HPLC-MS Detection of Nonylphenol Ethoxylates and Lauryl Ethoxylates in Foodstuffs and the Inner Coatings of High-Barrier Pouches
Abstract
1. Introduction
2. Materials and Methods
2.1. Analyzed Samples
2.2. Sample Preparation
2.3. High-Pressure Liquid Chromatography–Mass Spectrometry Analyses
3. Results and Discussion
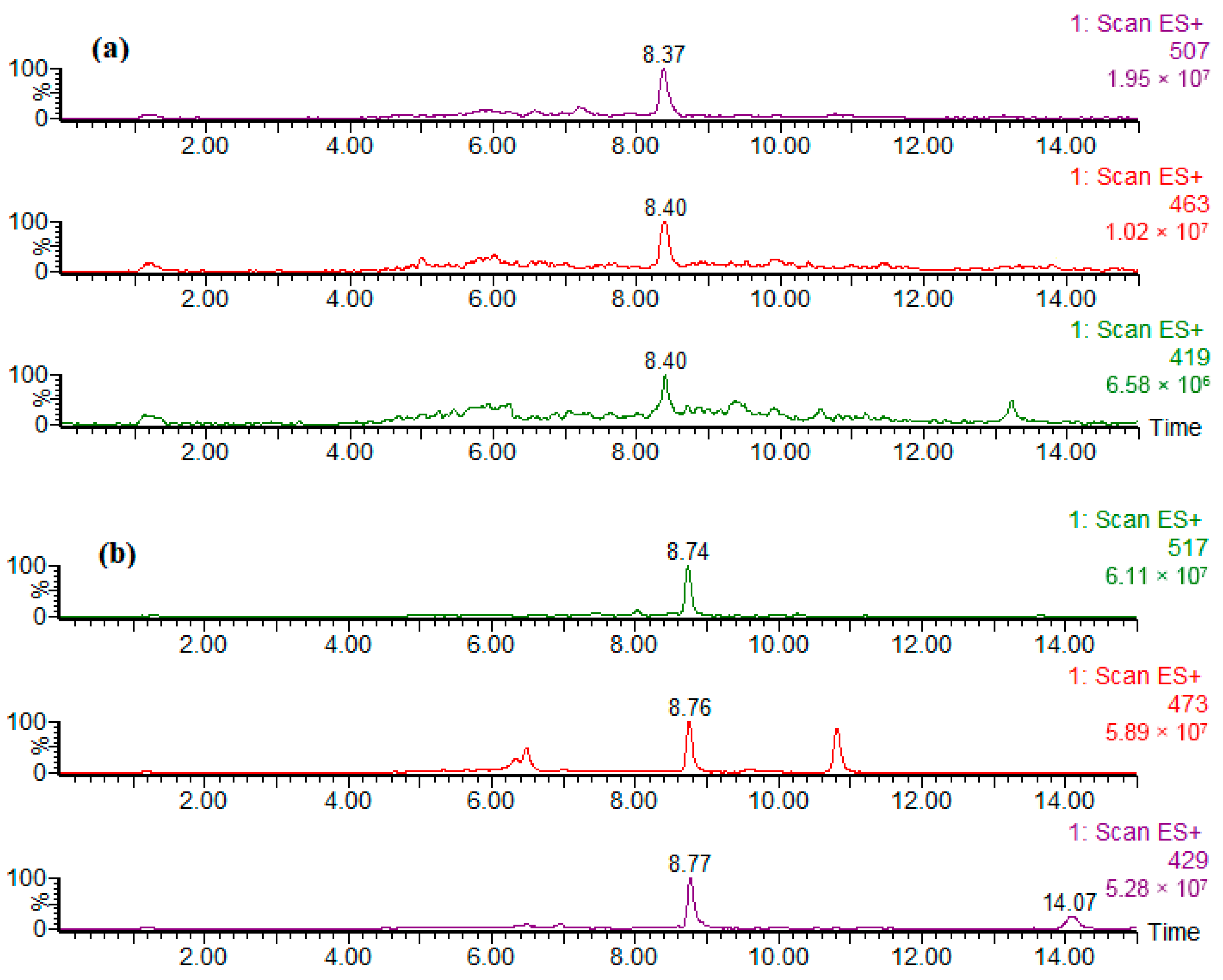
3.1. Qualitative Identification of Analyzed Ethoxylates
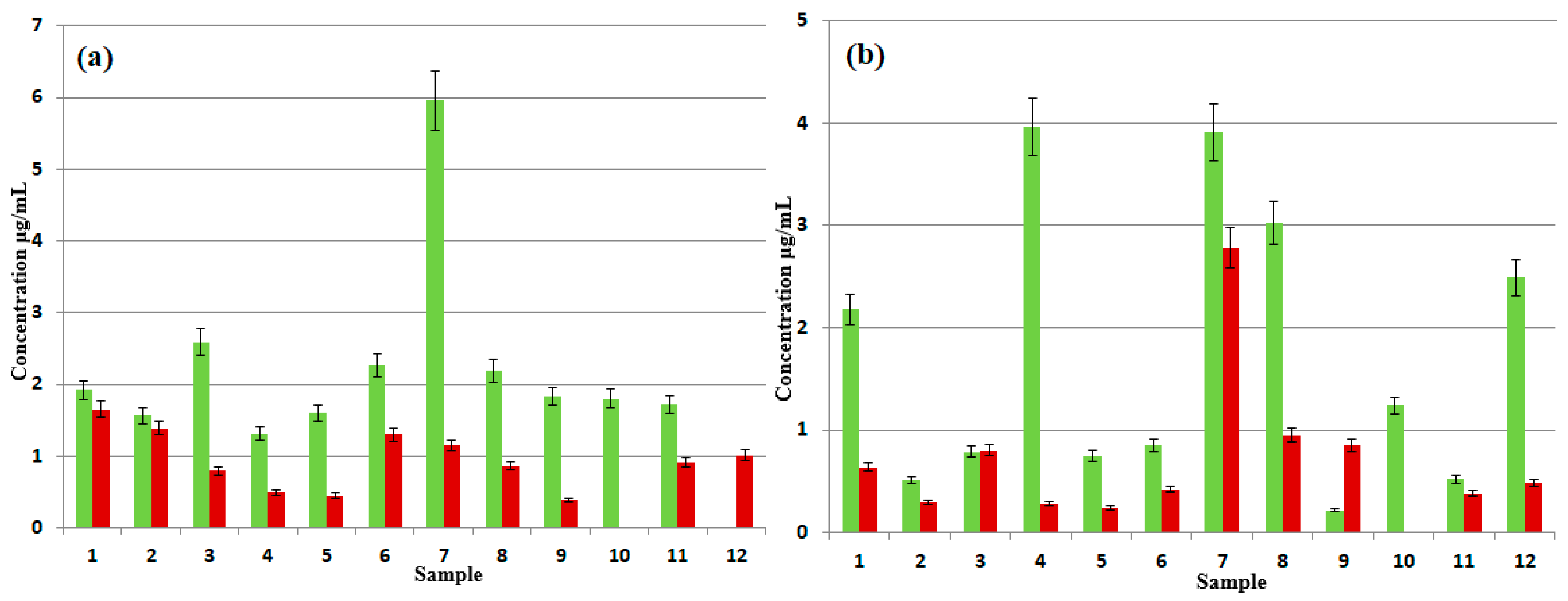
3.2. Semi-Quantitative Analysis of NPEOn and DDEOn
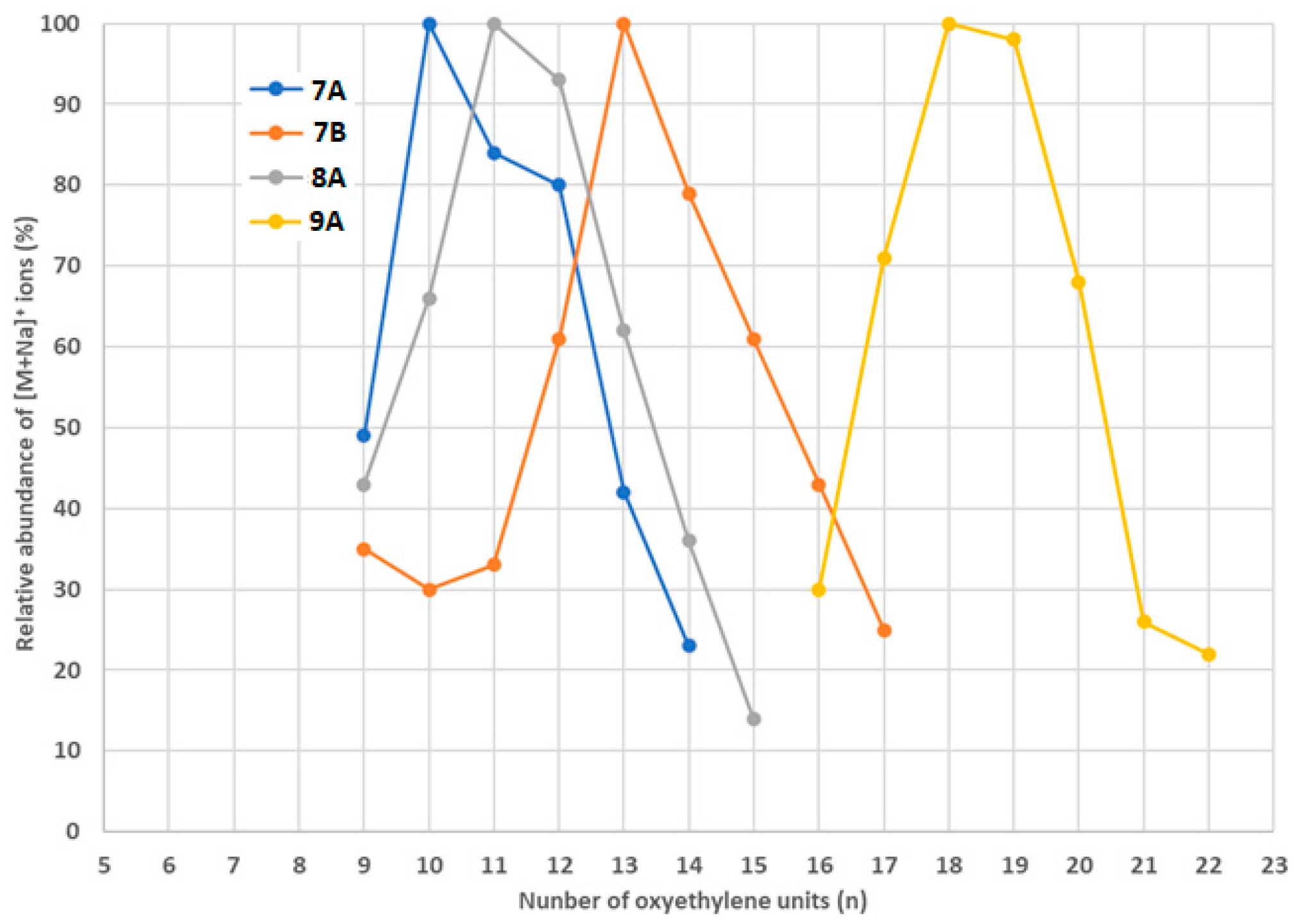
3.3. Comparison of the Oxyethylene Chains Length of the Ethoxylates Detected in the Analyzed Samples
3.4. Detection of Other Potential Migrants in the High-Barrier Pouch Coating Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beneito-Cambra, M.; Herrero-Martínez, J.M.; Ramis-Ramos, G. Analytical methods for the characterization and determination of nonionic surfactants in cosmetics and environmental matrices. Anal. Methods 2013, 5, 341–354. [Google Scholar] [CrossRef]
- Plata, M.R.; Contento, A.M.; Ríos, Á. Analytical characterization of alcohol-ethoxylate substances by instrumental separation techniques. Trends Anal. Chem. 2011, 30, 1018–1034. [Google Scholar] [CrossRef]
- Ali, A.A.; Bhat, G.; Al-Ghamdi, I.; Cao, W.; Kumar, A.; Iali, W.; Narayan, K.C.; Ghazwani, Q. A systematic derivatization technique for characterization of ethoxylates by GC and GCMS. J. Surfactants Deterg. 2024, 27, 605–612. [Google Scholar] [CrossRef]
- Lalonde, B.; Garron, C. Nonylphenol, octylphenol, and nonylphenol ethoxylates dissemination in the Canadian freshwater environment. Arch. Environ. Contam. Toxicol. 2021, 80, 319–330. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Rafiquzzaman, M. Determination of alkylphenols and alkylphenol ethoxylates in some of the textile wastewater samples in Dhaka division, Bangladesh. J. Chem. Health Risks 2023, 13, 249–257. [Google Scholar] [CrossRef]
- Lara-Martín, P.A.; Petrovic, M.; Gómez-Parra, A.; Barceló, D.; González-Mazo, E. Presence of surfactants and their degradation intermediates in sediment cores and grabs from the Cadiz Bay area. Environ. Pollut. 