1. Introduction
Based on a large-scale investigation of the Global Burden of Disease, as of 31 December 2021, the overall estimated prevalence of periodontitis in the global population was approximately 63%, with severe periodontitis estimated at 24.2%, and both trends are continuing to rise [
1].
Porphyromonas gingivalis, a Gram-negative anaerobe, is recognized as a keystone pathogen in periodontitis and has been implicated in systemic conditions such as cardiovascular disease, Alzheimer’s disease, and rheumatoid arthritis.
Research on different strains of
P. gingivalis has consistently shown that the virulence factors it secretes are key pathogenic factors leading to various diseases, including periodontitis. These virulence factors mainly include gingipains, fimbriae, lipopolysaccharides (LPSs), and outer membrane vesicles (OMVs), which play important roles in the survival, invasion, host immune regulation, and inflammation induction of
P. gingivalis. Among these, gingipains are considered the most critical virulence factors, and many complications caused by
P. gingivalis are closely related to their action [
2,
3]. Studies have shown that knocking out the gingipain genes in
P. gingivalis significantly reduces the virulence of the strain [
4]. Notably,
P. gingivalis requires the secretion of gingipains to obtain iron and protoporphyrin IX for its metabolism, presenting an important breakthrough point for research on treatment strategies against this bacterium [
5].
The survival and pathogenicity of
P. gingivalis are closely related to its virulence factors. Its cysteine proteases, gingipains—arginine-specific (RgpA and RgpB) and lysine-specific (KGP)—are essential for nutrient acquisition, host tissue destruction, and immune modulation [
6,
7,
8]. By degrading host structural proteins, cytokines, and complement factors, gingipains orchestrate both local and systemic inflammation, making them attractive therapeutic targets [
8,
9].
Food-derived natural products, characterized by structural diversity and favorable safety profiles, have emerged as promising gingipain inhibitors. In addition to directly inhibiting bacteria and biofilm formation, compounds such as epigallocatechin gallate, phloretin, and phlorizin can inhibit KGP and/or Rgp activity. Compounds such as proanthocyanidins and resveratrol can effectively reduce inflammation caused by P. gingivalis. However, most findings are based on in vitro assays, with limited mechanistic elucidation, scarce structure–activity relationship analyses, and few in vivo validations. Furthermore, challenges such as poor bioavailability, instability under physiological conditions, and a lack of clinical evidence hinder translation to therapeutic use. To date, no comprehensive review has focused specifically on food-derived gingipain inhibitors or their chemical diversity, mechanisms, and translational prospects.
This review aims to systematically summarize advances in food-derived natural products that inhibit P. gingivalis or gingipains, with an emphasis on chemical classes, potency, and mechanisms of action. By integrating recent evidence, including structure–activity relationship insights, this study critically evaluates their therapeutic potential and discusses strategies to overcome pharmacokinetic and delivery challenges. We provide a foundation for the future research and development of gingipain-targeted interventions derived from dietary sources.
2. Literature Search Strategy
A comprehensive literature search was conducted to identify relevant studies investigating the inhibitory effects of natural products on gingipains, key virulence factors of Porphyromonas gingivalis. The electronic databases PubMed, Web of Science, and Google Scholar were systematically searched for articles published between January 2000 and December 2024.
The search strategy combined MeSH terms and commonly used keywords, namely, “Porphyromonas gingivalis,” “gingipain,” “KGP,” and “periodontitis,” together with terms related to natural compounds, namely, “inhibitor”, “natural products,” “phytochemical,” “polyphenol,” “flavonoid,” “polysaccharide,” “oligosaccharide,” “alkaloid,” “terpenoid,” “quinone,” “saponin,” and “lignan.” Boolean operators (AND/OR) were used to refine the search.
Studies were included if they met the following criteria: (1) original research articles including in vitro or animal experimental studies; (2) a clearly defined chemical structure and natural source for the tested compounds or extracts; (3) evaluations of inhibitory effects on gingipain activity or related virulence mechanisms.
The exclusion criteria comprised the following: (1) non-English language articles; (2) review articles without experimental validation; (3) studies not involving specific natural products; (4) articles whose findings have been superseded by more recent and comprehensive publications.
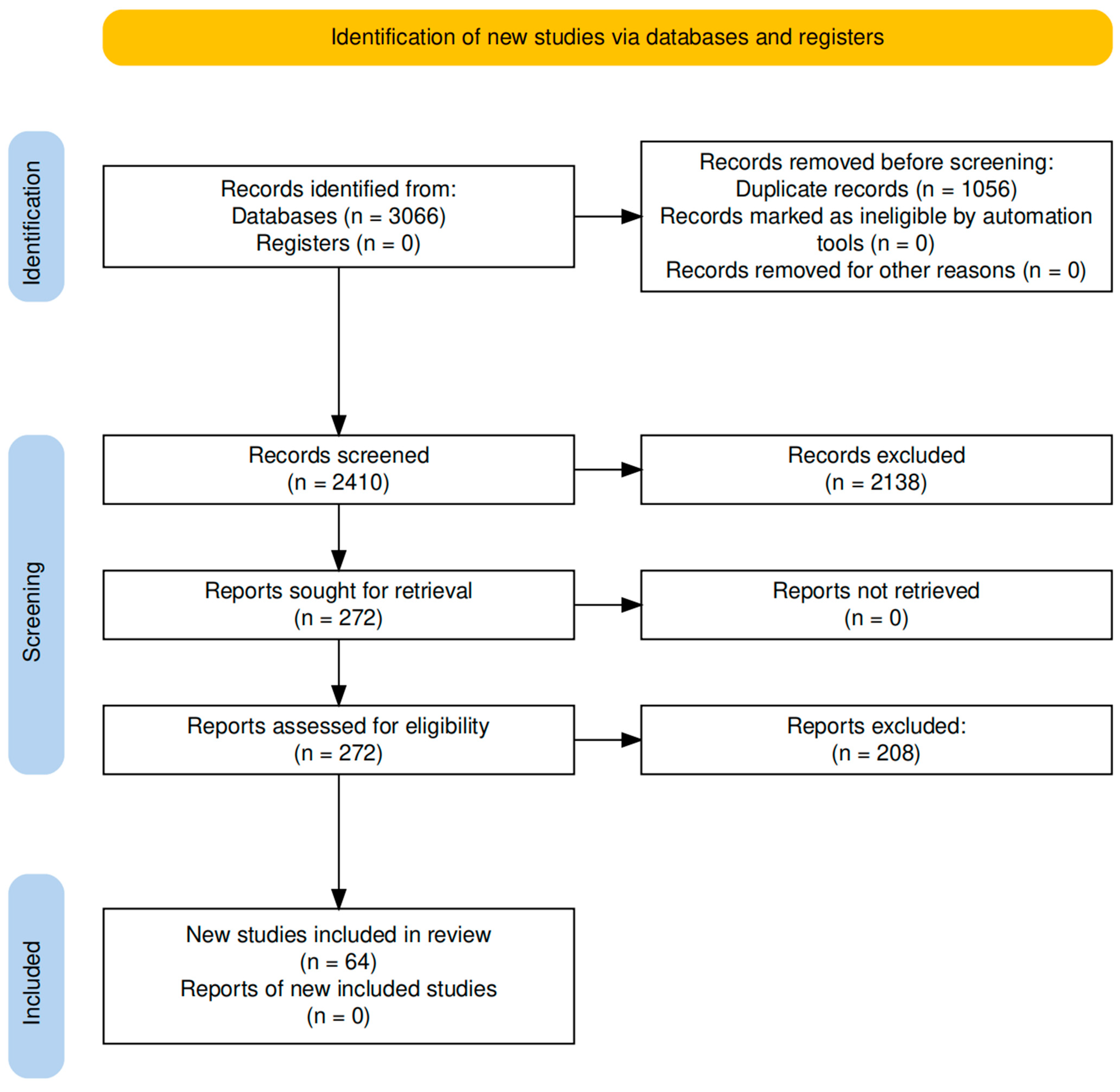
The study selection process is illustrated in
Figure 1, according to the PRISMA guidelines [
10].
3. Overview of Porphyromonas gingivalis
3.1. Biological Characteristics and Systemic Implications of P. gingivalis
P. gingivalis requires heme and vitamin K for growth, allowing it to thrive and rapidly proliferate in the presence of blood [
12]. Research findings suggest that
P. gingivalis has immune evasion abilities, enabling it to interfere with inflammatory signaling, disrupt the complement system, control apoptosis, and undermine host defense mechanisms, thereby creating conditions for other pathogenic factors to act [
13].
P. gingivalis selectively manipulates host responses and inhibits bactericidal mechanisms without suppressing inflammation. The tissue degradation products released during inflammation, such as peptides and heme, become important nutrient sources for
P. gingivalis and some oral bacteria [
14]. This increase in pro-inflammatory factors triggers bone loss and ultimately results in periodontal tissue destruction and alveolar bone resorption [
15].
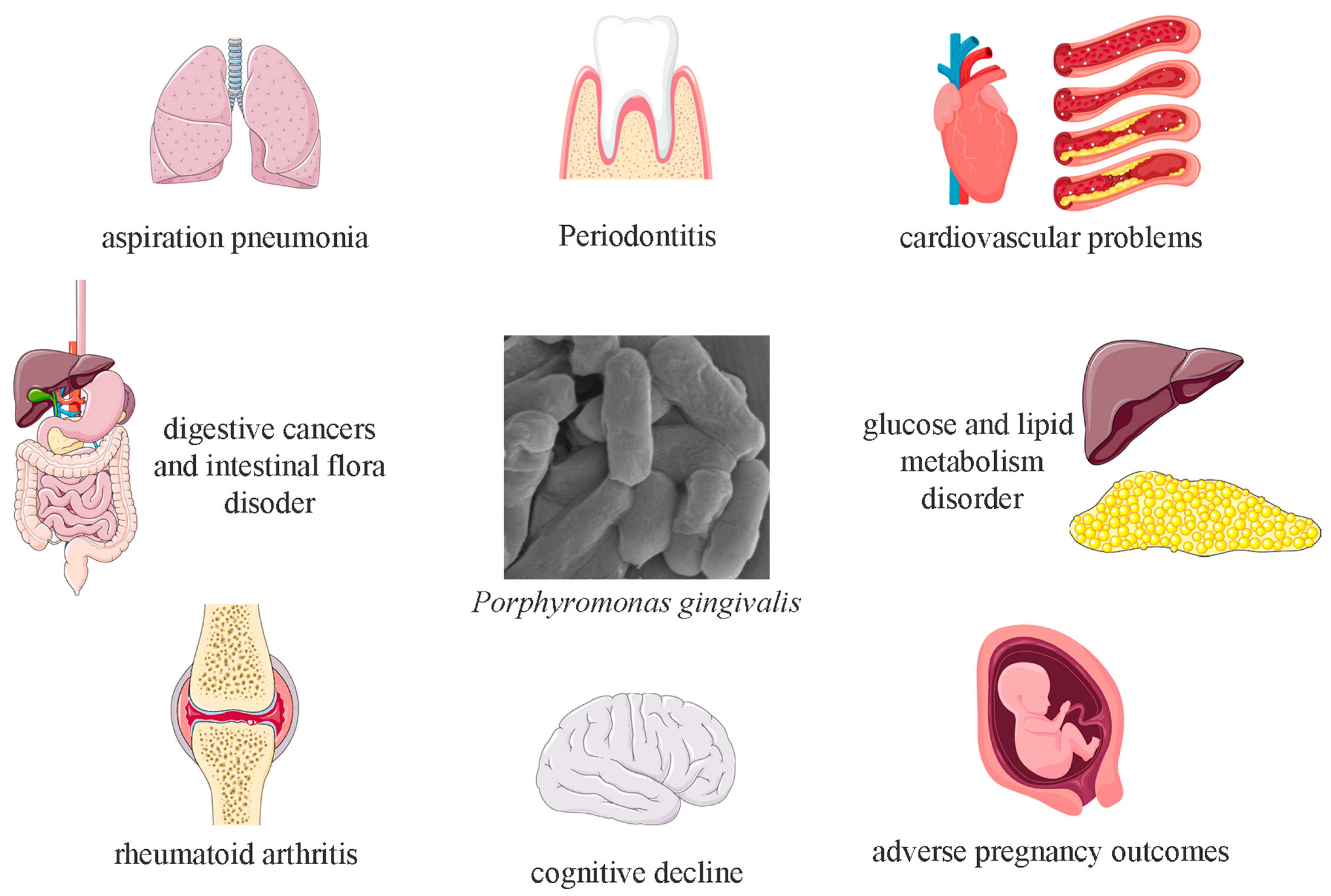
P. gingivalis possesses strong penetrative capabilities, with its virulence factors increasing the permeability of cellular barriers, allowing it to even penetrate the blood–brain barrier.
P. gingivalis can survive not only in the oral cavity but also in other organs, contributing to the onset and progression of systemic diseases such as cardiovascular disease, cognitive decline, adverse pregnancy outcomes, type 2 diabetes, and rheumatoid arthritis (
Figure 2) [
16,
17,
18,
19]. Additionally, eating behaviors can facilitate
P. gingivalis’s entry into the digestive tract along with food, potentially inducing gastrointestinal diseases and altering the composition of the gut microbiota [
20].
