Micronization Combined Ultrasound-Assisted Extraction Enhances the Sustainability of Polyphenols from Pineapple and Lemon Peels Utilizing Acidified Ethanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Pineapple and Lemon Peel Micropowder
2.2. Chemicals
2.3. Screening of Extraction Conditions for Micropowder
2.4. Physical Properties of Micropowder
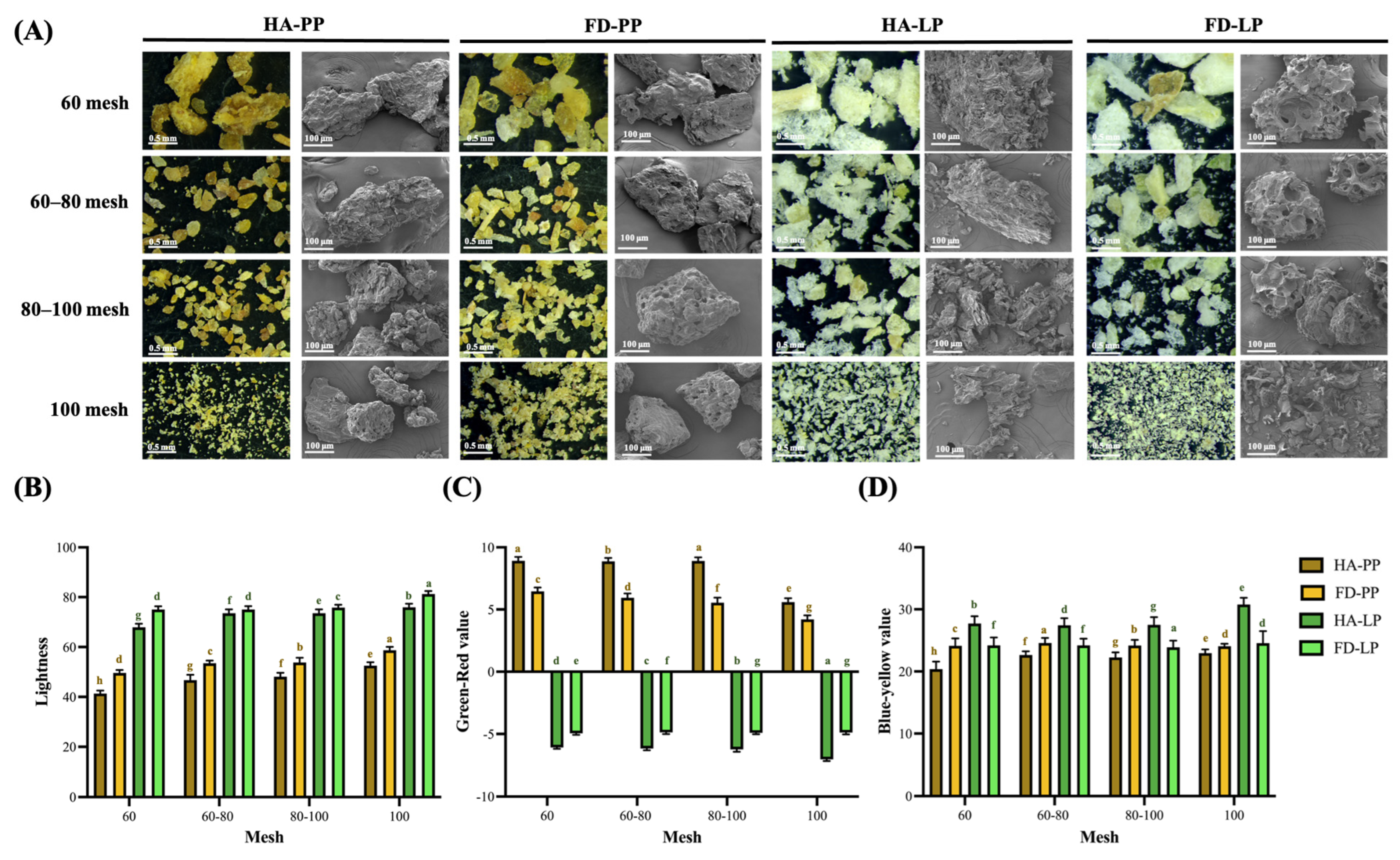
2.4.1. Imaging of Appearance
2.4.2. Color Analysis
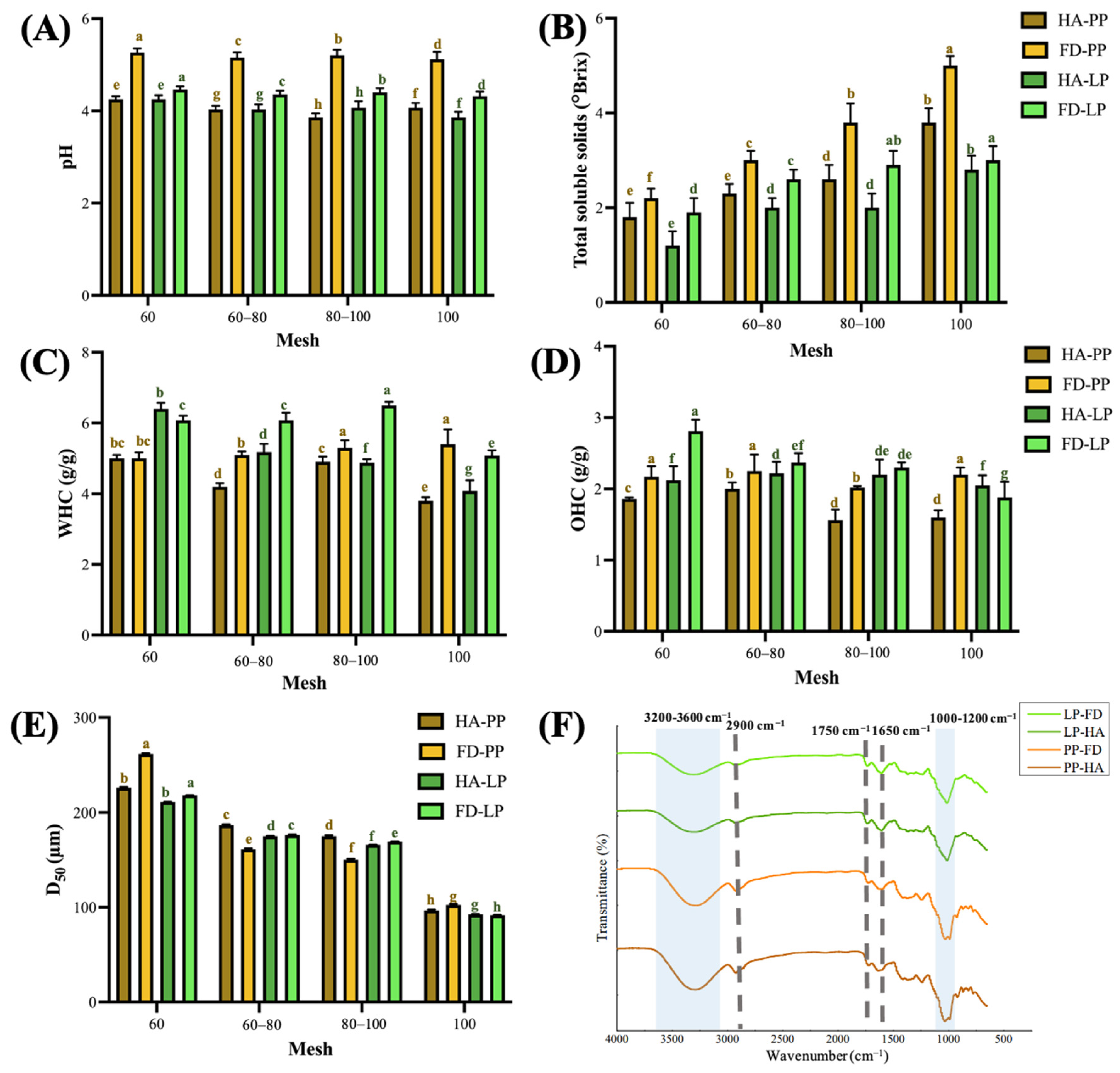
2.5. Determination of Physicochemical Properties of Micropowder
2.5.1. pH and Total Soluble Solids
2.5.2. Water-Holding Capacity (WHC) and Oil-Holding Capacity (OHC)
2.5.3. Particle Size
2.5.4. Fourier-Transform Infrared Spectroscopy Analysis
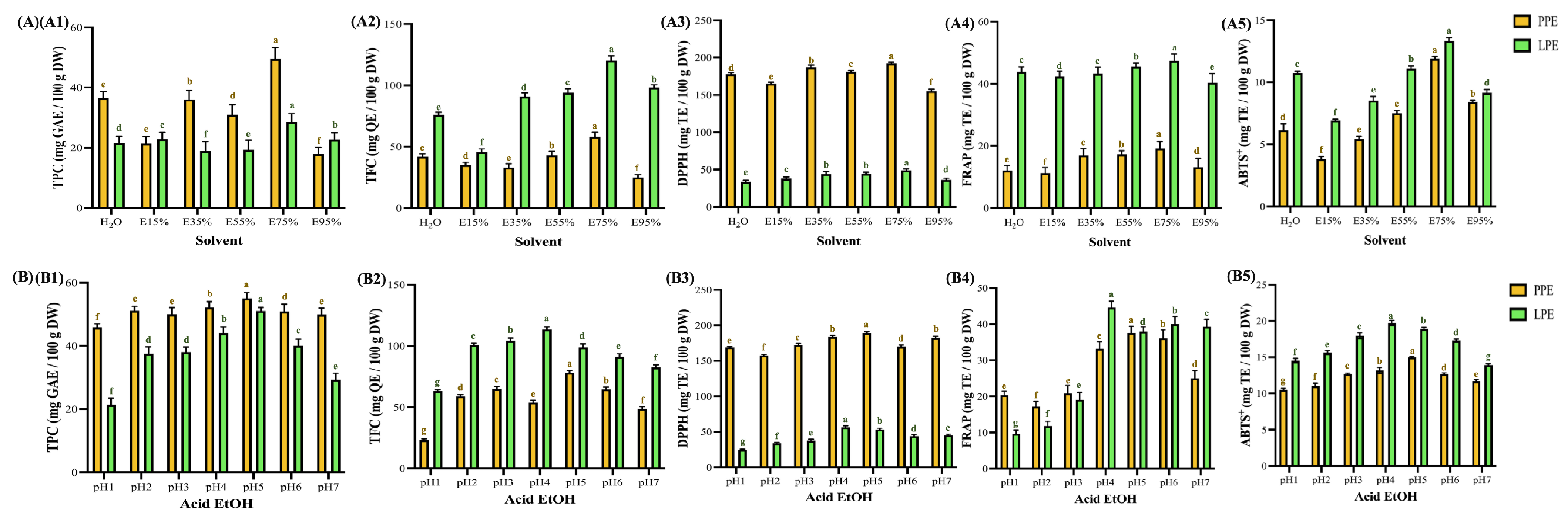
2.6. Antioxidant Characterization Evaluation
2.6.1. Total Polyphenols
2.6.2. Total Flavonoids
2.6.3. 2,2-Diphenyl-1-picrylhydrazyl Radical-Scavenging Activity
2.6.4. Ferric Ion Reducing Power
2.6.5. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic Acid) Free Radical Scavenging
2.7. Functional Component Assessment of Pineapple Peel (PPE) and Lemon Peel (LPE)
2.7.1. Phenolic Acid Analysis
2.7.2. Polyphenols Analysis
2.8. Contour Plot Visualization of Polyphenolic Compounds and Antioxidant Activities
2.9. Statistical Analysis
3. Results and Discussion
3.1. Appearance of PP and LP
3.2. Physicochemical Properties of PPP and LPP
3.3. Antioxidant Activities Under Different Extraction Parameters
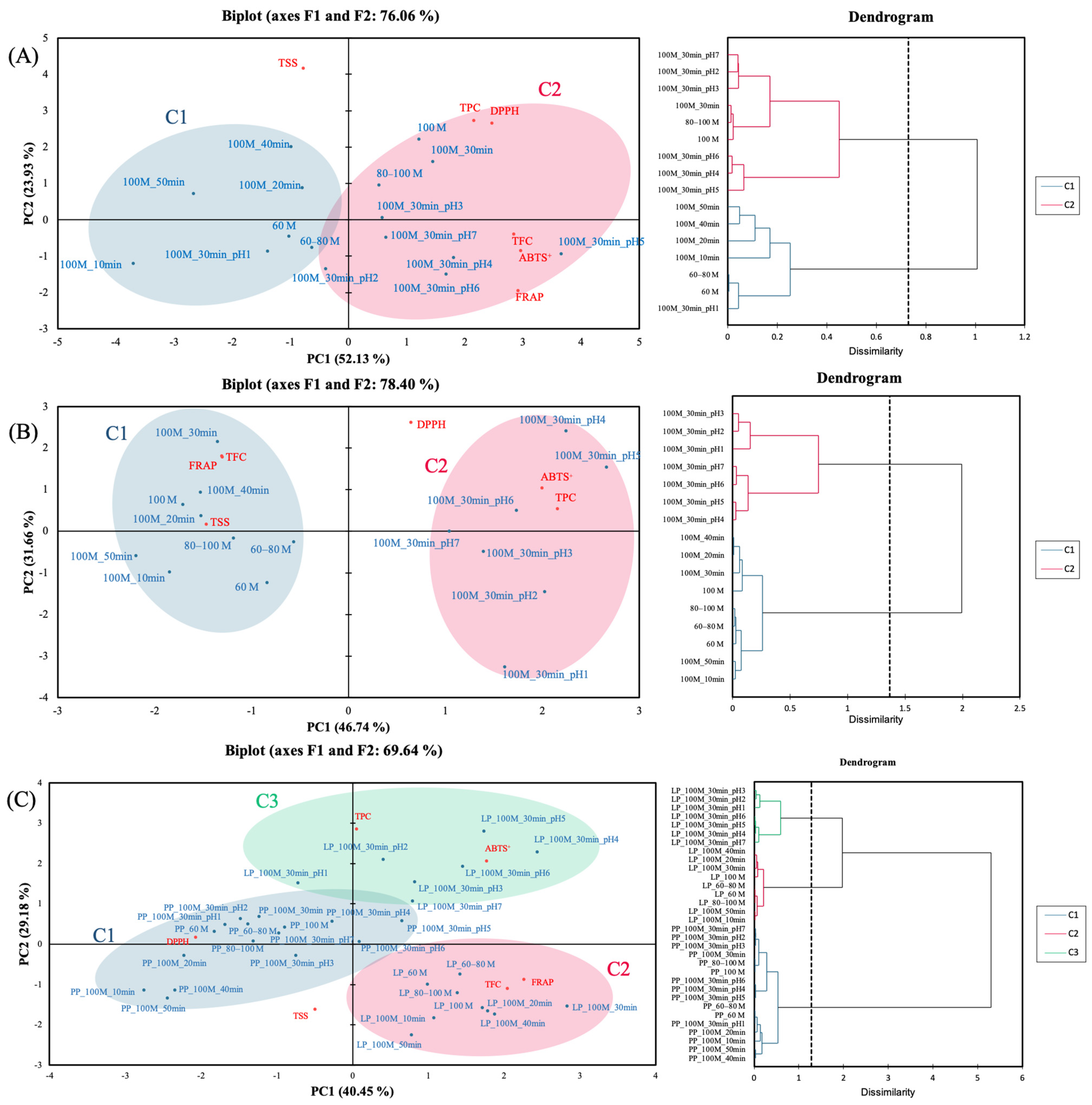
3.4. Principal Component Analysis
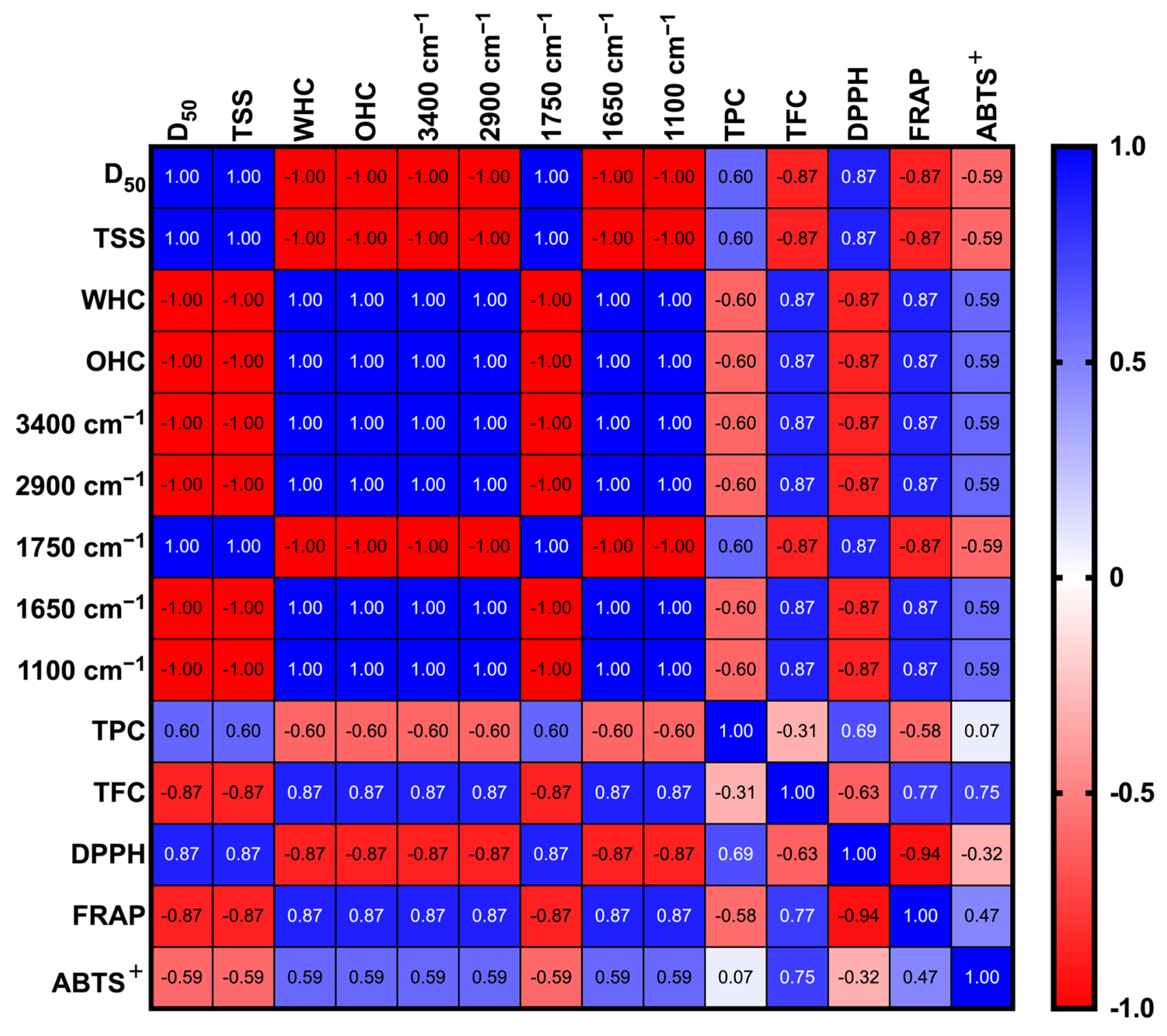
3.5. Pearson Correlation Analysis
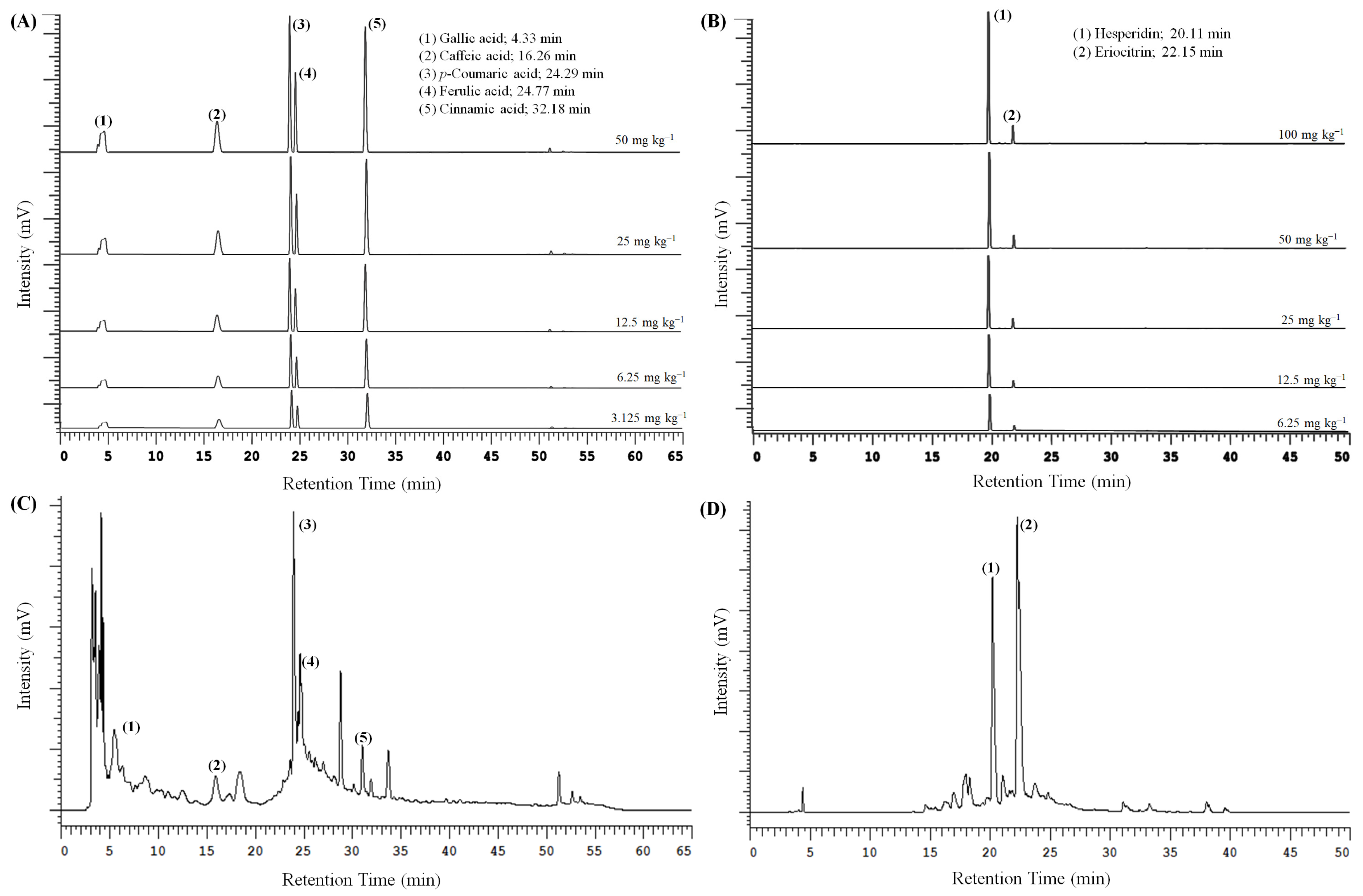
3.6. Bioactive Composition of Pineapple and Lemon Peel Extracts
| Compound | Source | Demonstrated Antioxidant Activity | Study |
|---|---|---|---|
| Cinnamic acid | Cinnamon, other plants | Scavenges free radicals and reactive oxygen species; contributes to oxidative stress reduction. | [63] |
| Gallic acid | Berries, tea, other plants | Strong antioxidant activity; increases total antioxidant capacity; inhibits lipid peroxidation. | [64] |
| Caffeic acid | Coffee, fruits, vegetables | Reduces oxidative stress in brain tissue, inhibits ROS accumulation, protects neurons from Aβ-induced damage. | [65] |
| p-Coumaric acid | Fruits, vegetables, cereals | Decreases lipid peroxidation, increases antioxidant enzyme activity (SOD, CAT), reduces oxidative stress in the liver and plasma. | [66] |
