Anti-Obesity and Hepatoprotective Effects of Herring–Saury Oil Fermented by Lactobacillus brevis KCCM13538P in High-Fat-Diet-Induced Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of GmO and GmOLb
2.2. Fatty Acid and Fat-Soluble Vitamin Analysis
2.3. T3-L1 Cell Culture and Adipogenic Differentiation
2.4. Cell Viability and Lipid Accumulation Assays
2.5. Animal Study Design
2.6. Serum Biochemical Analysis
2.7. Statistical Analysis
3. Results
3.1. Fatty Acid Composition of GmO and GmOLb
3.2. Contents of Fat-Soluble Vitamins
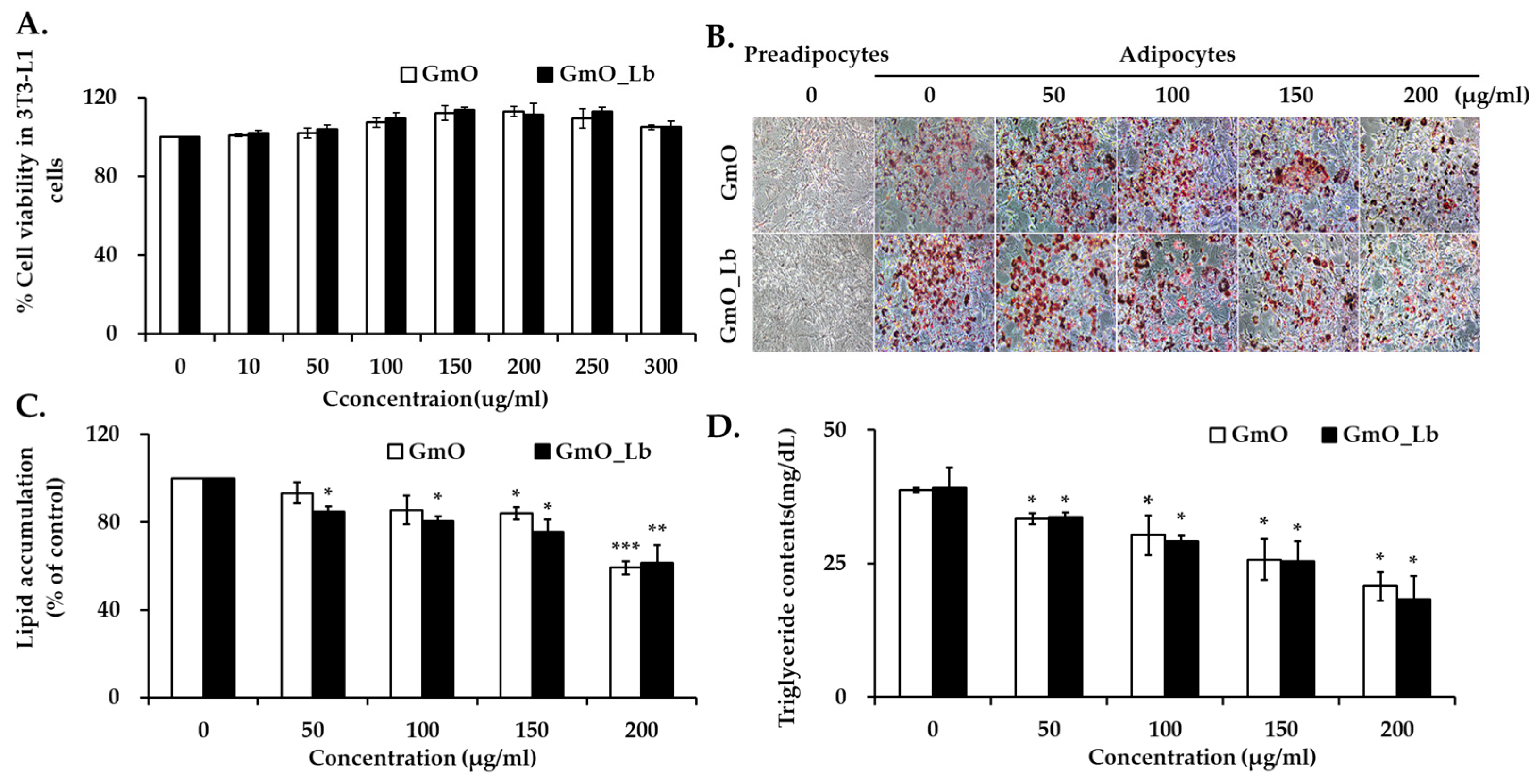
3.3. Effects on Lipid Accumulation in 3T3-L1 Adipocytes
3.4. Effects on Body Weight Gain and Adiposity in HFD-Fed Mice
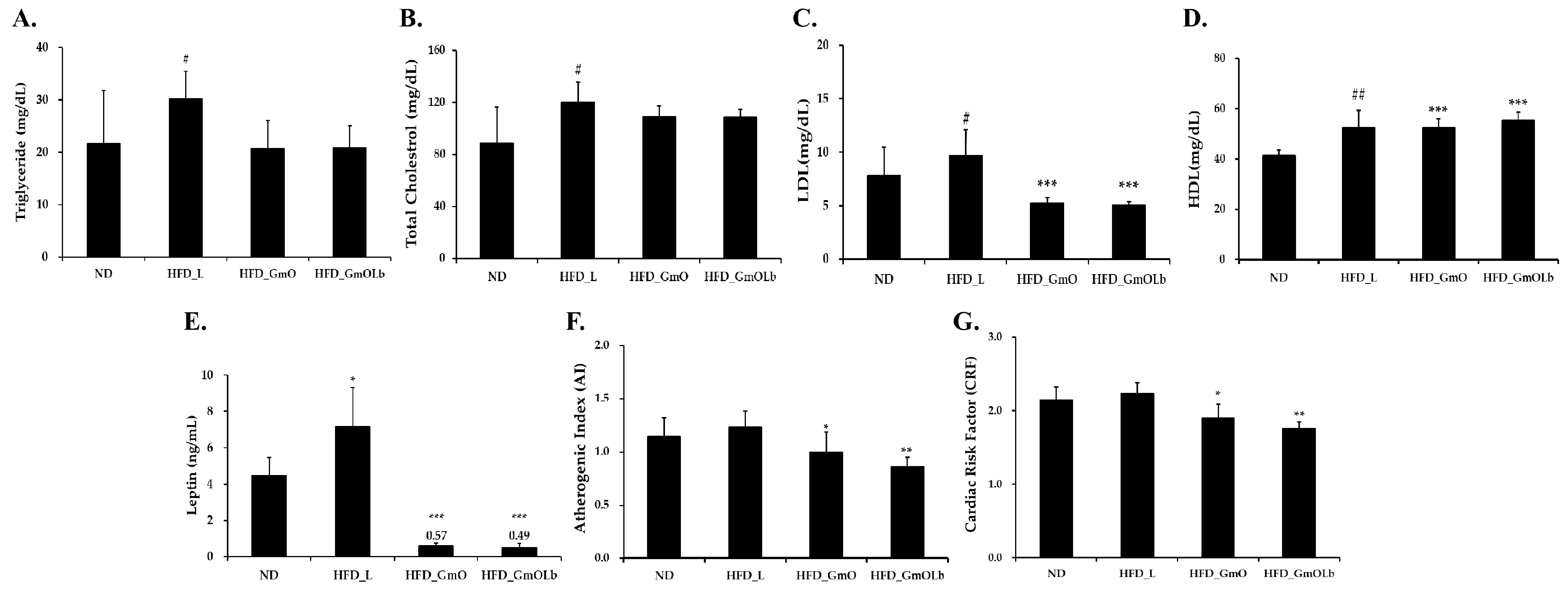
3.5. Effects on Serum Lipid Profiles and Cardiovascular Risk in HFD-Fed Mice
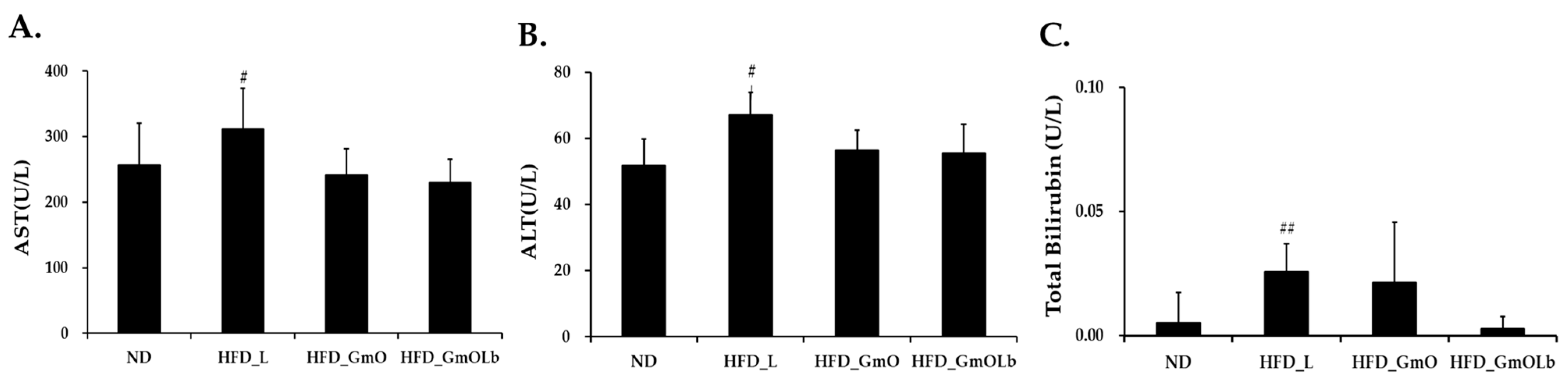
3.6. Effects on Hepatic Enzyme and Bilirubin Levels in HFD-Fed Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14, 21–28. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Jump, D.B.; Depner, C.M.; Tripathy, S. Omega-3 fatty acid supplementation and cardiovascular disease: Thematic review series: New lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J. Lipid Res. 2012, 53, 2525–2545. [Google Scholar] [CrossRef]
- Ross, A.C. Vitamin A and retinoic acid in T cell–related immunity. Am. J. Clin. Nutr. 2012, 96, 1166S–1172S. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; Booth, S.L.; van den Heuvel, E.G.; Stoecklin, E.; Baka, A.; Vermeer, C. The role of menaquinones (vitamin K2) in human health. Br. J. Nutr. 2013, 110, 1357–1368. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Metabolism and cell biology of vitamin K. Thromb. Haemost. 2008, 100, 530–559. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Teunissen, K.J.F.; Hamulyák, K.; Knapen, M.H.J.; Vik, H.; Vermeer, C. Vitamin K–containing dietary supplements: Comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2007, 109, 3279–3283. [Google Scholar] [CrossRef]
- Rai, A.K.; Jini, R.; Swapna, H.C.; Sachindra, N.M.; Bhaskar, N.; Baskaran, V. Application of native lactic acid bacteria (LAB) for fermentative recovery of lipids and proteins from fish processing wastes: Bioactivities of fermentation products. J. Aquat. Food Prod. Technol. 2011, 20, 32–44. [Google Scholar] [CrossRef]