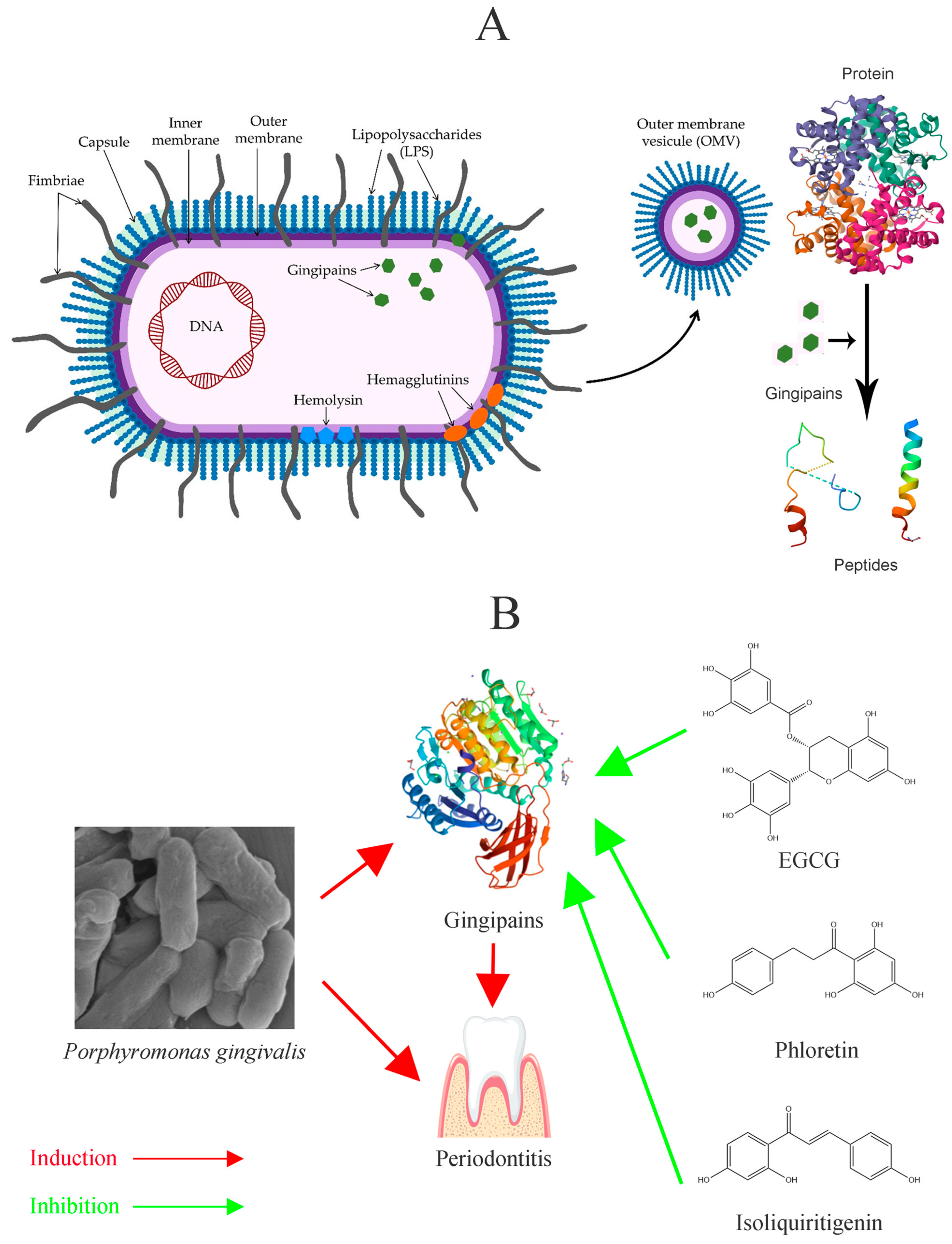
Gingipains are central to the colonization, disruption of host defenses, tissue destruction, and nutrient acquisition of
P. gingivalis (
Figure 3A) [
2,
12]. The survival and pathogenicity of
P. gingivalis are largely dependent on its virulence factors; a lack of gingipains significantly hampers its growth and reduces its pathogenic potential (
Figure 3B). Beyond gingipains, LPSs primarily induce inflammatory responses and bolster the bacterium’s immune evasion capabilities [
21,
22].
3.2. The Roles of Gingipains in the Survival and Pathogenicity of Porphyromonas gingivalis
3.2.1. Overview of Gingipains
Gingipains are multifunctional enzymes involved in the colonization, nutrient acquisition, and manipulation of host immune systems by
P. gingivalis, making them indispensable tools for this bacterium. They are considered its most critical virulence factors. Gene knockout, antigen immunization, and targeted inhibition of gingipains have all been shown to effectively reduce the pathogenicity of
P. gingivalis [
7].
Gingipains are the primary proteolytic enzymes secreted by
P. gingivalis. These cysteine proteases account for over 85% of its total proteolytic activity [
23]. Gingipains include arginine-specific gingipains (Rgp), divided into RgpA and RgpB, and lysine-specific gingipain (KGP). The genes encoding these enzymes are conserved across multiple strains of
P. gingivalis. These three enzymes can either function as membrane-bound proteins or be secreted into the external environment in a soluble form. RgpA and RgpB share essentially the same protease and immunoglobulin-like domains, but RgpB is secreted as a monomer, whereas RgpA forms a stable hemagglutinin–adhesin (HA) domain, similar to KGP [
24]. Inhibiting any of these functional domains would have a significant impact on the survival and pathogenicity of
P. gingivalis.
3.2.2. Role of Gingipains in the Survival of Porphyromonas gingivalis
P. gingivalis needs to adhere to the host surface to complete colonization, and it also needs to co-aggregate with other oral bacteria to colonize the biofilm. This adhesion requires the joint action of fimbriae and gingipains. The HA domains on RgpA and Kgp are required for the adhesion function of gingipains [
25]. When these domains are lost, or when gingipain is lacking, the co-aggregation ability of
P. gingivalis is significantly reduced.
In addition to adhesion, gingipains are crucial for
P. gingivalis in obtaining nutrients.
P. gingivalis is a porphyrin auxotroph, and heme is an ideal source for both iron and porphyrin. As such, the bacterium relies on gingipains to capture sufficient heme [
6]. Studies have shown that RgpA and KGP can bind hemoglobin with high affinity, and once hemoglobin is anchored near the protease domains, it is gradually degraded and releases heme [
6,
26]. This hemolytic property is directly linked to the activity of gingipains. During this process, Rgp and KGP work in tandem: Rgp facilitates the conversion of hemoglobin into a form more easily degraded by KGP, and KGP then efficiently completes the degradation. The absolutely conserved HA-2 domain on gingipains captures the degraded heme and directs the secreted gingipains to capture more hemoglobin [
27]. This function is lost in mutant strains in which the HA domain is knocked out.
Experimental evidence shows that when gingipains are inhibited,
P. gingivalis cannot grow in media supplemented with transferrin. However, when the supplement is switched to heme, the growth of
P. gingivalis is restored [
28]. This characteristic provides a potential direction for the development of
P. gingivalis inhibitors.
3.2.3. Role of Gingipains in the Pathogenicity of Porphyromonas gingivalis
Gingipains also play an important role in
P. gingivalis’s resistance to host immune system responses. The complement system is one of the main players in the human body’s resistance to oral bacterial invasion, and
P. gingivalis relies on gingipains to resist complement-mediated lysis [
29]. The HA domain of RgpA can bind to the human complement inhibitor C4b-binding protein to form protective capabilities [
30]. At the same time, all three gingipain types can degrade complements C3, C4, and C5, rendering their defense systems ineffective. In this process, RGP performs significantly better than KGP [
9]. In addition to antagonizing the complement system, gingipains can also protect
P. gingivalis from immune cell attacks and even destroy cell surface receptors and weaken the immune system’s communication network [
9].
In addition to ensuring
P. gingivalis viability, gingipains also play a critical role in
P. gingivalis aggressiveness. In a manner independent of proteolytic activity, RgpA and KGP manipulate cytokine networks to help
P. gingivalis evade host defenses and alter the local environment [
8]. This gives
P. gingivalis the ability to control inflammatory responses, which is beneficial to both offense and defense. The host’s coagulation cascade, protease-activated receptor cascade, and kinin pathway are inhibited by gingipains, paralyzing the host’s defense system, assisting
P. gingivalis in breaking through the vascular barrier to spread, and reversing the protective effect of the inflammatory response to the host’s own attacks [
31]. Although gingipains have proteolytic activity, they may not directly degrade periodontal tissue. In fact, they inactivate the host’s protease inhibitors, disrupt the balance between host proteases and endogenous protease inhibitors, and allow the host’s proteases to attack periodontal tissue regardless of friend or foe, ultimately leading to alveolar bone resorption and periodontal ligament destruction [
7]. Inducing host cell apoptosis with gingipain not only enhances its destructive effect on periodontal tissue but also enhances the invasion and spread ability of
P. gingivalis [
32].
Research has found that gingipains can enhance the bacterium’s ability to penetrate the blood–brain barrier by degrading intercellular junction proteins [
33,
34]. Peripheral infections play a significant role in cognitive decline and the progression of Alzheimer’s disease, and gingipains have been detected in substantial numbers in the brains of AD patients [
3].
3.3. The Structure of Gingipains
3.3.1. Structure of RGP
Gingipain-R is encoded by two genes,
rgpA and
rgpB, and divided into two types of enzymes, RgpA and RgpB [
35]. These enzymes are multidomain proteins: RgpA consists of a catalytic domain, plus four additional HA domains between the immunoglobulin superfamily domain (IgSF) and the C-terminal domain to form a non-covalent complex, HRgpA, and RgpB lacks the HA domain, only appears in the catalytic domain [
36]. The difference between RgpB and RgpA is not only related to the HA domain, although the catalytic domain of RgpB is similar to that of RpgA; four amino acid substitutions around the active site of RgpA allow it to degrade protein substrates more efficiently [
37]. The loss of RgpB has specific effects on RgpA maturation, and the loss of RGP can also cause abnormal KGP processing. Therefore, although alveolar bone loss is significantly induced only when
P. gingivalis expresses RgpA, the role of RgpB cannot be ignored [
37]. The structure of RgpB is the main direction of RGP structure research, and most studies related to the HA domain tend to analyze it in combination with KGP.
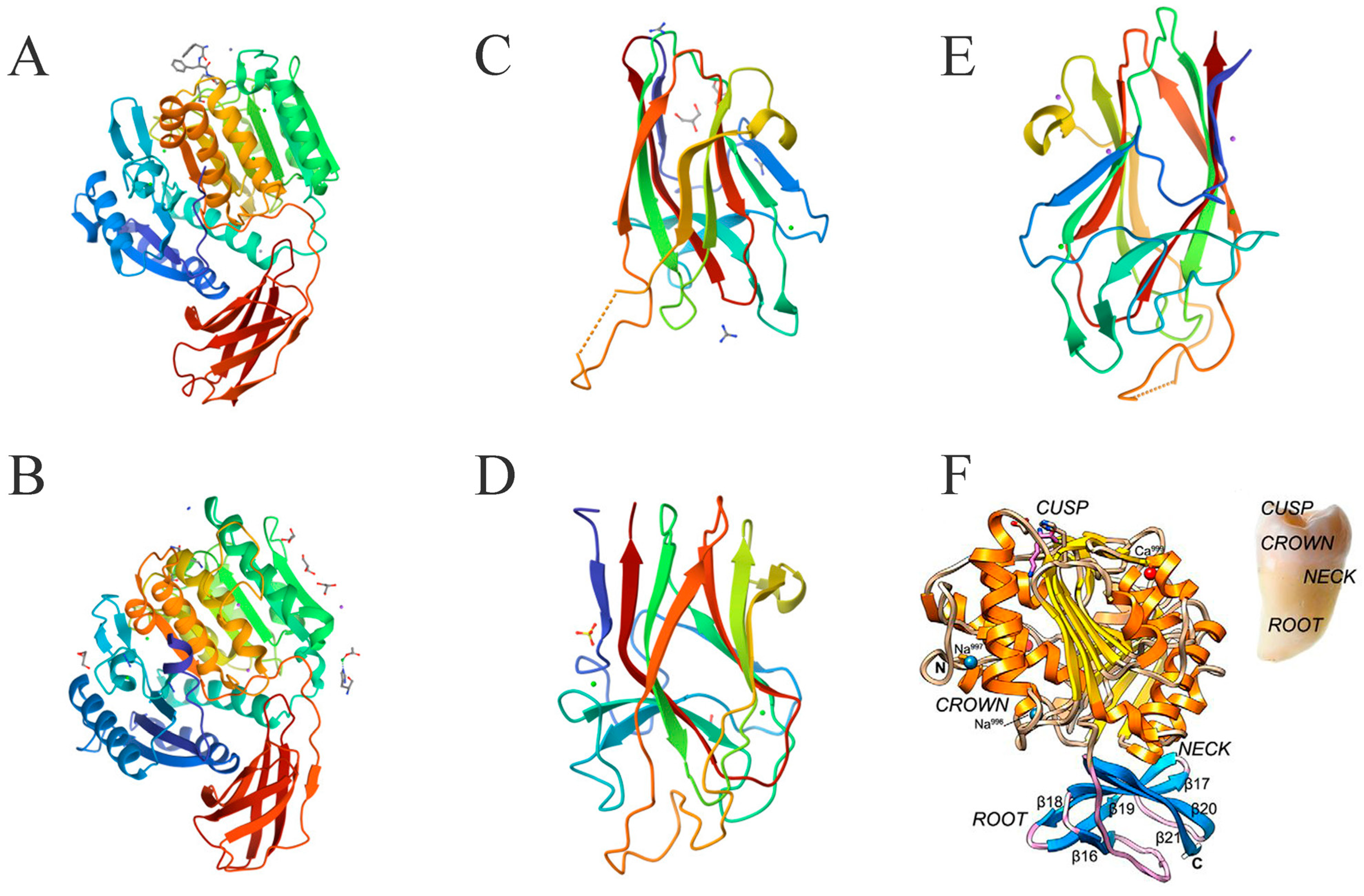
The structural shape of RgpB is similar to a single-rooted tooth. The crown formed by the 351 N-terminal residues is the catalytic domain, and the root formed by 84 residues is similar to the IgSF domain (
Figure 4A). The catalytic domain of RgpB consists of two subdomains, A and B, and the active center of RgpB is located on the “chewing surface” of the “tooth crown” [
38]. The center is exposed Cys244, surrounded by a pocket formed by His211-Glu214, Ala243-Val245, and Glu152-Asp163. The active site residues are inferred to be His211 and Cys244, and Glu152 also has a significant influence. The catalytic domain of RgpB is highly similar to that of caspase, and it is speculated that the catalytic mechanism of RgpB may be similar as well. The S1 pocket of RgpB can preferentially accommodate Arg side chains, and structural differences provide it with a higher optimal pH, necessitating an active anaerobic environment [
38]. The function of the IgSF domain for RgpB is less obvious because this domain is mainly responsible for anchoring the HA domain, so it would be more significant for RgpA [
36].
