Effect of Wine Yeast (Saccharomyces sp.) Strains on the Physicochemical, Sensory, and Antioxidant Properties of Plum, Apple, and Hawthorn Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Winemaking Process
2.2.1. Plum Wines
2.2.2. Apple Wines
2.2.3. Hawthorn Wines
2.3. Wine Color and pH Measurements
2.4. Ethanol Content Determination
2.5. Sensory Analyses
2.6. Total Polyphenol Content
2.7. Antioxidant Activity
2.8. Data Analyses
3. Results and Discussion
3.1. Physicochemcial Properties
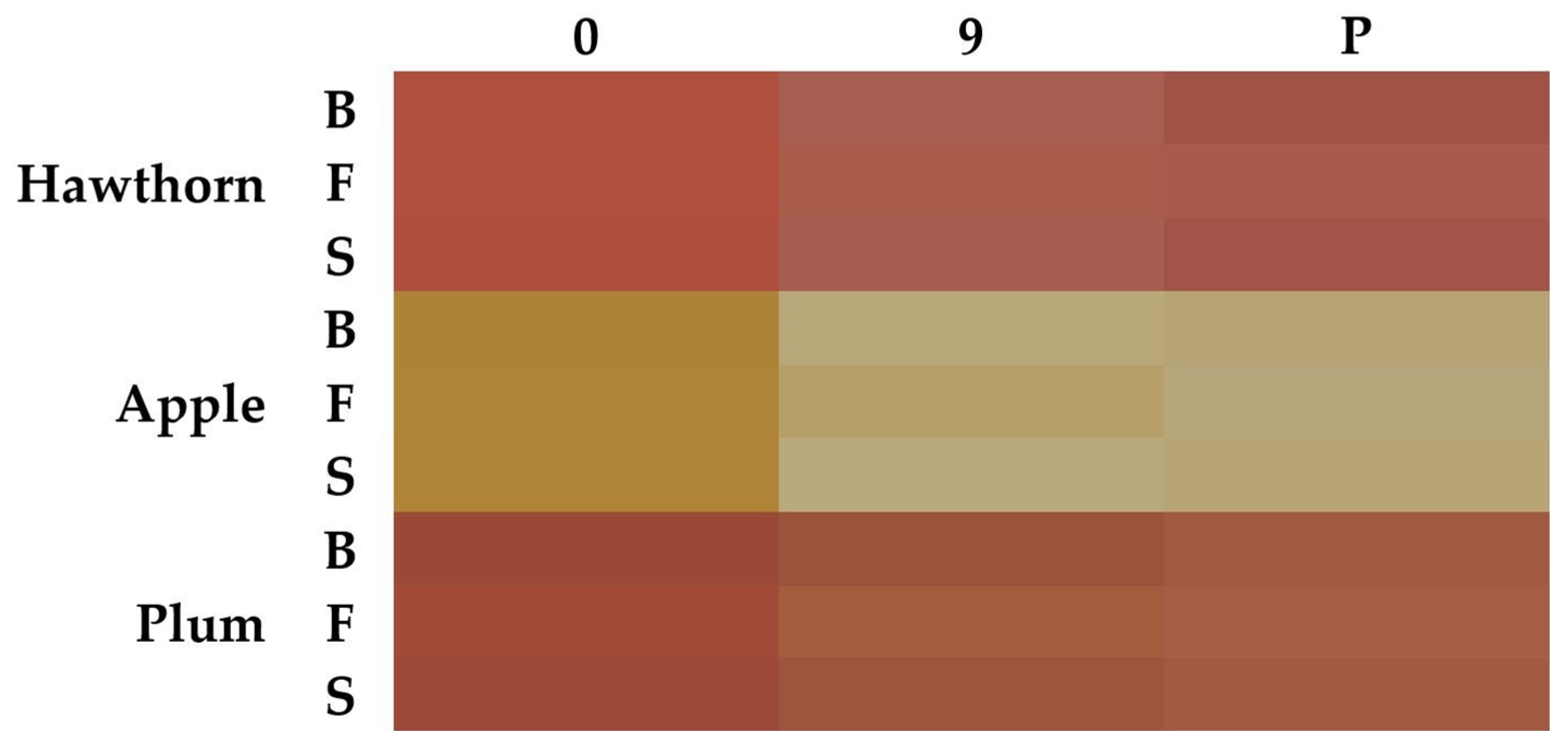
3.1.1. Color Evaluation
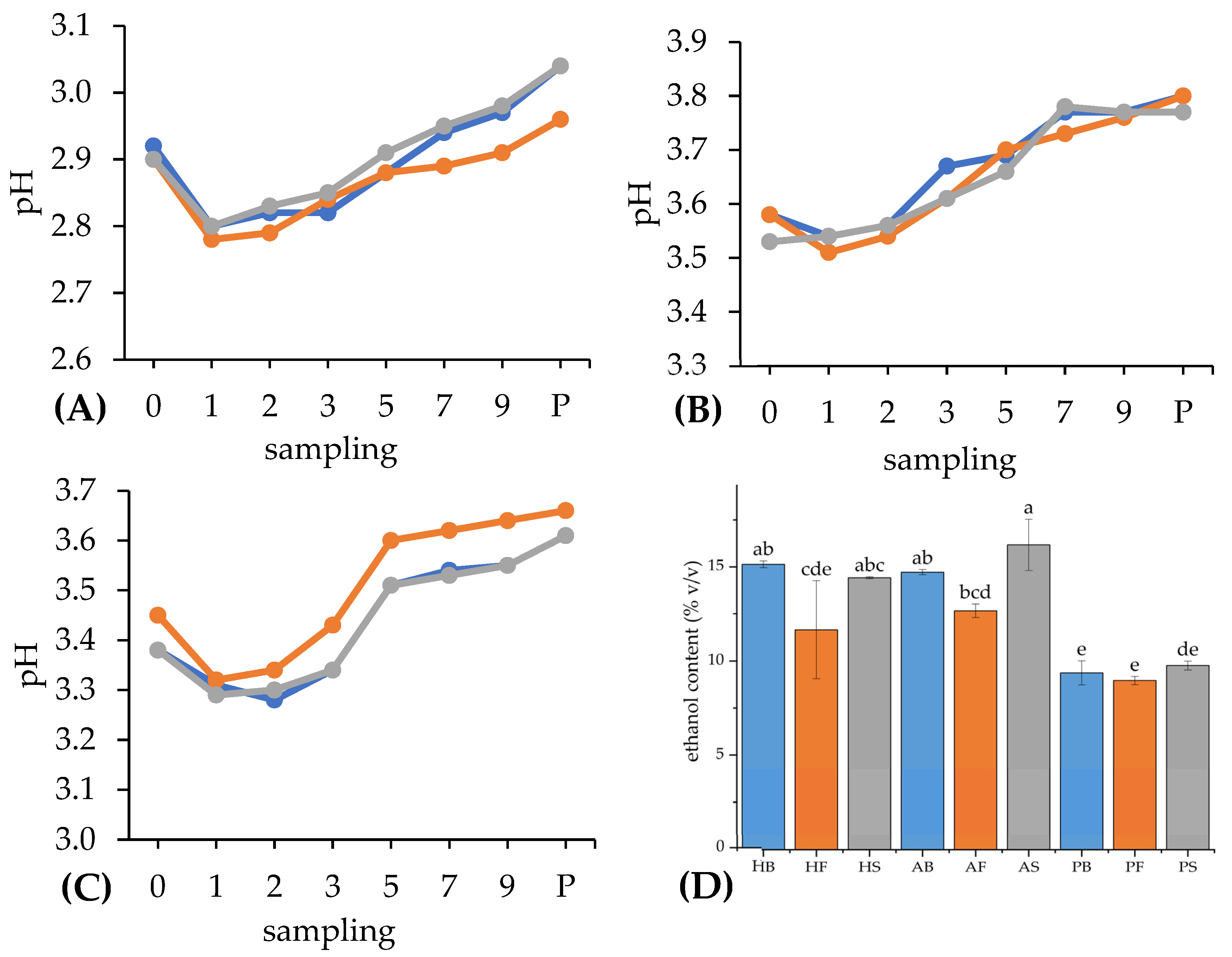
3.1.2. Changes in pH
3.1.3. Ethanol Content
3.2. Total Polyphenol Content and Antioxidant Activity
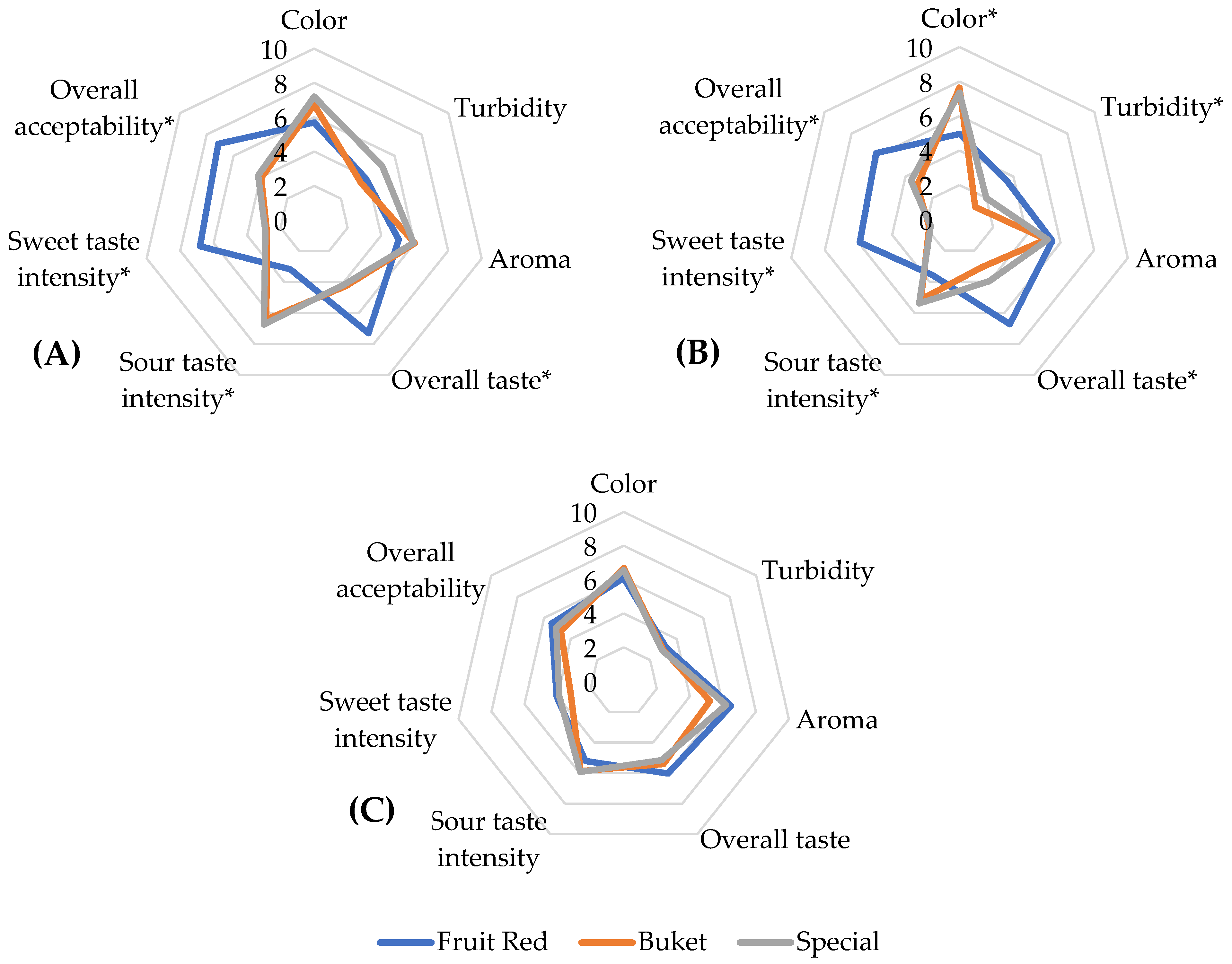
3.3. Sensory Analyses
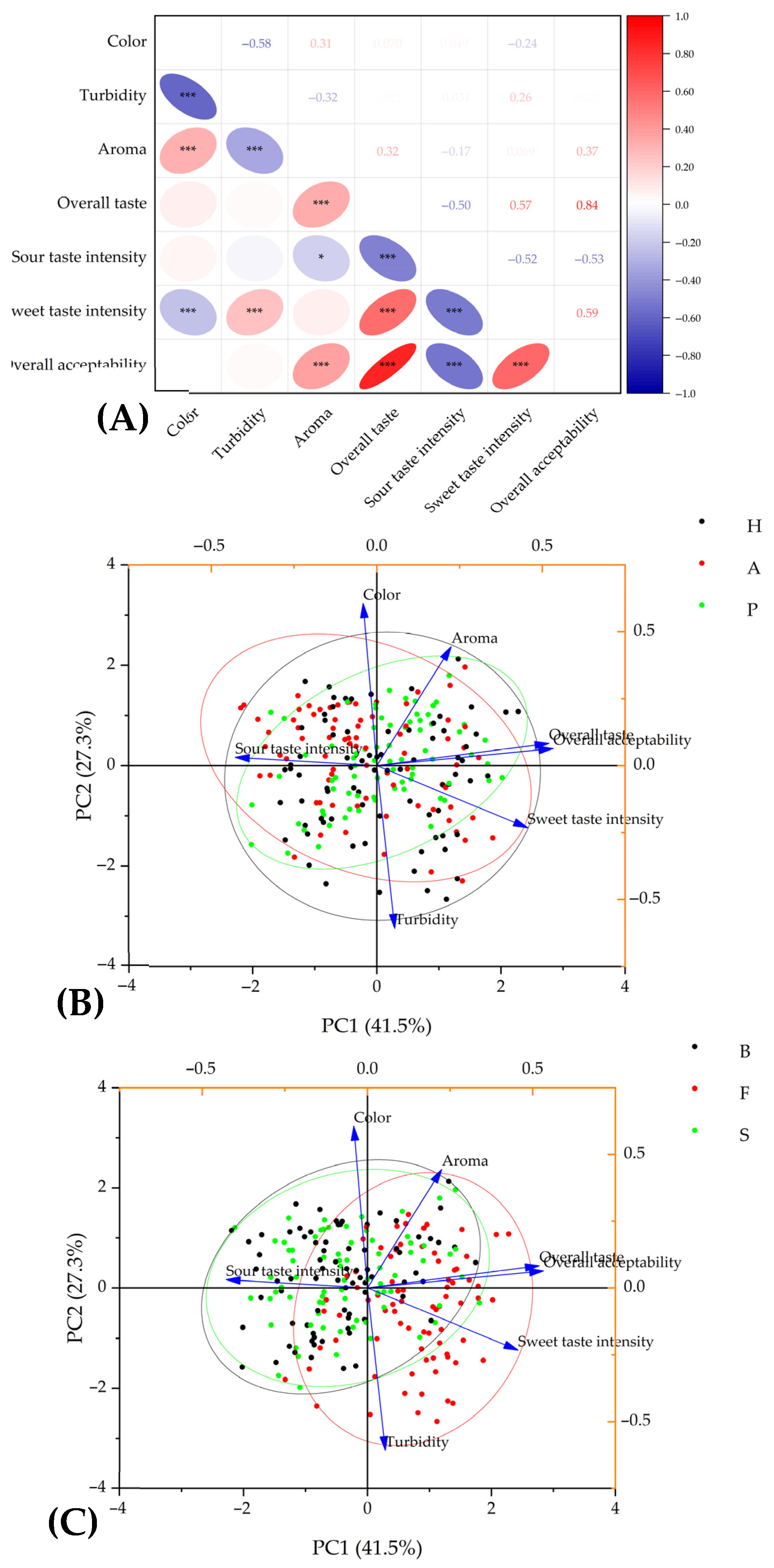
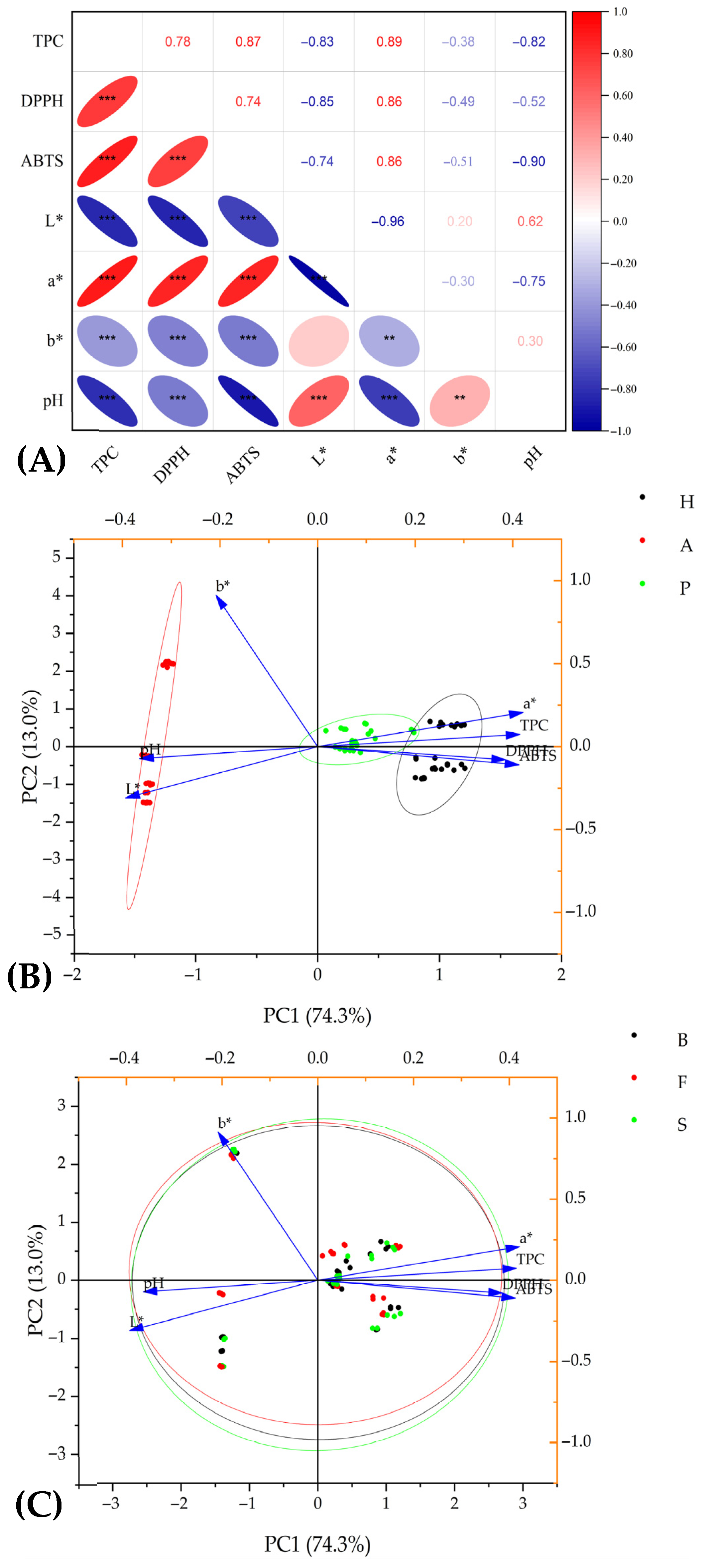
3.4. The Relationships Between Observed Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojić, N.; Prodanovic, R. By-Products of Wine Production in the Service of the Circular Economy. J. Agron. Technol. Eng. Manag. 2024, 7, 1245–1251. [Google Scholar] [CrossRef]
- He, L.; Yan, Y.; Wu, M.; Ke, L. Advances in the Quality Improvement of Fruit Wines: A Review. Horticulturae 2024, 10, 93. [Google Scholar] [CrossRef]
- Radeka, S.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganić, K.; Horvat, I.; Lukić, I.; Orbanić, F.; Zaninović Jurjević, T.; Dvornik, Š. Bioactive Compounds and Antioxidant Activity of Red and White Wines Produced from Autochthonous Croatian Varieties: Effect of Moderate Consumption on Human Health. Foods 2022, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Maksimović, V.; Dragišić Maksimović, J. Chapter 4—Composition, Nutritional, and Therapeutic Values of Fruit and Berry Wines. In Science and Technology of Fruit Wine Production; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 177–226. [Google Scholar]
- Jagtap, U.B.; Waghmare, S.R.; Lokhande, V.H.; Suprasanna, P.; Bapat, V.A. Preparation and evaluation of antioxidant capacity of Jackfruit (Artocarpus heterophyllus Lam.) wine and its protective role against radiation induced DNA damage. Ind. Crops Prod. 2011, 34, 1595–1601. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Phenolic Composition and Antioxidant Capacity of Wine Prepared from Custard Apple (Annona squamosa L.) Fruits. J. Food Process. Preserv. 2015, 39, 175–182. [Google Scholar] [CrossRef]
- Varakumar, S.; Kumar, Y.S.; Reddy, O.V.S. Carotenoid composition of mango (Mangifera indica L.) wine and its antioxidant activity. J. Food Biochem. 2011, 35, 1538–1547. [Google Scholar] [CrossRef]