- Özyurt, G.; Durmuş, M.; Sakarya, Y.; Uslu, L.; Kuley, E. Improved oxidative stability of functional oils with spirulina enhanced by probiotic fermentation and encapsulation. J. Appl. Phycol. 2024, 36, 1837–1847. [Google Scholar] [CrossRef]
- Eratte, D.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B. Survival, oxidative stability and surface characteristics of spray-dried co-microcapsules containing omega-3 fatty acids and probiotic bacteria. Dry. Technol. 2016, 34, 1926–1935. [Google Scholar] [CrossRef]
- Tingö, L.; Hutchinson, A.N.; Bergh, C.; Stiefvatter, L.; Schweinlin, A.; Jensen, M.G.; Krüger, K.; Bischoff, S.; Brummer, R.J. Potential modulation of inflammation by probiotic and omega-3 polyunsaturated fatty acid supplementation in elderly with chronic low-grade inflammation—A randomized, placebo-controlled trial. Nutrients 2022, 14, 3998. [Google Scholar] [CrossRef]
- Park, J.E.; Oh, S.H.; Cha, Y.S. Lactobacillus brevis OPK-3 from kimchi prevents obesity and modulates inflammatory gene expression in high-fat diet-induced obese mice. Nutrients 2020, 12, 604. [Google Scholar] [CrossRef]
- Feyereisen, M.; Mahony, J.; Kelleher, P.; Roberts, R.J.; O’Sullivan, T.; Geertman, J.M.A.; van Sinderen, D. Comparative genome analysis of the Lactobacillus brevis species. BMC Genom. 2019, 20, 416. [Google Scholar] [CrossRef] [PubMed]
- Lyberg, A.-M.; Adlercreutz, P. Lipase specificity towards Eicosapentaenoic acid and docosahexaenoic acid depends on substrate structure. Biochim. Biophys. Acta 2008, 1784, 343–350. [Google Scholar] [CrossRef]
- Kuo, C.H.; Huang, C.Y.; Lee, C.L.; Kuo, W.C.; Hsieh, S.L.; Shieh, C.J. Synthesis of DHA/EPA ethyl esters via lipase-catalyzed acidolysis using Novozym® 435: A kinetic study. Catalysts 2020, 10, 565. [Google Scholar] [CrossRef]
- Ichihara, K.; Shibahara, A.; Yamamoto, K.; Nakayama, T. An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids 1996, 31, 535–539. [Google Scholar] [CrossRef]
- Bošnir, J.; Bevardi, M.; Hećimović, I.; Budeč, M.; Cindrić, I.J.; Kober, R.; Jurak, G.; Lasić, D.; Brkić, D.; Racz, A. Optimization of sample preparation procedure for determination of fat-soluble vitamins in milk and infant food by HPLC technique. Processes 2024, 12, 1530. [Google Scholar] [CrossRef]