3.3.2. Structure of KGP
KGP is encoded by the
kgp gene and is a multi-domain protein consisting of a signal peptide, N-terminal pro-domain (NPD); a catalytic domain, IgSF; three to five HA domains; and a C-terminal domain. Abnormal function in the
kgp gene does not affect the expression, processing, or activity of RGP [
40]. KGP and RGP are sensitive to lysine and arginine, respectively, making them cleave proteins differently. The folding structure of the Kgp catalytic domain is similar to that of Rgp, especially in the NPD, but they share less than 23% sequence identity (
Figure 4A,B) [
41]. By contrast, the NPD sequence identity of RgpA and RgpB reaches 75% [
42]. KGP is similar to RgpA in that it forms a non-covalent complex with the HA domain during post-translational events, shaping its unique function [
43].
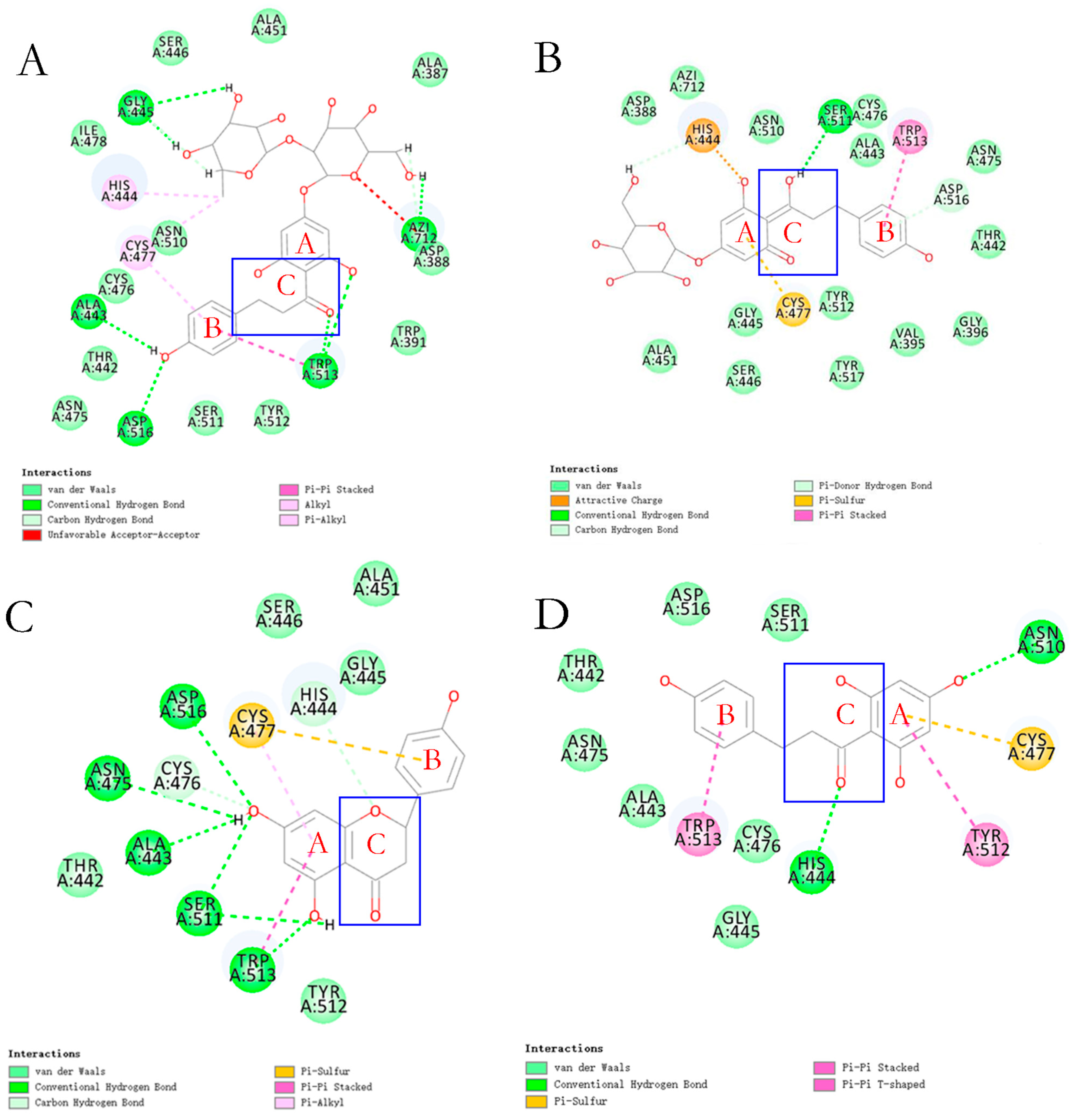
The catalytic domain of KGP consists of CD and IgSF. Interestingly, its structure is very similar to that of a tooth, with CD as the crown, IgSF as the root, and the active site located in the tooth cusp (
Figure 4F) [
39]. Structural analysis of the catalytic domain of KGP shows that the catalytic pocket of its active site is actually a catalytic triplet formed by Cys477-His444-Asp388, rather than a cysteine–histidine duplex [
39]. A structure–activity mechanism analysis of the targeted inhibitory effect of KYT-36 inhibitors on KGP has also proved this [
4]. The C-terminal domain of KGP has an extended solvent channel that passes through the inner core of the protein, which not only enhances the plasticity and flexibility of the enzyme but also increases its energy cost due to instability [
39]. This structural difference weakens the proteolytic ability of KGP, making its protein catalytic activity lower than that of RgpB [
44]. However, the actual role of KGP is far greater than that of RgpB, because the complex with the HA domain is the key for KGP to cover the functions of heme acquisition, delivery, and binding to the extracellular matrix in support of
P. gingivalis.
3.3.3. Structure of Haemagglutinin/Adhesin Domains
Structural differences in the modular composition and organization of the HA domain provide KGP with several structural variants, giving
P. gingivalis strains different functional properties. By contrast, the conservation of RgpA in different
P. gingivalis strains is as high as 99% [
45]. The HA domain contains three types, K1, K2, and K3, which can also form complexes with each other (
Figure 4C–E). Since the
P. gingivalis W83 strain can express all three HA domains, it is an excellent sample for studying HA domains and also one of the most virulent [
46].
The cleaved HA domains K1, K2, and K3 share 30% sequence identity, with the sequence identity of K1 and K3 being 71%. Their folds share significant similarities, especially in the core fold, and the short-loop end of their β-sandwich is highly conserved in sequence and conformation terms (
Figure 4B–D) [
46]. The loop conformations at the long-loop end are significantly different, but this seems to provide the opportunity for their interaction [
47]. Arg1109 of K1 in L9, Arg1280 of K2 in L8, and Arg1557 of K3 in L10 are their arginine anchor sites, and their spatial structures are very similar [
46]. Analysis of their surface electrostatic potential shows that the electron density of the Thr1098–Ser1104 region on loop L9 of K1 is weak or missing [
46]. The electron density of the three fragments in loop L8 of K2, Lys1276, Arg1280, and Lys1291, is weak or missing, and the lysis or truncation of this region severely abrogates the hemolytic capacity [
48]. K3 has weak or missing electron density in the Leu1544–Pro1553 region of L10, in which the density of Leu1544–Lys1547 is weak, and the electron density of Thr1551–Ala1552 and Ala1546–Pro1553 is missing [
47].
K1, K2, and K3 can all selectively bind hemoglobin with high affinity and induce hemolysis in vitro, but heat treatment renders this hemolytic ability ineffective [
46,
47,
48]. The lack of a catalytic domain indicates that hemolysis is not related to proteolytic activity. The anion transport inhibitor SITS can reduce the detectable hemolysis threshold of K2 by one order of magnitude and potentially the hemolysis mechanism of HA domains [
48]. Crystal structure and hemolytic performance tests show that there may be functionally relevant structural differences between K1, K2, and K3.
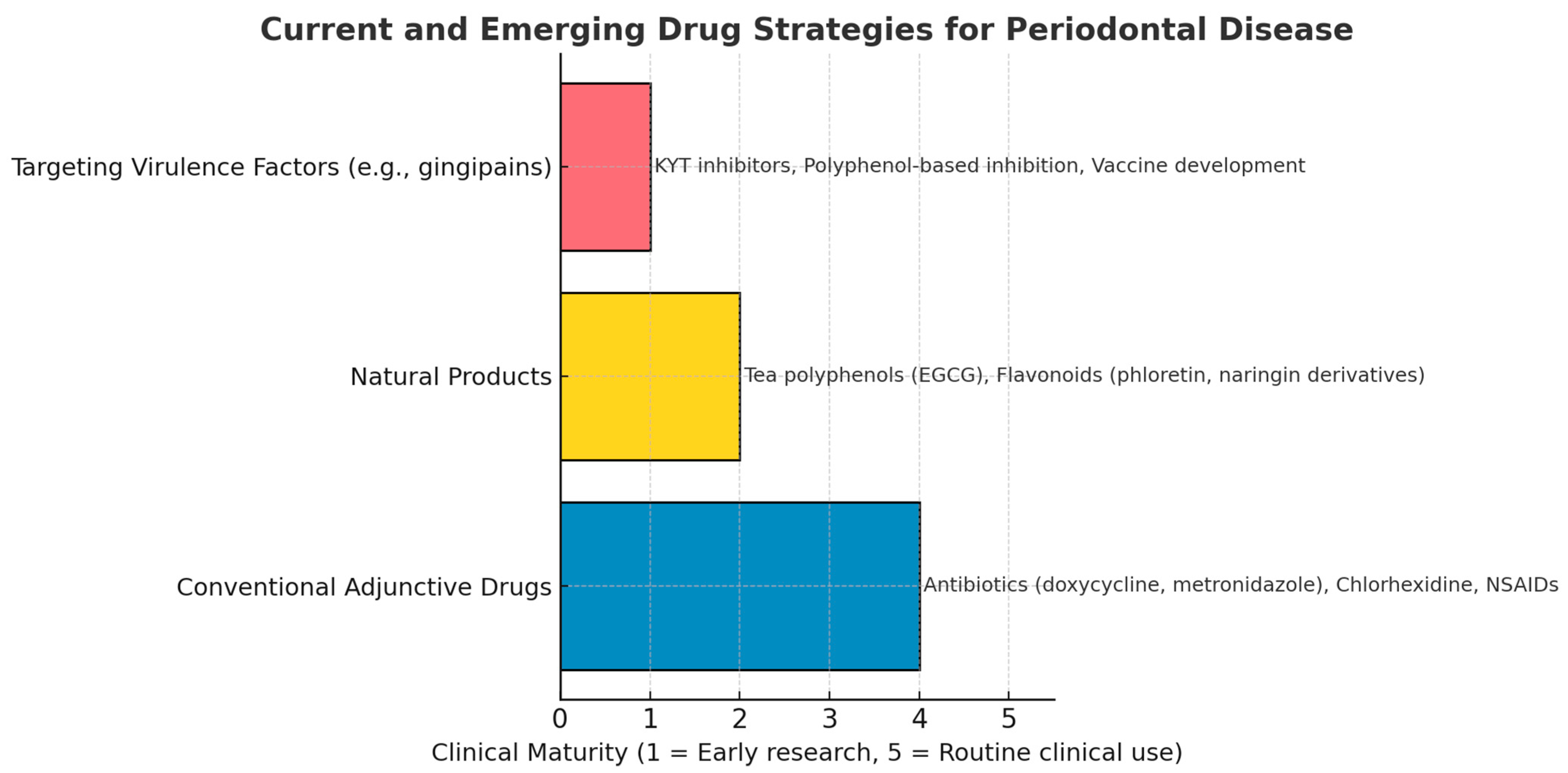
4. Current Treatment Options for Porphyromonas gingivalis Related Problems
Currently, the treatment of periodontal diseases associated with
P. gingivalis infections primarily involves physical debridement, irrigation therapy, and antibiotics [
49]. Chlorhexidine is a commonly used antibacterial mouthwash in clinical practice, and nonsteroidal anti-inflammatory drugs (NSAIDs) may be used to relieve inflammation. Metronidazole, doxycycline, minocycline, amoxicillin, and clindamycin are the most commonly used antibiotics [
50]. However, due to the long treatment cycles and high recurrence rates of periodontal diseases, antibiotics can only serve as a short-term treatment option. Prolonged antibiotic use can disrupt beneficial microbiota in the oral cavity or body and the development of antibiotic resistance. Moreover, when biofilms form subgingivally, the effectiveness of antibiotic therapy is significantly reduced compared with planktonic cells [
51]. Antibiotic resistance in
P. gingivalis has been observed in multiple regions and populations, making the development of new drugs that are effective, safe, and less prone to causing resistance an important measure to address these issues [
52].
Antibiotics and chemical drugs such as chlorhexidine have sufficient clinical data to support their use, but they are not routinely used in every case. Developing specific inhibitors targeting
P. gingivalis or discovering more effective broad-spectrum antimicrobial agents are two important approaches to addressing
P. gingivalis-related issues. Studies have shown that targeting gingipains can help reduce the risk of diseases associated with
P. gingivalis [
3,
53].
Given the crucial role of virulence factors such as gingipains in the pathogenicity of P. gingivalis, targeted inhibition of these virulence factors is also a viable option. However, there are no reports on the targeting inhibition of gingipain activity by antibiotics. The complex structure of gingipains also poses challenges to the development of their targeted inhibitors. This has led to slow progress in research on P. gingivalis and gingipain inhibitor discovery over the past two decades.
Studies have developed molecular-targeted inhibitors to solve the problem of
Porphyromonas gingivalis and its virulence factors, achieving effective results in animal experiments, such as with KTY inhibitors and COR inhibitors, but there have been no reports of successful clinical applications [
3,
4,
54].
Natural products, widely distributed in nature with a diverse range of types and biological activities, represent a treasure trove for the development of new functional food factors and novel drugs [
55]. Many natural products exhibit broad-spectrum antibacterial properties, including some with inhibitory effects against
P. gingivalis. Given this, there is great potential in discovering targeted inhibitors of gingipains. The safety of food-derived natural products also provides a strong guarantee for their long-term application.
However, to date, most natural products and inhibitors targeting virulence factors remain in animal experiments or early clinical trials (
Figure 5).
