of Fruits and Vegetables During Postharvest Period: A Review. Food Bioprocess. Technol. 2021, 14, 388–414. [Google Scholar] [CrossRef]
- Temoso, O.; Myeki, L.W.; Motlhabane, C.; Asante, B.O.; Villano, R.A. The role of commercial agriculture in meeting sustainable development goals in South Africa: Evidence from municipal-level total factor productivity analysis. J. Clean. Prod. 2024, 463, 142723. [Google Scholar] [CrossRef]
- Zwane, S.; Ferrer, S.R.D. Competitiveness analysis of the South African avocado industry. Agrekon 2024, 63, 277–302. [Google Scholar] [CrossRef]
- Rovetto, E.; La Spada, F.; Aloi, F.; Riolo, M.; Pane, A.; Garbelotto, M.; Cacciola, S.O. Green solutions and new technologies for sustainable management of fungus and oomycete diseases in the citrus fruit supply chain. J. Plant Pathol. 2024, 106, 411–437. [Google Scholar] [CrossRef]
- Liguori, J.; Trübswasser, U.; Pradeilles, R.; Le Port, A.; Landais, E.; Talsma, E.F.; Lundy, M.; Béné, C.; Bricas, N.; Laar, A.; et al. How do food safety concerns affect consumer behaviors and diets in low- and middle-income countries? A systematic review. Glob. Food Secur. 2022, 32, 100606. [Google Scholar] [CrossRef]
- Bento de Carvalho, T.; Silva, B.N.; Tomé, E.; Teixeira, P. Preventing Fungal Spoilage from Raw terials to Final Product: Innovative Preservation Techniques for Fruit Fillings. Foods 2024, 13, 2669. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, X.; Gao, Q.-H.; Geng, C.; Duan, K. Colletotrichum species pathogenic to strawberry: Discovery history, global diversity, prevalence in China, and the host range of top two species. Phytopathol. Res. 2022, 4, 42. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, T.; Chen, K.; Du, M.; Chen, G.; Chen, X.; Wang, K.; Zalan, Z.; Takács, K.; Kan, J. Antifungal activity of volatile organic compounds produced by Pseudomonas fluorescens ZX and potential biocontrol of blue mold decay on postharvest citrus. Food Control 2021, 120, 107499. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; Rossi, C.; Grande-Tovar, C.D.; Chaves-López, C. Green Management of Postharvest Anthracnose Caused by Colletotrichum gloeosporioides. J. Fungi 2023, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Ghooshkhaneh, N.G.; Mollazade, K. Optical Techniques for Fungal Disease Detection in Citrus Fruit: A Review. Food Bioprocess. Technol. 2023, 16, 1668–1689. [Google Scholar] [CrossRef]
- Dwiastuti, M.E.; Soesanto, L.; Aji, T.G.; Devy, N.N.; Harddiyanto. Biological control strategy for postharvest diseases of citrus, apples, grapes and strawberries fruits and application in Indonesia. Egypt. J. Biol. Pest. Control 2021, 31, 141. [Google Scholar] [CrossRef]
- Zakaria, L. An Overview of Aspergillus Species Associated with Plant Diseases. Pathogens 2024, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.H.; Ali, A.; Siddiqui, Y. Major fungal postharvest diseases of papaya: Current and prospective diagnosis methods. Crop Prot. 2023, 174, 106399. [Google Scholar] [CrossRef]
- Zakaria, L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops—A review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Neugebauer, K.A.; Mattupalli, C.; Hu, M.; Oliver, J.E.; VanderWeide, J.; Lu, Y.; Sullivan, K.; Stockwell, V.O.; Oudemans, P.; Miles, T.D. Managing fruit rot diseases of Vaccinium corymbosum. Front. Plant Sci. 2024, 15, 1428769. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Gupta, A.; Prusty, M.R.; Kumar, M. Elicitation of Fruit Fungi Infection and Its Protective Response to Improve the Postharvest Quality of Fruits. Stresses 2023, 3, 231–255. [Google Scholar] [CrossRef]
- Wenneker, M.; Thomma, B.P.H.J. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Zaccaron, A.Z.; Stergiopoulos, L. The dynamics of fungal genome organization and its impact on host adaptation and antifungal resistance. J. Genet. Genom. 2025, 52, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.E.; Derbyshire, M.C. The Evolutionary and Molecular Features of Broad Host-Range Necrotrophy in Plant Pathogenic Fungi. Front. Plant Sci. 2020, 11, 591733. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Teuber, P.; Rouxel, T.; Mason, A.S.; Soyer, J.L. Breeding and management of major resistance genes to stem canker/blackleg in Brassica crops. Theor. Appl. Genet. 2024, 137, 192. [Google Scholar] [CrossRef] [PubMed]
- Talhinhas, P.; Baroncelli, R. Hosts of Colletotrichum. Mycosphere 2023, 14, 158–261. [Google Scholar] [CrossRef]
- Dowling, M.; Peres, N.; Villani, S.; Schnabel, G. Managing Colletotrichum on Fruit Crops: A “Complex” Challenge. Plant Dis. 2020, 104, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Ciofini, A.; Negrini, F.; Baroncelli, R.; Baraldi, E. Management of Post-Harvest Anthracnose: Current Approaches and Future Perspectives. Plants 2022, 11, 1856. [Google Scholar] [CrossRef] [PubMed]
- Munhuweyi, K.; Mpai, S.; Sivakumar, D. Extension of avocado fruit postharvest quality using non-chemical treatments. Agronomy 2020, 10, 212. [Google Scholar] [CrossRef]
- McLaughlin, M.S.; Roy, M.; Abbasi, P.A.; Carisse, O.; Yurgel, S.N.; Ali, S. Why Do We Need Alternative Methods for Fungal Disease Management in Plants? Plants 2023, 12, 3822. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, X.; Liu, B.; Battino, M.; Meng, X.; Zhang, F. Blue light inhibits gray mold infection by inducing disease resistance in cherry tomato. Postharvest Biol. Technol. 2024, 215, 113006. [Google Scholar] [CrossRef]
- Verde-Yáñez, L.; Vall-llaura, N.; Usall, J.; Teixidó, N.; Torres, R. Phenotypic plasticity of Monilinia spp. in response to light wavelengths: From in vitro development to virulence on nectarines. Int. J. Food Microbiol. 2022, 373, 109700. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.T.; Romero, P.; Ballester, A.R. Coordinated activation of the metabolic pathways induced by LED blue light in citrus fruit. Food Chem. 2021, 341, 128050. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; Ghate, V.; Zhou, W.; Yuk, H.-G. Developing an LED preservation technology to minimize strawberry quality deterioration during distribution. Food Chem. 2022, 366, 130566. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, J.; Zhong, J.; Deng, W.; Zhang, Z.; Wu, Y.; Zheng, Q. Antifungal efficacy of LEDs against major postharvest pathogens of litchi fruit in vitro and in vivo. Food Control 2023, 154, 110019. [Google Scholar] [CrossRef]
