Widely Targeted Metabolomics Decodes Metabolic Remodeling and Functional Shifts in Ganoderma lucidum-Fermented Green Tea Infusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Green Tea Fermentation and Major Chemical Composition Determination
2.3. Determination of Non-Volatile Metabolites During the Fermentation Process of TFG
2.3.1. Sample Preparation
2.3.2. UPLC and ESI-Q TRAP-MS/MS Conditions
2.4. Analysis of the Volatile Metabolites Present in the TFG Samples
2.4.1. Sample Treatment
2.4.2. GC-MS Conditions
2.5. Sensory Evaluation, E-Tongue, and E-Nose Analysis
2.6. Analysis of Antioxidant Capacity and Lipid-Lowering Activities In Vitro
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes in Major Chemical Components
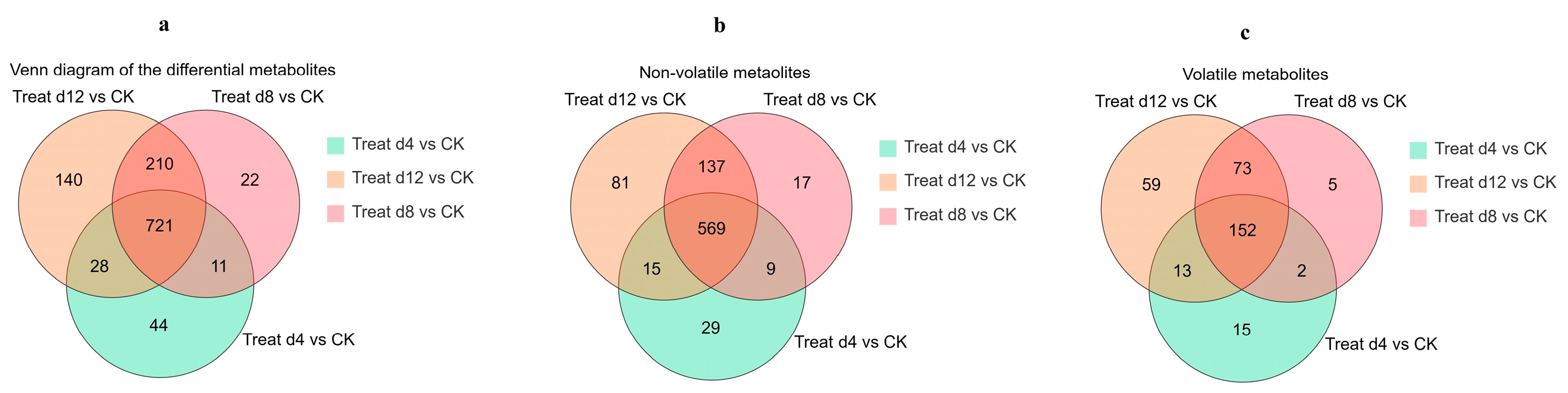
3.2. Analysis of Total Metabolites During the Fermentation
3.3. Evolution of the Differential Metabolites During Fermentation
3.3.1. Flavonoids
3.3.2. Amino Acids and Their Derivatives
3.3.3. Phenolic Acids
3.3.4. Nucleotides and Derivatives
3.4. Changes in the Volatile Metabolites During Fermentation
3.5. Taste and Aroma Characteristics of Green Tea Infusion
3.6. Analysis of Key Aroma-Active Compounds in TFG
3.7. Analysis of In Vitro Antioxidant and Lipid-Lowering Activities of TFG and Associated Metabolites
3.7.1. Changes in Antioxidant and Lipid-Lowering Activities of Green Tea Infusion After Fermentation
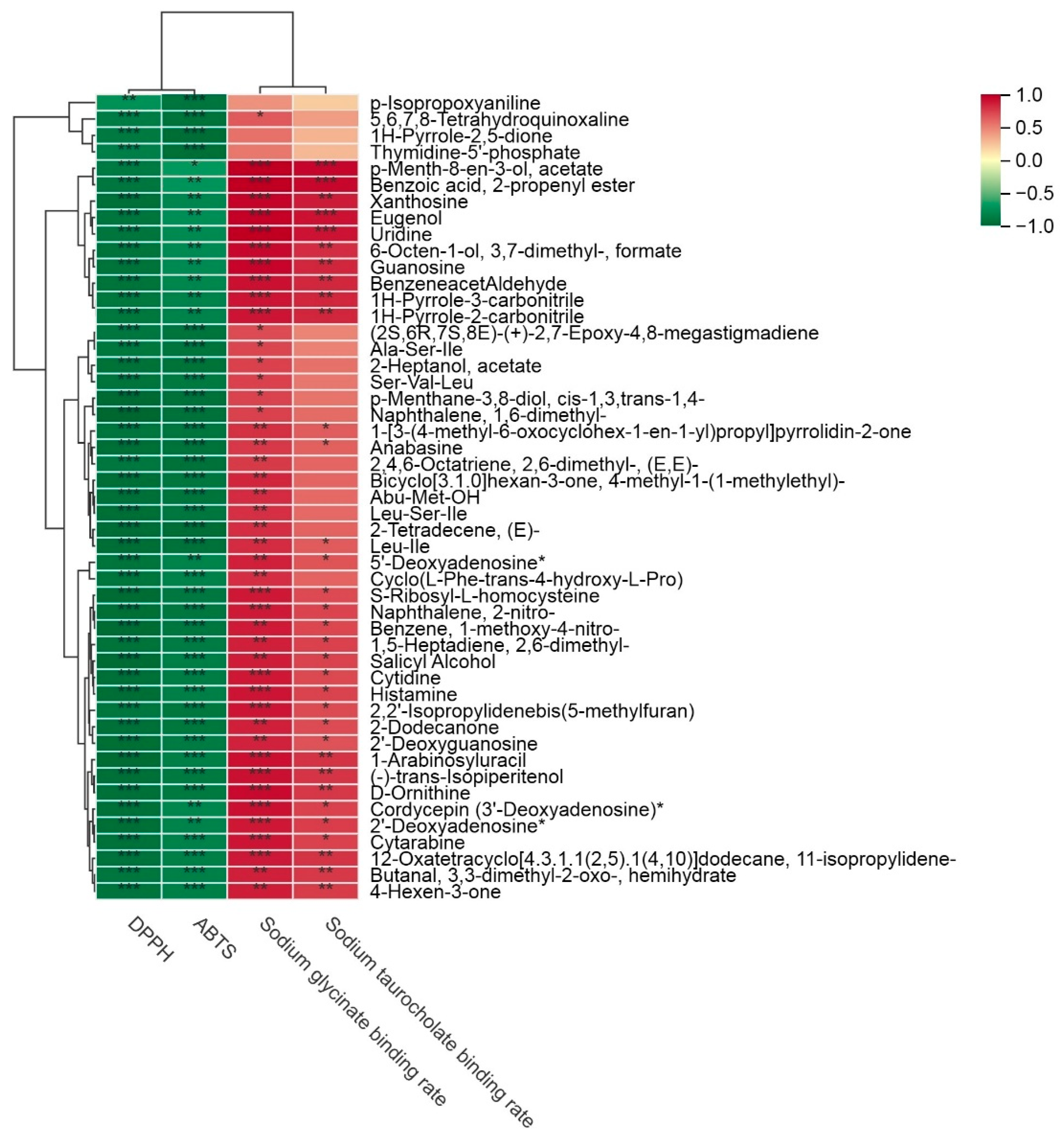
3.7.2. Association Between Differential Metabolites and Antioxidant and Lipid-Lowering Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS·+ | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GC–MS/MS | gas chromatography–tandem mass spectrometry |
| rOAVs | relative odor activity values |
| TFG | green tea infusions fermented by G. lucidum |
| UPLC–MS/MS | ultra-performance liquid chromatography–tandem mass spectrometry |