- Ahmed, S.; Mahmoud, A.M. A novel salting-out assisted extraction coupled with HPLC-fluorescence detection for trace determination of vitamin K homologues in human plasma. Talanta 2015, 144, 480–487. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Zacarias, J.L.; Castro-Munozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition Ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Wanasundara, U.N. Omega-3 fatty acid concentrates: Nutritional aspects and production technologies. Trends Food Sci. Technol. 1998, 9, 230–240. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 PUFA and Inflammation: From Membrane to Nucleus and from Bench to Bedside. Proc. Nutr. Soc. 2020, 79, 404–416. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Col. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free. Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, C. Vitamin K: The effect on health beyond coagulation—An overview. Food Nutr. Res. 2012, 56, 5329. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; De Giori, G.S.; Sesma, F.; Van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Tamura, A.; Makino, T.; Kudo, S. Production of menaquinones by lactic acid bacteria. J. Dairy Sci. 1999, 82, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Laiño, J.E.; Del Valle, M.J.; Vannini, V.; van Sinderen, D.; Taranto, M.P.; de Valdez, G.F.; de Giori, G.S.; Sesma, F. B-Group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef]
- Calvo, M.S.; Whiting, S.J.; Barton, C.N. Vitamin D intake: A global perspective of current status. J. Nutr. 2005, 135, 310–316. [Google Scholar] [CrossRef]
- Bayat, F.; Danafar, H.; Aminzare, M.; Mohseni, M. Investigating the stability of vitamin D3 and Bifidobacterium lactis nanoparticles coated with polycaprolactone-polyethylene glycol-polycaprolactone triblock copolymer in Iranian white cheese: Physicochemical, microbiological, and sensory properties. J. Agric. Food Res. 2024, 15, 101039. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E: Antioxidant activity, biokinetics, and bioavailability. Annu. Rev. Nutr. 1990, 10, 357–382. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, T.; O’Connor, C.; Barlow, J.W.; Walsh, J.; Scalabrino, G.; Xu, F.; Sheridan, H. The Biological Responses of Vitamin K2: A Comprehensive Review. Food Sci. Nutr. 2023, 11, 1634–1656. [Google Scholar] [CrossRef]