- Vashisht, P.; Sangeetha, K.; Ramesh, B.; Gowda, N.; Prasanna, A.; Singh, R.; Nisha, R.; Nickhil, C.; Charles, A.P.R.; Kenchanna, D.; et al. Harnessing light: The role of semiconductor technology in boosting phenolic compounds in fruit and vegetable. Crit. Rev. Food Sci. Nutr. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sena, S.; Kumari, S.; Kumar, V.; Husen, A. Light emitting diode (LED) lights for the improvement of plant performance and production: A comprehensive review. Curr. Res. Biotechnol. 2024, 7, 100184. [Google Scholar] [CrossRef]
- Filho, F.O.; de Oliveira Silva, E.; de Almeida Lopes, M.M.; Ribeiro, P.R.V.; Oster, A.H.; Guedes, J.A.C.; de Souza Zampieri, D.; do Nascimento Bordallo, P.; Zocolo, G.J. Effect of pulsed light on postharvest disease control-related metabolomic variation in melon (Cucumis melo) artificially inoculated with Fusarium pallidoroseum. PLoS ONE 2020, 15, e0220097. [Google Scholar] [CrossRef] [PubMed]
- Poonia, A.; Pandey, S.; Vasundhara. Application of light emitting diodes (LEDs) for food preservation, post-harvest losses and production of bioactive compounds: A review. Food Prod. Process. Nutr. 2022, 4, 8. [Google Scholar] [CrossRef]
- Prasad, A.; Du, L.; Zubair, M.; Subedi, S.; Ullah, A.; Roopesh, M.S. Applications of light-emitting diodes (LEDs) in food processing and water treatment. Food Eng. Rev. 2020, 12, 268–289. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, E.E.; Kulawik, P.; Szymkowiak, A.; Šimat, V. Application of UV-A and blue light emitting diodes within the range of 320–480 nm on quality and shelf-life extension of food products. Food Bioprod. Process. 2024, 148, 436–455. [Google Scholar] [CrossRef]
- Bi, X.; Xu, H.; Yang, C.; Zhang, H.; Li, W.; Su, W.; Zheng, M.; Lei, B. Investigating the influence of varied ratios of red and far-red light on lettuce (Lactuca sativa): Effects on growth, photosynthetic characteristics and chlorophyll fluorescence. Front. Plant Sci. 2024, 15, 1430241. [Google Scholar] [CrossRef] [PubMed]
- Neo, D.C.N.; Ong, M.M.X.; Lee, Y.Y.; Teo, J.E.; Ong, Q.; Tanoto, H.; Xu, J.; Ong, K.S.; Suresh, V. Shaping and Tuning Lighting Conditions in Controlled Environment Agriculture: A Review. ACS Agric. Sci. Technol. 2022, 2, 3–16. [Google Scholar] [CrossRef]
- Theparod, T.; Harnsoongnoen, S. Narrow-Band Light-Emitting Diodes (LEDs) Effects on Sunflower (Helianthus annuus) Sprouts with Remote Monitoring and Recording by Internet of Things Device. Sensors 2022, 22, 1503. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, T.; Ebong, A.; Raja, M.Y.A. A Review of Light-Emitting Diodes and Ultraviolet Light-Emitting Diodes and Their Applications. Photonics 2024, 11, 491. [Google Scholar] [CrossRef]
- Veloso, T.M.; de Souza, A.d.F.; Costa dos Santos, G. Effects of light-emitting diodes on cell biology. Front. Photonics 2022, 3, 101877. [Google Scholar] [CrossRef]
- Barceló-Muñoz, A.; Barceló-Muñoz, M.; Gago-Calderon, A. Effect of LED Lighting on Physical Environment and Microenvironment on In Vitro Plant Growth and Morphogenesis: The Need to Standardize Lighting Conditions and Their Description. Plants 2022, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Saadouni, I.; Douass, O.; Samoudi, B.; Araoud, Z.; Charrada, K.; Asselman, A.; Canale, L. Optimal Thermal Management Using the Taguchi Method for LED Lighting Squared Heat Sink, Including Statistical Approaches. Sustainability 2025, 17, 1811. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and opportunities of light-emitting diode (LED) as key to modulate antioxidant compounds in plants: A review. Antioxidants 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Plesken, C.; Pattar, P.; Reiss, B.; Noor, Z.N.; Zhang, L.; Klug, K.; Huettel, B.; Hahn, M. Genetic Diversity of Botrytis cinerea Revealed by Multilocus Sequencing, and Identification of B. cinerea Populations Showing Genetic Isolation and Distinct Host Adaptation. Front. Plant Sci. 2021, 12, 663027. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, T.B.; Muzhinji, N.; Halterman, D.; Louws, F.J. Genetic diversity and population structure of Alternaria species from tomato and potato in North Carolina and Wisconsin. Sci. Rep. 2021, 11, 17024. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Chen, T.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Molecular basis of pathogenesis of postharvest pathogenic Fungi and control strategy in fruits: Progress and prospect. Mol. Hortic. 2021, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, S.; Sheng, S.; Li, H. A Colletotrichum fructicola dual specificity phosphatase CfMsg5 is regulated by the CfAp1 transcription factor during oxidative stress and promotes virulence on Camellia oleifera. Virulence 2024, 15, 2413851. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Ning, N.; Wu, H.X.; Chen, X.L.; Li, Z.Q.; Liu, W.D. Glycosylphosphatidylinositol anchor biosynthesis pathway-related protein GPI7 is required for the vegetative growth and pathogenicity of Colletotrichum graminicola. Int. J. Mol. Sci. 2022, 23, 2985. [Google Scholar] [CrossRef] [PubMed]
- Mapuranga, J.; Zhang, N.; Zhang, L.; Chang, J.; Yang, W. Infection Strategies and Pathogenicity of Biotrophic Plant Fungal Pathogens. Front. Microbiol. 2022, 13, 799396. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Ruiz-Bedoya, T.; Breit-McNally, C.; Laflamme, B.; Desveaux, D.; Guttman, D.S. The ETS-ETI cycle: Evolutionary processes and metapopulation dynamics driving the diversification of pathogen effectors and host immune factors. Curr. Opin. Plant Biol. 2021, 62, 102011. [Google Scholar] [CrossRef] [PubMed]
- McCombe, C.L.; Greenwood, J.R.; Solomon, P.S.; Williams, S.J. Molecular plant immunity against biotrophic, hemibiotrophic, and necrotrophic fungi. Essays Biochem. 2022, 66, 581–593. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.D.; Crous, P.W.; Ades, P.K.; Hyde, K.D.; Taylor, P.W.J. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2017, 31, 155–168. [Google Scholar] [CrossRef]
