Widely Targeted Metabolomic and Network Pharmacology Analyses of Active Compounds Enriched from Ethanolic Extract of Oudemansiella raphanipes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
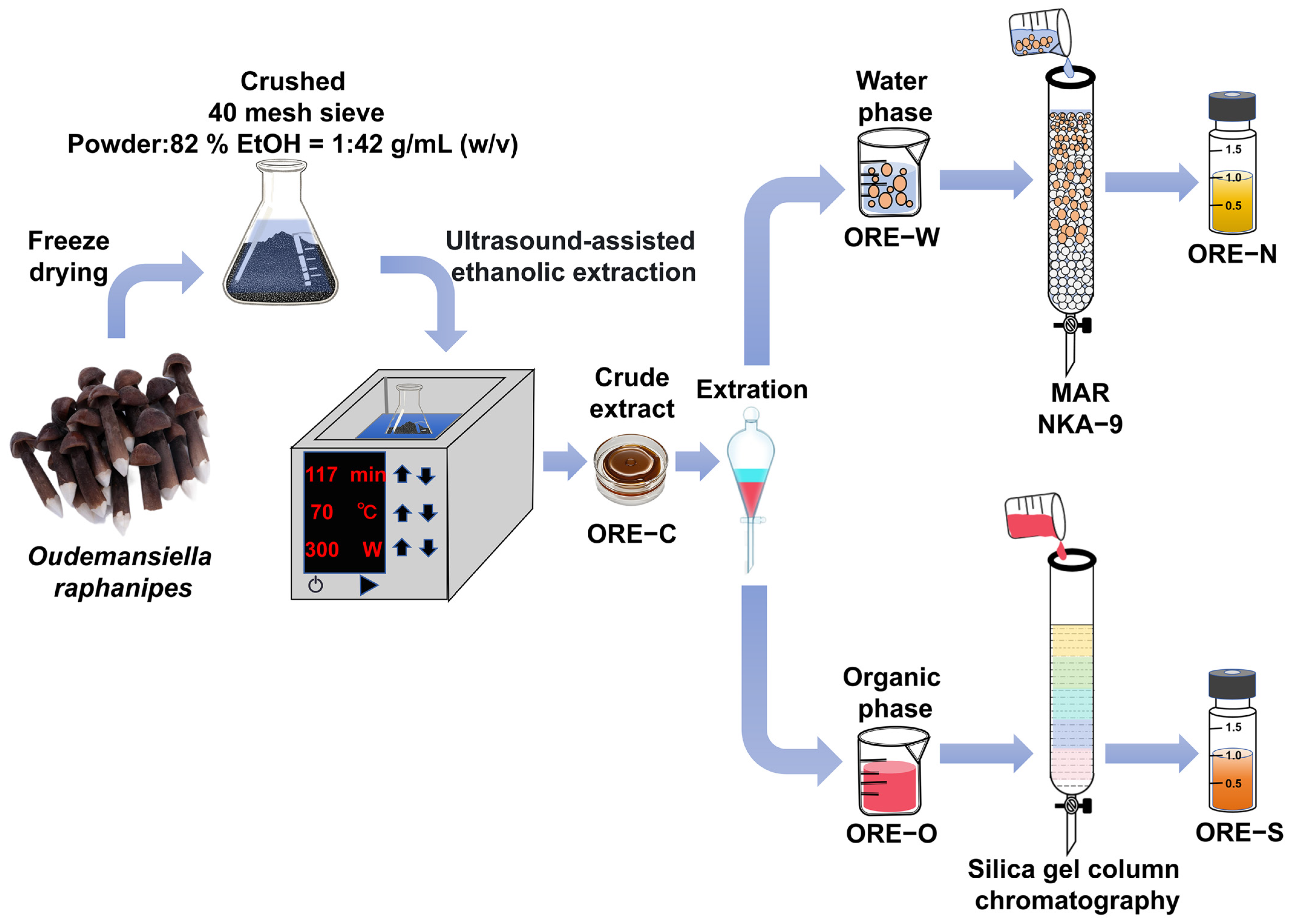
2.2. Preparation of ORE-N and ORE-S
2.3. Enrichment of Bioactive Components from ORE Using MAR Chromatography
2.3.1. MAR Activation
2.3.2. Static Experiments
2.3.3. Dynamic Experiments
2.4. Determination of Major Bioactive Components in ORE Series Products
2.5. Antioxidant Activities of ORE Series Products
2.5.1. ABTS Radical Scavenging ABILITY
2.5.2. DPPH Radical Scavenging Ability
2.5.3. Ferric Ion Reducing Antioxidant Power (FRAP)
2.5.4. Oxygen Radical Absorbance Capacity (ORAC)
2.6. Widely Targeted Metabolomic Analysis
2.6.1. Sample Preparation
2.6.2. Chromatography and Mass Spectrometry Conditions
2.6.3. Qualitative and Quantitative Analysis of Metabolites
2.7. Network Pharmacology Analysis
2.8. Data Processing and Multivariate Statistical Analysis
3. Results and Discussion
3.1. Enrichment of Bioactive Components from ORE
3.2. Comparative Analysis of Major Bioactive Components in ORE Series Products Using Different Enrichment Methods
3.3. Analysis of Antioxidant Activity of ORE Series Products
3.4. The Relationship Between Bioactive Components and Antioxidant Properties of ORE-N and ORE-S
3.5. Metabolites in ORE Series Products Identified by UPLC–MS/MS
3.6. Overview of Metabolomic Differences Between ORE-N and ORE-S
3.7. Selection of Differential Metabolites Between ORE-N and ORE-S and Their Enrichment in KEGG Pathways
3.8. Network Pharmacology-Based Elucidation of Antioxidant Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forman, H.J.; Zhang, H.Q. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Talebi, M.; Oskouie, A.A.; Mahboubi, A.; Khani, M.; Mojab, F. Analysis of Eremostachys hyoscyamoides essential oil composition and assessing the antibacterial and antioxidant properties of the ethanol extract. Heliyon 2024, 10, e38389. [Google Scholar] [CrossRef]
- Hamza, A.; Mylarapu, A.; Krishna, K.V.; Kumar, D.S. An insight into the nutritional and medicinal value of edible mushrooms: A natural treasury for human health. J. Biotechnol. 2024, 381, 86–99. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Wang, Y.Q.; Li, K.; Mazurenko, I. Effect of Oudemansiella raphanipies powder on physicochemical and textural properties, water distribution and protein conformation of lower-fat pork meat batter. Foods 2022, 11, 2623. [Google Scholar] [CrossRef]