- Rai, A.K.; Prakash, M.; Anu Appaiah, K.A. Production of Garcinia wine: Changes in biochemical parameters, organic acids and free sugars during fermentation of Garcinia must. Int. J. Food Sci. Technol. 2010, 45, 1330–1336. [Google Scholar] [CrossRef]
- Wang, Z.; Svyantek, A.; Miller, Z.; Watrelot, A.A.; Kapus, A. Juice Dilution Affects Haskap (Lonicera caerulea L.) Wine Fermentation Completion and Wine Chemistry. J. Food Process. Preserv. 2025, 2025, 5257507. [Google Scholar] [CrossRef]
- Kim, A.Y.; Jeong, Y.-J.; Park, Y.B.; Lee, M.-K.; Jeon, S.-M.; McGregor, R.A.; Choi, M.-S. Dose dependent effects of lycopene enriched tomato-wine on liver and adipose tissue in high-fat diet fed rats. Food Chem. 2012, 130, 42–48. [Google Scholar] [CrossRef]
- Matei, F. Chapter 14—Technical Guide for Fruit Wine Production. In Science and Technology of Fruit Wine Production; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 663–703. [Google Scholar]
- Ayub, H.; Nadeem, M.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Zubair Khalid, M.; Zongo, E.; Zarlasht, M.; et al. A comprehensive review on the availability of bioactive compounds, phytochemicals, and antioxidant potential of plum (Prunus domestica). Int. J. Food Prop. 2023, 26, 2388–2406. [Google Scholar] [CrossRef]
- Xiao, Q.; Ye, S.; Wang, H.; Xing, S.; Zhu, W.; Zhang, H.; Zhu, J.; Pu, C.; Zhao, D.; Zhou, Q.; et al. Soluble sugar, organic acid and phenolic composition and flavor evaluation of plum fruits. Food Chem. X 2024, 24, 101790. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Belay, Z.A.; Mditshwa, A.; Caleb, O.J. “An apple a day keeps the doctor away”: The potentials of apple bioactive constituents for chronic disease prevention. J. Food Sci. 2022, 87, 2291–2309. [Google Scholar] [CrossRef]
- Cebulj, A.; Vanzo, A.; Hladnik, J.; Kastelec, D.; Vrhovsek, U. Apple (Malus domestica Borkh.) Cultivar ‘Majda’, a Naturally Non-Browning Cultivar: An Assessment of Its Qualities. Plants 2021, 10, 1402. [Google Scholar] [CrossRef]
- Mignard, P.; Beguería, S.; Giménez, R.; Font i Forcada, C.; Reig, G.; Moreno, M.Á. Effect of Genetics and Climate on Apple Sugars and Organic Acids Profiles. Agronomy 2022, 12, 827. [Google Scholar] [CrossRef]
- Ma, J.-X.; Yang, W.; Ng, C.Y.J.; Tang, X.-D.; Wong, S.; Gan, R.-Y.; Zhong, L. The hawthorn (Crataegus pinnatifida Bge.) fruit as a new dietary source of bioactive ingredients with multiple beneficial functions. Food Front. 2024, 5, 1534–1558. [Google Scholar] [CrossRef]
- Wu, J.; Peng, W.; Qin, R.; Zhou, H. Crataegus pinnatifida: Chemical Constituents, Pharmacology, and Potential Applications. Molecules 2014, 19, 1685–1712. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Bu, A.; Wang, B.; Sun, M.; Wang, P.; Wang, B.; Wang, A. Sugar and organic acid metabolism and accumulation in different cultivars during fruit development in hawthorn (Crataegus pinnatifida). Food Qual. Saf. 2025, 9, fyaf010. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef]