2006, 144, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Sparham, C.; Rehman, N.; Melling, J.; Van Duynhoven, J.; Marshall, S. Biodegradability of highly ethoxylated nonionic surfactants: Determination of intermediates and pathways of biodegradation. Environ. Toxicol. Chem. 2008, 27, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Van de Plassche, E.J.; de Bruijn, J.H.; Stephenson, R.R.; Marshall, S.J.; Feijtel, T.C.; Belanger, S.E. Predicted no-effect concentrations and risk characterization of four surfactants: Linear alkyl benzene sulfonate, alcohol ethoxylates, alcohol ethoxylated sulfates, and soap. Environ. Toxicol. Chem. 1999, 18, 2653–2663. [Google Scholar] [CrossRef]
- Garcia, V.S.G.; Tominaga, F.K.; Rosa, J.M.; Borrely, S.I. Emerging pollutants in textile wastewater: An ecotoxicological assessment focusing on surfactants. Environ. Sci. Pollut. Res. 2024, 31, 27817–27828. [Google Scholar] [CrossRef]
- Mahalakshmi, R.; Pugazhendhi, A.; Brindhadevi, K.; Ramesh, N. Analysis of alkylphenol ethoxylates (APEOs) from tannery sediments using LC-MS and their environmental risks. Process Biochem. 2020, 97, 37–42. [Google Scholar] [CrossRef]
- Korsman, J.C.; Schipper, A.M.; de Vos, M.G.; van den Heuvel-Greve, M.J.; Vethaak, A.D.; de Voogt, P.; Hendriks, A.J. Modeling bioaccumulation and biomagnification of nonylphenol and its ethoxylates in estuarine-marine food chains. Chemosphere 2015, 138, 33–39. [Google Scholar] [CrossRef]
- De la Parra-Guerra, A.C.; Acevedo-Barrios, R. Studies of endocrine disruptors: Nonylphenol and isomers in biological models. Environ. Toxicol. Chem. 2023, 42, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ji, J.; Wu, Y.; Chen, S.; Xu, M.; Cao, X.; Liu, H.; Wang, Z.; Bi, H.; Guan, G.; et al. Nonylphenol and its derivatives: Environmental distribution, treatment strategy, management and future perspectives. Chemosphere 2024, 352, 141377. [Google Scholar] [CrossRef]
- Chung, S.W. The development of isomer-specific analysis of branched 4-nonylphenol in food for dietary exposure-a critical review of analytical methods and occurrence in foodstuffs. Food Addit. Contam. Part A 2021, 38, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Farhat, J.; Mokbel, I.; Bassil, G.; Sari-Ali, B.F.; Negadi, L.; Jose, J.; Saab, J. Experimental and predicted aqueous solubility and vapor pressures of food packaging migrants: 4-n-octylphenol, 4-tert-octylphenol and 4-n-nonylphenol. J. Chem. Thermodyn. 2025, 201, 107410. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Rose, M.; Charlton, C. 4-Nonylphenol (NP) in food-contact materials: Analytical methodology and occurrence. Food Addit. Contam. 2008, 25, 364–372. [Google Scholar] [CrossRef]