- Qiu, J.Q.; Shi, W.; Miao, J.N.; Hu, H.; Gao, Y.A. Extraction, isolation, screening, and preliminary characterization of polysaccharides with anti-oxidant activities from Oudemansiella raphanipies. Polymers 2023, 15, 2917. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.T.; Wang, R.L.; Zhou, F.; Wu, Y.L.; Li, S.J.; Huo, G.M.; Ye, J.C.; Hua, C.; Wang, Z.J. Preparation, physicochemical characterization, and cytotoxicity of selenium nanoparticles stabilized by Oudemansiella raphanipies polysaccharide. Int. J. Biol. Macromol. 2022, 211, 35–46. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, C.; Liu, H.; Zhu, Y.F.; Ren, Z.Z.; Jing, H.J.; Li, S.S.; Zhang, J.J.; Liu, X.T.; Jia, L. The characteristics and antioxidation of Oudemansiella radicata selenium polysaccharides on lipopolysaccharide-induced endo-toxemic mice. Int. J. Biol. Macromol. 2018, 116, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Lai, Y.; Zhang, D.; Lei, H.; Wang, F.; Guo, X.R.; Song, C. Gut microbiota regulation and prebiotic properties of polysaccharides from Oudemansiella raphanipes mushroom. World J. Microbiol. Biotechnol. 2023, 39, 167. [Google Scholar] [CrossRef]
- Liu, Y.T.; Li, Y.W.; Ke, Y.; Li, C.; Zhang, Z.Q.; Wu, Y.L.; Hu, B.; Liu, A.P.; Luo, Q.Y.; Wu, W.J. In vitro saliva-gastrointestinal digestion and fecal fermentation of Oudemansiella radicata polysaccharides reveal its digestion profile and effect on the modulation of the gut microbiota. Carbohydr. Polym. 2021, 251, 117041. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.J.; Shi, L.L.; Ren, Y.R.; Yao, C.Y.; Lu, Y.M.; Chen, Y. Polysaccharide ORP-1 isolated from Oudemansiella raphanipes ameliorates age-associated intestinal epithelial barrier dysfunction in Caco-2 cells monolayer. Food Res. Int. 2022, 162, 112038. [Google Scholar] [CrossRef]
- Chen, P.Y.; Li, X.X.; Zhang, Y.W.; Wang, H.P.; Yu, Y.K.; Wu, C.; Jia, L.; Zhang, J.J. Oudemansiella radicata polysaccharides alleviated LPS-induced liver damage via regulating TLR4/NF-κB and Bax/Bcl-2 signaling pathways. Int. J. Biol. Macromol. 2024, 282, 137370. [Google Scholar] [CrossRef]
- Wang, W.S.; Zhang, Y.H.; Liu, X.C.; Liu, Z.H.; Jia, L.; Zhang, J.J. Polysaccharides from Oudemansiella radicata residues attenuate carbon tetrachloride-induced liver injury. Int. J. Biol. Macromol. 2023, 242, 124823. [Google Scholar] [CrossRef]
- Gao, Z.; Li, J.; Song, X.L.; Zhang, J.J.; Wang, X.X.; Jing, H.J.; Ren, Z.Z.; Li, S.S.; Zhang, C.; Jia, L. Antioxidative, anti-inflammation and lung-protective effects of mycelia selenium polysaccharides from Oudemansiella radicata. Int. J. Biol. Macromol. 2017, 104, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, C.; Tian, C.Y.; Ren, Z.Z.; Song, X.L.; Wang, X.X.; Xu, N.; Jing, H.J.; Li, S.S.; Liu, W.R.; et al. Characterization, antioxidation, anti-inflammation and renoprotection effects of selenized mycelia polysaccharides from Oudemansiella radicata. Carbohydr. Polym. 