| VOCs | volatile organic compounds |
References
- Wang, R.; Sun, J.C.; Lassabliere, B.; Yu, B.; Liu, S.Q. Green tea fermentation with Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299V. LWT 2022, 157, 113081. [Google Scholar] [CrossRef]
- Rigling, M.; Heger, F.; Graule, M.; Liu, Z.B.; Zhang, C.; Ni, L.; Zhang, Y.Y. A Robust Fermentation Process for Natural Chocolate-like Flavor Production with Mycetinis scorodonius. Molecules 2022, 27, 2503. [Google Scholar] [CrossRef]
- Li, M.Y.; Xiao, Y.; Zhong, K.; Wu, Y.P.; Gao, H. Delving into the Biotransformation Characteristics and Mechanism of Steamed Green Tea Fermented by Aspergillus niger PW-2 Based on Metabolomic and Proteomic Approaches. Foods 2022, 11, 865. [Google Scholar] [CrossRef]
- Xu, H.L.; Hong, J.H.; Kim, D.; Jin, Y.H.; Pawluk, A.M.; Mah, J.H. Evaluation of Bioactive Compounds and Antioxidative Activity of Fermented Green Tea Produced via One- and Two-Step Fermentation. Antioxidants 2022, 11, 1425. [Google Scholar] [CrossRef]
- Deng, X.J.; Hou, Y.; Zhou, H.J.; Li, Y.L.; Xue, Z.Q.; Xue, X.T.; Huang, G.H.; Huang, K.L.; He, X.Y.; Xu, W.T. Hypolipidemic, anti-inflammatory, and anti-atherosclerotic effects of tea before and after microbial fermentation. Food Sci. Nutr. 2021, 9, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, S.; Ohmachi, K.; Miyata, Y.; Tanaka, T.; Kubayasi, T.; Nagata, Y.; Tanaka, K. Hypotriglyceridemic potential of fermented mixed tea made with third-crop green tea leaves and camellia (Camellia japonica) leaves in Sprague-Dawley rats. J. Agric. Food Chem. 2013, 61, 5817–5823. [Google Scholar] [CrossRef]
- Wu, S.J.; Zhang, S.Y.; Peng, B.; Tan, D.C.; Wu, M.Y.; Wei, J.C.; Wang, Y.T.; Luo, H. Ganoderma lucidum: A comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness 2024, 13, 568–596. [Google Scholar] [CrossRef]