- Eldarov, M.A.; Kishkovskaia, S.A.; Tanaschuk, T.N.; Mardanov, A.V. Genomics and biochemistry of Saccharomyces cerevisiae wine yeast strains. Biochemistry 2016, 81, 1650–1668. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Troianou, V.; Toumpeki, C.; Gosselin, Y.; Dorignac, É.; Kotseridis, Y. Effect of strains from different Saccharomyces species used in different inoculation schemes on chemical composition and sensory characteristics of Sauvignon blanc wine. OENO One 2020, 54, 745–759. [Google Scholar] [CrossRef]
- Orlić, S.; Arroyo-López, F.N.; Huić-Babić, K.; Lucilla, I.; Querol, A.; Barrio, E. A comparative study of the wine fermentation performance of Saccharomyces paradoxus under different nitrogen concentrations and glucose/fructose ratios. J. Appl. Microbiol. 2010, 108, 73–80. [Google Scholar] [CrossRef]
- Patrignani, F.; Siesto, G.; Gottardi, D.; Vigentini, I.; Toffanin, A.; Englezos, V.; Blaiotta, G.; Grieco, F.; Lanciotti, R.; Speranza, B.; et al. Impact of Two Commercial S. cerevisiae Strains on the Aroma Profiles of Different Regional Musts. Beverages 2022, 8, 59. [Google Scholar] [CrossRef]
- Bordet, F.; Roullier-Gall, C.; Ballester, J.; Vichi, S.; Quintanilla-Casas, B.; Gougeon, R.D.; Julien-Ortiz, A.; Kopplin, P.S.; Alexandre, H. Different Wines from Different Yeasts? “Saccharomyces cerevisiae Intraspecies Differentiation by Metabolomic Signature and Sensory Patterns in Wine”. Microorganisms 2021, 9, 2327. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, W.; Chen, Z.; Peng, Z.; Ma, M.; Zhang, J.; Wu, D.; Xie, G.; Lu, J. Exploring the Impact of Different Saccharomyces cerevisiae Strains on the Flavor Profile of Greengage Alcoholic Beverage Using GC-E-Nose, HS-GC-IMS, and HS-SPME-GC-MS. Foods 2024, 13, 3984. [Google Scholar] [CrossRef] [PubMed]
- Fugelsang, K.C.; Edwards, C.G. Yeasts. In Wine Microbiology: Practical Applications and Procedures; Fugelsang, K.C., Edwards, C.G., Eds.; Springer: Boston, MA, USA, 2007; pp. 3–28. [Google Scholar]
- Milani, E.A.; Silva, F.V.M. Pasteurization of Beer by Non-Thermal Technologies. Front. Food. Sci. Technol. 2022, 1, 798676. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 942.06 Alcohol by Volume in Distilled Liquors. In AOAC Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Bedrníček, J.; Lorenc, F.; Jarošová, M.; Bártová, V.; Smetana, P.; Kadlec, J.; Jirotková, D.; Kyselka, J.; Petrášková, E.; Bjelková, M.; et al. Milk Thistle Oilseed Cake Flour Fractions: A Source of Silymarin and Macronutrients for Gluten-Free Bread. Antioxidants 2022, 11, 2022. [Google Scholar] [CrossRef]