5. Discovery of Gingipain Targeted Inhibitors and Potential Structure-Activity Relationships
Given the critical importance of gingipains to the survival and pathogenicity of P. gingivalis, the development of targeted inhibitors is essential. RgpA, RgpB, and KGP are the three most important gingipains, with RgpA and KGP exhibiting significantly higher toxicity, likely due to their HA domains. Overall, Rgp tends to play a supportive role, while KGP is the primary contributor to virulence and is crucial for the hemoglobin cleavage that allows P. gingivalis to acquire heme. Targeting the structure of gingipains to inhibit their function is an effective way to reduce the pathogenicity of P. gingivalis and even decrease its population, with research on KGP being the most critical direction. The structure–activity relationships found in studies related to gingipain inhibition can also help to discover targeted inhibitors.
One study examined the effects of catechins and their derivatives on Rgp and KGP activities. It found that catechin derivatives, including EGCG, (−)-epicatechin gallate (ECG), (−)-gallocatechin gallate (GCG), and (−)-catechin gallate (CG), significantly inhibit Rgp activity; however, only GCG and CG significantly inhibit KGP activity, and their performance is worse in inhibiting Rgp [
56]. In this experiment, only tea catechin derivatives containing the galloyl moiety inhibited Rgp and KGP activities, indicating that this mechanism may be associated with the presence of a galloyl moiety linked to 3-OH. This provides an important reference for studying the structure–activity relationship of catechins in inhibiting gingipain, but further evidence is still needed to advance research in this area.
Another study tested the anti-inflammatory effects of 18 dihydrochalcones and structurally related compounds on
P. gingivalis LPS-induced periodontitis. The structure–activity analysis results indicated the importance of electronegative atoms on the A and B-linker chains for the anti-inflammatory effect of flavonoids, suggesting that, in addition to interactions with NF-κB transcription factors, these molecules may have direct molecular interactions with virulence enzymes, providing a reference for the discovery and structural improvement of leading compounds [
57].
In recent studies based on the reported structure of KGP crystals, 14 flavonoids were identified with the ability to inhibit gingipain activity through molecular docking and virtual screening, demonstrating a significant structure–activity relationship (
Figure 6 and
Figure 7) [
28]. Among these, flavonoids with a dihydrochalcone structure demonstrated stronger inhibitory effects and interfered with
P. gingivalis’s utilization of hemoglobin [
28]. However, when the dihydrochalcone structure transformed into a flavonoid structure, as the C ring closed, this interference effect disappeared, and their antibacterial effect was significantly reduced. Among these flavonoids, phloretin and phlorizin performed best. However, the effect of naringin dihydrochalcone was poor, which may be due to steric hindrance caused by the diglycoside group.
6. Potential Antibacterial Agents Against Porphyromonas gingivalis from Food-Derived Natural Products
Some natural products that are abundant in common foods could inhibit
P. gingivalis, presenting the possibility of substituting medication with dietary intake (
Table 1 and
Table 2). However, most studies on the antibacterial effects of natural products on
P. gingivalis have not discussed the role of gingipains. Problems related to
P. gingivalis may be solved by inhibiting virulence factors such as gingipains and lipopolysaccharide, and it is worth further analyzing the specific mechanism. A comprehensive analysis of related studies will facilitate the identification of lead compounds within natural products that could resolve issues associated with
P. gingivalis and gingipains, potentially discovering structure–activity relationships.
6.1. Polyphenols
In research on
P. gingivalis-related natural products, polyphenols such as flavonoids occupy a considerable proportion and have obvious effects. Among them, tea polyphenols, proanthocyanidins, resveratrol, and several flavonoids have significant effects. The structures of polyphenols with inhibitory effects are summarized in
Figure 8.
6.1.1. Tea Polyphenols
Epidemiological surveys have revealed a modest inverse association between green tea intake and the incidence of periodontal disease [
58]. Daily green tea intake was significantly correlated with multiple periodontal health indicators; the more frequently the subjects drank green tea, the better their periodontal condition. Antibacterial experiments on clinically isolated strains have also verified that
P. gingivalis is sensitive to green tea extract, and 12.5 mg/mL green tea extract can have a significant inhibitory effect on the growth of
P. gingivalis [
59]. The main component of green tea polyphenols is epigallocatechin-3-gallate (EGCG). Confirmed by several studies, EGCG has a direct antibacterial effect, damaging the cell membrane and biofilm of
P. gingivalis. However, when the biofilm is constructed, the inhibitory effect of EGCG may be greatly reduced [
60].
Administering EGCG to mice with periodontitis can effectively inhibit the activation of the NF-κB signaling pathway through
P. gingivalis virulence factors, downregulate the levels of various inflammatory mediators, and alleviate alveolar bone loss caused by inflammation [
61]. In one experiment, EGCG administration for 40 days reduced elevated levels of IL-1β, IL-6, IL-9, and IL-12p70 induced by
P. gingivalis; it also inhibited RANKL-induced activation of the JNK/c-Jun and NF-κB pathways, IL-1β-induced bone resorption, and osteoclast formation but had no significant regulatory effect on IL-17, which can induce RANKL activation. However, whether the regulatory effects were associated with any virulence factors was not verified.
Studies on osteoclasts and gingival fibroblasts have shown that, by inhibiting IL-6 and blocking the activation of the MAPK/ERK signaling pathway, EGCG can reduce the expression and activity of matrix metalloproteinase-1 (MMP-1), MMP-2, and MMP-9 induced by
P. gingivalis LPS, thereby preventing alveolar bone resorption [
62]. A study on ligature-induced periodontitis SD rats also demonstrated that, by reducing the level of IL-6, systemic administration of EGCG for more than 2 weeks can reduce osteoclast activity and inhibit collagen destruction, providing it with a potential therapeutic effect on damaged periodontal tissue [
63]. In-depth research on the anti-infection mechanism showed that EGCG can induce gingival epithelial cells to secrete the antimicrobial peptide human β-defensin and protect them from degradation caused by gingipains, enhancing β-defensin’s establishment of innate immune defense against gingival cells and its role in promoting wound healing [
64]. The use of specific protein kinase inhibitors has demonstrated that the mechanism by which EGCG induces β-defensin secretion involves activating the ERK/MAPK pathway.
The anti-inflammatory effect can also reduce the systemic inflammation problems caused by
P. gingivalis virulence factors and reduce various health risks, such as atherosclerosis [
65]. This effect mainly involves inhibiting EGCG regarding increased levels of inflammatory factors and peroxidation damage caused by
P. gingivalis’s virulence factors [
65]. This means that the antioxidant function of dietary polyphenols may help alleviate oxidative damage induced by
P. gingivalis virulence factors, thereby contributing to the relief of symptoms.
Some studies have used total catechins or green tea extracts for
P. gingivalis research instead of pure EGCG. Besides reducing IL-1β by inhibiting NF-κB, MAPK, and the TLR pathway and activating NLRP3 and AIM2 inflammasomes to reveal anti-inflammatory effects, the total catechin in green tea also synergizes with traditional antibiotics such as metronidazole to enhance its ability to inhibit
P. gingivalis, interfering with AI-2 signaling [
66,
67]. Moreover, the expression of several important genes involved in host colonization (
fimA,
hagA, and
hagB), tissue destruction (
rgpA and
kgp), and heme acquisition (
hem) has shown significant catechin dose-dependent inhibition [
66].
Given the research results for green tea, the inhibitory effects of white tea, oolong tea, and black tea extracts on
P. gingivalis have also been studied. No significant differences have been observed, although the catechin content and proportion of catechin derivatives in these four teas are different [
68]. They can all interfere with the growth and toxicity of
P. gingivalis and exert anti-inflammatory effects. All four tea extracts strongly inhibit the collagenase activity of
P. gingivalis, which may interfere with its acquisition of protein nutrition.
Research results for black tea show that theaflavins also attenuate
P. gingivalis toxicity, inhibit the activity of KGP and Rgp, inhibit inflammatory signaling pathway activation, promote β-defensin secretion, and inhibit MMP-1 and MMP-2 expression [
69,
70,
71].
The systemic bioavailability of small-molecule catechins is high, that of galloyl catechins is low, and the oligomers and polymers in black tea are extremely low or nonexistent, which provides guidance for the utilization and structural modification of tea polyphenols [
72].
6.1.2. Berry Polyphenols
Most of the research related to
P. gingivalis and proanthocyanidins comes from studies of cranberry polyphenols. In addition to green tea, berry extract has also been found to have mitigating effects on
P. gingivalis-related issues. In a study using cranberry, wild blueberry, and strawberry complex extracts, the growth, adhesion, and toxicity of
P. gingivalis were inhibited [
73]. Although the berry-mixed extract had poor antibacterial performance, with an MIC of more than 2000 mg/L, the hemolytic activity of
P. gingivalis was completely inhibited at a concentration of 31.25 μg/mL, and the activities of collagenase, Rgp, and KGP were reduced by 35.8%, 84.2%, and 67.6%, respectively. Analysis of the components of the mixed extract showed that phenolic acids, flavonoids (flavonols, anthocyanins, and flavan-3-ols), and proanthocyanidins accounted for 10.71%, 19.76%, and 5.29%, respectively. This provides an important molecular structure reference for discovering targeted inhibitors of gingipains.
Several studies have tested the effects of polyphenolic components in cranberry extract on
P. gingivalis-related issues. They found that these polyphenols can interfere with the formation of biofilms and bacterial coaggregation, reduce adhesion capability, inhibit the activation of inflammatory factors such as IL-6 caused by
P. gingivalis virulence factors, suppress the secretion of MMP-2 and MMP-9, and exhibit inhibitory effects on gingipains [
74,
75,
76]. Sánchez et al. found that the amount of
Porphyromonas gingivalis was reduced by 39.3% after exposure to 20 mg/mL of cranberry extract for 60 s, while the use of 0.2 mg/mL cranberry extract for 6 h could reduce colonization by 97.2% [
74]. However, an analysis of the extract components showed that its main components were benzoic acid, p-coumaric acid, protocatechuic acid, and flavan-3-ol A-type trimers; the anthocyanin content was not high, and the experiment did not test the efficacy of virulence factors. Neto et al. conducted an experiment to classify the components of cranberry polyphenols and concluded that the antibacterial and anti-adhesion effects of cranberry on
P. gingivalis mainly came from the combined effects of its high-molecular-weight nondialyzable material (NDM) fraction and other polyphenols, rather than from proanthocyanidins [
75]. Yamanaka et al. found that the total cranberry polyphenol components, other than NDM, inhibited the activity of Rgp and KGP at 1 mg/L; furthermore, the inhibition rate could reach 87–91% at a concentration of 100 mg/L, but they believed that the activity mainly came from the EGCG component rather than proanthocyanidins [
77]. Tanabe et al. demonstrated that the effect of cranberry extract on
P. gingivalis LPS-induced osteoclast formation and bone resorption originated from proanthocyanidins (especially A-type cranberry proanthocyanidins), which could inhibit RANKL-dependent osteoclast differentiation by 95% and inhibit the secretion of MMP-2 and MMP-9 [
76]. Notably, the anti-inflammatory effects of proanthocyanidins have been widely confirmed in past studies. Research on
Pelargonium sidoides DC extracts has verified the inhibitory effect of proanthocyanidins on
P. gingivalis [
78,
79]. These benefits may come mainly from proanthocyanidins in the berry, but they lack the ability to inhibit proteases. However, considering that the method used to extract the NDM component increases proanthocyanidins up to 125 times compared with cranberry juice, the inhibitory effects of proanthocyanidins on gingipain cannot be ruled out, and more evidence is needed [
80]. Currently, there is a lack of structure–activity relationship research on the inhibition of
P. gingivalis using berry polyphenols, and we look forward to future progress in this field.
In vitro and in vivo studies have shown that berry polyphenols have low bioavailability and are particularly susceptible to conversion into other metabolites caused by intestinal microbiota [
81]. The high variability in data between subjects suggests that their bioavailability depends on the intestinal microbiota, the food matrix, and possible interactions with other components of the diet.
6.1.3. Resveratrol
Resveratrol has also shown promising results for
P. gingivalis-related problems. Resveratrol is an antitoxin naturally produced by plants and present in various foods, and it is an important mechanism of bacterial resistance. Many studies have confirmed the anti-inflammatory and antioxidant effects of resveratrol and its good safety profile [
82].
Resveratrol has a direct antibacterial effect on
P. gingivalis, destroying biofilms and reducing the adhesion ability of bacteria [
83]. According to Ben Lagha et al., at a concentration of 250 µg/mL, resveratrol can completely inhibit
P. gingivalis, inhibiting 51.59% of biofilm activity and 59.91% of adhesion ability. At the same time, 40 μg/mL of resveratrol treatment for 2 h can inhibit 48.84% of the collagen degradation caused by
P. gingivalis. O’Connor et al. found that resveratrol inhibited the anaerobic bacteria
P. gingivalis and
Actinobacillus actinomycetemcomitans but not other aerobic microorganisms [
84]. This means that resveratrol’s antibacterial effect may be a result of its antioxidant properties, which interfere with the relevant metabolic pathways of anaerobic bacteria that shape the anaerobic environment [
84]. At the same time, pterostilbene, an analog of resveratrol, can increase intracellular reactive oxygen species (ROS), and this conclusion may provide new insights into its antibacterial mechanism and structure–activity relationship [
82]. The specificity of resveratrol against anaerobic bacteria makes it less likely to cause drug resistance in oral bacteria.