- Wang, C.F.; Liu, X.M.; Lian, C.L.; Ke, J.Y.; Liu, J.Q. Triterpenes and aromatic meroterpenoids with antioxidant activity and neuroprotective effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef]
- Li, C.Q.; Cui, Y.P.; Lu, J.; Meng, L.J.; Ma, C.Y.; Liu, Z.H.; Zhang, Y.; Kang, W.Y. Spectrum-effect relationship of immunologic activity of Ganoderma lucidum by UPLC-MS/MS and component knockout method. Food Sci. Hum. Wellness 2021, 10, 278–288. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, F.; He, Y.M.; Zhang, Q.; Zhang, Y.; Zhou, G.R.; Yang, H.J.; Zhou, P. A novel PTP1B inhibitor extracted from Ganoderma lucidum ameliorates insulin resistance by regulating IRS1-GLUT4 cascades in the insulin signaling pathway. Food Funct. 2018, 9, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.R.; Sheng, Z.L.; Wang, J.P.; Jiang, Y.; Yang, B. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem. 2022, 373, 131374. [Google Scholar] [CrossRef]
- Fu, Y.L.; Shi, L.; Ding, K. Structure elucidation and anti-tumor activity in vivo of a polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Int. J. Biol. Macromol. 2019, 141, 693–699. [Google Scholar] [CrossRef]
- Wang, H.J.; Hua, J.J.; Yu, Q.Y.; Li, J.; Wang, J.J.; Deng, Y.L.; Yuan, H.B.; Jiang, Y.W. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chem. 2021, 363, 130131. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, W.J.; Wang, C.P.; Wu, W.L.; Tian, J.; Zhang, Y.; Shi, Y.L.; Wang, J.T.; Peng, Q.H.; Lin, Z.; et al. Impact of Various Microbial-Fermented Methods on the Chemical Profile of Dark Tea Using a Single Raw Tea Material. J. Agric. Food Chem. 2021, 69, 4210–4222. [Google Scholar] [CrossRef] [PubMed]
- GB/T 30483-2013; Determination of Theaflavins in Tea-High Performance Liquid Chromatography. Standardization Administration of China: Beijing, China, 2013.