- Li, C.-T.; Cheng, C.-Y.; Ding, W.-H. Determination of alkylphenol residues in baby-food purees by steam distillation extraction and gas chromatography-mass spectrometry. Food Chem. Toxicol. 2008, 46, 803–807. [Google Scholar] [CrossRef]
- Al Rashed, N.; Gerlach, C.; Guenther, K. Determination of nonylphenol in selected foods and identification of single isomers in a coffee sample by comprehensive two-dimensional gas chromatography-time of flight mass spectrometry. Anal. Lett. 2023, 56, 2586–2604. [Google Scholar] [CrossRef]
- Lee, S.M.; Cheong, D.; Kim, M.; Kim, Y.-S. Analysis of endocrine disrupting nonylphenols in foods by gas chromatography-mass spectrometry. Foods 2023, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Guenther, K.; Heinke, V.; Thiele, B.; Kleist, E.; Prast, H.; Raecker, T. Endocrine disrupting nonylphenols are ubiquitous in food. Environ. Sci. Technol. 2002, 36, 1676–1680. [Google Scholar] [CrossRef]
- Günther, K.; Räcker, T.; Böhme, R. An isomer-specific approach to endocrine-disrupting nonylphenol in infant food. J. Agric. Food Chem. 2017, 65, 1247–1254. [Google Scholar] [CrossRef]
- Casajuana, N.; Lacorte, S. New methodology for the determination of phthalate esters, bisphenol A, bisphenol A diglycidyl ether, and nonylphenol in commercial whole milk samples. J. Agric. Food Chem. 2004, 52, 3702–3707. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Stoffella, P.J.; Wilson, P.C. Analysis of bisphenol A, nonylphenol, and natural estrogens in vegetables and fruits using gas chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2013, 61, 84–89. [Google Scholar] [CrossRef]
- Deng, H.; Su, X.; Wang, H. Simultaneous determination of aflatoxin B1, bisphenol A, and 4-nonylphenol in peanut oils by liquid-liquid extraction combined with solid-phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Anal. Methods 2018, 11, 1303–1311. [Google Scholar] [CrossRef]
- Loyo-Rosales, J.E.; Rosales-Rivera, G.C.; Lynch, A.M.; Rice, C.P.; Torrents, A. Migration of nonylphenol from plastic containers to water and a milk surrogate. J. Agric. Food Chem. 2004, 52, 2016–2020. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jin, F.; Snyder, S.A. Recent advancements and future trends in analysis of nonylphenol ethoxylates and their degradation product nonylphenol in food and environment. Trends Anal. Chem. 2018, 107, 78–90. [Google Scholar] [CrossRef]