The anti-inflammatory effect is the main way resveratrol relieves health problems caused by
P. gingivalis. The increased levels of inflammatory factors IL-1β, IL-6, IL-8, IL-12, TNF-α, and NO induced by
P. gingivalis LPS can be inhibited after applying resveratrol; the reduction ranges from 40% to 80.2%, and the mRNA expressions of IL-6, IL-8, IL-1β, IL-12, and TNF-α are highly inhibited, reflecting its powerful anti-inflammatory ability [
85]. At the same time, the inflammatory pathway signal transduction triggered by
P. gingivalis virulence factors, such as LPS, can also be significantly inhibited and partially blocked by resveratrol. The expression of TLR4 decreases, and the phosphorylation of downstream factors NF-κB p65, p38MAPK, and STAT3 is inhibited [
86]. Furthermore, resveratrol and its derivative activate the downstream expression of ERK1/2, AMPK, and SIRT1, inhibiting the activation of the NF-κB pathway and the expression of inflammatory factors [
87]. This effect can be achieved by treating the target cells with low concentrations (1–25 μM) of resveratrol for 30 min. Interestingly, although SIRT1 activation is one of the mechanisms by which resveratrol exerts its therapeutic effect, knocking out SIRT1 does not counteract its anti-inflammatory effect [
88]. The inhibition of the NF-κB signaling pathway with resveratrol may be the most important mechanism, but it is independent of SIRT1. Activating NF-κB induces osteoclast differentiation, and resveratrol contributes to inhibiting alveolar bone loss caused by osteoclast differentiation [
89]. In one study, resveratrol preferentially inhibited pathological bone resorption but did not impair physiological bone metabolism, as the expression of related genes did not differ between the blank and treatment groups, whereas the expression of
Il1b,
Ptgs2, and
Tnfsf11, which are related to osteoclast differentiation and activation, was significantly inhibited.
This powerful anti-inflammatory effect helps alleviate symptoms other than periodontal disease caused by
P. gingivalis, including rheumatoid arthritis and enhanced vascular permeability caused by VEGF overexpression [
90]. The effect of resveratrol on rheumatoid arthritis may be due to the fact that resveratrol can restore the level of IL-4, which is decreased by antagonizing the elevated IL-17 levels induced by
P. gingivalis [
90]. This is not only beneficial for protecting periodontal tissues but can also alleviate joint inflammation and bone destruction.
In addition to anti-inflammation effects, the expression of virulence-factor-related genes
fimA,
kgp, and
rgpA has also been inhibited by resveratrol [
91]. This not only helps reduce the colonization of
P. gingivalis but also indirectly reduces the problems caused by gingipain toxicity. However, further evidence is still needed to prove whether resveratrol has the ability to inhibit the activity of Rgp and KGP directly.
In vitro and in vivo studies have accumulated extensive data on the bioavailability of resveratrol. Resveratrol’s poor water solubility and rapid metabolism contribute to its low bioavailability [
92]. Human studies have shown that the amount of free resveratrol entering the bloodstream increases linearly with increasing doses, while the
Tmax value remains constant [
93]. This suggests that a specific dose of resveratrol is appropriate, taking into account its bioavailability and the low risk of potential side effects.
6.1.4. Flavonoids
Flavonoids are polyphenolic compounds derived from plants. Past studies have reported their anti-inflammatory, antioxidant, and antibacterial applications. There are also studies on the use of a variety of flavonoids on
P. gingivalis [
94]. These effects cover many factors and include various issues related to
P. gingivalis, including antibacterial qualities, biofilm inhibition, inhibiting the activation of inflammatory pathways, reducing inflammatory factors, repairing oxidative damage, and regulating the differentiation of osteoblasts/osteoclasts to protect alveolar bone.
Studies have reported the effects of quercetin, genistein, luteolin, and quercetagetin on
P. gingivalis LPS-induced inflammation, showing that they can inhibit the expression of the MAPK, ERK1/2, p38, and JNK pathways, and reduce multiple inflammatory factors, especially cyclooxygenase-2 (COX-2), with quercetagetin performing the best [
95]. Research on quercetin has shown that quercetin not only directly inhibits
P. gingivalis but also reverses the inhibition of osteoblast differentiation caused by LPS by regulating MAPK signaling [
96]. Luteolin inhibits the activation of the MAPK, AKT, and NF-κB signaling pathways and NOS expression, abolishes LPS’s effects on NF-κB translocation, and reduces alveolar bone resorption [
97,
98]. Similarly to luteolin, fisetin and myricetin can also inhibit the activation of these signaling pathways and block LPS-induced COX-2 expression [
99,
100]. According to Grenier et al., 100 μg/mL of myricetin can decrease the expression of
fimA,
hagA, and
hagB by 81%, 93%, and 64%, respectively, and the expression of
rgpA,
rgpB, and
kgp by 68%, 49%, and 96%, respectively. This could highly inhibit collagen degradation [
100].
In addition to regulating MAPK, NF-κB, and TLR signaling, baicalin also regulates macrophage polarization to clear LPS-induced inflammation by inhibiting the RhoA/ROCK signaling pathway; it also has a good protective effect on osteoblasts and alveolar bone [
101,
102]. Hesperidin, widely found in citrus fruits, can also inhibit
P. gingivalis and reduce inflammation [
103]. Maquera-Huacho et al. reported that hesperidin dose-dependently inhibits
P. gingivalis-induced ROS production and the secretion of IL-1β, TNF-α, IL-8, MMP-2, and MMP-9; it also attenuates the activation of NF-κB in
P. gingivalis-stimulated macrophages, thereby protecting the gingival epithelial barrier [
104].
Isoliquiritigenin, licoricidin, and licorisoflavan A isolated from licorice can also reduce the toxicity of
P. gingivalis, and licochalcone A can synergize with A-type cranberry proanthocyanidins to reduce the secretion of pro-inflammatory mediators [
103,
105,
106]. Notably, isoliquiritigenin and liquiritigenin are very similar in structure, but the opening on the C ring of isoliquiritigenin makes its anti-inflammatory properties superior to those of liquiritigenin. Applying 5 μg/mL of isoliquiritigenin can reduce TNF-α by 87% and block LPS-induced CCL5, IL-6, and IL-1β secretion, while the same concentration of liquiritigenin can only inhibit LPS-induced TNF-α, IL-1β, IL-6, IL-8, and CCL5 by 17%, 34%, 23%, 11%, and 24%, respectively. This indicates a potential structure–activity relationship. Another study investigated the antibacterial effects of prunin laurate and its analogs on
P. gingivalis [
107]. The lauroyl chain moiety of prunin laurate is essential for growth inhibition and could be associated with the bacterial membrane, and physical damage to the cell membrane is not the cause of growth inhibition.
Recent studies have demonstrated the significant potential of phlorizin and phloretin in inhibiting
P. gingivalis and gingipains [
28,
108]. In addition to targeted inhibition of gingipains, transcriptomics-based studies have revealed that phlorizin, phloretin, and naringenin can all inhibit the transposition function of
P. gingivalis [
108]. This provides important guidance for dealing with the drug resistance of
P. gingivalis.
In summary, flavonoids have great potential in reducing P. gingivalis toxicity and inhibiting inflammatory reactions, and they have an obvious structure–activity relationship, which deserves further exploration.
Flavonoids readily interact with other food components, complicating the study of their absorption mechanisms. Furthermore, flavonoids are utilized by intestinal microbiota and then metabolize into other products, reducing their blood concentrations [
109]. In vitro and in vivo studies have shown that flavonoid aglycones have higher bioavailability than glycosides, which are often metabolized to aglycones before exerting their effects. However, the natural concentration of aglycones in plants is low, making dietary intake difficult.
6.2. Polysaccharides and Oligosaccharides
There are few studies on the inhibitory effects of polysaccharides and oligosaccharides on
P. gingivalis. The findings are mostly focused on reducing adhesion ability, and only a few studies report direct antibacterial effects and enzyme inhibitory abilities. Polysaccharides extracted from
Panax ginseng exert strong anti-adhesive activity against oral pathogens in a dose-dependent manner at concentrations of 0.1–2.0 mg/mL; partial hydrolysis of the polysaccharide into oligosaccharides retains anti-adhesive activity, but enzymatic hydrolysis results in a complete loss [
110]. This anti-adhesive activity has also been observed in
P. gingivalis-related studies on
Glycyrrhiza glabra polysaccharides and
Rhododendron ferrugineum leaf polysaccharides, reducing the adhesion of
P. gingivalis by 60% and 75%, respectively [
111,
112]. In addition,
Rhododendron ferrugineum polysaccharide has a time- and dose-dependent inhibitory effect on the activity of Rgp but had no effect on KGP, and an inhibitory effect on hemagglutinin was also observed. Exopolysaccharides (EPSs) from
Lactobacillus plantarum EIR/IF-1 postbiotics inhibit the auto- and co-aggregation of
P. gingivalis, exhibiting antibiofilm activity, which may be due to reduced bacterial hydrophobicity, thereby inhibiting bacterial aggregation [
113]. In addition to having an IC
50 of 5 mg/mL against
P. gingivalis, Jujube polysaccharides (JPs) not only reduce the adhesion ability but also the invasion ability and cytotoxicity of
P. gingivalis. This can lead to significant changes in the composition of the oral microbiome, potentially reshaping the oral microbiota [
114]. However, the complex structure of polysaccharides makes it difficult to explore structure–activity relationships.
Interestingly, drug-free and non-crosslinked chitosan scaffolds can induce the formation of
P. gingivalis cell clusters and exhibit direct antibacterial activity. As chitosan can be used to make antibacterial films and hydrocolloids and enhance the antibacterial properties of materials, the composite material formed by cross-linking chitosan and small-molecule inhibitors may represent a new development direction for
Porphyromonas gingivalis antibacterial agents [
115,
116,
117].
These polysaccharides have only been reported in in vitro experimental data, and no data related to bioavailability and pharmacokinetics are available.
6.3. Others
Berberine extracted from
Coptidis rhizoma has a direct antibacterial effect on
P. gingivalis but can also reverse the downregulation of osteogenesis-related gene expression in bone mesenchymal stem cells (BMSCs) caused by
P. gingivalis [
118,
119]. It can also inhibit collagenase activity. The expression of osteogenic-related genes such as OSX, COLI, ALP, OCN, and OPN significantly increases after berberine treatment, showing a combination of antibacterial effects and promotion of osteogenic differentiation. Wnt signaling-specific inhibitor DKK-1 can block the effect of berberine, indicating that its mechanism of action is involved in regulating the Wnt signaling pathway. Berberine is already a registered drug, and the results of related pharmacokinetic studies have been made public. However, due to its poor fat solubility and poor gastrointestinal absorption, its bioavailability is low, and increasing the dosage may lead to toxicity risks.
Corydalis saxicola bunting total alkaloids (CSBTA) can block the pyroptosis of macrophages caused by
P. gingivalis LPS [
120]. The alkali-transformed saponin extracted from quinoa shell has an antibacterial effect on
P. gingivalis, and reducing the polarity of the saponin can improve its antibacterial performance. In addition, the leakage of cell components suggests that its mechanism of action may be the destruction of bacterial cell membranes [
121].
Some studies have explored the effects of natural extracts on
P. gingivalis-related issues but not the specific components responsible for their effects. For example, aqueous extract from the leaves of
Rhododendron ferrugineum has demonstrated the ability to inhibit Rgp, and
Copaifera reticulata oleoresin has demonstrated antibacterial activity against various oral pathogens, including
P. gingivalis [
112,
122]. The acetone–water extract of
Limonium brasiliense (Lb) roots can inhibit gingipain activity and adhesion; the reduced bacterial adhesion is due to a strong interaction between proanthocyanidins and gingipains [
123]. Natural products extracted from
Boswellia trees (
B. serrata) have also shown antimicrobial activity against
P. gingivalis growth and biofilm formation [
124]. The peel extract of
Garcinia mangostana L. not only has an antibacterial effect but also has a significant inhibitory effect on biofilm formation. The author of one study speculates that this function may come from its xanthone derivatives, flavonoids, and saponins [
125].
Table 1.
Parts of polyphenols that are shown to be active against P. gingivalis and its virulence factors.
Table 1.
Parts of polyphenols that are shown to be active against P. gingivalis and its virulence factors.
| Nature Products | Type | Food Source | Mechanism | Virulence Factors | Model | References |
|---|
| EGCG | Polyphenol | Tea | Antibacterial, anti-inflammation, reduces virulence, alleviates alveolar bone loss, reduces invasion ability, enhances immune system defense, inhibits gingipain expression | LPS, KGP, Rgp, kgp, rgp, fimA | A, O | [60,61,62,63,64,65,66,126] |
| Catechin | Polyphenol | Tea | Anti-inflammation | / | A | [67] |
| Theaflavins | Polyphenol | Tea | Antibacterial, anti-inflammation, reduces virulence, alleviates alveolar bone loss, enhances immune system defense | KGP/Rgp | O | [69,70,71] |
| Proanthocyanidin | Polyphenol | Berry | Anti-inflammation, reduces virulence, alleviates alveolar bone loss | LPS | O | [74,75,76,77,78,127] |
| Resveratrol | Polyphenol | Grapes, peanuts | Anti-inflammation, inhibits virulence factor expression | LPS | A, O | [85,86,87,88,91,128] |
Quercetin/
Quercetagetin | Flavonoids | Potato leaves, pea leaves | Antibacterial, regulates MAPK pathway, protect alveolar bone | LPS | A, O | [95,96,129,130,131] |
| Luteolin | Flavonoids | Peanut shells | Inhibits MAPK, AKT, and NF-κB signaling pathways; reduces alveolar bone resorption | LPS | A, O | [97,98] |
Fisetin/
Myricetin | Flavonoids | Berry, apple | Inhibits MAPK, AKT, and NF-κB signaling pathways; blocks COX-2 expression | LPS, kgp, rgp, fimA | O | [98,99,100] |
| Baicalin | Flavonoids | Scutellaria baicalensis | Inhibits MAPK, NF-κB, TLR, and RhoA/ROCK signaling pathways; protects osteoblasts and alveolar bone | LPS | A, O | [101,102,132] |
| Hesperidin | Flavonoids | Citrus peel | Antibacterial, anti-inflammation | LPS | O | [104] |
Isoliquiritigenin/
Licoricidin/
Licorisoflavan A | Flavonoids | Licorice | Inhibits NF-κB p65 and activator protein-1 | LPS | O | [103,105,106] |
Phloretin/
Phlorizin | Flavonoids | Apple | Antibacterial, inhibits KGP activities | KGP | O | [28] |
| Naringenin | Flavonoids | Citrus peel | Antibacterial, inhibits KGP activities | KGP | O | [28] |
Table 2.
Parts of natural products (excluding polyphenols) that are shown to be active against P. gingivalis and its virulence factors.
Table 2.