| Ferulic acid | Whole grains (wheat, rice bran), corn | Strong antioxidant; inhibits lipid peroxidation; stabilizes cell membranes; protects from UV-induced oxidative stress. | [67] |
| Hesperidin | Citrus fruits (oranges, lemons) | Reduces inflammation; scavenges ROS; protects tissues | [68] |
| Eriocitrin | Lemon peel, citrus fruits | Protects mitochondria; reduces oxidative stress | [69] |
3.7. Contour Plot Visualization
3.8. Study Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soni, S.; Noor, U.; Gupta, E. Ananas comosus peel waste, a novel substrate of therapeutic potential: Evidences and prospects. Indian J. Nat. Prod. Resour. 2022, 13, 129–143. [Google Scholar] [CrossRef]
- M’hiri, N.; Ghali, R.; Ben Nasr, I.; Boudhrioua, N. Effect of different drying processes on functional properties of industrial lemon byproduct. Process Saf. Environ. Protect. 2018, 116, 450–460. [Google Scholar] [CrossRef]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple peel extract incorporated poly(vinyl alcohol)-corn starch film for active food packaging: Preparation, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef]
- Zhuo, L.C.; Abang Zamhari, D.N.J.b.; Yong, A.S.K.; Shapawi, R.; Lin, Y.H. Effects of fermented lemon peel supplementation in diet on growth, immune responses, and intestinal morphology of Asian sea bass, Lates calcarifer. Aquacult. Rep. 2021, 21, 100801. [Google Scholar] [CrossRef]
- Li, T.; Peiyi, S.; Wei, L.; Chengmei, L.; Ruihong, L.; Na, Y.; Chen, J. Major Polyphenolics in Pineapple Peels and their Antioxidant Interactions. Int. J. Food Prop. 2014, 17, 1805–1817. [Google Scholar] [CrossRef]
- Chuang, K.C.; Chiang, Y.C.; Chang, Y.J.; Lee, Y.C.; Chiang, P.Y. Evaluation of Antioxidant and Anti-Glycemic Characteristics of Aged Lemon Peel Induced by Three Thermal Browning Models: Hot-Air Drying, High Temperature and Humidity, and Steam-Drying Cycle. Foods 2024, 13, 3053. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, S.; Wu, X.; He, Y.; Shen, H.; Tang, S.; Zhu, F.; Luo, Y. Phytochemical Profile and Antioxidant Activity of the Tuber and Peel of Pachyrhizus erosus. Antioxidants 2025, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.A.; Wahab, R.A.; Xine, T.L.S.; Hamid, M.A. Ultrasound-assisted extraction of polyphenols from pineapple skin. AIP Conf. Proc. 2019, 2155, 02002-1–02002-5. [Google Scholar] [CrossRef]