- Jiang, Z.-J.; Cao, X.-L.; Li, H.; Zhang, C.; Abd El-Aty, A.M.; Jin, F.; Shao, H.; Jin, M.-J.; Wang, S.-S.; She, Y.-X.; et al. Fast determination of alkylphenol ethoxylates in leafy vegetables using a modified quick, easy, cheap, effective, rugged, and safe method and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1525, 161–172. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Wang, J.; Zheng, Y.; Cao, W.; Wang, R.; Dong, F.; Liu, X.; Qian, M.; Zhang, H.; Wu, L. Determination of nonylphenol ethoxylate metabolites in vegetables and crops by high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2012, 132, 502–507. [Google Scholar] [CrossRef]
- Cai, Q.-Y.; Huang, H.-J.; Lü, H.; Mo, C.-H.; Zhang, J.; Zeng, Q.-Y.; Tian, J.-J.; Li, Y.-W.; Wu, X.-L. Occurrence of nonylphenol and nonylphenol monoethoxylate in soil and vegetables from vegetable farms in the Pearl River Delta, South China. Arch. Environ. Contam. Toxicol. 2012, 63, 22–28. [Google Scholar] [CrossRef]
- Chen, J.; Mullin, C.A. Determination of nonylphenol ethoxylate and octylphenol ethoxylate surfactants in beehive samples by high performance liquid chromatography coupled to mass spectrometry. Food Chem. 2014, 158, 473–479. [Google Scholar] [CrossRef]
- Barahona, F.; Turiel, E.; Martín-Esteban, A. Determination of nonylphenol and nonylphenol ethoxylates in powdered milk infant formula by HPLC-FL. J. Chromatogr. Sci. 2011, 49, 243–248. [Google Scholar] [CrossRef]
- Viñas, P.; Pastor-Belda, M.; Torres, A.; Campillo, N.; Hernández-Córdoba, M. Use of oleic-acid functionalized nanoparticles for the magnetic solid-phase microextraction of alkylphenols in fruit juices using liquid chromatography-tandem mass spectrometry. Talanta 2016, 151, 217–223. [Google Scholar] [CrossRef]
- Al Rashed, N.; Guenther, K. Determination of endocrine-disrupting nonylphenols and nonylphenol carboxylates by high-performance liquid chromatography-tandem mass spectrometry: Levels in German food after restriction. Anal. Lett. 2022, 55, 634–647. [Google Scholar] [CrossRef]
- Albahr, Z.; Ganjyal, G.M.; Tang, J.; Sablani, S.S. Storage stability of selected vegetable purees in high barrier pouches processed with pressure-assisted thermal sterilization. J. Food Process Eng. 2025, 48, e70137. [Google Scholar] [CrossRef]