Parts of natural products (excluding polyphenols) that are shown to be active against P. gingivalis and its virulence factors.
| Nature Products | Type | Food Source | Mechanism | Virulence Factors | Model | References |
|---|
| PG-F2/PG-HMW | Polysaccharide | Panax ginseng | Anti-adhesion | / | O | [110] |
| Glycyrrhiza glabra polysaccharides | Polysaccharide | Glycyrrhiza glabra | Anti-adhesion | / | O | [111] |
| RF-RPS | Polysaccharide | Rhododendron ferrugineum | Anti-adhesion | KGP, Rgp, kgp, rgp, fimA | O | [112] |
| EIR/IF-1 EPSs | Polysaccharides | Lactobacillus plantarum EIR/IF-1 | Anti-adhesion | / | O | [113] |
| JP | Polysaccharide | Jujube | Anti-adhesion, reduces invasion ability and cytotoxicity, reshapes oral microbiota | LPS | O | [114] |
| CS | Polysaccharide | Shrimp shells, crab shells | Adsorbs bacterial cells | / | O | [115] |
| Berberine | Alkaloid | Coptidis rhizoma | Antibacterial, restores osteogenesis-related gene expression | / | O | [118] |
| CSBTA | Alkaloid | Corydalis saxicola Bunting | Blocks macrophage pyroptosis | LPS | O | [120] |
| QS/ATS | Saponin | Quinoa | Destroys bacterial cell membrane | / | O | [121] |
7. Conclusions
This study is an overview of P. gingivalis and gingipains and their hazards, summarizing relevant research on natural products for treating related problems. The structural characteristics of gingipain indicate that targeting specific domains to inhibit its function is an effective way to reduce the pathogenicity and abundance of P. gingivalis.
Antibiotic treatment represented by metronidazole is currently the main treatment for P. gingivalis related problems. Substitution with food-derived natural products can effectively complement the shortcomings of antibiotic treatment. A survey of relevant studies shows that polyphenols, represented by tea polyphenols, cranberry polyphenols, resveratrol, and various flavonoids, have good therapeutic effects, especially in inhibiting inflammatory reactions and alleviating alveolar bone loss. Epigallocatechin gallate, phloretin, and phlorizin can inhibit KGP and/or Rgp activity, and structure–activity relationships suggest that galloyl moieties and dihydrochalcone scaffolds enhance gingipain inhibition. Clear structure–activity relationships will make research on gingipains more realistic.
To address the common problem of low bioavailability in polyphenol compounds, esterification modification may be considered to improve their bioavailability; nanoparticle encapsulation may also be used to enhance delivery performance and reduce the impact of intestinal microbiota.
Notably, few relevant studies of natural products have explored the structure–activity relationship between their structures and gingipain inhibition. These products represent promising gingipain inhibitors, with potential structure–activity relationships indicating that food-derived natural products have considerable research prospects. Understanding of structure–activity relationships will inspire structure-based virtual screening and greatly improve the efficiency of researchers in discovering potential inhibitors.
However, due to a lack of evidence from clinical practice, the summary of natural products in this article mainly focuses on in vitro tests and animal studies. The complex in vivo environment and differences between species will have a great impact on the practical application of these natural products. More original research is needed to prove the feasibility of developing inhibitors derived from natural products. Future research should prioritize structure-based discovery and structure optimization to realize their therapeutic potential, focusing on the in vivo application of these natural products.
Author Contributions
Conceptualization, D.W. and X.L. (Xiaofeng Li); methodology, D.W. and G.Z.; software, D.W., G.Z. and L.H.; validation, D.W., X.L. (Xiaofeng Li), G.Z., L.H. and X.L. (Xiaohan Liu); formal analysis, D.W., L.H. and X.L. (Xiaohan Liu); investigation, D.W. and X.L. (Xiaohan Liu); resources, X.L. (Xiaofeng Li) and G.Z.; data curation, D.W. and X.L. (Xiaohan Liu); writing—original draft preparation, D.W.; writing—review and editing, D.W. and X.L. (Xiaofeng Li); visualization, D.W., L.H. and X.L. (Xiaohan Liu); supervision, X.L. (Xiaofeng Li) and G.Z.; project administration, X.L. (Xiaofeng Li); funding acquisition, D.W. and X.L. (Xiaofeng Li). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 22278159), the Natural Science Foundation of Guangdong Province (Grant No. 2024A1515011105), the Natural Science Foundation of Fujian Province (Grant No. 2025J08136), and the Doctoral Research Start-up Fund of Liming Vocational University (Grant No. LZB202501).
Conflicts of Interest
The authors have no conflicts of interest to declare.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Aleksijević, L.H.; Aleksijević, M.; Škrlec, I.; Šram, M.; Šram, M.; Talapko, J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens 2022, 11, 1173. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Guevara, T.; Rodríguez-Banqueri, A.; Lasica, A.M.; Ksiazek, M.; Potempa, B.A.; Potempa, J.; Gomis-Rüth, F.X. Structural determinants of inhibition of Porphyromonas gingivalis gingipain K by KYT-36, a potent, selective, and bioavailable peptidase inhibitor. Sci. Rep. 2019, 9, 4935. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, X.; Song, Z.; Li, L.; Chang, H.; Li, S.; Zhou, W. Quercetin inhibits virulence properties of Porphyromas gingivalis in periodontal disease. Sci. Rep. 2020, 10, 18313. [Google Scholar] [CrossRef] [PubMed]
- Śmiga, M.; Olczak, T. Exploring heme and iron acquisition strategies of Porphyromonas gingivalis—Current facts and hypotheses. FEMS Microbiol. Rev. 2025, 49, fuaf019. [Google Scholar] [CrossRef]
- Guo, Y.; Nguyen, K.A.; Potempa, J. Dichotomy of gingipains action as virulence factors: From cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol. 2000 2010, 54, 15–44. [Google Scholar] [CrossRef]
- Ciaston, I.; Budziaszek, J.; Satala, D.; Potempa, B.; Fuchs, A.; Rapala-Kozik, M.; Mizgalska, D.; Dobosz, E.; Lamont Richard, J.; Potempa, J.; et al. Proteolytic Activity-Independent Activation of the Immune Response by Gingipains from Porphyromonas gingivalis. mBio 2022, 13, e03787-21. [Google Scholar] [CrossRef]
- Olsen, I.; Singhrao, S.K. Is there a link between genetic defects in the complement cascade and Porphyromonas gingivalis in Alzheimer’s disease? J. Oral Microbiol. 2020, 12, 1676486. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and Its Systemic Impact: Current Status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Bhat, S.G.; Sivaraman, K. Porphyromonas gingivalis adopts intricate and unique molecular mechanisms to survive and persist within the host: A critical update. J. Oral Microbiol. 2020, 12, 1801090. [Google Scholar] [CrossRef]
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G.; et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 2014, 15, 768–778. [Google Scholar] [CrossRef]
- Landzberg, M.; Doering, H.; Aboodi, G.M.; Tenenbaum, H.C.; Glogauer, M. Quantifying oral inflammatory load: Oral neutrophil counts in periodontal health and disease. J. Periodontal Res. 2015, 50, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, C.; Zhang, X.; Chen, H.; Dong, J.; Lu, W.; Song, Z.; Zhou, W. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J. Neuroinflamm. 2018, 15, 37. [Google Scholar] [CrossRef]
- Sanz, M.; Kornman, K.; on behalf of working group 3 of the joint, EFP/AAP. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S164–S169. [Google Scholar] [CrossRef]
- Graziani, F.; Gennai, S.; Solini, A.; Petrini, M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes An update of the EFP-AAP review. J. Clin. Periodontol. 2018, 45, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Bretz, W.A.; Abramson, S.B. Periodontal disease and subgingival microbiota as contributors for rheumatoid arthritis pathogenesis: Modifiable risk factors? Curr. Opin. Rheumatol. 2014, 26, 424–429. [Google Scholar] [CrossRef]
- Nakajima, M.; Arimatsu, K.; Kato, T.; Matsuda, Y.; Minagawa, T.; Takahashi, N.; Ohno, H.; Yamazaki, K. Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLoS ONE 2015, 10, e0134234. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, W.; Wang, H.; Liang, S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Struct. Biol. 2020, 120, 45–84. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Herrera, D.; Kozarov, E.; Roldán, S.; Progulske-Fox, A. Periodontal bacterial invasion and infection: Contribution to atherosclerotic pathology. J. Clin. Periodontol. 2013, 40, S30–S50. [Google Scholar] [CrossRef]
- Chow, Y.C.; Yam, H.C.; Gunasekaran, B.; Lai, W.Y.; Wo, W.Y.; Agarwal, T.; Ong, Y.Y.; Cheong, S.L.; Tan, S.-A. Implications of Porphyromonas gingivalis peptidyl arginine deiminase and gingipain R in human health and diseases. Front. Cell. Infect. Microbiol. 2022, 12, 987683. [Google Scholar] [CrossRef]
- Lunar Silva, I.; Cascales, E. Molecular Strategies Underlying Porphyromonas gingivalis Virulence. J. Mol. Biol. 2021, 433, 166836. [Google Scholar] [CrossRef]
- Ito, R.; Ishihara, K.; Shoji, M.; Nakayama, K.; Okuda, K. Hemagglutinin/Adhesin domains of Porphyromonas gingivalis play key roles in coaggregation with Treponema denticola. FEMS Immunol. Med. Microbiol 2010, 60, 251–260. [Google Scholar] [CrossRef]
- Sroka, A.; Sztukowska, M.; Potempa, J.; Travis, J.; Genco, C.A. Degradation of host heme proteins by lysine- and arginine-specific cysteine proteinases (gingipains) of Porphyromonas gingivalis. J. Bacteriol. 2001, 183, 5609–5616. [Google Scholar] [CrossRef]
- Lei, Z.; Ma, Q.; Tu, Y.; Qiu, Y.; Gong, T.; Lin, Y.; Zhou, X.; Li, Y. Nicotinamide employs a starvation strategy against Porphyromonas gingivalis virulence by inhibiting the heme uptake system and gingipain activities. Mol. Oral Microbiol. 2024, 39, 321–333. [Google Scholar] [CrossRef]
- Wu, D.; Liu, X.; Li, X.; Hao, L.; Zhao, G. Discovery of specific inhibitors of Porphyromonas gingivalis gingipains from food-derived flavonoids by high-throughput virtual screening and molecular simulations. Food Biosci. 2024, 61, 104596. [Google Scholar] [CrossRef]
- Zheng, S.; Yu, S.; Fan, X.; Zhang, Y.; Sun, Y.; Lin, L.; Wang, H.; Pan, Y.; Li, C. Porphyromonas gingivalis survival skills: Immune evasion. J. Periodontal Res. 2021, 56, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Potempa, M.; Potempa, J.; Okroj, M.; Popadiak, K.; Eick, S.; Nguyen, K.A.; Riesbeck, K.; Blom, A.M. Binding of complement inhibitor C4b-binding protein contributes to serum resistance of Porphyromonas gingivalis. J. Immunol. 2008, 181, 5537–5544. [Google Scholar] [CrossRef] [PubMed]
- de Jongh, C.A.; de Vriesb, T.J.; Bikker, F.J.; Susan, G.; Krom, B.P. Mechanisms of Porphyromonas gingivalis to translocate over the oral mucosa and other tissue barriers. J. Oral Microbiol. 2023, 15, 2205291. [Google Scholar] [CrossRef]
- Nakayama, M.; Ohara, N. Molecular mechanisms of Porphyromonas gingivalis-host cell interaction on periodontal diseases. Jpn. Dent. Sci. Rev. 2017, 53, 134–140. [Google Scholar] [CrossRef]
- Pritchard, A.B.; Fabian, Z.; Lawrence, C.L.; Morton, G.; Crean, S.; Alder, J.E. An Investigation into the Effects of Outer Membrane Vesicles and Lipopolysaccharide of Porphyromonas gingivalis on Blood-Brain Barrier Integrity, Permeability, and Disruption of Scaffolding Proteins in a Human in vitro Model. J. Alzheimer’s Dis. 2022, 86, 343–364. [Google Scholar] [CrossRef]
- Nonaka, S.; Kadowaki, T.; Nakanishi, H. Secreted gingipains from Porphyromonas gingivalis increase permeability in human cerebral microvascular endothelial cells through intracellular degradation of tight junction proteins. Neurochem. Int. 2022, 154, 105282. [Google Scholar] [CrossRef] [PubMed]
- Movilla, S.; Martí, S.; Roca, M.; Moliner, V. Unrevealing the Proteolytic Activity of RgpB Gingipain from Computational Simulations. J. Chem. Inf. Model. 2021, 61, 4582–4593. [Google Scholar] [CrossRef] [PubMed]
- Yongqing, T.; Potempa, J.; Pike, R.N.; Wijeyewickrema, L.C. The lysine-specific gingipain of Porphyromonas gingivalis: Importance to pathogenicity and potential strategies for inhibition. Adv. Exp. Med. Biol. 2011, 712, 15–29. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, J.; Wu, J.; Sun, W.-B.; Liu, Y. Effect of deletion of the rgpA gene on selected virulence of Porphyromonas gingivalis. J. Dent. Sci. 2016, 11, 279–286. [Google Scholar] [CrossRef]