- Arya, G.C.; Sutanni, S.; Ekaterina, M.; Asaph, A.; Hagai, C. The Plant Cuticle: An Ancient Guardian Barrier Set Against Long-Standing Rivals. Front. Plant Sci. 2021, 12, 663165. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, V.; Fadanavis, S.V.; Panwar, J. Revisiting the architecture, biosynthesis and functional aspects of the plant cuticle: There is more scope. Environ. Exp. Bot. 2021, 183, 104364. [Google Scholar] [CrossRef]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2025, 15, 1507628. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, T.; Liu, H. How plants coordinate their development in response to light and temperature signals. Plant Cell 2022, 34, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Rab, A.; Ahmad, N. Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert). J. Photochem. Photobiol. B Biol. 2016, 154, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Obajuluwa, A.O.; Babafemi, L.J.; Ndianefo, O.J.; Obajuluwa, T.M.; Lech, J.C.; Okiki, P.A. Red Light Therapy Attenuates Prolonged LED light Exposure-Associated Neuropathology and Mediates Circadian Clock Genes-Per1 and Bmal1 Expression in Rats’ Basal Ganglia. Alzheimer’s Dement. 2024, 20, e093626. [Google Scholar] [CrossRef]
- Yu, W.; Pei, R.; Zhang, Y.; Tu, Y.; He, B. Light regulation of secondary metabolism in fungi. J. Biol. Eng. 2023, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, M.; Sanz, C.; Zhang, W. Editorial: Light Regulation of Metabolic Networks in Microbes. Front. Microbiol. 2022, 13, 829106. [Google Scholar] [CrossRef] [PubMed]
- Tisch, D.; Schmoll, M. Light regulation of metabolic pathways in fungi. Appl. Microbiol. Biotechnol. 2010, 85, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Piao, J.; Li, Y.; Guan, H.; Hao, J.; Zhou, R. Transcriptome Analysis Reveals Candidate Genes for Light Regulation of Elsinochrome Biosynthesis in Elsinoë arachidis. Microorganisms 2024, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Rasiukevičiūtė, N.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Valiuškaitė, A. Different LED Light Wavelengths and Photosynthetic Photon Flux Density Effect on Colletotrichum acutatum Growth. Plants 2022, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, Y.; Zhang, Z.; Li, T.; Jiang, Y.; Gao, H.; Yun, Z. Effect of blue light on primary metabolite and volatile compound profiling in the peel of red pitaya. Postharvest Biol. Technol. 2020, 160, 111059. [Google Scholar] [CrossRef]
- Smith, B.J.; Rezazadeh, A.; Stafne, E.T.; Sakhanokho, H.F. Effect of Light-emitting Diodes, Ultraviolet-B, and Fluorescent Supplemental Greenhouse Lights on Strawberry Plant Growth and Response to Infection by the Anthracnose Pathogen Colletotrichum gloeosporioides. HortScience 2022, 57, 856–863. [Google Scholar] [CrossRef]
- Thiery, T.; Beney, L.; Grangeteau, C.; Dupont, S. Sporicidal efficiency of an ultra-high irradiance (UHI) near UV/visible light treatment: An example of application to infected mandarins. Food Control 2023, 147, 109568. [Google Scholar] [CrossRef]
- Yamaga, I.; Takahashi, T.; Ishii, K.; Kato, M.; Kobayashi, Y. Suppression of Blue Mold Symptom Development in Satsuma Mandarin Fruits Treated by Low-Intensity Blue LED Irradiation. Food Sci. Technol. Res. 2015, 21, 347–351. [Google Scholar] [CrossRef]
- Mpai, S.; Sivakumar, D. Stimulation of light-emitting diode treatment on defence system and changes in mesocarp metabolites of avocados cultivars (Hass and Fuerte) during simulated market shelf conditions. Agronomy 2020, 10, 1654. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, A.C.; Castro, V.S. Phenolic Compounds in Bacterial Inactivation: A Perspective from Brazil. Antibiotics 2023, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wei, L.; Miao, C.; Zhang, Q.; Yan, J.; Li, S.; Qin, W. Application of Exogenous Phenolic Compounds in Improving Postharvest Fruits Quality: Classification, Potential Biochemical Mechanisms and Synergistic Treatment. Food Rev. Int. 2023, 40, 1776–1795. [Google Scholar] [CrossRef]
- Meng, L.; Van Labeke, M.-C.; Höfte, M. Timing of light quality affects susceptibility to Botrytis cinerea in strawberry leaves. J. Photochem. Photobiol. B Biol. 2020, 211, 111988. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Piccolo, E.L.; Ceccanti, C.; Guidi, L.; Bernardi, R.; Araniti, F.; Cotrozzi, L.; Pellegrini, E.; Moriconi, M.; Giordani, T.; et al. Supplemental red LED light promotes plant productivity, “photomodulates” fruit quality and increases Botrytis cinerea tolerance in strawberry. Postharvest Biol. Technol. 2023, 198, 112253. [Google Scholar] [CrossRef]
- Ghate, V.; Yew, I.; Zhou, W.; Yuk, H.-G. Influence of temperature and relative humidity on the antifungal effect of 405 nm LEDs against Botrytis cinerea and Rhizopus stolonifer and their inactivation on strawberries and tomatoes. Int. J. Food Microbiol. 2021, 359, 109427. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Sun, J.; Tian, Z.; Cheng, Y.; Long, C. Effect of blue light treatments on Geotrichum citri-aurantii and the corresponding physiological mechanisms of citrus. Food Control 2023, 145, 109468. [Google Scholar] [CrossRef]
- Lena, A.; Marino, M.; Manzano, C.; Comuzzi, C.; Maifreni, M. An Overview of the Application of Blue Light-Emitting Diodes as a Non-Thermic Green Technology for Microbial Inactivation in the Food Sector. Food Eng. Rev. 2024, 16, 59–84. [Google Scholar] [CrossRef]
- Wu, J.; Chu, Z.; Ruan, Z.; Wang, X.; Dai, T.; Hu, X. Changes of Intracellular Porphyrin, Reactive Oxygen Species, and Fatty Acids Profiles During Inactivation of Methicillin-Resistant Staphylococcus aureus by Antimicrobial Blue Light. Front. Physiol. 2018, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Bumah, V.V.; Morrow, B.N.; Cortez, P.M.; Bowman, C.R.; Rojas, P.; Masson-Meyers, D.S.; Suprapto, J.; Tong, W.G.; Enwemeka, C.S. The importance of porphyrins in blue light suppression of Streptococcus agalactiae. J. Photochem. Photobiol. B Biol. 2020, 212, 111996. [Google Scholar] [CrossRef] [PubMed]