- Haque, M.A.; Peterson, A.M.; Froio-Blumsack, D.; Ratto, J.A.; Chen, W.T. Exploring acidic sauce permeation on high-barrier packaging film properties. Packag. Technol. Sci. 2025, 38, 407–423. [Google Scholar] [CrossRef]
- Parhi, A.; Maya, D.; Sablani, S.S. Pioneering high barrier packaging for pressure assisted thermal sterilization of low-acid food products. Food Res. Int. 2024, 196, 115126. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Parhi, A.; Tang, Z.; Tang, J.; Sablani, S.S. Storage stability of vitamin C fortified purple mashed potatoes processed with microwave-assisted thermal sterilization system. Food Innov. Adv. 2023, 2, 106–114. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Sonar, C.R.; Albahr, Z.; Alqahtani, O.; Collins, B.A.; Sablani, S.S. Pressure-assisted thermal sterilization of avocado puree in high barrier polymeric packaging. LWT 2022, 155, 112960. [Google Scholar] [CrossRef]
- Dunno, K.D.; Whiteside, S.; Thomas, R.; Cooksey, K.; Gerard, P. Effects of transportation hazards on barrier properties of gas flushed retort pouches. Packag. Technol. Sci. 2016, 29, 431–436. [Google Scholar] [CrossRef]
- Martín, M.P.; Nepote, V.; Grosso, N.R. Chemical, sensory, and microbiological stability of stored raw peanuts packaged in polypropylene ventilated bags and high barrier plastic bags. LWT 2016, 68, 174–182. [Google Scholar] [CrossRef]
- Henríquez, C.; Córdova, A.; Lutz, M.; Saavedra, J. Storage stability test of apple peel powder using two packaging materials: High-density polyethylene and metalized films of high barrier. Ind. Crops Prod. 2013, 45, 121–127. [Google Scholar] [CrossRef]
- Mexis, S.F.; Riganakos, K.A.; Kontominas, M.G. Effect of irradiation, active and modified atmosphere packaging, container oxygen barrier and storage conditions on the physicochemical and sensory properties of raw unpeeled almond kernels (Prunus dulcis). J. Sci. Food Agric. 2011, 91, 634–649. [Google Scholar] [CrossRef]
- Ayvaz, H.; Schirmer, S.; Parulekar, Y.; Balasubramaniam, V.M.; Somerville, J.A.; Daryaei, H. Influence of selected packaging materials on some quality aspects of pressure-assisted thermally processed carrots during storage. LWT 2012, 46, 437–447. [Google Scholar] [CrossRef]