- GB/T 8313-2018; Determination of Total Polyphenols and Catechins Content in Tea. Standardization Administration of China: Beijing, China, 2018.
- NY/T 3675-2020; Determination of Thearubigin and Theabrownine in Black Tea-Spectrophotometry. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2020.
- GB/T 8314-2013; Tea-Determination of Free Amino Acids Content. Standardization Administration of China: Beijing, China, 2013.
- NY/T 2742-2015; Determination of Soluble Sugar in Fruits and Derived Products 3,5-Dinitrosalicylic Acid Colorimetry. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2015.
- SN/T 4592-2016; Determination of Total Flavonoids in Export Food. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2016.
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. Standardization Administration of China: Beijing, China, 2018.
- Wen, S.; Jiang, R.; An, R.; Ouyang, J.; Liu, C.; Wang, Z.; Chen, H.; Ou, X.; Zeng, H.; Chen, J.; et al. Effects of pile-fermentation on the aroma quality of dark tea from a single large-leaf tea variety by GC × GC-QTOFMS and electronic nose. Food Res. Int. 2023, 174, 113643. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.J.; Wei, Y.; Deng, W.W.; Wan, X.; Bao, G.H.; Ning, J. Metabolomics based on UHPLC-orbitrap-MS and global natural product social molecular networking reveals effects of time scale and environment of storage on the metabolites and taste quality of raw Pu-erh tea. J. Agric. Food Chem. 2019, 67, 12084–12093. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef]
- Dong, X.; Cai, Y.C.; Liao, H.; Wang, Y.; Chen, Z.H.; Zhou, Y.; Wu, J.R.; Luo, Y.; Sheng, F.Y.; Zhou, Z.M.; et al. Biological transformation of medicine and food homology hawthorn with Monascus ruber to enhance lipid-lowering function. Food Biosci. 2024, 58, 103825. [Google Scholar] [CrossRef]
- Judelson, D.A.; Preston, A.G.; Miller, D.L.; Muãoz, C.X.; Kellogg, M.D.; Lieberman, H.R. Effects of theobromine and caffeine on mood and vigilance. J. Clin. Psychopharmacol. 2013, 33, 499–506. [Google Scholar] [CrossRef]
- Huang, Y.X.; Chen, R.Y.; Chen, Y.L.; Ho, C.T.; Hou, A.X.; Zhang, X.L.; Zhu, M.Z.; Zhang, C.Y.; Wang, Y.L.; Liu, Z.H.; et al. Dynamics changes in volatile profile, non-volatile metabolites and antioxidant activities of dark tea infusion during submerged fermentation with Eurotium cristatum. Food Biosci. 2023, 55, 102966. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Luo, Y.H.; Wang, P.P.; Zhao, M.Y.; Li, L.; Hu, X.S.; Chen, F. Simultaneous determination of free amino acids in Pu-erh tea and their changes during fermentation. Food Chem. 2016, 194, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.X.; Liu, M.M.; Hu, Y.J.; Xue, Q.; Yao, F.; Sun, J.; Sun, L.W.; Liu, Y.J. Systemic characteristics of biomarkers and differential metabolites of raw and ripened pu-erh teas by chemical methods combined with a UPLC-QQQ-MS-based metabolomic approach. LWT 2021, 136, 110316. [Google Scholar] [CrossRef]