- Bonaldo, F.; Leroy, F. Bacterially produced vitamin K2 and its potential to generate health benefits in humans. Trends Food Sci. Technol. 2024, 147, 104461. [Google Scholar] [CrossRef]
- Brooijmans, R.J.W.; Smit, B.A.; dos Santos, F.; Van Riel, J.W.; de Vos, W.M.; Hugenholtz, J. Heme and menaquinone induced electron transport in lactic acid bacteria. Microb. Cell Fact 2009, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Plutzky, J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab. J. 2016, 40, 12–21. [Google Scholar] [CrossRef]
- Kim, H.K.; Della-Fera, M.A.; Lin, J.; Baile, C.A. Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes. J. Nutr. 2006, 136, 2965–2969. [Google Scholar] [CrossRef]
- Clarke, S.D. Polyunsaturated fatty acid regulation of gene transcription: A molecular mechanism to improve the metabolic syndrome. J. Nutr. 2001, 131, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.D.; Howe, P.R. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes. Rev. 2009, 10, 648–659. [Google Scholar] [CrossRef]
- Flachs, P.; Horáková, O.; Brauner, P.; Rossmeisl, M.; Pecina, P.; Franssen-van Hal, N.; Růžičková, J.; Šponarová, J.; Drahota, Z.; Vlček, C.; et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005, 48, 2365–2375. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Jones, P.J. Physiological effects of medium-chain triglycerides: Potential agents in the prevention of obesity. J. Nutr. 2002, 132, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chuang, C.C.; Martinez, K.; Reid, T.; Brown, J.M.; Xi, L.; Hixson, L.; Hopkins, R.; Starnes, J.; McIntosh, M. Conjugated linoleic acid reduces adiposity and increases markers of browning and inflammation in white adipose tissue of mice. J. Lipid Res. 2013, 54, 909–922. [Google Scholar] [CrossRef]
- Den Hartigh, L.J.; Han, C.Y.; Wang, S.; Omer, M.; Chait, A. 10E,12Z-conjugated linoleic acid impairs adipocyte triglyceride storage by enhancing fatty acid oxidation, lipolysis, and mitochondrial reactive oxygen species. J. Lipid Res. 2013, 54, 2964–2978. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.L. Roles for vitamin K beyond coagulation. Annu. Rev. Nutr. 2009, 29, 89–110. [Google Scholar] [CrossRef]