- Mahalingaiah, L.; Venkateshaiah, B.V.; Kulkarni, S.; Rao, K.J. Study on the effect of packaging materials on the physico-chemical, microbiological and sensory quality of kunda. J. Food Sci. Technol. 2014, 51, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Scarsella, J.B.; Hartman, T.G. Identification and quantitation studies of migrants from BPA alternative food-contact metal can coatings. Polymers 2020, 12, 2846. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V. Migration phenomenon in food packaging. Food–package interactions, mechanisms, types of migrants, testing and relative legislation—A review. Food Bioprocess Technol. 2014, 7, 21–36. [Google Scholar] [CrossRef]
- Oldring, P.K.T.; Castle, L.; Hart, A.; Holmes, M.J. Migrants from food cans revisited–application of a stochastic model for a more realistic assessment of exposure to bisphenol A diglycidyl ether (BADGE). Packag. Technol. Sci. 2006, 19, 121–137. [Google Scholar] [CrossRef]
- Vázquez Loureiro, P.; Lestido-Cardama, A.; Sendón, R.; Bustos, J.; Paseiro Losada, P.; Rodríguez Bernaldo de Quirós, A. Identification of potential migrants from epoxy and organosol coatings used in metal food cans. Food Addit. Contam. Part A 2023, 40, 597–611. [Google Scholar] [CrossRef]
- Frańska, M.; Ginter-Kramarczyk, D.; Szymański, A.; Kozik, T.; Frański, R. Resistance of alkylphenol ethoxylate containing six ethoxylene units to biodegradation under the conditions of OECD (Organization for Economic Co-operation and Development) screening test. Int. Biodeterior. Biodegrad. 2009, 63, 1066–1069. [Google Scholar] [CrossRef]
- Fonseca-Corona, C.; Vera-Avila, L.E.; Gallegos-Pérez, J.L. Use of mass spectrometry for identification and quantitation of tensoactive agents in synthetic latex samples. J. Mex. Chem. Soc. 2014, 58, 444–451. [Google Scholar] [CrossRef]
- Amelin, V.G.; Bol’shakov, D.S. Identification and determination of nonionic surfactants by ultrahigh-performance liquid chromatography–high-resolution mass spectrometry. J. Anal. Chem. 2021, 76, 226–242. [Google Scholar] [CrossRef]
- Beszterda-Buszczak, M.; Frański, R. Oligoester identification in the inner coatings of metallic cans by high-pressure liquid chromatography-mass spectrometry with cone voltage-induced fragmentation. Materials 2024, 17, 2771. [Google Scholar] [CrossRef]