- Harbowy, M.E.; Balentine, D.A.; Davies, A.P.; Cai, Y. Tea chemistry. Crit. Rev. Plant Sci. 1997, 16, 415–480. [Google Scholar] [CrossRef]
- Engelhardt, U.H.; Lakenbrink, C.; Lapczynski, S. Antioxidative Phenolic Compounds in Green-Black Tea and Other Methylxanthine-Containing Beverages. Caffeinated Beverages. In ACS National Meeting Book of Abstracts; American Chemical Society: Washington, DC, USA, 1999; Volume 217, pp. 111–118. [Google Scholar]
- Liu, Z.Y.; Ran, Q.S.; Li, Q.; Yang, T.; Dai, Y.Q.; Zhang, T. Interaction between major catechins and umami amino acids in green tea based on electronic tongue technology. J. Food Sci. 2023, 88, 2339–2352. [Google Scholar] [CrossRef]
- Sambandam, T.; Mahadevan, A. Degradation of catechin and purification and partial characterization of catechin oxygenase from Chaetomium cupreum. World J. Microbiol. Biotechnol. 1993, 9, 37–44. [Google Scholar] [CrossRef]
- Cruz-Hernańdez, M.; Contreras-Esquivel, J.C.; Lara, F.; Rodríguez, R.; Aguilar, C.N. Isolation and evaluation of tannin-degrading fungal strains from the Mexican desert. Z. Naturforsch. C J. Biosci. 2005, 60, 844–848. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Chen, J.J.; Ji, X.M.; Hu, X.; Ling, T.J.; Zhang, Z.Z.; Bao, G.H.; Wan, X.C. Changes of major tea polyphenols and production of four new B-ring fission metabolites of catechins from post-fermented Jing-Wei Fu brick tea. Food Chem. 2015, 170, 110–117. [Google Scholar] [CrossRef]
- Cheng, L.Z.; Wei, Y.; Peng, L.L.; Wei, K.; Liu, Z.H.; Wei, X.L. State-of-the-art review of theabrownins: From preparation, structural characterization to health-promoting benefits. Crit. Rev. Food Sci. Nutr. 2024, 64, 11321–11340. [Google Scholar] [CrossRef]
- Lv, H.P.; Zhang, Y.; Shi, J.; Lin, Z. Phytochemical profiles and antioxidant activities of Chinese dark teas obtained by different processing technologies. Food Res. Int. 2017, 100 Pt 3, 486–493. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.Y.; Zhang, Z.; Xiao, J.B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Dai, W.D.; Xie, D.C.; Lu, M.L.; Li, P.L.; Lv, H.P.; Yang, C.; Peng, Q.H.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef]
- Chu, C.; Du, Y.M.; Yu, X.T.; Shi, J.; Yuan, X.L.; Liu, X.M.; Liu, Y.H.; Zhang, H.B.; Zhang, Z.F.; Yan, N. Dynamics of antioxidant activities, metabolites, phenolic acids, flavonoids, and phenolic biosynthetic genes in germinating Chinese wild rice (Zizania latifolia). Food Chem. 2020, 318, 126483. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, X.Q.; Jassbi, A.R.; Xiao, J.B. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.P.; Zhong, Q.S.; Lin, Z.; Wang, L.; Tan, J.F.; Guo, L. Aroma characterization of Pu-erh tea using headspace-solid phase microextraction combined with GC/MS and GC-olfactometry. Food Chem. 2012, 130, 1074–1081. [Google Scholar] [CrossRef]