2018, 181, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Li, H.F.; Chen, P.; Wang, Q. Chemical constituents and anticancer activity of the petroleum ether extract from the fruiting of Oudemansiella raphanipes. Chem. Nat. Compd. 2021, 57, 976–977. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Tarone, A.G.; Tosi, M.M.; Maróstica Júnior, M.R.; Meireles, M.A.A. Extraction of bioactive compounds from genipap (Genipa americana L.) by pressurized ethanol: Iridoids, phenolic content and antioxidant activity. Food Res. Int. 2017, 102, 595–604. [Google Scholar] [CrossRef]
- Perrier, A.; Delsart, C.; Boussetta, N.; Grimi, N.; Citeau, M.; Vorobiev, E. Effect of ultrasound and green solvents addition on the oil extraction efficiency from rapeseed flakes. Ultrason. Sonochem. 2017, 39, 58–65. [Google Scholar] [CrossRef]
- Martínez-Inda, B.; Jiménez-Moreno, N.; Esparza, I.; Ancín-Azpilicueta, C. Coffee and cocoa by-products as valuable sources of bioactive compounds: The Influence of ethanol on extraction. Antioxidants 2025, 14, 42. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jayakody, J.T.M.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Ryu, B. The influence of solvent choice on the extraction of bioactive compounds from asteraceae: A comparative review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef]
- Marques, F.; Pinho, M.; Guerra, I.M.S.; Conde, T.A.; Silva, J.; Cardoso, H.; Domingues, M.R. Unlocking functional lipid ingredients from algae by food-grade biosolvents and ultrasound-assisted extraction for nutritional applications. LWT 2024, 200, 116136. [Google Scholar] [CrossRef]
- Lesa, K.N.; Khandaker, M.U.; Iqbal, F.M.R.; Sharma, R.; Islam, F.; Mitra, S.; Bin Emran, T. Nutritional value, medicinal importance, and health-promoting effects of dietary mushroom (Pleurotus ostreatus). J. Food Qual. 2022, 2022, 2454180. [Google Scholar] [CrossRef]
- Liu, X.; Cui, S.Y.; Li, W.L.; Xie, H.Q.; Shi, L.E. Elucidation of the anti-colon cancer mechanism of Phellinus baumii polyphenol by an integrative approach of network pharmacology and experimental verification. Int. J. Biol. Macromol. 2023, 253, 127429. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Shi, C.; Yang, Y.; Fang, Y.; Sheng, L.; Li, N. Medicinal mushroom Phellinus igniarius induced cell apoptosis in gastric cancer SGC-7901 through a mitochondria-dependent pathway. Biomed. Pharmacother. 2018, 102, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, W.J.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.Y.