- Artes-Hernandez, F.; Castillejo, N.; Martinez-Zamora, L. UV and visible spectrum LED lighting as abiotic elicitors of bioactive compounds in sprouts, microgreens, and baby leaves—A comprehensive review including their mode of action. Foods 2022, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, S.; Chen, B.; Sangsoy, K.; Luengwilai, K.; Albornoz, K.; Beckles, D.M. Integrative analysis of the methylome and transcriptome of tomato fruit (Solanum lycopersicum L.) induced by postharvest handling. Hortic. Res. 2024, 11, uhae095. [Google Scholar] [CrossRef] [PubMed]
- Ganganelli, I.; Agostini, M.C.A.; Galatro, A.; Grozeff, G.E.G. Specific wavelength LED light pulses modify vitamin C and organic acids content in raspberry and blackberry fruit during postharvest. J. Hortic. Sci. Biotechnol. 2023, 98, 649–661. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light Emitting Diodes (LEDs) as Agricultural Lighting: Impact and Its Potential on Improving Physiology, Flowering, and Secondary Metabolites of Crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Ang, M.C.-Y.; Saju, J.M.; Porter, T.K.; Mohaideen, S.; Sarangapani, S.; Khong, D.T.; Wang, S.; Cui, J.; Loh, S.I.; Singh, G.P.; et al. Decoding early stress signaling waves in living plants using nanosensor multiplexing. Nat. Commun. 2024, 15, 2943. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Guang, Y.; Lin, J.; Zhou, Y.; Yu, T.; Ding, F.; Wang, Y.; Chen, J.; Zhou, Y.; et al. Red light induces salicylic acid accumulation by activating CaHY5 to enhance pepper resistance against Phytophthora capsici. Hortic. Res. 2023, 10, uhad213. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Ding, Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Gallé, Á.; Czékus, Z.; Tóth, L.; Galgóczy, L.; Poór, P. Pest and disease management by red light. Plant Cell Environ. 2021, 44, 3197–3210. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.B.; Sionov, E. Special Issue “Interplay between Fungal Pathogens and Harvested Crops and Fruits”. Microorganisms 2021, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. N. Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Shine, M.B.; Xiao, X.; Kachroo, P.; Kachroo, A. Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci. 2019, 279, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.M.; Zhu, S.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Gao, H.; Zhang, Z.; Li, T.; Qu, H.; Jiang, Y.; Yun, Z. Deciphering the Metabolic Pathways of Pitaya Peel after Postharvest Red Light Irradiation. Metabolites 2020, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Luo, D.; Ba, L. Advances in the Understanding of Postharvest Physiological Changes and the Storage and Preservation of Pitaya. Foods 2024, 13, 1307. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, M.R.; Roosta, H.R.; Kalaji, H.M. Enhancing strawberry resilience to saline, alkaline, and combined stresses with light spectra: Impacts on growth, enzymatic activity, nutrient uptake, and osmotic regulation. BMC Plant Biol. 2024, 24, 1038. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive compounds in lettuce: Highlighting the benefits to human health and impacts of preharvest and postharvest practices. Compr. Rev. Food Sci. Food Saf. 2021, 21, 4–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schouten, R.E.; Tikunov, Y.; Liu, X.; Visser, R.G.F.; Tan, F.; Bovy, A.; Marcelis, L.F.M. Blue light increases anthocyanin content and delays fruit ripening in purple pepper fruit. Postharvest Biol. Technol. 2022, 192, 112024. [Google Scholar] [CrossRef]
- Xu, Y.; You, C.; Xu, C.; Zhang, C.; Hu, X.; Li, X.; Ma, H.; Gong, J.; Sun, X. Red and blue light promote tomato fruit coloration through modulation of hormone homeostasis and pigment accumulation. Postharvest Biol. Technol. 2024, 207, 112588. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kwon, S.-J.; Eom, S.H. Red and blue light-specific metabolic changes in soybean seedlings. Front. Plant Sci. 2023, 14, 1128001. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.M.; Ha, T.W.; Park, C.H.; Kim, N.S.; Yeo, H.J.; Chun, S.W.; Kim, C.; Park, S.U. Effects of LED lights on expression of genes involved in phenylpropanoid biosynthesis and accumulation of phenylpropanoids in wheat sprout. Agronomy 2019, 9, 307. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.A.; Iqbal, Z.; Ge, Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications-A Review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef] [PubMed]
- Sbodio, A.O.; Mesquida-Pesci, S.D.; Yip, N.; Alvarez-Rojo, I.; Gutierrez-Baeza, E.; Tay, S.; Bello, P.; Wang, L.; Blanco-Ulate, B. Non-wounding contact-based Inoculation of fruits with fungal pathogens in postharvest. Plant. Methods 2024, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Omwange, K.A.; Saito, Y.; Kuramoto, M.; Kondo, N. Monitoring strawberry (Fragaria × ananassa) quality changes during storage using UV-excited fluorescence imaging. J. Food Eng. 2023, 353, 111553. [Google Scholar] [CrossRef]
- Santos, L.S.; Fernandes, C.C.; Santos, L.S.; Dias, A.L.B.; Souchie, E.L.; Miranda, M.L.D. Phenolic compounds and antifungal activity of ethyl acetate extract and methanolic extract from Capsicum chinense Jacq. ripe fruit. Braz. J. Biol. 2024, 84, e258084. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.; Franco, O.L. Pathogenesis-Related Proteins (PRs) with Enzyme Activity Activating Plant Defense Responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef] [PubMed]
- Osondu, H.A.A.; Akinola, S.A.; Shoko, T.; Sivakumar, D. Phenolic compounds suppress anthracnose decay by enhancing antifungal properties and biochemical defence responses in avocado fruit. J. Plant Pathol. 2022, 104, 711–720. [Google Scholar] [CrossRef]
- Jin, J.; Qi, L.; Shen, S.; Yang, S.; Yuan, H.; Wang, A. Violet LED light-activated MdHY5 positively regulates phenolic accumulation to inhibit fresh-cut apple fruit browning. Hortic. Res. 2025, 12, uhae276. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Kitaya, Y.; Seoka, M.; Kudaka, R.; Yahata, M.; Yamawaki, K.; Shimada, T.; Fujii, H.; Endo, T.; et al. Blue LED Light Induces Regreening in the Flavedo of Valencia Orange In Vitro. Food Chem. 2021, 335, 1276211. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qiu, K.; Gao, J.; Kuai, B. Molecular and Physiological Analyses of the Effects of Red and Blue LED Light Irradiation on Postharvest Senescence of Pak Choi. Postharvest Biol. Technol. 2020, 164, 111155. [Google Scholar] [CrossRef]