- Kumar, M.; Selvasekaran, P.; Chidambaram, R.; Zhang, B.H.; Hasan, M.; Gupta, O.P.; Rais, N.; Sharma, K.; Sharma, A.; Lorenzo, J.M.; et al. Tea (Camellia sinensis (L.) Kuntze) as an emerging source of protein and bioactive peptides: A narrative review. Food Chem. 2023, 428, 136783. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumazawa, K.; Masuda, H.; Henze, A.; Hofmann, T. Molecular and sensory studies on the umami taste of Japanese green tea. J. Agric. Food Chem. 2006, 54, 2688–2694. [Google Scholar] [CrossRef]
- Phan, C.W.; Wang, J.K.; Cheah, S.C.; Naidu, M.; David, P.; Sabaratnam, V. A review on the nucleic acid constituents in mushrooms: Nucleobases, nucleosides and nucleotides. Crit. Rev. Biotechnol. 2018, 38, 762–777. [Google Scholar] [CrossRef]
- Peng, J.L.; Peng, Q.X.; Lin, L.M.; Dong, W.W.; Liu, T.S.; Xia, X.H.; Yang, D.J. Simultaneous determination of 13 nucleosides and nucleobases in Ganoderma lucidum and related species by HPLCDAD. Asian J. Chem. 2014, 26, 3477–3482. [Google Scholar] [CrossRef]
- Chen, Y.; Bicker, W.; Wu, J.Y.; Xie, M.Y.; Lindner, W. Simultaneous determination of 16 nucleosides and nucleobases by hydrophilic interaction chromatography and its application to the quality evaluation of Ganoderma. J. Agric. Food Chem. 2012, 60, 4243–4252. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, T.Y.; Sun, C.; Han, T.T.; Zang, M.W.; Wang, D.Y.; Xu, W.M. An electrochemical platform for guanosine-5’-monophosphate detection using gold doped polypyrrole nanocomposite embedded on graphitic carbon nitride. Electrochim. Acta 2022, 415, 140271. [Google Scholar] [CrossRef]
- Schlimme, E.; Martin, D.; Meisel, H. Nucleosides and nucleotides: Natural bioactive substances in milk and colostrum. Br. J. Nutr. 2000, 84 (Suppl. 1), S59–S68. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Wang, M.F.; Adjei, A.A.; Ameho, C.K. Role of nucleosides and nucleotides in the immune system, gut reparation after injury, and brain function. Nutrition 1997, 13, 372–374. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compound in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Ho, C.T.; Zheng, X.; Li, S.M. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Xu, X.Q.; Mo, H.Z.; Yan, M.C.; Zhu, Y. Analysis of characteristic aroma of fungal fermented Fuzhuan brick-tea by gas chromatography/mass spectrophotometry. J. Sci. Food Agric. 2007, 87, 1502–1504. [Google Scholar] [CrossRef]
- Huang, D.Z.; Li, M.R.; Wang, H.; Fu, M.Y.; Hu, S.D.; Wan, X.C.; Wang, Z.C.; Chen, Q. Combining gas chromatography-ion mobility spectrometry and olfactory analysis to reveal the effect of filled-N2 anaerobic treatment duration on variation in the volatile profiles of gabaron green tea. LWT 2023, 179, 114630. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, S.K. Studies on the free and bound aroma compounds in green and fermented teas. Korean J. Food Sci. Technol. 2011, 43, 407–412. [Google Scholar] [CrossRef]
- Huang, W.J.; Fang, S.M.; Wang, J.; Zhuo, C.; Luo, Y.H.; Yu, Y.L.; Li, L.Q.; Wang, Y.J.; Deng, W.W.; Ning, J.M. Sensomics analysis of the effect of the withering method on the aroma components of Keemun black tea. Food Chem. 2022, 395, 133549. [Google Scholar] [CrossRef]