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Yu, X.; Chen, H.M.; Sha, Z.G.; Hu, Y.W.; Yan, M.X.; Xin, J.H.; Cao, X.J.; Wan, J.F. Highly selective separation and purification of lincomycin by macroporous adsorption resin column chromatography. J. Chromatogr. 2024, 1735, 465282. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Dong, S.H.; Zhang, W.Y.; Qi, Z.L.; Liu, X.L. Study on adsorption/resolution properties, enrichment and purification of phenolic substances of Inonotus hispidus by macroporous adsorption resin. Ind. Crops Prod. 2024, 216, 118661. [Google Scholar] [CrossRef]
- Susanti, I.; Pratiwi, R.; Rosandi, Y.; Hasanah, A.N. Separation Methods of Phenolic Compounds from Plant Extract as Antioxidant Agents Candidate. Plants 2024, 13, 965. [Google Scholar] [CrossRef]
- Zhou, J.N.; Hou, D.H.; Zou, W.Q.; Wang, J.H.; Luo, R.; Wang, M.; Yu, H. Comparison of widely targeted metabolomics and untargeted metabolomics of wild Ophiocordyceps sinensis. Molecules 2022, 27, 3645. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Zhang, W.Y.; Du, M.H.; Zhong, H.Y.; Fang, X.Z. Widely targeted metabolomic and KEGG analyses of natural deep eutectic solvent-based saponins extraction from Camellia oleifera Abel.: Effects on composition. Food Chem. 2024, 450, 139333. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, H.; Li, X.Z.; Wang, C.X.; Li, Q.H.; Xu, M.; Guan, X.L.; Lan, Z.H.; Ni, Y.Q.; Zhang, Y. Widely targeted metabolomics analysis of enriched secondary metabolites and determination of their corresponding antioxidant activities in Elaeagnus angustifolia var. orientalis (L.)Kuntze fruit juice enhanced by Bifidobacterium animalis subsp. Lactis HN-3 fermentation. Food Chem. 2022, 374, 131568. [Google Scholar] [CrossRef]
- Liu, H.; Lan, Z.; Zhang, Y.; Zhao, Z.; Wu, Y.; Tang, X.; Nie, J. Metabolomics combined with network pharmacology reveals the effects of ripening stages and edible parts on bioactive ingredients of Luohan Guo (Siraitia grosvenorii). Food Res. Int. 2025, 203, 115896. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Qi, Z.X.; Li, Y.; Yue, L.; Zhao, G.P.; Gui, X.Y.; Dong, P.; Wang, Y.; Zhang, B.; Li, X. A new species and new records of Hymenopellis and Xerula (Agaricales, Physalacriaceae) from China. Peerj 2023, 11, e16681. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Yan, J.J.; Miao, R.Y.; Lin, J.B.; Li, X.; Zhou, X.R.; Zhang, P.; Gan, B.C. Optimization of extraction process of total flavonoids from Oudemansiella raphanipes by response surface methodology. China Condiment 2022, 47, 138–143. Available online: https://oversea.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&filename=ZGTW202211026&dbname=CJFDLAST2022 (accessed on 11 August 2025).