- Molina-Hernandez, J.B.; Grande-Tovar, C.D.; Neri, L.; Delgado-Ospina, J.; Rinaldi, M.; Cordero-Bueso, G.A.; Chaves-López, C. Enhancing postharvest food safety: The essential role of non-thermal technologies in combating fungal contamination and mycotoxins. Front. Microbiol. 2025, 16, 1543716. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Zheng, Y.; Watkins, C.B.; Ma, L.; Jiang, Y.; Chen, S.; Wang, H.; He, X.; Han, L.; Zhou, X.; et al. Multiomics analyses of the effects of LED white light on the ripening of apricot fruits. J. Adv. Res. 2024, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zuo, J.; Zhou, F.; Shi, J.; Xu, D.; Hu, W.; Jiang, A.; Liu, Y.; Wang, Q. Integrated analysis of transcriptomic and metabolomic data reveals the mechanism by which LED light irradiation extends the postharvest quality of pak-choi (Brassica campestris L. ssp. chinensis (L.) makino var. communis tsen et lee). Biomolecules 2020, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zheng, Y. Complex Signaling Networks Underlying Blue-Light-Mediated Floral Transition in Plants. Plants 2025, 14, 1533. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Yu, Z.; Zhang, Y.; Yang, H.; Wu, Y.; Ying, Z.; Qi, X.; Zhang, S. Influence of color bagging on anthocyanin and sugar in bayberry fruit. Sci. Hortic. 2025, 345, 114141. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, L.; Wang, Y.; Zhang, L.; Xu, S.; Wang, X.; He, W.; Zhang, Y.; Lin, Y.; Wang, Y.; et al. The Blue Light Signal Transduction Module FaCRY1-FaCOP1-FaHY5 Regulates Anthocyanin Accumulation in Cultivated Strawberry. Front. Plant Sci. 2023, 14, 1144273. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Galián, M.V.; Torres, M.; Sanchez-Pagán, J.D.; Navarro, P.J.; Weiss, J.; Egea-Cortines, M. Enhancement of strawberry production and fruit quality by blue and red LED lights in research and commercial greenhouses. South. Afr. J. Bot. 2021, 140, 269–275. [Google Scholar] [CrossRef]
- Nadalini, S.; Zucchi, P.; Andreotti, C. Effects of blue and red LED lights on soilless cultivated strawberry growth performances and fruit quality. Eur. J. Hortic. Sci. 2017, 82, 12–20. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; Pérez-López, A.J.; Acosta-Motos, J.R. Strategies to Delay Ethylene-Mediated Ripening in Climacteric Fruits: Implications for Shelf Life Extension and Postharvest Quality. Horticulturae 2024, 10, 840. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Pola, W.; Sugaya, S.; Photchanachai, S. Color Development and Phytochemical Changes in Mature Green Chili (Capsicum annuum L.) Exposed to Red and Blue Light-Emitting Diodes. J. Agric. Food Chem. 2020, 68, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, H.; Shi, J.; Duan, Y.; Wu, W.; Lyu, L.; Li, W. Effects of Different Light Wavelengths on Fruit Quality and Gene Expression of Anthocyanin Biosynthesis in Blueberry (Vaccinium corymbosum). Cells 2023, 12, 1225. [Google Scholar] [CrossRef] [PubMed]
- Kamol, P.; Nukool, W.; Pumjaroen, S.; Inthima, P.; Kongbangkerd, A.; Suphrom, N.; Buddhachat, K. Harnessing Postharvest Light Emitting Diode (LED) Technology of Centella asiatica (L.) Urb. to Improve Centelloside Content by Up-Regulating Gene Expressions in the Triterpenoid Pathway. Heliyon 2024, 10, e23639. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Zuo, J.; Zheng, Q.; Guo, L.; Gao, L.; Zhao, S.; Wang, Q.; Hu, W. Red LED Irradiation Maintains the Postharvest Quality of Broccoli by Elevating Antioxidant Enzyme Activity and Reducing the Expression of Senescence-Related Genes. Sci. Hortic. 2019, 251, 73–79. [Google Scholar] [CrossRef]
- Mi, H.; Zhou, X.; Yang, J.; Chen, J.; Liu, B. LED White Light Treatment Delays Postharvest Senescence of ‘Zaosu’ Pear Fruit with Inhibited Chlorophyll Degradation. Horticulturae 2024, 10, 32. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, S.; Chen, L.; Wang, X.-M.; Cheng, G.-X. Effect of Different Light Emitting Diode (LED) Light Quality Parameters on the Maturation Period and Development of Flowers in Hot Pepper (Capsicum annuum L.). Sci. Hortic. 2024, 338, 113673. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Artés–Hernández, F. Effect of postharvest visible spectrum LED lighting on quality and bioactive compounds of tomatoes during shelf life. LWT 2023, 174, 114420. [Google Scholar] [CrossRef]
- Kavipriya, S.; Beaulah, A.; Sundharaiya, K.; Anitha, T.; Sivakumar. The role of LED lighting in enhancing post-harvest fruit and vegetablequality. Plant Sci. Today, 2025; early access. [Google Scholar] [CrossRef]
- García-Villegas, A.; Rojas-García, A.; Villegas-Aguilar, M.d.C.; Fernández-Moreno, P.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.d.l.L.; Arráez-Román, D.; Segura-Carretero, A. Cosmeceutical Potential of Major Tropical and Subtropical Fruit By-Products for a Sustainable Revalorization. Antioxidants 2022, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as Natural Phenolic Compounds and Their Role in Therapeutics: An Overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, X.; Gu, X.; Deng, M.; Li, X.; Zhou, A.; Suo, M.; Gao, W.; Lin, Y.; Wang, Y.; et al. Light Quality and Sucrose-Regulated Detached Ripening of Strawberry with Possible Involvement of Abscisic Acid and Auxin Signaling. Int. J. Mol. Sci. 2023, 24, 5681. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Joel, J.M.; Anjitha, K.S.; Tóth, S.Z.; Puthur, J.T. Ascorbate, as a Versatile Regulator of Plant Development: Practical Implications for Enhancing Crop Productivity, Quality, and Postharvest Storage. Hortic. Plant J. 2024. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The Multiple Roles of Ascorbate in the Abiotic Stress Response of Plants: Antioxidant, Cofactor, and Regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Hameed, L.; Qadri, R.; Ejaz, S.; Aslam, A.; Khan, M.I.; Shen, J.; Zhang, J.; Nafees, M.; Ahmad, I.; et al. Postharvest Ascorbic Acid Application Maintained Physiological and Antioxidant Responses of Guava (Psidium guajava L.) at Ambient Storage. Food Sci. Technol. 2020, 41, 748–754. [Google Scholar] [CrossRef]