- Durmus, N.; Kilic-Akyilmaz, M. Bioactivity of non-extractable phenolics from lemon peel obtained by enzyme and ultrasound assisted extractions. Food Biosci. 2023, 53, 102571. [Google Scholar] [CrossRef]
- Chew, K.K.; Khoo, M.Z.; Ng, S.Y.; Thoo, Y.Y.; Mustapha, W.A.W.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427–1435. [Google Scholar]
- Zampar, G.G.; Zampar, I.C.; Beserra da Silva de Souza, S.; da Silva, C.; Bolanho Barros, B.C. Effect of solvent mixtures on the ultrasound-assisted extraction of compounds from pineapple by-product. Food Biosci. 2022, 50, 102098. [Google Scholar] [CrossRef]
- Chen, P.C.; Lin, C.; Chen, M.H.; Chiang, P.Y. The micronization process for improving the dietary value of okara (soybean residue) by planetary ball milling. LWT 2020, 132, 109848. [Google Scholar] [CrossRef]
- Chupin, L.; Maunu, S.L.; Reynaud, S.; Pizzi, A.; Charrier, B.; Charrier-El Bouhtoury, F. Microwave assisted extraction of maritime pine (Pinus pinaster) bark: Impact of particle size and characterization. Ind. Crop. Prod. 2015, 65, 142–149. [Google Scholar] [CrossRef]
- Ruenroengklin, N.; Zhong, J.; Duan, X.; Yang, B.; Li, J.; Jiang, Y. Effects of Various Temperatures and pH Values on the Extraction Yield of Phenolics from Litchi Fruit Pericarp Tissue and the Antioxidant Activity of the Extracted Anthocyanins. Int. J. Mol. Sci. 2008, 9, 1333–1341. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez-Salazar, J.A.; Jaime-Patlán, M.; Sosa-Morales, M.E.; Lorenzo, J.M. Plant Extracts Obtained with Green Solvents as Natural Antioxidants in Fresh Meat Products. Antioxidants 2021, 10, 181. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. The influence of immersion and contact ultrasound treatment on selected properties of the apple tissue. Appl. Acoust. 2016, 103, 136–142. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Astráin-Redín, L.; Alejandre, M.; Raso, J.; Cebrián, G.; Álvarez, I. Direct contact ultrasound in food processing: Impact on food quality. Front. Nutr. 2021, 8, 633070. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chiang, P.Y. Accentuation of the browning characteristics and functional properties of aged tomatoes (Solanum lycopersicum cv.). Food Chem. X 2024, 22, 101499. [Google Scholar] [CrossRef]
- Hsu, T.Y.; Yang, K.M.; Chiang, Y.C.; Lin, L.Y.; Chiang, P.Y. The browning properties, antioxidant activity, and α-glucosidase inhibitory improvement of aged oranges (Citrus sinensis). Foods 2024, 13, 1093. [Google Scholar] [CrossRef]