- Medic-Saric, M.; Jasprica, L.; Mornar, A.; Smolcic-Bubalo, A.; Golja, P. Quantitative analysis of flavonoids and phenolic acids in propolis by two-dimensional thin layer chromatography. J. Planar Chromatogr. 2004, 17, 459–463. [Google Scholar] [CrossRef]
- Wang, X.Y.; Su, J.Q.; Chu, X.L.; Zhang, X.Y.; Kan, Q.B.; Liu, R.X.; Fu, X. Adsorption and desorption characteristics of total flavonoids from Acanthopanax senticosus on macroporous adsorption resins. Molecules 2021, 26, 4162. [Google Scholar] [CrossRef]
- Yang, Y.H.; Liang, Q.M.; Zhang, B.; Zhang, J.M.; Fan, L.; Kang, J.H.; Lin, Y.Q.; Huang, Y.; Tan, T.C.; Ho, L.H. Adsorption and desorption characteristics of flavonoids from white tea using macroporous adsorption resin. J. Chromatogr. 2024, 1715, 464621. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Tang, H.T.; Shao, Q.; Bilia, A.R.; Wang, Y.; Zhao, X.P. Enrichment and purification of the bioactive flavonoids from flower of Abelmoschus manihot (L.) medic using macroporous resins. Molecules 2018, 23, 2649. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, W.Y. Adsorption and desorption characteristics of a phenolic compound from Ecklonia cava on macroporous. Food Chem. 2021, 338, 128150. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Liu, X.; Wang, N.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; Ouyang, K.H.; et al. Investigation of chemical composition, antioxidant activity, and the effects of alfalfa flavonoids on growth performance. Oxid. Med. Cell. Longev. 2020, 2020, 8569237. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, J.J.; Cao, F.W.; Chen, F.J. Anti-inflammatory properties of extracts from Chimonanthus nitens Oliv. leaf. PLoS ONE 2017, 12, e0181094. [Google Scholar] [CrossRef] [PubMed]
- Bobinaite, R.; Viskelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, Y.L.; Gao, M.X.; Chen, S.; Li, L.; Liu, Y.B.; Gu, T.; Zhang, J.L. Mechanisms of the increase triterpenoids content of Morchella eximia induced by salicylic acid and magnetic field. Food Bioprod. Process. 2024, 145, 21–31. [Google Scholar] [CrossRef]
- Li, L.Y.; Long, W.F.; Wan, X.L.; Ding, Q.; Zhang, F.; Wan, D.R. Studies on quantitative determination of total alkaloids and berberine in five origins of crude medicine “Sankezhen”. J. Chromatogr. Sci. 2015, 53, 307–311. [Google Scholar] [CrossRef]
- Birhanie, Z.M.; Xiao, A.P.; Yang, D.W.; Huang, S.Q.; Zhang, C.; Zhao, L.N.; Liu, L.L.; Li, J.J.; Chen, A.G.; Tang, H.J.; et al. Polysaccharides, total phenolic, and flavonoid content from different Kenaf (Hibiscus cannabinus L.) genotypes and their antioxidants and antibacterial properties. Plants 2021, 10, 1900. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Hamidian, Y.; Behrouzifar, F.; Mostafazadeh, R.; Ghorbani-HasanSaraei, A.; Alizadeh, M.; Mortazavi, S.M.; Janbazi, M.; Asrami, P.N. An applicable method for extraction of whole seeds protein and its determination through Bradford’s method. Food Chem. Toxicol. 2022, 164, 113053. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.G.; Li, H.Y.; Liu, Y.; Li, C.; Fang, Z.F.; Hu, B.; Li, X.L.; Zeng, Z.; Liu, Y.T. Changes in flavor and biological activities of Lentinula edodes hydrolysates after maillard reaction. Food Chem. 2024, 431, 137138. [Google Scholar] [CrossRef]
- Lin, G.; Dou, X.; Gao, N.B.; Hu, D.J.; Zhang, L.H. Antiradical activity studies of ultrasound-assisted extraction of total flavonoids from Sanghuangporus vaninii fruit bodies. J. Food Biochem. 2023, 2023, 5683207. [Google Scholar] [CrossRef]
- Ren, X.L.; Wang, S.L.; Wang, J.Y.; Xu, D.; Ye, Y.; Song, Y.B. Widely targeted metabolome profiling of different plateau raspberries and berry parts provides innovative insight into their antioxidant activities. Front. Plant Sci. 2023, 14, 1143439. [Google Scholar] [CrossRef]
- Chen, X.C.; Xu, Y.T.; Du, X.P.; Li, Z.P.; Yang, Y.F.; Jiang, Z.D.; Ni, H.; Li, Q.B. Effect of Porphyra haitanensis polyphenols from different harvest periods on hypoglycaemic activity based on in vitro digestion and widely targeted metabolomic analysis. Food Chem. 2024, 437, 137793. [Google Scholar] [CrossRef]