- Jarošová, M.; Lorenc, F.; Bedrníček, J.; Petrášková, E.; Bjelková, M.; Bártová, V.; Jarošová, E.; Zdráhal, Z.; Kyselka, J.; Smetana, P.; et al. Comparison of Yield Characteristics, Chemical Composition, Lignans Content and Antioxidant Potential of Experimentally Grown Six Linseed (Linum usitatissimum L.) Cultivars. Plant Foods Hum. Nutr. 2024, 79, 159–165. [Google Scholar] [CrossRef]
- Tan, X.; Ding, M.; Wang, C.; Huang, L.; Bai, J. The Transformation of Pigment in Fruit Wine, Precise Control of Pigment Formation, and Their Effect on Product Quality. Foods 2025, 14, 2207. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of Ethanol Concentration on the Extraction of Color and Phenolic Compounds from the Skin and Seeds of Tempranillo Grapes at Different Stages of Ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, B.; Yu, L.; Yuan, G.; Ma, Y.; Zhang, J.; Lin, F. Differential Analysis of Anthocyanins in Red and Yellow Hawthorn (Crataegus pinnatifida) Peel Based on Ultra-High Performance Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry. Molecules 2025, 30, 1149. [Google Scholar] [CrossRef]
- Shi, C.; Liu, L.; Wei, Z.; Liu, J.; Li, M.; Yan, Z.; Gao, D. Anthocyanin Accumulation and Molecular Analysis of Correlated Genes by Metabolomics and Transcriptomics in Sister Line Apple Cultivars. Life 2022, 12, 1246. [Google Scholar] [CrossRef] [PubMed]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; González, C.; Escott, C. Non-Saccharomyces as Biotools to Control the Production of Off-Flavors in Wines. Molecules 2021, 26, 4571. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G. Effects of pasteurization on the physicochemical, sensory properties and microbiological quality of beetroot (Beta vulgaris L.) wine during storage. Sci. Tech. Dev. J. 2023, 26, 2950–2958. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, K.; Kim, S.; Park, K.; Park, S.; Kim, J.; Kang, S.; Cheong, C.; Jang, K. Physicochemical characteristics and electric conductivity of various fruit wines. Int. Food Res. J. 2013, 20, 2987. [Google Scholar]
- Choi, K.T.; Lee, S.B.; Choi, J.S.; Park, H.D. Influence of different pretreatments and chaptalization types on the physiological characteristics and antioxidant activity of apricot (Prunus armeniaca L.) wine. Ital. J. Food Sci. 2020, 32, 912–927. [Google Scholar] [CrossRef]
- Lu, Y.; Voon, M.K.W.; Huang, D.; Lee, P.-R.; Liu, S.-Q. Combined effects of fermentation temperature and pH on kinetic changes of chemical constituents of durian wine fermented with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 3005–3014. [Google Scholar] [CrossRef]