- Hong, H.Y.; Chiang, P.Y. Effects of Drying and Micronization Treatment on Quality of Okara. Taiwan. J. Agric. Chem. Food Sci. 2022, 60, 73–84. [Google Scholar] [CrossRef]
- Pei, W.; Hu, C.; Sun, X.; Yang, X.; Zhang, X.; Liu, Y.; Chen, Y.; Yu, Q. Functional redundancy as an indicator for evaluating functional diversity of macrobenthos under the mussel raft farm near Gouqi Island. Aquaculture 2024, 578, 740024. [Google Scholar] [CrossRef]
- Das, S.; Deb, T.; Mottola, F.; Madhu, N.R.; Revanaiah, Y.; Rosas, I.M.; Ray, S.D.; Roychoudhury, S. Phytochemical Screening, Pharmacognostic Characterization, Antioxidant Activity, and Hepatoprotective Effects of Abroma augustum (L.) Lf on Human Hepatocellular Carcinoma (HepG2) Cells and Goat Liver Homogenate. Antioxidants 2025, 14, 472. [Google Scholar] [CrossRef]
- Hernández-Montesinos, I.Y.; Carreón-Delgado, D.F.; Lazo-Zamalloa, O.; Tapia-López, L.; Rosas-Morales, M.; Ochoa-Velasco, C.E.; Hernández-Carranza, P.; Cruz-Narváez, Y.; Ramírez-López, C. Exploring Agro-Industrial By-Products: Phenolic Content, Antioxidant Capacity, and Phytochemical Profiling via FI-ESI-FTICR-MS Untargeted Analysis. Antioxidants 2024, 13, 925. [Google Scholar] [CrossRef]
- Bavaro, A.R.; De Bellis, P.; Linsalata, V.; Rucci, S.; Predieri, S.; Cianciabella, M.; Tamburino, R.; Cardinali, A. Valorization of Artichoke Bracts in Pasta Enrichment: Impact on Nutritional, Technological, Antioxidant, and Sensorial Properties. Antioxidants 2025, 14, 475. [Google Scholar] [CrossRef]
- Lubaina, A.S.; Renjith, P.R.; Roshni, A.S.; Thompson, J. Identification and quantification of polyphenols from pineapple peel by high performance liquid chromatography analysis. Adv. Zool. Bot. 2020, 8, 431–438. [Google Scholar] [CrossRef]
- Kumar, R.; Flint-Garcia, S.; Salazar Vidal, M.N.; Channaiah, L.; Vardhanabhuti, B.; Sommer, S.; Wan, C.; Somavat, P. Optimization of Polyphenol Extraction from Purple Corn Pericarp Using Glycerol/Lactic Acid-Based Deep Eutectic Solvent in Combination with Ultrasound-Assisted Extraction. Antioxidants 2025, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xing, B.; Yi, H.; Li, Y.; Zheng, B.; Wang, Y.; Shao, Q. Effects of different drying methods on appearance, microstructure, bioactive compounds and aroma compounds of saffron (Crocus sativus L.). LWT-Food Sci. Technol. 2020, 120, 108913. [Google Scholar] [CrossRef]