- Tang, Y.C.; Liu, Y.J.; He, G.R.; Cao, Y.W.; Bi, M.M.; Song, M.; Yang, P.P.; Xu, L.F.; Ming, J. Comprehensive analysis of secondary metabolites in the extracts from different lily bulbs and their antioxidant ability. Antioxidants 2021, 10, 1634. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Aljawarneh, R.Y.A.; Zain, M.S.C.; Zakaria, F. Macroporous polymeric resin for the purification of flavonoids from medicinal plants: A review. J. Sep. Sci. 2024, 47, 2400372. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.F.; Nie, W.; Cui, Y.P.; Yue, F.L.; Fan, X.T.; Sun, R.Y. Purification with macroporous resin and antioxidant activity of polyphenols from sweet potato leaves. Chem. Pap. 2024, 78, 181–188. [Google Scholar] [CrossRef]
- Zain, M.S.C.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption and desorption properties of total flavonoids from oil palm (Elaeis guineensis Jacq.) mature leaf on macroporous adsorption resins. Molecules 2020, 25, 778. [Google Scholar] [CrossRef]
- Chen, X.M.; Wang, H.; Huang, X.J.; Xia, S.K.; Chen, C.H.; Nie, Q.X.; Nie, S.P. Efficient enrichment of total flavonoids from kale (Brassica oleracea L. var. acephala L.) extracts by NKA-9 resin and antioxidant activities of flavonoids extract In Vitro. Food Chem. 2022, 374, 131508. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Saini, A.; Seni, K.; Chawla, P.A.; Chawla, V.; Ganti, S.S. An insight into recent updates on analytical techniques for bioactive alkaloids. Phytochem. Anal. 2024, 35, 423–444. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Bin Emran, T.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. 2022, 13, 100217. [Google Scholar] [CrossRef]
- Yahia, E.M.; Gutiérrez-Orozco, F.; Moreno-Pérez, M.A. Identification of phenolic compounds by liquid chromatography-mass spectrometry in seventeen species of wild mushrooms in Central Mexico and determination of their antioxidant activity and bioactive compounds. Food Chem. 2017, 226, 14–22. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Fraga, L.N.; Oliveira, A.K.D.; Aragao, B.P.; de Souza, D.A.; dos Santos, E.W.P.; Melo, J.A.; Silva, A.M.D.E.; Wisniewski, A.; Corrêa, C.B.; Wartha, E.R.S.D.; et al. Mass spectrometry characterization, antioxidant activity, and cytotoxicity of the peel and pulp extracts of Pitomba. Food Chem. 2021, 340, 127929. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Bhagwat, S.A. USDA database for the oxygen radical capacity (ORAC) of selected foods, release 2. In USDA National Nutrient Database for Standard Reference; USDA: Washington, DC, USA, 2010. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 15 March 2025).
- González, E.A.; Nazareno, M.A. Antiradical action of flavonoid-ascorbate mixtures. LWT 2011, 44, 558–564. [Google Scholar] [CrossRef]
- Hidalgo, M.; Sánchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid-flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Myint, K.Z.; Zhou, Z.Y.; Xia, Y.M.; Fang, Y.; Wu, M.N.; Zhu, S.; Shen, J. Stevia polyphenols: A stable antioxidant that presents a synergistic effect with vitamin C. J. Food Process. Preserv. 2021, 45, e15317. [Google Scholar] [CrossRef]
- Otero, C.; Miranda-Rojas, S.; Llancalahuén, F.M.; Fuentes, J.A.; Atala, C.; González-Silva, G.; Verdugo, D.; Sierra-Rosales, P.; Moreno, A.; Gordillo-Fuenzalida, F. Biochemical characterization of Peumus boldus fruits: Insights of its antioxidant properties through a theoretical approach. Food Chem. 2022, 370, 131012. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, T.Y.; Xu, J.X.; Xi, W.J.; Shang, E.; Xiao, P.; Duan, J.A. The relationship between polysaccharide structure and its antioxidant activity needs to be systematically elucidated. Int. J. Biol. Macromol. 2024, 270, 132391. [Google Scholar] [CrossRef]