- Tian, H.Y.; Zhang, H.; Sun, B.G. A straightforward synthesis of 5-ethyl-3-hydroxy-4-methyl -2 (5H)-furanone. Flavour Fragr. J. 2009, 24, 234–237. [Google Scholar] [CrossRef]
- Tominaga, T.; Guimbertau, G.; Dubourdieu, D. Contribution of benzenemethanethiol to smoky aroma of certain Vitis vinifera L. wines. J. Agric. Food Chem. 2003, 51, 1373–1376. [Google Scholar] [CrossRef]
- Kang, S.Y.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.P.; Zhang, Y.; Dai, W.D.; Guo, L.; Tan, J.F.; Peng, Q.H.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef]
- Sharma, P.; Ghosh, A.; Tudu, B.; Bhuyan, L.P.; Tamuly, P.; Bhattacharyya, N.; Bandyopadhyay, R.; Das, U. A quartz crystal microbalance sensor for detection of geraniol in black tea. IEEE Sens. J. 2015, 15, 1178–1185. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Feng, W.; Shen, S.; Wei, Y.; Jia, H.; Wang, Y.; Deng, W.; Ning, J. Effects of Three Different Withering Treatments on the Aroma of White Tea. Foods 2022, 11, 2502. [Google Scholar] [CrossRef]
- Hong, L.; Wang, Y.; Zhang, Q.; Wang, Y.; Chen, M.; Li, M.; Huang, Y.X.; Wu, Z.Y.; Ye, J.H.; Wang, H.B. Effects of processing procedures on the formation of aroma intensity and odor characteristic of Benshan tea (Oolong tea, Camellia sentences). Heliyon 2023, 9, e14855. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.Y.; Kim, Y.J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Kuo, K.L.; Weng, M.S.; Chiang, C.T.; Tsai, Y.J.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the hypolipidemic and growth suppressive effects of oolong, black, Pu-erh, and green tea leaves in rats. J. Agric. Food Chem. 2005, 53, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.S.; Peng, C.X.; Chen, T.; Gao, B.; Zhou, H. Effects of theabrownin from pu-erh tea on the metabolism of serum lipids in rats: Mechanism of action. J. Food Sci. 2010, 75, H182–H189. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.J.; Zheng, X.J.; Ma, X.H.; Jiang, R.Q.; Zhou, W.Y.; Zhou, S.P.; Zhang, Y.J.; Lei, S.; Wang, S.L.; Kuang, J.L.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971–4987. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, M.Y.; Wu, Y.P.; Zhong, K.; Gao, H. Structural characteristics and hypolipidemic activity of theabrownins from dark tea fermented by single species Eurotium cristatum PW-1. Biomolecules 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]



| Item | CK | TFG |
|---|---|---|
| Tea polyphenol, g/100 mL | 3.06 ± 0.83 | 1.01 ± 0.12 |
| Total flavonoids, g/100 mL | 0.486 ± 0.091 | 0.0627 ± 0.006 |
| Caffeine, mg/L | 905.87 ± 26.24 | 702.19 ± 49.08 |
| Soluble sugar, g/100 mL | 0.36 ± 0.07 | 0.21 ± 0.03 |
| Free amino acid, g/100 mL | 0.093 ± 0.005 | 0.238 ± 0.028 |
| Theaflavins, mg/L | 3183.07 ± 180.85 | 1165.85 ± 33.83 |
| Theabrownins, mg/100 mL | 1.44 ± 0.08 | 14.70 ± 1.50 |
| Constituent | CK | Treat-d4 | Treat-d8 | Treat-d12 (TFG) | SEM | p-Value | Linear |
|---|---|---|---|---|---|---|---|
| Epigallocatechin gallate, EGCG | 36,077.55 ± 4763.05 a | 802.40 ± 407.13 b | 306.83 ± 76.34 b | 113.07 ± 5.07 b | 1656.12 | <0.01 | <0.01 |