- Akin, H.; Brandam, C.; Meyer, X.-M.; Strehaiano, P. A model for pH determination during alcoholic fermentation of a grape must by Saccharomyces cerevisiae. Chem. Eng. Process. Process Intensif. 2008, 47, 1986–1993. [Google Scholar] [CrossRef]
- Thammasittirong, S.N.-R.; Thirasaktana, T.; Thammasittirong, A.; Srisodsuk, M. Improvement of ethanol production by ethanol-tolerant Saccharomyces cerevisiae UVNR56. Springerplus 2013, 2, 583. [Google Scholar] [CrossRef]
- Pereira, V.; Albuquerque, F.; Cacho, J.; Marques, J.C. Polyphenols, Antioxidant Potential and Color of Fortified Wines during Accelerated Ageing: The Madeira Wine Case Study. Molecules 2013, 18, 2997–3017. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Varo, M.A.; Serratosa, M.P.; Martín-Gómez, J.; Moyano, L.; Mérida, J. Influence of Fermentation Time on the Phenolic Compounds, Vitamin C, Color and Antioxidant Activity in the Winemaking Process of Blueberry (Vaccinium corymbosum) Wine Obtained by Maceration. Molecules 2022, 27, 7744. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, L.; Huang, X.; Ding, Z.; Wang, C. Evolution of polyphenolic, anthocyanin, and organic acid components during coinoculation fermentation (simultaneous inoculation of LAB and yeast) and sequential fermentation of blueberry wine. J. Food Sci. 2022, 87, 4878–4891. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chen, S.; Pu, Y.; Wang, T.; Xu, H.; Li, H.; Ma, P.; Hou, X. Effect of Winemaking on Phenolic Compounds and Antioxidant Activities of Msalais Wine. Molecules 2023, 28, 1250. [Google Scholar] [CrossRef] [PubMed]
- Hensen, J.-P.; Hoening, F.; Bogdanovic, T.; Schieber, A.; Weber, F. Pectin forms polymeric pigments by complexing anthocyanins during red winemaking and ageing. Food Res. Int. 2024, 188, 114442. [Google Scholar] [CrossRef] [PubMed]
- Weilack, I.; Mehren, L.; Schieber, A.; Weber, F. Grape-derived pectic polysaccharides alter the tannin and pigment composition of Cabernet Sauvignon red wines. Curr. Res. Food Sci. 2023, 6, 100506. [Google Scholar] [CrossRef]
- Hensen, J.-P.; Hoening, F.; Weilack, I.; Damm, S.; Weber, F. Influence of Grape Cell Wall Polysaccharides on the Extraction of Polyphenols during Fermentation in Microvinifications. J. Agric. Food Chem. 2022, 70, 9117–9131. [Google Scholar] [CrossRef]
- Razmkhab, S.; Lopez-Toledano, A.; Ortega, J.M.; Mayen, M.; Merida, J.; Medina, M. Adsorption of Phenolic Compounds and Browning Products in White Wines by Yeasts and Their Cell Walls. J. Agric. Food Chem. 2002, 50, 7432–7437. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Soszka, A. Changes in Phenolic Compounds and Antioxidant Activity of Fruit Musts and Fruit Wines during Simulated Digestion. Molecules 2020, 25, 5574. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Clegg, S. Total antioxidant capacity, total phenolic content, mineral elements, and histamine concentrations in wines of different fruit sources. J. Food Compos. Anal. 2007, 20, 133–137. [Google Scholar] [CrossRef]