- Habibi, F.; Shahid, M.A.; Jacobson, T.; Voiniciuc, C.; Brecht, J.K.; Sarkhosh, A. Postharvest Quality and Biochemical Changes in Blood Orange Fruit Exposed to Various Non-Chilling Storage Temperatures. Horticulturae 2025, 11, 493. [Google Scholar] [CrossRef]
- Salama, A.-M.; Abdelsalam, M.A.; Rehan, M.; Elansary, M.; El-Shereif, A. Anthocyanin Accumulation and Its Corresponding Gene Expression, Total Phenol, Antioxidant Capacity, and Fruit Quality of ‘Crimson Seedless’ Grapevine (Vitis vinifera L.) in Response to Grafting and Pre-Harvest Applications. Horticulturae 2023, 9, 1001. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Dwivedi, S.L.; Dutt, S.; Singh, B.; Garg, M.; Ortiz, R. Anthocyanin-Rich Vegetables for Human Consumption—Focus on Potato, Sweetpotato and Tomato. Int. J. Mol. Sci. 2022, 23, 2634. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.H.; Jung, S. Effects of Light-Emitting Diode Irradiation on Growth Characteristics and Regulation of Porphyrin Biosynthesis in Rice Seedlings. Int. J. Mol. Sci. 2017, 18, 641. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Zare Mehrjerdi, M.; Aliniaeifard, S. Alteration of Bioactive Compounds in Two Varieties of Basil (Ocimum basilicum) Grown under Different Light Spectra. J. Essent. Oil Bear. Plants 2018, 21, 913–923. [Google Scholar] [CrossRef]
- Panjai, L.; Noga, G.; Hunsche, M.; Fiebig, A. Optimal Red Light Irradiation Time to Increase Health-Promoting Compounds in Tomato Fruit Postharvest. Sci. Hortic. 2019, 251, 189–196. [Google Scholar] [CrossRef]
- Song, Y.; Teakle, G.; Lillywhite, R. Unravelling Effects of Red/Far-Red Light on Nutritional Quality and the Role and Mechanism in Regulating Lycopene Synthesis in Postharvest Cherry Tomatoes. Food Chem. 2023, 414, 135690. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jin, N.; Jin, L.; Xiao, X.; Hu, L.; Liu, Z.; Wu, Y.; Xie, Y.; Zhu, W.; Lyu, J.; et al. Response of Tomato Fruit Quality Depends on Period of LED Supplementary Light. Front. Nutr. 2022, 9, 833723. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhang, W.; Li, J.; Huang, Z.; Tao, Y.; Hong, J.; Zhang, L.; Zhou, Y. Pre-Harvest Short-Term Continuous LED Lighting Improves the Nutritional Quality and Flavor of Hydroponic Purple-Leaf Lettuce. Sci. Hortic. 2024, 334, 113304. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Mesa, T.; Romero, A.; Bosch, S.M. Blue LED Light Improves the Antioxidant Composition of Valencia Oranges during Postharvest: Impact on Orange Juice, Pulp Portion and Peel Residue. Sci. Hortic. 2024, 338, 113679. [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Spinelli, M.; Fontanarosa, C.; Paradiso, R.; Amoresano, A.; Arena, C. Modulation of Antioxidant Compounds in Fruits of Citrus reticulata Blanco Using Postharvest LED Irradiation. Biology 2023, 12, 1029. [Google Scholar] [CrossRef] [PubMed]
- Ntagkas, N.; de Vos, R.C.H.; Woltering, E.J.; Nicole, C.C.S.; Labrie, C.; Marcelis, L.F.M. Modulation of the Tomato Fruit Metabolome by LED Light. Metabolites 2020, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Xu, H.; Zhou, W. Effect of LED Irradiation on the Ripening and Nutritional Quality of Postharvest Banana Fruit. J. Sci. Food Agric. 2018, 98, 5486–5493. [Google Scholar] [CrossRef] [PubMed]
- He, X.; He, R.; Li, Y.; Liu, K.; Tan, J.; Chen, Y.; Liu, X.; Liu, H. Effect of Ratios of Red and White Light on the Growth and Quality of Pak Choi. Agronomy 2022, 12, 2322. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Crepaldi, A.; Fernandez, J.A.; Orsini, F.; Artes-Hanandez, F. Postharvest LED Lighting: Effect of Red, Blue and Far Red on Quality of Minimally Processed Broccoli Sprouts. J. Sci. Food Agric. 2021, 101, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Pintos, F.M.; Hasperué, J.H.; Vicente, A.R.; Rodoni, L.M. Role of White Light Intensity and Photoperiod during Retail in Broccoli Shelf-Life. Postharvest Biol. Technol. 2020, 163, 111121. [Google Scholar] [CrossRef]
- Roosta, H.R.; Bikdeloo, M.; Ghorbanpour, M. The growth, nutrient uptake and fruit quality in four strawberry cultivars under different Spectra of LED supplemental light. BMC Plant Biol. 2024, 24, 179. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Orsini, F.; Castillejo, N.; Gómez, P.A.; Crepaldi, A.; Fernández, J.; Egea-Gilabert, C.; Artés-Hernández, F.; Gianquinto, G. Spectral composition from led lighting during storage affects nutraceuticals and safety attributes of fresh-cut red chard (Beta vulgaris) and rocket (Diplotaxis tenuifolia) leaves. Postharvest Biol. Technol. 2021, 175, 111500. [Google Scholar] [CrossRef]
| Fruit | Pathogen | LED Types and Treatment | Results/Observations | References |
|---|---|---|---|---|
| Strawberries | Colletotrichum acutatum | Blue [450 nm], green [530 nm], red [630 nm], far red [735 nm], and white [5700 k] at fluence rates of 50 μmol m−2 s−1, 100 μmol m−2 s−1, and 200 μmol m−2 s−1 | High inhibition of C. acutatum under green, red, and blue LED lights | [67] |
| Strawberries | Botrytis. cinerea | White [300–800 nm], blue [460 nm], and red [660 nm] at a fluence rate of 10 µmol m−2 s−1 | High inhibition of B. cinerea sporulation by red light, while blue, blue + red, and white lights inhibited sclerotia formation | [76] |
| Avocados | Colletotrichum. gloeosporioides | Blue and Red | Significantly lower anthracnose incidence [25%] in red LED light and [50%] in blue LED light | [72] |
| Late oranges | Penicillium digitatum | Blue [450 nm] at a fluence rate of 60 µmol m−2 s−1 for 2 days | Significantly reduced rot disease incidence [99–100%] and disease severity reduction [67–70%] in LED blue light-elicited fruits | [30] |
| Strawberries | Botrytis cinerea | Blue [450 nm], green [520 nm], and red [660 nm] at a fluence rate of 250 μmol m−2 s−1 for 5 h/day | Lower AUDPC values [46.9 ± 8.4] at 36 h post-inoculation with red light | [77] |