- Aaseth, J.; Alexander, J.; Alehagen, U. The Importance of Vitamin K and the Combination with Vitamin D3 for Bone and Cardiovascular Health. Nutrients 2024, 16, 2420. [Google Scholar] [CrossRef]
- Knapen, M.H.J.; Jardon, K.M.; Vermeer, C. Vitamin K-induced effects on body fat and weight: Results from a 3-year vitamin K2 intervention study. Eur. J. Clin. Nutr. 2018, 72, 136–141. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H.; Hirahara, K.; Sone, H.; Kamiyama, S.; Komai, M. Menaquinone-4 amplified glucose-stimulated insulin secretion in isolated mouse pancreatic islets and INS-1 rat insulinoma cells. Int. J. Mol. Sci. 2019, 20, 1995. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Wei, C.; Wang, X.; Li, R.; Xu, X.; Zhang, Y.; Geng, G.; Dang, K.; Ming, Z.; et al. Vitamin K2 supplementation improves impaired glycemic homeostasis and insulin sensitivity for type 2 diabetes through gut microbiome and fecal metabolites. BMC Med. 2023, 21, 174. [Google Scholar] [CrossRef]
- Adeli, S.; Gargari, B.P.; Karamzad, N. The effects of vitamin K2 (menaquinone-7) on leptin and adiponectin levels in overweight/obese type 2 diabetes patients: A randomized clinical trial. Pharm. Sci. 2023, 29, 346–354. [Google Scholar] [CrossRef]
- Choi, H.J.; Yu, J.; Choi, H.; An, J.H.; Kim, S.W.; Park, K.S.; Jang, H.C.; Kim, S.Y.; Shin, C.S. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: A placebo-controlled trial. Diabetes Care 2011, 34, e147. [Google Scholar] [CrossRef] [PubMed]
- Van Ballegooijen, A.J.; Pilz, S.; Tomaschitz, A.; Grübler, M.; Verheyen, N. The synergistic interplay between vitamins D and K for bone and cardiovascular health: A narrative review. Int. J. Endocrinol. 2017, 2017, 7454376. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Jain, S.; Prakash, S.; Thakur, M. Studies on the synergistic interplay of vitamin D and K for improving bone and cardiovascular health. Curr. Res. Nutr. Food Sci. 2022, 10, 840–857. [Google Scholar] [CrossRef]