- Karunasena, C.; Hesami, P.; Senadeera, W.; Gu, Y.; Brown, R.; Oloyede, A. Scanning Electron Microscopic Study of Microstructure of Gala Apples During Hot Air Drying. Dry. Technol. 2014, 32, 455–468. [Google Scholar] [CrossRef]
- Zhang, J.; Mei, Z.; Huang, X.; Ding, Y.; Liang, Y.; Mei, Y. Inhibition of Maillard reaction in production of low-molecular-weight chitosan by enzymatic hydrolysis. Carbohydr. Polym. 2020, 236, 116059. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Ni, K.; Wu, T.; Luo, X.; Liang, B.; Zhang, M. Reduction of particle size based on superfine grinding: Effects on structure, rheological and gelling properties of whey protein concentrate. J. Food Eng. 2016, 186, 69–76. [Google Scholar] [CrossRef]
- Guclu, G.; Polat, S.; Kelebek, H.; Capanoglu, E.; Selli, S. Elucidation of the impact of four different drying methods on the phenolics, volatiles, and color properties of the peels of four types of citrus fruits. J. Sci. Food Agric. 2022, 102, 6036–6046. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.; Liu, C.; Al-Rejaie, S.; Elmasry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Yi, J.Y.; Lyu, J.; Bi, J.F.; Zhou, L.Y.; Zhou, M. Hot air drying and freeze drying pre-treatments coupled to explosion puffing drying in terms of quality attributes of mango, pitaya, and papaya fruit chips. J. Food Process Preserv. 2017, 41, e13300. [Google Scholar] [CrossRef]
- Zain, N.; Ghani, M.; Kasim, Z.; Hashim, H. Effects of Different Drying Methods on the Functional Properties and Physicochemical Characteristics of Chia Mucilage Powder (Salvia hispanica L.). Sains Malays. 2021, 50, 3603–3615. [Google Scholar] [CrossRef]
- Borchani, C.; Besbes, S.; Masmoudi, M.; Bouaziz, M.A.; Blecker, C.; Attia, H. Influence of Oven-Drying Temperature on Physicochemical and Functional Properties of Date Fibre Concentrates. Food Bioprocess Technol. 2012, 5, 1541–1551. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Huang, Y.; Huang, H. Utilization of pineapple peel for production of nanocellulose and film application. Cellulose 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Mandavgane, S.; Pathak, P.; Kulkarni, B. Fruit Peel Waste:Characterization and its Potential Uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Santos, R.M.d.; Flauzino Neto, W.P.