- de Diego, I.; Veillard, F.T.; Guevara, T.; Potempa, B.; Sztukowska, M.; Potempa, J.; Gomis-Rüth, F.X. Porphyromonas gingivalis virulence factor gingipain RgpB shows a unique zymogenic mechanism for cysteine peptidases. J. Biol. Chem. 2013, 288, 14287–14296. [Google Scholar] [CrossRef]
- de Diego, I.; Veillard, F.; Sztukowska, M.N.; Guevara, T.; Potempa, B.; Pomowski, A.; Huntington, J.A.; Potempa, J.; Gomis-Rüth, F.X. Structure and mechanism of cysteine peptidase gingipain K (Kgp), a major virulence factor of Porphyromonas gingivalis in periodontitis. J. Biol. Chem. 2014, 289, 32291–32302. [Google Scholar] [CrossRef] [PubMed]
- Pomowski, A.; Usón, I.; Nowakowska, Z.; Veillard, F.; Sztukowska, M.N.; Guevara, T.; Goulas, T.; Mizgalska, D.; Nowak, M.; Potempa, B.; et al. Structural insights unravel the zymogenic mechanism of the virulence factor gingipain K from Porphyromonas gingivalis, a causative agent of gum disease from the human oral microbiome. J. Biol. Chem. 2017, 292, 5724–5735. [Google Scholar] [CrossRef]
- Gorman, M.A.; Seers, C.A.; Michell, B.J.; Feil, S.C.; Huq, N.L.; Cross, K.J.; Reynolds, E.C.; Parker, M.W. Structure of the lysine specific protease Kgp from Porphyromonas gingivalis, a target for improved oral health. Protein Sci. 2015, 24, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Veillard, F.; Sztukowska, M.; Mizgalska, D.; Ksiazek, M.; Houston, J.; Potempa, B.; Enghild, J.J.; Thogersen, I.B.; Gomis-Rüth, F.X.; Nguyen, K.A.; et al. Inhibition of gingipains by their profragments as the mechanism protecting Porphyromonas gingivalis against premature activation of secreted proteases. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4218–4228. [Google Scholar] [CrossRef]
- Sztukowska, M.; Veillard, F.; Potempa, B.; Bogyo, M.; Enghild, J.J.; Thogersen, I.B.; Nguyen, K.A.; Potempa, J. Disruption of gingipain oligomerization into non-covalent cell-surface attached complexes. Biol. Chem. 2012, 393, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Robles, A.; Roy, F.; Aruni, A.W.; Sandberg, L.; Nothnagel, E.; Fletcher, H.M. The roles of RgpB and Kgp in late onset gingipain activity in the vimA-defective mutant of Porphyromonas gingivalis W83. Mol. Oral Microbiol. 2015, 30, 347–360. [Google Scholar] [CrossRef]
- Mahmud, A.S.M.; Seers, C.A.; Huq, N.L.; Zhang, L.; Butler, C.A.; Moore, C.; Cross, K.J.; Reynolds, E.C. Production and properties of adhesin-free gingipain proteinase RgpA. Sci. Rep. 2023, 13, 10780. [Google Scholar] [CrossRef]
- Ganuelas, L.; Li, N.; Yun, P.; Hunter, N.; Collyer, C.A. The lysine gingipain adhesin domains from Porphyromonas gingivalis interact with erythrocytes and albumin: Structures correlate to function. Eur. J. Microbiol. Immunol. 2013, 3, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yun, P.; Jeffries, C.M.; Langley, D.; Gamsjaeger, R.; Church, W.B.; Hunter, N.; Collyer, C.A. The modular structure of haemagglutinin/adhesin regions in gingipains of Porphyromonas gingivalis. Mol. Microbiol. 2011, 81, 1358–1373. [Google Scholar] [CrossRef]
- Li, N.; Yun, P.; Nadkarni, M.A.; Ghadikolaee, N.B.; Nguyen, K.-A.; Lee, M.; Hunter, N.; Collyer, C.A. Structure determination and analysis of a haemolytic gingipain adhesin domain from Porphyromonas gingivalis. Mol. Microbiol. 2010, 76, 861–873. [Google Scholar] [CrossRef]
- Ye, P.; Chang, J.; Foo, L.F.; Yap, B.C.M. An early report: A modified porphyrin-linked metronidazole targeting intracellular Porphyromonas gingivalis in cultured oral epithelial cells. Int. J. Oral Sci. 2017, 9, 167–173. [Google Scholar] [CrossRef]
- Rams, T.E.; Sautter, J.D.; van Winkelhoff, A.J. Emergence of antibiotic-resistant Porphyromonas gingivalis in United States periodontitis patients. Antibiotics 2023, 12, 1584. [Google Scholar] [CrossRef]
- Ng, E.; Tay, J.R.H.; Boey, S.K.; Laine, M.L.; Ivanovski, S.; Seneviratne, C.J. Antibiotic resistance in the microbiota of periodontitis patients: An update of current findings. Crit. Rev. Microbiol. 2024, 50, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Granada, M.I.; Guzmán, I.C. Antibiotic resistance of subgingival species in chronic periodontitis patients. J. Periodontal Res. 2010, 45, 557–563. [Google Scholar] [CrossRef]
- Benedyk, M.; Mydel, P.M.; Delaleu, N.; Płaza, K.; Gawron, K.; Milewska, A.; Maresz, K.; Koziel, J.; Pyrc, K.; Potempa, J. Gingipains: Critical Factors in the Development of Aspiration Pneumonia Caused by Porphyromonas gingivalis. J. Innate Immun. 2015, 8, 185–198. [Google Scholar] [CrossRef]
- Kataoka, S.; Baba, A.; Suda, Y.; Takii, R.; Hashimoto, M.; Kawakubo, T.; Asao, T.; Kadowaki, T.; Yamamoto, K. A novel, potent dual inhibitor of Arg-gingipains and Lys-gingipain as a promising agent for periodontal disease therapy. FASEB J. 2014, 28, 3564–3578. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Sugimoto, A.; Leung, K.P.; Nakayama, K.; Kamaguchi, A.; Maeda, N. Inhibitory effect of green tea catechins on cysteine proteinases in Porphyromonas gingivalis. Oral Microbiol. Immunol. 2004, 19, 118–120. [Google Scholar] [CrossRef]
- Schueller, K.; Hans, J.; Pfeiffer, S.; Walker, J.; Ley, J.P.; Somoza, V. Identification of Interleukin-8-Reducing Lead Compounds Based on SAR Studies on Dihydrochalcone-Related Compounds in Human Gingival Fibroblasts (HGF-1 cells) In Vitro. Molecules 2020, 25, 1382. [Google Scholar] [CrossRef] [PubMed]
- Kushiyama, M.; Shimazaki, Y.; Murakami, M.; Yamashita, Y. Relationship Between Intake of Green Tea and Periodontal Disease. J. Periodontol. 2009, 80, 372–377. [Google Scholar] [CrossRef]
- Araghizadeh, A.; Kohanteb, J.; Fani, M.M. Inhibitory Activity of Green Tea (Camellia sinensis) Extract on Some Clinically Isolated Cariogenic and Periodontopathic Bacteria. Med. Princ. Pract. 2013, 22, 368–372. [Google Scholar] [CrossRef]
- Asahi, Y.; Noiri, Y.; Miura, J.; Maezono, H.; Yamaguchi, M.; Yamamoto, R.; Azakami, H.; Hayashi, M.; Ebisu, S. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J. Appl. Microbiol. 2014, 116, 1164–1171. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Z.; Liu, H.; Xuan, Y.; Wang, X.; Luan, Q. Green tea epigallocatechin-3-gallate alleviates Porphyromonas gingivalis-induced periodontitis in mice. Int. Immunopharmacol. 2015, 29, 839–845. [Google Scholar] [CrossRef]
- Wen, W.-C.; Kuo, P.-J.; Chiang, C.-Y.; Chin, Y.-T.; Fu, M.M.J.; Fu, E. Epigallocatechin-3-Gallate Attenuates Porphyromonas gingivalis Lipopolysaccharide-Enhanced Matrix Metalloproteinase-1 Production Through Inhibition of Interleukin-6 in Gingival Fibroblasts. J. Periodontol. 2014, 85, 868–875. [Google Scholar] [CrossRef]
- Cho, A.R.; Kim, J.H.; Lee, D.E.; Lee, J.S.; Jung, U.W.; Bak, E.J.; Yoo, Y.J.; Chung, W.G.; Choi, S.H. The effect of orally administered epigallocatechin-3-gallate on ligature-induced periodontitis in rats. J. Periodontal Res. 2013, 48, 781–789. [Google Scholar] [CrossRef]
- Lombardo Bedran, T.B.; Feghali, K.; Zhao, L.; Palomari Spolidorio, D.M.; Grenier, D. Green tea extract and its major constituent, epigallocatechin-3-gallate, induce epithelial beta-defensin secretion and prevent beta-defensin degradation by Porphyromonas gingivalis. J. Periodontal Res. 2014, 49, 615–623. [Google Scholar] [CrossRef]
- Cai, Y.; Kurita-Ochiai, T.; Hashizume, T.; Yamamoto, M. Green tea epigallocatechin-3-gallate attenuates Porphyromonas gingivalis -induced atherosclerosis. Pathog. Dis. 2013, 67, 76–83. [Google Scholar] [CrossRef]
- Fournier-Larente, J.; Morin, M.-P.; Grenier, D. Green tea catechins potentiate the effect of antibiotics and modulate adherence and gene expression in Porphyromonas gingivalis. Arch. Oral Biol. 2016, 65, 35–43. [Google Scholar] [CrossRef]
- Lee, H.A.; Song, Y.R.; Park, M.H.; Chung, H.-Y.; Na, H.S.; Chung, J. Catechin ameliorates Porphyromonas gingivalis-induced inflammation via the regulation of TLR2/4 and inflammasome signaling. J. Periodontol. 2020, 91, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; La, V.D.; Grenier, D. Antibacterial, Antiadherence, Antiprotease, and Anti-Inflammatory Activities of Various Tea Extracts: Potential Benefits for Periodontal Diseases. J. Med. Food 2013, 16, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Grenier, D. Black tea theaflavins attenuate Porphyromonas gingivalis virulence properties, modulate gingival keratinocyte tight junction integrity and exert anti-inflammatory activity. J. Periodontal Res. 2017, 52, 458–470. [Google Scholar] [CrossRef]
- Kong, L.; Qi, X.; Huang, S.; Chen, S.; Wu, Y.; Zhao, L. Theaflavins inhibit pathogenic properties of P. gingivalis and MMPs production in P. gingivalis-stimulated human gingival fibroblasts. Arch. Oral Biol. 2015, 60, 12–22. [Google Scholar] [CrossRef]
- Lombardo Bedran, T.B.; Morin, M.-P.; Palomari Spolidorio, D.; Grenier, D. Black Tea Extract and Its Theaflavin Derivatives Inhibit the Growth of Periodontopathogens and Modulate Interleukin-8 and β-Defensin Secretion in Oral Epithelial Cells. PLoS ONE 2015, 10, e0143158. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Yang, C.S. Bioavailability of Tea Polyphenols: A Key Factor in Understanding Their Mechanisms of Action In Vivo and Health Effects. J. Agric. Food Chem. 2025, 73, 3816–3825. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, K.; Ben Lagha, A.; Grenier, D. Effects of a Berry Polyphenolic Fraction on the Pathogenic Properties of Porphyromonas gingivalis. Front. Oral Health 2022, 3, 923663. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Ribeiro-Vidal, H.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.V.; Sanz, M.; Herrera, D. New Evidences of Antibacterial Effects of Cranberry Against Periodontal Pathogens. Foods 2020, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C.; Penndorf, K.A.; Feldman, M.; Meron-Sudai, S.; Zakay-Rones, Z.; Steinberg, D.; Fridman, M.; Kashman, Y.; Ginsburg, I.; Ofek, I.; et al. Characterization of non-dialyzable constituents from cranberry juice that inhibit adhesion, co-aggregation and biofilm formation by oral bacteria. Food Funct. 2017, 8, 1955–1965. [Google Scholar] [CrossRef]
- Tanabe, S.; Santos, J.; La, V.D.; Howell, A.B.; Grenier, D. A-Type Cranberry Proanthocyanidins Inhibit the RANKL-Dependent Differentiation and Function of Human Osteoclasts. Molecules 2011, 16, 2365–2374. [Google Scholar] [CrossRef]
- Yamanaka, A.; Kouchi, T.; Kasai, K.; Kato, T.; Ishihara, K.; Okuda, K. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J. Periodontal Res. 2007, 42, 589–592. [Google Scholar] [CrossRef]
- Savickiene, N.; Jekabsone, A.; Raudone, L.; Abdelgeliel, A.S.; Cochis, A.; Rimondini, L.; Makarova, E.; Grinberga, S.; Pugovics, O.; Dambrova, M. Efficacy of proanthocyanidins from Pelargonium sidoides root extract in reducing P. gingivalis viability while preserving oral commensal S. salivarius. Materials 2018, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Jekabsone, A.; Sile, I.; Cochis, A.; Makrecka-Kuka, M.; Laucaityte, G.; Makarova, E.; Rimondini, L.; Bernotiene, R.; Raudone, L.; Vedlugaite, E.; et al. Investigation of Antibacterial and Antiinflammatory Activities of Proanthocyanidins from Pelargonium sidoides DC Root Extract. Nutrients 2019, 11, 2829. [Google Scholar] [CrossRef]
- Bodet, C.; Grenier, D.; Chandad, F.; Ofek, I.; Steinberg, D.; Weiss, E.I. Potential Oral Health Benefits of Cranberry. Crit. Rev. Food Sci. Nutr. 2008, 48, 672–680. [Google Scholar] [CrossRef]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- Lim, Y.R.I.; Preshaw, P.M.; Lin, H.; Tan, K.S. Resveratrol and Its Analogs as Functional Foods in Periodontal Disease Management. Front. Dent. Med. 2021, 2, 636423. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Andrian, E.; Grenier, D. Resveratrol attenuates the pathogenic and inflammatory properties of Porphyromonas gingivalis. Mol. Oral Microbiol. 2019, 34, 118–130. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Wong, R.W.K.; Rabie, A.B.M. Resveratrol Inhibits Periodontal Pathogens In Vitro. Phytother. Res. 2011, 25, 1727–1731. [Google Scholar] [CrossRef]
- Rizzo, A.; Bevilacqua, N.; Guida, L.; Annunziata, M.; Romano Carratelli, C.; Paolillo, R. Effect of resveratrol and modulation of cytokine production on human periodontal ligament cells. Cytokine 2012, 60, 197–204. [Google Scholar] [CrossRef]