- Zúñiga, J.; Cancino, M.; Medina, F.; Varela, P.; Vargas, R.; Tapia, G.; Videla, L.A.; Fernandez, V. n-3 PUFA supplementation triggers PPAR-α activation and PPAR-α/NF-κB interaction: Anti-inflammatory implications in liver ischemia-reperfusion injury. PLoS ONE 2011, 6, e28502. [Google Scholar] [CrossRef]
- Kieronska-Rudek, A.; Kij, A.; Kaczara, P.; Tworzydlo, A.; Napiorkowski, M.; Sidoryk, K.; Chlopicki, S. Exogenous vitamins K exert anti-inflammatory effects dissociated from their role as substrates for synthesis of endogenous MK-4 in murine macrophages cell line. Cells 2021, 10, 1571. [Google Scholar] [CrossRef]
- El-Sherbiny, M.; Atef, H.; Helal, G.M.; Al-Serwi, R.H.; Elkattawy, H.A.; Shaker, G.A.; Said, E.; Abulfaraj, M.; Albalawi, M.A.; Elsherbiny, N.M. Vitamin K2 (MK-7) intercepts the Keap-1/Nrf-2/HO-1 pathway and attenuates inflammatory/apoptotic signaling and liver aging in naturally aging rats. Antioxidants 2022, 11, 2150. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sun, X.; Luo, X.; Zheng, W.; Yin, L.; Zhang, Y.; Fu, Y. A Review on Marine Microbial Docosahexaenoic Acid Production through Circular Economy, Fermentation Engineering, and Antioxidant Technology. Mar. Drugs 2025, 23, 256. [Google Scholar] [CrossRef] [PubMed]

| Fatty Acid (g/100 g) | Lipid Number | GmO | GmOLb | Type |
|---|---|---|---|---|
| SFA (Saturated Fatty Acid) | ||||
| Myristic acid | C14:0 | 4.28 ± 0.21 | 4.33 ± 0.22 | |
| Pentadecanoic acid | C15:0 | 0.37 ± 0.02 | 0.37 ± 0.03 | |
| Palmitic acid | C16:0 | 13.03 ± 0.65 | 12.32 ± 0.62 | |
| Heptadecanoic acid | C17:0 | 0.31 ± 0.02 | 0.35 ± 0.02 | |
| Stearic acid | C18:0 | 2.74 ± 0.14 | 2.6 ± 0.13 | |
| Arachidic acid | C20:0 | 0.23 ± 0.01 | 0.21 ± 0.01 | |
| Eicosanoic acid | C20:0 | 3.33 ± 0.17 | 3.37 ± 0.17 | |
| Heneicosylic acid | C21:0 | 0.07 ± 0.01 | 0.07 ± 0.01 | |
| Behenic acid | C22:0 | 0.08 ± 0.03 | 0.08 ± 0.03 | |
| Total SFAs (g/100 g) | 24.44 ± 0.72 | 23.7 ± 0.69 | ||
| USFA (Unsaturated Fatty Acid) | ||||
| Myristoleic acid | C14:2 | 0.07 ± 0.01 | 0.0 7± 0.02 | MUFA (ω-5) |
| Palmitoleic acid | C16:1 | 6.71 ± 0.34 | 6.92 ± 0.35 | MUFA (ω-7) |
| Oleic acid | C18:1 | 19.2 ± 0.96 | 20.03 ± 1.02 | MUFA (ω-9) |
| Linoleic acid | C18:2 | 2.74 ± 0.14 | 2.79 ± 0.14 | PUFA (ω-6) |
| α-Linolenic acid | C18:3 | 1.33 ± 0.07 | 1.39 ± 0.07 | PUFA (ω-3) |
| γ-Linolenic acid | C18:3 | 0.01 ± 0.00 | 0.15 ± 0.01 | PUFA (ω-6) |