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crop. Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Haafiz, M.K.M.; Hassan, A.; Zakaria, Z.; Inuwa, I.M. Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr. Polym. 2014, 103, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ernawita; Wahyuono, R.A.; Hesse, J.; Hipler, U.C.; Elsner, P.; Böhm, V. In Vitro Lipophilic Antioxidant Capacity, Antidiabetic and Antibacterial Activity of Citrus Fruits Extracts from Aceh, Indonesia. Antioxidants 2017, 6, 11. [Google Scholar] [CrossRef]
- Bhagia, S.; Ďurkovič, J.; Lagaňa, R.; Kardošová, M.; Kačík, F.; Cernescu, A.; Schäfer, P.; Yoo, C.G.; Ragauskas, A.J. Nanoscale FTIR and Mechanical Mapping of Plant Cell Walls for Understanding Biomass Deconstruction. ACS Sustain. Chem. Eng. 2022, 10, 3016–3026. [Google Scholar] [CrossRef]
- Hayat, K.; Zhang, X.; Chen, H.; Xia, S.; Jia, C.; Zhong, F. Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Sep. Purif. Technol. 2010, 73, 371–376. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef]
- Asem, N.; Adilah, A.G.N.; Hussaini, A.H.N.; Omar, E.A. Correlation between total phenolic and flavonoid contents with antioxidant activity of Malaysian stingless bee propolis extract. J. Apic. Res. 2020, 59, 437–442. [Google Scholar] [CrossRef]
- Yang, S.X.; Liu, B.; Tang, M.; Yang, J.; Kuang, Y.; Zhang, M.Z.; Zhang, C.Y.; Wang, C.Y.; Qin, J.C.; Guo, L.P.; et al. Extraction of flavonoids from Cyclocarya paliurus (Juglandaceae) leaves using ethanol/salt aqueous two-phase system coupled with ultrasonic. J. Food Process Preserv. 2020, 44, e14469. [Google Scholar] [CrossRef]
- Romdhane, M.; Gourdon, C. Investigation in solid–liquid extraction: Influence of ultrasound. Chem. Eng. J. 2002, 87, 11–19. [Google Scholar] [CrossRef]
- Lai, Y.J.; Chiang, Y.C.; Jhan, Y.S.; Song, T.Y.; Cheng, M.C. Extraction Effects on Roselle Functionalities: Antioxidant, Antiglycation, and Antibacterial Capacities. Foods. 2024, 13, 2172. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, F. Ultrasound-assisted extraction of rutin and quercetin from Euonymus alatus (Thunb.) Sieb. Ultrason. Sonochem. 2008, 15, 308–313. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Li, D.; Wang, L.J.; Ozkan, N.; Chen, X.D.; Mao, Z.H.; Yang, H.Z. Optimization of ethanol–water extraction of lignans from flaxseed. Sep. Purif. Technol. 2007, 57, 17–24. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, C.; Chen, M.H.; Chiang, P.Y. Stability and Quality of Anthocyanin in Purple Sweet Potato Extracts. Foods 2019, 8, 393. [Google Scholar] [CrossRef]