- He, G.; Sui, J.; Du, J.; Lin, J. Characteristics and antioxidant capacities of five hawthorn wines fermented by different wine yeasts. J. Inst. Brew. 2013, 119, 321–327. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; You, L.; Guo, Z.; Chang, X. Changes in anthocyanin profile, color, and antioxidant capacity of hawthorn wine (Crataegus pinnatifida) during storage by pretreatments. LWT 2018, 95, 179–186. [Google Scholar] [CrossRef]
- Liu, S.; Chang, X.; Liu, X.; Shen, Z. Effects of pretreatments on anthocyanin composition, phenolics contents and antioxidant capacities during fermentation of hawthorn (Crataegus pinnatifida) drink. Food Chem. 2016, 212, 87–95. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Zeng, Y.; Cai, D.; Teng, Z.; Wu, Q.; Sun, J.; Bai, W. Color Stability Enhancement and Antioxidation Improvement of Sanhua Plum Wine under Circulating Ultrasound. Foods 2022, 11, 2435. [Google Scholar] [CrossRef]
- Liu, G.; Wei, P.; Tang, Y.; Pang, Y.; Sun, J.; Li, J.; Rao, C.; Wu, C.; He, X.; Li, L.; et al. Evaluation of Bioactive Compounds and Bioactivities in Plum (Prunus salicina Lindl.) Wine. Front. Nutr. 2021, 8, 766415. [Google Scholar] [CrossRef]
- Miljić, U.; Puškaš, V.; Cvejić Hogervorst, J.; Torović, L. Phenolic compounds, chromatic characteristics and antiradical activity of plum wines. Int. J. Food Prop. 2017, 20, 2022–2033. [Google Scholar] [CrossRef][Green Version]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Costa, A.M.; Pinto, C.A.; Silva, A.M.S.; Saraiva, J.A.; Cardoso, S.M. Impact of Fermentation and Pasteurization on the Physico-Chemical and Phytochemical Composition of Opuntia ficus-indica Juices. Foods 2023, 12, 2096. [Google Scholar] [CrossRef]
- Miljić, U.; Puškaš, V.; Vučurović, V.; Muzalevski, A. Fermentation Characteristics and Aromatic Profile of Plum Wines Produced with Indigenous Microbiota and Pure Cultures of Selected Yeast. J. Food Sci. 2017, 82, 1443–1450. [Google Scholar] [CrossRef]