- Cariou, R.; Riviére, M.; Hutinet, S.; Tebbaa, A.; Dubreuil, D.; Mathé-Allainmat, M.; Lebreton, J.; Le Bizec, B.; Tessier, A.; Dervilly, G. Thorough investigation of non-volatile substances extractible from inner coatings of metallic cans and their occurrence in the canned vegetables. J. Hazard. Mater. 2022, 435, 129026. [Google Scholar] [CrossRef] [PubMed]
- Lestido-Cardama, A.; Vázquez-Loureiro, P.; Sendón, R.; Bustos, J.; Santillana, M.I.; Paseiro Losada, P.; Rodríguez Bernaldo de Quirós, A. Characterization of polyester coatings intended for food contact by different analytical techniques and migration testing by LC-MSn. Polymers 2022, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Zar, T.; Graeber, C.; Perazella, M.A. Reviews: Recognition, treatment, and prevention of propylene glycol toxicity. Semin. Dial. 2007, 20, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Beszterda-Buszczak, M.; Kasperkowiak, M.; Teżyk, A.; Augustynowicz, N.; Frański, R. Mass spectrometric study of the most common potential migrants extractible from the inner coatings of metallic beverage cans. Foods 2024, 13, 2025. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; Rodríguez Bernaldo de Quirós, A. Tentative identification of BADGE derivatives in epoxy type coatings in a model sample: A beverage can. J. Coat. Technol. Res. 2022, 19, 1893–1900. [Google Scholar] [CrossRef]
- Uematsu, Y.; Ogimoto, M.; Kabashima, J.; Suzuki, K.; Kaneko, R.; Funayama, K.; Haneishi, N.; Yasuno, T.; Ogino, S. Simulation of migration from a multi-layer laminated film intended for retort foods. J. Food Hyg. Soc. Jpn. 2005, 46, 133–138. [Google Scholar] [CrossRef]




| Sample | Description | Packaging Type | Protein (%) | Fat (%) | Ingredients |
|---|---|---|---|---|---|
| 1A, 1B | Fruit puree | Spouted high-barrier pouch | <0.5 | <0.5 | Apple, banana, lemon juice, ascorbic acid |
| 2A, 2B | Fruit puree | Spouted high-barrier pouch | 0.5 | 0.4 | Apple, peach, mango, ascorbic acid |
| 3A, 3B | Fruit puree | Spouted high-barrier pouch | 1.6 | 1.1 | Banana, mango, yoghurt (milk, water, skimmed milk powder, pectin, milk yoghurt cultures), water, rice flour, oat flour, lemon juice, ascorbic acid |
| 4A, 4B | Tomato puree | Retort pouch | 1.3 | 0 | Tomato purée, salt |
| 5A, 5B | Mustard | Spouted high-barrier pouch | 6.9 | 7.8 | Water, mustard seed, sugar, white mustard seed, mustard powder, table salt, acetic acid, refined deodorized sunflower oil, curcumin, xanthan gum, sodium benzoate, mustard flavor, sodium metabisulfite, paprika extract, ground cloves, turmeric extract |