| Gallocatechin gallate, GCG | 21,914.94 ± 988.83 a | 363.05 ± 137.75 b | 82.04 ± 6.32 b | 51.74 ± 5.24 b | 337.31 | <0.01 | <0.01 |
| Epicatechin gallate, ECG | 12,763.01 ± 1409.09 a | 845.99 ± 537.58 b | 518.93 ± 6.07 b | 198.74 ± 12.88 b | 586.33 | <0.01 | <0.01 |
| Catechin gallate, CG | 14,008.53 ± 173.37 a | 961.45 ± 623.34 b | 543.04 ± 27.76 b | 196.56 ± 10.81 b | 247.69 | <0.01 | <0.01 |
| Epigallocatechin, EGC | 35,537.91 ± 2319.60 a | 102.07 ± 68.12 b | 43.55 ± 34.40 b | 48.34 ± 13.11 b | 831.66 | <0.01 | <0.01 |
| Gallocatechin, GC | 1598.29 ± 108.90 a | 4.50 ± 1.01 b | 0.40 ± 0.19 b | 0.94 ± 0.76 b | 38.70 | <0.01 | <0.01 |
| Epicatechin, EC | 11,937.85 ± 585.05 a | 2886.10 ± 176.90 b | 946.55 ± 151.08 c | 870.15 ± 15.64 c | 199.26 | <0.01 | <0.01 |
| Catechin, C | 10,602.68 ± 546.43 a | 5185.98 ± 1046.12 b | 3301.63 ± 403.64 c | 2821.21 ± 523.60 c | 434.34 | <0.01 | <0.01 |
| Total | 144,440.76 ± 2303.22 a | 11,151.55 ± 2728.33 b | 5742.97 ± 576.62 c | 4300.75 ± 493.52 c | 1308.23 | <0.01 | <0.01 |
| Item | Liquor Color | Aroma | Taste |
|---|---|---|---|
| CK | yellowish-green, bright | slightly grassy | fresh, slightly astringent |
| TFG | reddish-brown, bright | woody, sweet | mellow, floral |
| Item | CK | Treat-d4 | Treat-d8 | Treat-d12 (TFG) | SEM | p-Value |
|---|---|---|---|---|---|---|
| DPPH (μmol/mL) | 1434.85 ± 1.80 a | 820.49 ± 66.99 b | 630.33 ± 24.06 b | 667.31 ± 133.06 b | 57.48 | <0.001 |
| ABTS (μmol/mL) | 37.70 ± 3.23 a | 9.53 ± 0.38 b | 11.61 ± 1.52 b | 14.70 ± 3.79 b | 2.05 | <0.001 |
| Sodium glycinate binding rate (%) | 17.92 ± 1.82 b | 29.94 ± 1.19 c | 34.45 ± 4.27 b | 51.58 ± 1.17 a | 1.20 | <0.001 |
| Sodium taurocholate binding rate (%) | 18.45 ± 0.70 c | 21.33 ± 0.44 c | 25.06 ± 1.50 b | 39.90 ± 2.36 a | 0.88 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ju, Y.; Wen, S.; Zeng, H.; Wang, C.; Jiang, M.; Tian, B.; Huang, J.; Liu, Z. Widely Targeted Metabolomics Decodes Metabolic Remodeling and Functional Shifts in Ganoderma lucidum-Fermented Green Tea Infusion. Foods 2025, 14, 2855. https://doi.org/10.3390/foods14162855
Liu X, Ju Y, Wen S, Zeng H, Wang C, Jiang M, Tian B, Huang J, Liu Z. Widely Targeted Metabolomics Decodes Metabolic Remodeling and Functional Shifts in Ganoderma lucidum-Fermented Green Tea Infusion. Foods. 2025; 14(16):2855. https://doi.org/10.3390/foods14162855
Chicago/Turabian StyleLiu, Xuzhou, Ying Ju, Shuai Wen, Hongzhe Zeng, Chao Wang, Mingguo Jiang, Bingchuan Tian, Jianan Huang, and Zhonghua Liu. 2025. "Widely Targeted Metabolomics Decodes Metabolic Remodeling and Functional Shifts in Ganoderma lucidum-Fermented Green Tea Infusion" Foods 14, no. 16: 2855. https://doi.org/10.3390/foods14162855
APA StyleLiu, X., Ju, Y., Wen, S., Zeng, H., Wang, C., Jiang, M., Tian, B., Huang, J., & Liu, Z. (2025). Widely Targeted Metabolomics Decodes Metabolic Remodeling and Functional Shifts in Ganoderma lucidum-Fermented Green Tea Infusion. Foods, 14(16), 2855. https://doi.org/10.3390/foods14162855