- Coelho, E.; Vilanova, M.; Genisheva, Z.; Oliveira, J.M.; Teixeira, J.A.; Domingues, L. Systematic approach for the development of fruit wines from industrially processed fruit concentrates, including optimization of fermentation parameters, chemical characterization and sensory evaluation. LWT—Food Sci. Technol. 2015, 62, 1043–1052. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Liu, J.; Lan, Y.; Qian, X.; Zhu, B. Comprehensive study of chemical and sensory profiles of hawthorn wines from China. Food Chem. X 2025, 26, 102277. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, Q.; Jiao, J.; Kelanne, N.; Kortesniemi, M.; Xu, X.; Zhu, B.; Laaksonen, O. Exploring the Sensory Properties and Preferences of Fruit Wines Based on an Online Survey and Partial Projective Mapping. Foods 2023, 12, 1844. [Google Scholar] [CrossRef]
- Sena-Esteves, M.M.; Mota, M.; Malfeito-Ferreira, M. Patterns of sweetness preference in red wine according to consumer characterisation. Food Res. Int. 2018, 106, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ailer, Š.; Valšíková, M.; Jedlička, J.; Mankovecký, J.; Baroň, M. Influence of Sugar and Ethanol Content and Color of Wines On the Sensory Evaluation: From Wine Competition “Nemčiňany Wine Days” in Slovak Republic (2013–2016). Erwerbs-Obstbau 2020, 62, 9–16. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Teliban, I.V.; Zamfir, C.I.; Luchian, C.E.; Colibaba, L.C.; Niculaua, M.; Cotea, V.V. Effect of Different Winemaking Conditions on Organic Acids Compounds of White Wines. Foods 2021, 10, 2569. [Google Scholar] [CrossRef] [PubMed]
- Čakar, U.; Čolović, M.; Milenković, D.; Pagnacco, M.; Maksimović, J.; Krstić, D.; Đorđević, B. Strawberry and Drupe Fruit Wines Antioxidant Activity and Protective Effect Against Induced Oxidative Stress in Rat Synaptosomes. Antioxidants 2025, 14, 155. [Google Scholar] [CrossRef]
- Ferri, M.; Gianotti, A.; Tassoni, A. Optimisation of assay conditions for the determination of antioxidant capacity and polyphenols in cereal food components. J. Food Compos. Anal. 2013, 30, 94–101. [Google Scholar] [CrossRef]
- Kalkan Yildirim, H. Evaluation of colour parameters and antioxidant activities of fruit wines. Int. J. Food Sci. Nutr. 2006, 57, 47–63. [Google Scholar] [CrossRef]
- Ştefănuţ, M.N.; Căta, A.; Pop, R.; Tănasie, C.; Boc, D.; Ienaşcu, I.; Ordodi, V. Anti-hyperglycemic Effect of Bilberry, Blackberry and Mulberry Ultrasonic Extracts on Diabetic Rats. Plant Foods Hum. Nutr. 2013, 68, 378–384. [Google Scholar] [CrossRef]
| TPC | DPPH | ABTS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | Y | Hawthorn | Apple | Plum | Hawthorn | Apple | Plum | Hawthorn | Apple | Plum |
| 0 | B | 2.14 ± 0.20 Aabc | 0.81 ± 0.02 Ba | 1.41 ± 0.43 Bb | 1.09 ± 0.06 Aab | 0.36 ± 0.05 Bb | 1.08 ± 0.27 Aab | 2.82 ± 0.08 Ab | 0.58 ± 0.02 Cd | 1.73 ± 0.02 Ba |
| F | 2.44 ± 0.11 Aa | 0.82 ± 0.05 Ca | 1.86 ± 0.04 Bab | 1.14 ± 0.07 Aab | 0.33 ± 0.03 Cb | 0.92 ± 0.08 Bb | 2.97 ± 0.04 Aa | 0.45 ± 0.01 Cf | 1.61 ± 0.03 Bcd | |