- Costa, V.C.; Nascimento Guedes, W.; de Santana Santos, A.; Nascimento, M. Multivariate Optimization for the Development of a Fast and Simple Ultrasound-Assisted Extraction Procedure for Multielemental Determination in Tea Leaves by Inductively Coupled Plasma Optical Emission Spectrometry (ICP OES). Food Anal. Meth. 2018, 11, 2004–2012. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapamtula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Alasalvar, H.; Yildirim, Z. Ultrasound-assisted extraction of antioxidant phenolic compounds from Lavandula angustifolia flowers using natural deep eutectic solvents: An experimental design approach. Sustain. Chem. Pharm. 2021, 22, 100492. [Google Scholar] [CrossRef]
- Minato, K.I.; Miyake, Y.; Fukumoto, S.; Yamamoto, K.; Kato, Y.; Shimomura, Y.; Osawa, T. Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci. 2003, 72, 1609–1616. [Google Scholar] [CrossRef]
- Alasalvar, H.; Kaya, M.; Berktas, S.; Basyigit, B.; Cam, M. Pressurised hot water extraction of phenolic compounds with a focus on eriocitrin and hesperidin from lemon peel. Int. J. Food Sci. Technol. 2023, 58, 2060–2066. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Torregiani, E.; Bonacucina, G.; Cespi, M.; Palmieri, G.F.; Gabbianelli, R. Antioxidant Properties of Ester Derivatives of Cinnamic and Hydroxycinnamic Acids in Nigella sativa and Extra-Virgin Olive Oils-Based Emulsions. Antioxidants 2022, 11, 194. [Google Scholar] [CrossRef]
- Pantoja, R.K.; Albuquerque, C.F.B.; Nascimento, R.A.d.; De Faria, L.J.G.; Maia, J.G.S.; Setzer, W.N.; Gratieri, T.; da Silva, J.K.R. Stability and Antioxidant Activity of Pouteria macrophylla Fruit Extract, a Natural Source of Gallic Acid. Molecules 2023, 28, 3477. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Park, J.S.; Kang, M.H.; Lee, H.J.; Ali, J.; Tahir, M.; Choe, K.; Kim, M.O. Caffeic Acid, a Polyphenolic Micronutrient Rescues Mice Brains against Aβ-Induced Neurodegeneration and Memory Impairment. Antioxidants 2023, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Steinbrecher, R.; Metzsch-Zilligen, E.; Pfaendner, R. Antioxidant Activity of Biogenic Cinnamic Acid Derivatives in Polypropylene. Polymers 2023, 15, 3621. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Lee, S.R.; Yoon, J.G.; Moon, H.R.; Zhang, J.; Park, E.; Yoon, S.; Cho, J.A. Ferulic Acid as a Protective Antioxidant of Human Intestinal Epithelial Cells. Antioxidants 2022, 11, 1448. [Google Scholar] [CrossRef]