| Strawberries | Rhizopus stolonifer and Botrytis cinerea | Violet [405 nm] at a fluence rate of 2.68 ± 0.5 mW/cm for 12 days | R. stolonifer: 3.4 CFU/g reduction B. cinerea: 1.9 log CFU/g reduction | [31] |
| Litchi | Geotrichum candidum and Fusarium sp. | Violet [410–420 nm], blue [460–470 nm], and green [520–530 nm] at fluence rates of 32.0 ± 0.15 W/m2, 49.2 ± 0.40 W/m2, and 60.4 ± 0.56 W/m2, respectively, scheduled at 2, 4, 6, 8, and 10 h illumination times | Reduced the population of G. candidum and Fusarium sp. by more than 2 log CFU/g [∼99%] | [32] |
| Nectarines | Monilinia spp. [M. laxa, M. fructicola, M. fructigen] | Blue [460 nm], red [660 nm], far-red [740 nm], UV-A [370 nm], and broad-spectrum white [400–700 nm] | M. fructicola growth rate was significantly reduced under red light wavelength | [29] |
| Strawberries | Botrytis cinerea, Rhizopus stolonifer | LED light [405 nm] | 67% reduction of B. cinerea and 19% reduction of R. stolonifer population | [78] |
| Tomatoes | Botrytis cinerea, Rhizopus stolonifer | LED light [405 nm] | 79% reduction of B. cinerea and 70% R. stolonifer population | [78] |
| Satsuma mandarins | Penicillium italicum | Blue LED [465 nm] at a fluence rate of 80 µmol m−2 s−1 [high] and 8 µmol m−2 s−1 [low] | Significant reduction of blue mold sporulation at both high and low fluence intensities | [71] |
| Cherry tomatoes | Botrytis cinerea | Purple [405 nm], blue [470 nm], green [530 nm], or red [660 nm] light at an intensity of 40 W m−2 | Significant 17% and 12% gray mold incidence reduction in treated blue and green irradiated fruit compared to control | [28] |
| Citrus [Satsuma mandarins] | Geotrichum. citri-aurantii | Blue [455 nm] in varied photoperiods [negative control-darkness DD, 8 h light/16 h dark [8 LD], 16 h light/8 h dark [16 LD], and 24 h constant light [24 LL] at fluence rates of 50, 100, 150, and 200 μmol m−2 s −1 | Significant reduction of sour rot decay to 0%, 3.33% and 41.67% in Citrus unshiu, Citrus sinensis L. Osbeck, and Citrus reticulata Blanco cv. Ponkan, respectively, with blue light treatment at a fluence rate of 200 μmol m−2 s−1 | [79] |
| Study | Fruit Type | LED Type and Exposure | Genes Involved in Phenolics Production | Enrichment Pathways |
|---|---|---|---|---|
| [118] | Valencia oranges | Blue [470 nm] | Upregulation of chlorophyll biosynthesis genes [CitGGDR, CitCHLH, CitCHLM, CitCHL27, CitPORA, and CitCAO] | Chlorophyll synthesis, color enhancement, and increased reactive species scavenging capacity |
| [125] | Strawberries | Red [660 nm] and blue [450 nm] for 96 h | Blue light upregulated anthocyanin biosynthetic enzyme genes [FaC4H, FaCHS, FaF3H, FaDFR2, FaANS] and anthocyanin transport gene [FaRAP], while red light upregulated FaCHS, FaCHI1, and FaUFGT1 | Transcriptional chaperones of anthocyanin structural genes, signalling and synthesis, phenylpropanoid biosynthesis |
| [131] | Blueberries | Red [660 nm], blue [460 nm], yellow [590 nm], and white [380–800 nm] | Upregulation of anthocyanin biosynthesis genes—VcC4H, Vc4CL, VcCHI, VcLDOX, VcDFR, VcUFGT | Anthocyanin biosynthesis |
| [106] | Purple capsicum | Red [660 nm] | Upregulation of biosynthetic genes—CaMYB, CaCHS, CaDFR, CaANS, and CaUFGT | Anthocyanin biosynthesis |
| [121] | Ripe apricot fruits | White [450–460 nm] at a fluence of 5 μmol m−2 s−1 for 12 days | Upregulation of lipoxygenase [LOX 6], endoglucanase [CEL- CEL6, CEL9, CEL10, CEL11], peroxidase [POD—PODA2, POD4, POD31, POD42], while malate dehydrogenase [MDH], 1-aminocyclopropane-1-carboxylate synthase [ACS], 1-aminocyclopropane-1-carboxylate oxidase [ACO], and hexokinase [HK] genes were downregulated | Ascorbate and aldarate metabolism, ethylene and flavonoid biosynthesis |
| [122] | Pak choi | White [448–549 nm], red [600–700 nm], green [500–599 nm], blue [400–499 nm], And far-red [701–780 nm] at fluence rates of 10 μmol m−2 s−1, 22.2 μmol m−2 s−1, 43.3 μmol m−2 s−1, 25.5 μmol m−2 s−1, and 2.3 μmol m−2 s−1, respectively | Distinct upregulation of HemA-related and chlorophyll synthesis genes—chlI, chlE, and por of the total of 2733 upregulated genes | Selenocompound metabolism, monoterpenoid biosynthesis, indole alkaloid biosynthesis, C5-branched dibasic acid metabolism, monobactam biosynthesis, glycosphingolipid biosynthesis, porphyrin and chlorophyll metabolism, nitrogen metabolism, amino sugar and nucleotide sugar metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, circadian rhythm, carbon metabolism, ascorbate and aldarate metabolism, carbon fixation, amino acid biosynthesis, sulphur metabolism, glycosylate biosynthesis, glyoxylate and dicarboxylate metabolism, and photosynthesis |
| [119] | Pak choi | Red [65 μmol m−2 s−1], blue [50 μmol m−2 s−1], and red + blue [45 μmol m−2 s−1] | Upregulation of ethylene signaling gene [BraEIN3] | Ethylene biosynthesis |
| [132] | Pennywort | White LED [27 μmol m−2 s−1], dark, red LED [24.7 μmol m−2 s−1 at 650 nm], blue LED [29.5 μmol m−2 s−1 at 450 nm], and green LED [30.5 μmol m−2 s−1 at 530 nm] for three days. In this study, white, red, blue, and green LEDs at an intensity range of 25–30 μmol m−2 s−1 | Expression of triterpenoid biosynthesis genes, including C. asiatica squalene synthase [CaSQS], C. asiatica β-amyrin synthase [CabAS], and C. asiatica UDP gluclosyltransferase-73AH1 [CaUGT73AH1; CaUGT] | Triterpenoid biosynthesis |
| [133] | Broccoli | Red LED at a fluence rate of 50 μmol m−2 s−1 | Suppression of chlorophyll degrading genes, chlorophyllase II [BoCLH2], chlorophyllase III [BoCLH3], and pheophorbide a oxygenase [BoPAO] | Porphyrin and chlorophyll metabolism |
| [134] | Pears | White LED [1200 lumens] at a fluence rate of 151 μmol/m2 s | Decreased relative expression of chlorophyll degradation-related genes [PbASC4, PbACO1, PbETR1] and increased expression of ethylene receptor genes PbETR2, PbERS1, and PbERS2 | Chlorophyll metabolism and ethylene biosynthesis |
| [135] | Peppers [Capsicum annuum L.] | Red [700 nm], blue [465 nm], and full-spectrum white light, in different ratios at a fluence rate of 240 ± 30 µmol m−2 s−1 | Increased expression of ERF021, FAD2, ERF1B, ERF026, TM9SF7, ERF091, ERF012, TM9SF2, and ERF110 genes | Flavonoid [vitexin and cyanin] biosynthesis and ethylene-responsive factors |