| Elaidic acid | C18:1 | 0.16 ± 0.01 | 0.74 ± 0.04 | Trans-MUFA (ω-9) |
| Linolelaidic acid | C18:2 | 0 | 0.15 ± 0.02 | Trans-PUFA (ω-6) |
| Eicosadienoic acid | C20:2n-6 | 0.28 ± 0.01 | 0.16 ± 0.21 | PUFA (ω-6) |
| Eicosatetraenoic acid | C20:4 | 0.12 ± 0.01 | 0.09 ± 0.02 | PUFA (ω-3) |
| Eicosatrienoic acid | C20:3n-3 | 0.15 ± 0.01 | 0.13 ± 0.01 | PUFA (ω-3) |
| Arachidonic acid | C20:4 | 0.61 ± 0.03 | 0.64 ± 0.02 | PUFA (ω-6) |
| Eicosapentaenoic acid (EPA) | C20:5 | 7.56 ± 0.38 | 7.66 ± 0.38 | PUFA (ω-3) |
| Erucic acid | C22:1 | 0.54 ± 0.03 | 0.48 ± 0.02 | MUFA (ω-9) |
| Docosapentaenoic acid | C22:5 | 0.59 ± 0.03 | 0.62 ± 0.03 | PUFA (ω-3) |
| Docosahexaenoic acid (DHA) | C22:6 | 8.92 ± 0.45 | 9.13 ± 0.46 | PUFA (ω-3) |
| Nervonic acid | C24:1 | 0.44 ± 0.02 | 0.51 ± 0.03 | MUFA (ω-9) |
| Total USFAs (g/100 g) | 49.43 ± 1.19 | 51.66 ± 1.26 |
| Vitamin | Abbreviation | Unit (/100 g) | GmO | GmOLb |
|---|---|---|---|---|
| Vitamin A | Vit A | μg RE | 6999.41 ± 34.99 | 7579.27 ± 37.89 |
| Vitamin D | Vit D | μg | 56.91 ± 0.28 | 67.95 ± 0.34 |
| Vitamin E | Vit E | μg α-TE | 1070 ± 6.85 | 4780 ± 23.91 |
| Vitamin K2 | MK-4 | μg | 42.19 ± 3.26 | 64.13 ± 3.07 |
| MK-7 | μg | 19.28 ± 1.48 | 46.56 ± 1.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.-S.; Goo, T.-W.; Hong, S.-M. Anti-Obesity and Hepatoprotective Effects of Herring–Saury Oil Fermented by Lactobacillus brevis KCCM13538P in High-Fat-Diet-Induced Mice. Foods 2025, 14, 2862. https://doi.org/10.3390/foods14162862
Jo H-S, Goo T-W, Hong S-M. Anti-Obesity and Hepatoprotective Effects of Herring–Saury Oil Fermented by Lactobacillus brevis KCCM13538P in High-Fat-Diet-Induced Mice. Foods. 2025; 14(16):2862. https://doi.org/10.3390/foods14162862
Chicago/Turabian StyleJo, Hyun-Sol, Tae-Won Goo, and Sun-Mee Hong. 2025. "Anti-Obesity and Hepatoprotective Effects of Herring–Saury Oil Fermented by Lactobacillus brevis KCCM13538P in High-Fat-Diet-Induced Mice" Foods 14, no. 16: 2862. https://doi.org/10.3390/foods14162862
APA StyleJo, H.-S., Goo, T.-W., & Hong, S.-M. (2025). Anti-Obesity and Hepatoprotective Effects of Herring–Saury Oil Fermented by Lactobacillus brevis KCCM13538P in High-Fat-Diet-Induced Mice. Foods, 14(16), 2862. https://doi.org/10.3390/foods14162862