- Denaro, M.; Smeriglio, A.; Trombetta, D. Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion. Antioxidants 2021, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]


| Variables | Conditions |
|---|---|
| Ultrasound parameter | 40 kHz, 500 ± 50 W |
| Extraction time | 10, 20, 30, 40, 50 min |
| Particle size | >60, 60–80, 80–100, <100 mesh |
| Ethanol ratio in deionized water | 0, 15, 35, 55, 75, 95% |
| Acidified ethanol † | pH 1, 2, 3, 4, 5, 6, 7 |
| Sample | Phenolic Acids (µg (mg DW)−1) | Flavonoids (µg mg (DW)−1) | |||||
|---|---|---|---|---|---|---|---|
| Gallic Acid MW 170.12 (g mol−1) | Caffeic Acid MW 180.16 (g mol−1) | p-Coumaric Acid MW 164.05 (g mol−1) | Ferulic Acid MW 194.18 (g mol−1) | Cinnamic Acid MW 148.15 (g mol−1) | Hesperidin MW 610.56 (g mol−1) | Eriocitrin MW 596.53 (g mol−1) | |
| PP_100 M_ 30 min_pH5 | 9.164 ± 0.021 | 6.988 ± 0.031 | 4.733 ± 0.025 | 10.355 ± 0.049 | 0.663 ± 0.022 | ND | ND |
| LP_100 M_ 30 min_pH4 | ND | ND | ND | ND | ND | 65.685 ± 0.018 | 23.195 ± 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Chiang, Y.-C.; Chen, M.-H.; Chiang, P.-Y. Micronization Combined Ultrasound-Assisted Extraction Enhances the Sustainability of Polyphenols from Pineapple and Lemon Peels Utilizing Acidified Ethanol. Foods 2025, 14, 2872. https://doi.org/10.3390/foods14162872
Lee Y-C, Chiang Y-C, Chen M-H, Chiang P-Y. Micronization Combined Ultrasound-Assisted Extraction Enhances the Sustainability of Polyphenols from Pineapple and Lemon Peels Utilizing Acidified Ethanol. Foods. 2025; 14(16):2872. https://doi.org/10.3390/foods14162872
Chicago/Turabian StyleLee, Yen-Chieh, Yi-Chan Chiang, Min-Hung Chen, and Po-Yuan Chiang. 2025. "Micronization Combined Ultrasound-Assisted Extraction Enhances the Sustainability of Polyphenols from Pineapple and Lemon Peels Utilizing Acidified Ethanol" Foods 14, no. 16: 2872. https://doi.org/10.3390/foods14162872
APA StyleLee, Y.-C., Chiang, Y.-C., Chen, M.-H., & Chiang, P.-Y. (2025). Micronization Combined Ultrasound-Assisted Extraction Enhances the Sustainability of Polyphenols from Pineapple and Lemon Peels Utilizing Acidified Ethanol. Foods, 14(16), 2872. https://doi.org/10.3390/foods14162872