| [28] | Cherry tomatoes | Purple [405 nm], blue [470 nm], green [530 nm], or red [660 nm] light at an intensity of 40 W m−2 | Upregulated the genes encoding six defense-related enzymes, namely LeCHI, LeGLU, LePAL, LeSOD, LePOD, and LeCAT | PAL and secondary metabolite biosynthesis |
| Fruit Type | LED Type and Exposure | Phenolic Compounds | Enhanced Fruit Qualities | Study Reference |
|---|---|---|---|---|
| Strawberries | Blue [460 nm], red [660 nm], and a combination of red and blue LEDs | Increased anthocyanin levels | Increased fruit mass, length, total chlorophyll, and total soluble solids. Improved potassium, iron, and magnesium levels | [162] |
| Valencia oranges | Blue [470 nm] | Increased lutein and decreased 9-cis-Violaxanthin | Two-times higher chlorophyll accumulation compared to non-treated orange fruits, enhanced color [regreening], and delayed senescence | [118] |
| Strawberries | Violet [405 nm] at 2.68 ± 0.5 mW/cm for 12 days | Significant increase in total phenolic content, anthocyanin content, and vitamin C content | Higher antioxidant levels and nutritive values | [31] |
| Red dragon fruit | Blue [450 nm] at 300 Lx for 2 h | Decreased ROS generation, reduced cell-wall monosaccharides, terpenes, and esters, and increased the activity of antioxidant enzymes | Improved fruit disease resistance and delayed fruit senescence by enhancing enzymatic antioxidant systems | [68] |
| Mandarin oranges | Broad-spectrum white [410–700 nm] at 150 ± 20 μmol photons m−2 s−1 for 7 days | Increased flavonoid quercetin rutinoside, chlorogenic acid, sinensetin, rutin, and naringin | Improved shelf life and nutritional quality of fruits | [156] |
| Dragon fruit | Red LED light [660 nm, 100 Lux for 24 h | Increased titratable acid [TA], total soluble solids [TSS], TSS-TA ratio, and DPPH scavenging potentials | Radically increased nutritive values and delayed fruit senescence | [102] |
| Strawberries | White, blue [450 nm], or red [730 nm] light during storage, stored for 16 h at a fluence of 100 μmolm−2 s−1 and 8 h of dark for 5 d | Modulation of anthocyanin and abscisic acid and regulation of auxin | Improved firmness, color, and taste | [140] |
| Strawberries | Red LED [660 nm] and blue [450 nm] for 96 h | Induced anthocyanin accumulation | Improved nutritive value, color, and taste | [125] |
| Pak choi | White [448 nm and 549 nm] at a fluence rate of 10 μmol m−2 s−1 | Induced higher vitamin C and chlorophyll content | Improved shelf life and color | [122] |
| Blueberries | Red [660 nm], blue [ 460 nm], yellow [590 nm], and white [380–800 nm] | Accumulation of anthocyanin, higher total phenol content, including ascorbic acid and glutathione | Increased fruit width, height, and weight of blueberry fruits, enhanced cell membrane integrity resulting in improved firmness | [131] |
| Valencia oranges | Blue LED light [462 nm, at a fluence of 6.8 μmol m−2 s−1] every 10 days for a period of 30 days. | Increased vitamin C and total phenol contents increased by 30% in the orange juice | Increased total antioxidant capacity of the peel | [155] |
| Tomatoes | Blue [450 nm], green [520 nm], white, red [638 nm], and far-red [740 nm] | Fast accumulation of carotenoids, flavonoids, tocopherols, and phenolic acids; faster color development | Improved nutritive value and color and postharvest physiology | [157] |
| Bananas | Blue [464–474 nm], green [515–525 nm], and red [617–627 nm] for 8 days at fluence rates of 3920, 4340, and 5200 µmol photon m−2 s−1, respectively | Enhanced ethylene production, ascorbic acid, and total phenols | Ripening promotion, enhanced peel color, firmness, and taste | [158] |
| Broccoli sprouts | White [610 nm], yellow [600 nm], and green [517 nm] at a fluence rate of 35 ± 2.5 μmol m−2 s−1 | Increased total phenolic content and total glucosinolate content under yellow and white LED lighting | Increased nutritive value and extended shelf life | [160] |
| Broccoli | Red [50 μmol m−2 s−1] for 5 days | Chlorophyll content modulation | Enhanced taste, higher sensory score, color, and weight | [133] |
| Red chard [Beta vulgaris] | Red [660 nm], green [ 517 nm], yellow [600 nm], white [610 nm], blue [465 nm] or far-red [730 nm] at a fluence rate of 35 ± 2.5 μmol m−2 | Modulation of total phenol content and enhanced antioxidant capacity | Increased nutritive value and reduced microbiological load | [163] |
| Broccoli | White LED lights at 3.6 W m−2, 7.5 W m−2, 19.0 W m−2 intensities | Carotenoid biosynthesis, reduction of soluble sugars, and ascorbic acid degradation | Enhanced shelf life and total antioxidant profile | [161] |
| Citrus | Blue [455 nm] in varied photoperiods [negative control—darkness DD, 8 h light/16 h dark [8 LD], 16 h light/8 h dark [16 LD], and 24 h constant light [24 LL] at fluence rates of 50, 100, 150, and 200 μmol m−2 s −1 | Carotenoid biosynthesis, titratable acidity, and total soluble solids [at 50 μmol m−2 s−1] | Improved firmness, color, and sensory properties | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obajuluwa, A.O.; Sivakumar, D. Light-Emitting Diode [LED]-Driven Mechanisms for Postharvest Decay Control and Functional Quality Improvement in Fruits and Vegetables. Foods 2025, 14, 2924. https://doi.org/10.3390/foods14172924
Obajuluwa AO, Sivakumar D. Light-Emitting Diode [LED]-Driven Mechanisms for Postharvest Decay Control and Functional Quality Improvement in Fruits and Vegetables. Foods. 2025; 14(17):2924. https://doi.org/10.3390/foods14172924
Chicago/Turabian StyleObajuluwa, Adejoke O., and Dharini Sivakumar. 2025. "Light-Emitting Diode [LED]-Driven Mechanisms for Postharvest Decay Control and Functional Quality Improvement in Fruits and Vegetables" Foods 14, no. 17: 2924. https://doi.org/10.3390/foods14172924
APA StyleObajuluwa, A. O., & Sivakumar, D. (2025). Light-Emitting Diode [LED]-Driven Mechanisms for Postharvest Decay Control and Functional Quality Improvement in Fruits and Vegetables. Foods, 14(17), 2924. https://doi.org/10.3390/foods14172924