- Zhen, L.; Fan, D.-S.; Zhang, Y.; Cao, X.-M.; Wang, L.-M. Resveratrol ameliorates experimental periodontitis in diabetic mice through negative regulation of TLR4 signaling. Acta Pharmacol. Sin. 2015, 36, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-T.; Cheng, G.-Y.; Shih, Y.-J.; Lin, C.-Y.; Lin, S.-J.; Lai, H.-Y.; Whang-Peng, J.; Chiu, H.-C.; Lee, S.-Y.; Fu, E.; et al. Therapeutic applications of resveratrol and its derivatives on periodontitis. Ann. N. Y. Acad. Sci. 2017, 1403, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, T.; Okui, T.; Takahashi, N.; Nakajima, T.; Tabeta, K.; Murakami, S.; Yamazaki, K. Resveratrol suppresses the inflammatory responses of human gingival epithelial cells in a SIRT1 independent manner. J. Periodontal Res. 2015, 50, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Minagawa, T.; Okui, T.; Yamazaki, K. Resveratrol suppresses the alveolar bone resorption induced by artificial trauma from occlusion in mice. Oral Dis. 2018, 24, 412–421. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Pires, P.R.; Ribeiro, F.V.; Pimentel, S.P.; Cirano, F.R.; Napimoga, M.H.; Casati, M.Z.; Casarin, R.C.V. Systemic treatment with resveratrol reduces the progression of experimental periodontitis and arthritis in rats. PLoS ONE 2018, 13, e0204414. [Google Scholar] [CrossRef]
- Kugaji, M.S.; Kumbar, V.M.; Peram, M.R.; Patil, S.; Bhat, K.G.; Diwan, P.V. Effect of Resveratrol on biofilm formation and virulence factor gene expression of Porphyromonas gingivalis in periodontal disease. APMIS 2019, 127, 187–195. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Zhang, Q.; Luo, M.; Lu, F.; He, Z.; Jiang, Q.; Zhang, T. Dual Strategy for Improving the Oral Bioavailability of Resveratrol: Enhancing Water Solubility and Inhibiting Glucuronidation. J. Agric. Food Chem. 2021, 69, 9249–9258. [Google Scholar] [CrossRef]
- Szymkowiak, I.; Marcinkowska, J.; Kucinska, M.; Regulski, M.; Murias, M. Resveratrol Bioavailability After Oral Administration: A Meta-Analysis of Clinical Trial Data. Phytother. Res. 2025, 39, 453–464. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Gutiérrez-Venegas, G. Flavonoids exert multiple periodontic benefits including anti-inflammatory, periodontal ligament-supporting, and alveolar bone-preserving effects. Life Sci. 2018, 209, 435–454. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Torras-Ceballos, A.; Gómez-Mora, J.A.; Fernández-Rojas, B. Luteolin, quercetin, genistein and quercetagetin inhibit the effects of lipopolysaccharide obtained from Porphyromonas gingivalis in H9c2 cardiomyoblasts. Cell Mol. Biol. Lett. 2017, 22, 19. [Google Scholar] [CrossRef]
- Wang, X.-C.; Zhao, N.-J.; Guo, C.; Chen, J.-T.; Song, J.-L.; Gao, L. Quercetin reversed lipopolysaccharide-induced inhibition of osteoblast differentiation through the mitogen-activated protein kinase pathway in MC3T3-E1 cells. Mol. Med. Rep. 2014, 10, 3320–3326. [Google Scholar] [CrossRef]
- Kariu, T.; Hamada, N.; Lakshmyya, K. Luteolin inhibits Porphyromonas gingivalis growth and alleviates alveolar bone destruction in experimental murine periodontitis. Biosci. Biotechnol. Biochem. 2024, 88, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Venegas, G.; Contreras-Sánchez, A. Luteolin and fisetin inhibit the effects of lipopolysaccharide obtained from Porphyromonas gingivalis in human gingival fibroblasts. Mol. Biol. Rep. 2013, 40, 477–485. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Contreras-Sánchez, A.; Ventura-Arroyo, J.A. Anti-inflammatory activity of fisetin in human gingival fibroblasts treated with lipopolysaccharide. J. Asian Nat. Prod. Res. 2014, 16, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Grenier, D.; Chen, H.; Amel Ben, L.; Fournier-Larente, J.; Morin, M.-P. Dual Action of Myricetin on Porphyromonas gingivalis and the Inflammatory Response of Host Cells: A Promising Therapeutic Molecule for Periodontal Diseases. PLoS ONE 2015, 10, e0131758. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Shi, Y.; Dai, Y.; Wang, F.; Chen, X.; Li, X. Baicalin clears inflammation by enhancing macrophage efferocytosis via inhibition of RhoA/ROCK signaling pathway and regulating macrophage polarization. Int. Immunopharmacol. 2022, 105, 108532. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Wang, B.; Zhang, F.; Niu, Z.; Shi, S.; Cannon, R.D.; Mei, L. Response of Human Periodontal Ligament Cells to Baicalin. J. Periodontol. 2014, 85, 1283–1290. [Google Scholar] [CrossRef]
- Feldman, M.; Santos, J.; Grenier, D. Comparative Evaluation of Two Structurally Related Flavonoids, Isoliquiritigenin and Liquiritigenin, for Their Oral Infection Therapeutic Potential. J. Nat. Prod. 2011, 74, 1862–1867. [Google Scholar] [CrossRef]
- Maquera-Huacho, P.M.; Spolidorio, D.P.; Manthey, J.; Grenier, D. Effect of Hesperidin on Barrier Function and Reactive Oxygen Species Production in an Oral Epithelial Cell Model, and on Secretion of Macrophage-Derived Inflammatory Mediators during Porphyromonas gingivalis Infection. Int. J. Mol. Sci. 2023, 24, 10389. [Google Scholar] [CrossRef]
- Gafner, S.; Bergeron, C.; Villinski, J.R.; Godejohann, M.; Kessler, P.; Cardellina, J.H., II; Ferreira, D.; Feghali, K.; Grenier, D. Isoflavonoids and Coumarins from Glycyrrhiza uralensis: Antibacterial Activity against Oral Pathogens and Conversion of Isoflavans into Isoflavan-Quinones during Purification. J. Nat. Prod. 2011, 74, 2514–2519. [Google Scholar] [CrossRef]
- Feldman, M.; Grenier, D. Cranberry proanthocyanidins act in synergy with licochalcone A to reduce Porphyromonas gingivalis growth and virulence properties, and to suppress cytokine secretion by macrophages. J. Appl. Microbiol. 2012, 113, 438–447. [Google Scholar] [CrossRef]
- Wada, E.; Ito, C.; Shinohara, M.; Handa, S.; Maetani, M.; Yasugi, M.; Miyake, M.; Sakamoto, T.; Yazawa, A.; Kamitani, S. Prunin Laurate Derived from Natural Substances Shows Antibacterial Activity against the Periodontal Pathogen Porphyromonas gingivalis. Foods 2024, 13, 1917. [Google Scholar] [CrossRef]
- Wu, D.; Hao, L.; Liu, X.; Li, X.; Zhao, G. Comparative transcriptomics reveals the mechanism of antibacterial activity of fruit-derived dihydrochalcone flavonoids against Porphyromonas gingivalis. Food Funct. 2024, 15, 9734–9749. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shim, J.S.; Chung, M.-S.; Lim, S.-T.; Kim, K.H. Inhibition of Pathogen Adhesion to Host Cells by Polysaccharides from Panax ginseng. Biosci. Biotechnol. Biochem. 2009, 73, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Wittschier, N.; Faller, G.; Hensel, A. Aqueous extracts and polysaccharides from Liquorice roots (Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter pylori to human gastric mucosa. J. Ethnopharmacol. 2009, 125, 218–223. [Google Scholar] [CrossRef]
- Löhr, G.; Beikler, T.; Hensel, A. Inhibition of in vitro adhesion and virulence of Porphyromonas gingivalis by aqueous extract and polysaccharides from Rhododendron ferrugineum L. A new way for prophylaxis of periodontitis? Fitoterapia 2015, 107, 105–113. [Google Scholar] [CrossRef]
- Karaca, B.; Haliscelik, O.; Gursoy, M.; Fadime, K.; Loimaranta, V.; Söderling, E.; Ulvi Kahraman, G. Analysis of Chemical Structure and Antibiofilm Properties of Exopolysaccharides from Lactiplantibacillus plantarum EIR/IF-1 Postbiotics. Microorganisms 2022, 10, 2200. [Google Scholar] [CrossRef]
- Xu, D.; Xiao, J.; Jiang, D.; Liu, Y.; Gou, Z.; Li, J.; Shi, M.; Wang, X.; Guo, Y.; Ma, L.; et al. Inhibitory effects of a water-soluble jujube polysaccharide against biofilm-forming oral pathogenic bacteria. Int. J. Biol. Macromol. 2022, 208, 1046–1062. [Google Scholar] [CrossRef]
- Li, Y.; Chi, Y.-Q.; Yu, C.-H.; Xie, Y.; Xia, M.-Y.; Zhang, C.-L.; Han, X.; Peng, Q. Drug-free and non-crosslinked chitosan scaffolds with efficient antibacterial activity against both Gram-negative and Gram-positive bacteria. Carbohydr. Polym. 2020, 241, 116386. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Xiao, X.; Duan, Q.; Bai, H.; Cao, Y.; Zhang, Y.; Alee, M.; Yu, L. Improved hydrophobicity, antibacterial and mechanical properties of polyvinyl alcohol/quaternary chitosan composite films for antibacterial packaging. Carbohydr. Polym. 2023, 312, 120755. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cao, Y.; Ren, J.; Xie, Y.; Xiao, X.; Zou, Y.; Bai, H.; Zhang, X.; Chen, Y. Optimization of starch foam extrusion through PVA polymerization, moisture content control, and CMCS incorporation for enhanced antibacterial cushioning packaging. Carbohydr. Polym. 2025, 347, 122763. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, J.; Wu, J.; Xiao, L.; Miao, L.; Qi, X.; Li, Y.; Sun, W. Berberine promotes osteogenic differentiation of mesenchymal stem cells with therapeutic potential in periodontal regeneration. Eur. J. Pharmacol. 2019, 851, 144–150. [Google Scholar] [CrossRef]
- Hu, J.P.; Takahashi, N.; Yamada, T. Coptidis Rhizoma inhibits growth and proteases of oral bacteria. Oral Dis. 2000, 6, 297–302. [Google Scholar] [CrossRef]
- Feng, C.; Yong, X.; Jiang, Q.; Su, Z.; Liu, Z.; Wu, T.; Tao, R. Inhibitory Effects of Corydalis saxicola Bunting Total Alkaloids on Macrophage Pyroptosis. Chem. Biodivers. 2023, 20, e202201255. [Google Scholar] [CrossRef]
- Sun, X.; Yang, X.; Xue, P.; Zhang, Z.; Ren, G. Improved antibacterial effects of alkali-transformed saponin from quinoa husks against halitosis-related bacteria. BMC Complement. Altern. Med. 2019, 19, 46. [Google Scholar] [CrossRef]
- Bardají, D.K.R.; da Silva, J.J.M.; Bianchi, T.C.; de Souza Eugênio, D.; de Oliveira, P.F.; Leandro, L.F.; Rogez, H.L.G.; Venezianni, R.C.S.; Ambrosio, S.R.; Tavares, D.C.; et al. Copaifera reticulata oleoresin: Chemical characterization and antibacterial properties against oral pathogens. Anaerobe 2016, 40, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Isolani, R.; Pilatti, F.; de Paula, M.N.; Valone, L.; da Silva, E.L.; de Oliveira Caleare, A.; Seixas, F.A.V.; Hensel, A.; Mello, J.C.P.D. Limonium brasiliense rhizomes extract against virulence factors of Porphyromonas gingivalis: Inhibition of gingipains, bacterial adhesion, and biofilm formation. Fitoterapia 2024, 177, 106120. [Google Scholar] [CrossRef] [PubMed]
- Vang, D.; Moreira-Souza, A.C.; Zusman, N.; Moncada, G.; Matshik Dakafay, H.; Asadi, H.; Ojcius, D.M.; Almeida-da-Silva, C.L. Frankincense (Boswellia serrata) Extract Effects on Growth and Biofilm Formation of Porphyromonas gingivalis, and Its Intracellular Infection in Human Gingival Epithelial Cells. Curr. Issues Mol. Biol. 2024, 46, 2991–3004. [Google Scholar] [CrossRef]
- Widyarman, A.S.; Lay, S.H.; Wendhita, I.P.; Tjakra, E.E.; Murdono, F.I.; Binartha, C.T.O. Indonesian Mangosteen Fruit (Garcinia mangostana L.) Peel Extract Inhibits Streptococcus mutans and Porphyromonas gingivalis in Biofilms In vitro. Contemp. Clin. Dent. 2019, 10, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Pang, E.-K.; Kim, C.-S.; Yoo, Y.-J.; Cho, K.-S.; Chai, J.-K.; Kim, C.-K.; Choi, S.-H. Inhibitory effects of green tea polyphenol (–)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J. Periodontal Res. 2004, 39, 300–307. [Google Scholar] [CrossRef]
- Bodet, C.; Piché, M.; Chandad, F.; Grenier, D. Inhibition of periodontopathogen-derived proteolytic enzymes by a high-molecular-weight fraction isolated from cranberry. J. Antimicrob. Chemother. 2006, 57, 685–690. [Google Scholar] [CrossRef]
- Park, H.-J.; Jeong, S.-K.; Kim, S.-R.; Bae, S.-K.; Kim, W.-S.; Jin, S.-D.; Koo, T.H.; Jang, H.-O.; Yun, I.; Kim, K.-W.; et al. Resveratrol inhibits Porphyromonas gingivalis lipopolysaccharide-induced endothelial adhesion molecule expression by suppressing NF-κB activation. Arch. Pharmacal Res. 2009, 32, 583–591. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Jiménez-Estrada, M.; Maldonado, S. The effect of flavonoids on transduction mechanisms in lipopolysaccharide-treated human gingival fibroblasts. Int. Immunopharmacol. 2007, 7, 1199–1210. [Google Scholar] [CrossRef]
- Geoghegan, F.; Wong, R.W.K.; Rabie, A.B.M. Inhibitory effect of quercetin on periodontal pathogens in vitro. Phytother. Res. 2010, 24, 817–820. [Google Scholar] [CrossRef]
- Cheng, W.C.; Huang, R.Y.; Chiang, C.Y.; Chen, J.K.; Liu, C.H.; Chu, C.L.; Fu, E. Ameliorative effect of quercetin on the destruction caused by experimental periodontitis in rats. J. Periodontal Res. 2010, 45, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Cun-Yu, W.; Jin, L. Baicalin Downregulates Porphyromonas gingivalis Lipopolysaccharide-Upregulated IL-6 and IL-8 Expression in Human Oral Keratinocytes by Negative Regulation of TLR Signaling. PLoS ONE 2012, 7, e51008. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).