| S | 2.32 ± 0.06 Aab | 0.77 ± 0.02 Ba | 2.32 ± 0.41 Aa | 1.06 ± 0.06 Aab | 0.32 ± 0.05 Bb | 1.22 ± 0.23 Aab | 2.94 ± 0.03 Aab | 0.53 ± 0.00 Ce | 1.35 ± 0.01 Bf | |
| 9 | B | 2.02 ± 0.06 Abc | 0.50 ± 0.01 Cc | 1.45 ± 0.07 Bb | 1.13 ± 0.01 Aab | 0.41 ± 0.02 Bab | 1.16 ± 0.08 Aab | 2.41 ± 0.01 Ac | 0.63 ± 0.01 Cbc | 1.71 ± 0.08 Ba |
| F | 2.25 ± 0.15 Aabc | 0.63 ± 0.03 Cb | 1.65 ± 0.04 Bb | 1.07 ± 0.13 Aab | 0.32 ± 0.06 Bb | 1.29 ± 0.07 Aab | 2.25 ± 0.02 Ad | 0.62 ± 0.01 Cc | 1.55 ± 0.02 Bde | |
| S | 2.14 ± 0.06 Aabc | 0.48 ± 0.02 Cc | 1.45 ± 0.02 Bb | 0.97 ± 0.06 Bb | 0.41 ± 0.03 Cab | 1.20 ± 0.09 Aab | 2.51 ± 0.05 Ac | 0.63 ± 0.00 Cbc | 1.67 ± 0.09 Bab | |
| P | B | 2.36 ± 0.17 Aab | 0.48 ± 0.01 Cc | 1.67 ± 0.07 Bb | 1.14 ± 0.07 Aab | 0.43 ± 0.02 Bab | 1.16 ± 0.19 Aab | 2.93 ± 0.03 Aab | 0.68 ± 0.01 Ca | 1.52 ± 0.02 Be |
| F | 1.91 ± 0.06 Ac | 0.52 ± 0.02 Cc | 1.57 ± 0.02 Bb | 1.16 ± 0.03 Bab | 0.34 ± 0.04 Cb | 1.41 ± 0.03 Aa | 2.87 ± 0.01 Aab | 0.58 ± 0.02 Cd | 1.64 ± 0.01 Bbc | |
| S | 2.24 ± 0.24 Aabc | 0.51 ± 0.03 Cc | 1.66 ± 0.03 Bb | 1.24 ± 0.06 Aa | 0.48 ± 0.01 Ca | 1.01 ± 0.13 Bab | 2.93 ± 0.06 Aab | 0.66 ± 0.01 Cab | 1.69 ± 0.03 Bab | |
| 9 (RSA) | B | - | - | - | 37.5 ± 0.2 Aa | 14.8 ± 0.6 Ba | 38.3 ± 2.6 Aa | 52.1 ± 0.3 Ab | 30.8 ± 0.3 Ca | 38.9 ± 0.4 Ba |
| F | - | - | - | 35.5 ± 4.1 Aa | 12.2 ± 1.9 Ba | 42.3 ± 2.1 Aa | 49.1 ± 0.4 Ac | 30.2 ± 0.4 Ca | 36.1 ± 0.4 Bb | |
| S | - | - | - | 32.5 ± 1.8 Ba | 14.9 ± 0.9 Ca | 39.7 ± 2.8 Aa | 53.9 ± 0.9 Aa | 30.7 ± 0.1 Ca | 38.3 ± 0.3 Ba | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenc, F.; Jarošová, M.; Bedrníček, J.; Nohejl, V.; Míková, E.; Smetana, P. Effect of Wine Yeast (Saccharomyces sp.) Strains on the Physicochemical, Sensory, and Antioxidant Properties of Plum, Apple, and Hawthorn Wines. Foods 2025, 14, 2844. https://doi.org/10.3390/foods14162844
Lorenc F, Jarošová M, Bedrníček J, Nohejl V, Míková E, Smetana P. Effect of Wine Yeast (Saccharomyces sp.) Strains on the Physicochemical, Sensory, and Antioxidant Properties of Plum, Apple, and Hawthorn Wines. Foods. 2025; 14(16):2844. https://doi.org/10.3390/foods14162844
Chicago/Turabian StyleLorenc, František, Markéta Jarošová, Jan Bedrníček, Vlastimil Nohejl, Eliška Míková, and Pavel Smetana. 2025. "Effect of Wine Yeast (Saccharomyces sp.) Strains on the Physicochemical, Sensory, and Antioxidant Properties of Plum, Apple, and Hawthorn Wines" Foods 14, no. 16: 2844. https://doi.org/10.3390/foods14162844
APA StyleLorenc, F., Jarošová, M., Bedrníček, J., Nohejl, V., Míková, E., & Smetana, P. (2025). Effect of Wine Yeast (Saccharomyces sp.) Strains on the Physicochemical, Sensory, and Antioxidant Properties of Plum, Apple, and Hawthorn Wines. Foods, 14(16), 2844. https://doi.org/10.3390/foods14162844