| 6A, 6B | Hungarian goulash soup | Retort pouch | 3.6 | 1.6 | Water, paprika, potatoes, onion, pork, modified starch, tomato concentrate, wheat flour, salt, pepper, thyme, parsley, yeast extract, sodium ascorbate |
| 7A, 7B | Ketchup | Spouted high-barrier pouch | 1.2 | 0 | Water, tomato paste, sugar, modified corn starch, salt, acetic acid, sweet paprika, onion, garlic, chili pepper, potassium sorbate, cinnamon, black pepper, cloves |
| 8A, 8B | Roasted coffee beans | Multilayer bag with degassing valve | 0 | 0 | Roasted Arabica coffee beans |
| 9A, 9B | Roasted coffee beans—Bagdrip | High-barrier pouch/filter paper | 0 | 0 | Roasted Arabica coffee beans |
| 10A, 10B | Freeze-dried fruit smoothie | High-barrier pouch | 5.9 | 2.0 | Apple, kiwi, pineapple, spinach, nettle, ginger |
| 11A, 11B | Freeze-dried granola with strawberries | High-barrier zipper pouch | 11 | 5.1 | Gluten-free oatmeal, agave syrup, strawberry, cocoa |
| 12A, 12B | Freeze-dried wholemeal bread | Retort pouch | 5.2 | 1.2 | Wholemeal rye flour, water, salt, yeast |
| Sample | Potential Migrants |
|---|---|
| 5A | Cyclic cooligoesters, (NPG-AA)n-(NPG-iPA)m, n, m = 0–5 |
| 9A | Polypropylene glycol, HO-(CH2-CH(CH3)-O)n-H, n = 4–15 |
| 12A | BAD(M)GE conjugates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beszterda-Buszczak, M.; Frańska, M.; Frański, R. HPLC-MS Detection of Nonylphenol Ethoxylates and Lauryl Ethoxylates in Foodstuffs and the Inner Coatings of High-Barrier Pouches. Foods 2025, 14, 2842. https://doi.org/10.3390/foods14162842
Beszterda-Buszczak M, Frańska M, Frański R. HPLC-MS Detection of Nonylphenol Ethoxylates and Lauryl Ethoxylates in Foodstuffs and the Inner Coatings of High-Barrier Pouches. Foods. 2025; 14(16):2842. https://doi.org/10.3390/foods14162842
Chicago/Turabian StyleBeszterda-Buszczak, Monika, Magdalena Frańska, and Rafał Frański. 2025. "HPLC-MS Detection of Nonylphenol Ethoxylates and Lauryl Ethoxylates in Foodstuffs and the Inner Coatings of High-Barrier Pouches" Foods 14, no. 16: 2842. https://doi.org/10.3390/foods14162842
APA StyleBeszterda-Buszczak, M., Frańska, M., & Frański, R. (2025). HPLC-MS Detection of Nonylphenol Ethoxylates and Lauryl Ethoxylates in Foodstuffs and the Inner Coatings of High-Barrier Pouches. Foods, 14(16), 2842. https://doi.org/10.3390/foods14162842