- Li, Y.T.; He, D.; Li, B.; Lund, M.N.; Xing, Y.F.; Wang, Y.; Li, F.X.; Cao, X.; Liu, Y.J.; Chen, X.Y.; et al. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends Food Sci. Technol. 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.F.; Liu, Y.F.; Li, J.W. Effects of antioxidants, proteins, and their combination on emulsion oxidation. Crit. Rev. Food Sci. Nutr. 2022, 62, 8137–8160. [Google Scholar] [CrossRef]
- Ko, H.H.; Weng, J.R.; Tsao, L.T.; Yen, M.H.; Wang, J.P.; Lin, C.N. Anti-inflammatory flavonoids and pterocarpanoid from Crotalaria pallida and C. assamica. Bioorg. Med. Chem. Lett. 2004, 14, 1011–1014. [Google Scholar] [CrossRef]
- Hou, X.Y.; Bai, X.P.; Gou, X.L.; Zeng, H.; Xia, C.; Zhuang, W.; Chen, X.M.; Zhao, Z.X.; Huang, M.; Jin, J. 3′,4′,5′,5,7-pentamethoxyflavone sensitizes Cisplatin-resistant A549 cells to Cisplatin by inhibition of Nrf2 pathway. Mol. Cells 2015, 38, 396–401. [Google Scholar] [CrossRef]
- Cui, P.P.; Cai, M.J.; Meng, Y.A.; Yang, Y.; Song, H.J.; Liu, Y.X.; Wang, Q.M. Design, synthesis and biological activities of echinopsine derivatives containing acylhydrazone moiety. Sci. Rep. 2022, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, L.; Asghari-Varzaneh, M.; Alavi, S.S.; Halvaei-Varnousfaderani, M.; Laher, I. Cardiovascular protective effects of cinnamic acid as a natural phenolic acid: A review. Arch. Physiol. Biochem. 2024, 131, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M.; Taniguchi, M.; Baba, K. Antitumor and antimetastatic actions of anthrone-C-glucoside, cassialoin isolated from Cassia garrettiana heartwood in colon 26-bearing mice. Cancer Sci. 2008, 99, 2336–2348. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.J.; Ryoo, I.J.; Kim, K.C.; Na, M.; Jang, J.H.; Ahn, J.S.; Yoo, I.D. Hypo-pigmenting effect of sesquiterpenes from Inula britannica in B16 melanoma cells. Arch. Pharmacal Res. 2014, 37, 567–574. [Google Scholar] [CrossRef]
- Zhang, X.A.; Li, X.; Su, M.S.; Du, J.H.; Zhou, H.J.; Li, X.W.; Ye, Z.W. A comparative UPLC-Q-TOF/MS-based metabolomics approach for distinguishing peach (Prunus persica (L.) Batsch) fruit cultivars with varying antioxidant activity. Food Res. Int. 2020, 137, 109531. [Google Scholar] [CrossRef]
- Kang, C.D.; Zhang, Y.Y.; Zhang, M.Y.; Qi, J.; Zhao, W.T.; Gu, J.; Guo, W.P.; Li, Y.Y. Screening of specific quantitative peptides of beef by LC-MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with potential health benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The roles of serotonin in neuropsychiatric disorders. Cell. Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Xu, P.Y.; Huang, S.J.; Zhang, H.B.; Mao, C.Y.; Zhou, X.E.; Cheng, X.; Simon, I.A.; Shen, D.D.; Yen, H.Y.; Robinson, C.V.; et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 2021, 592, 469–473. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, J.; Cui, Z.K.; Li, Y.; Gao, Q.; Miao, Y.L.; Xiong, B. Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging. Nat. Aging 2023, 3, 1372–1386. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.A.; Zhao, Z.Y.; Li, L.; Min, Y.; Zhang, D.Y.; Ji, A.Y.; Jiang, C.; Wei, X.T.; Wu, X. A comprehensive review of spermidine: Safety, health effects, absorption and metabolism, food materials evaluation, physical and chemical processing, and bioprocessing. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2820–2842. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, W.; Su, J.J.; Zhou, B.S.; Han, Q.J. Spermidine retarded the senescence of multipotent mesenchymal stromal cells in vitro and in vivo through SIRT3-mediated antioxidation. Stem Cells Int. 2023, 2023, 9672658. [Google Scholar] [CrossRef]
- Chantarawong, W.; Kuncharoen, N.; Tanasupawat, S.; Chanvorachote, P. Lumichrome inhibits human lung cancer cell growth and induces apoptosis via a p53-dependent mechanism. Nutr. Cancer 2019, 71, 1390–1402. [Google Scholar] [CrossRef]
- Liu, J.X.; Li, C.Y.; Zhang, C.Y.; Huang, R.; Qu, L.H.; Ge, Y.H. 2-hydroxy-3-phenylpropanoic acid suppressed the growth of Alternaria alternata through damaging cell membrane integrity and modulating reactive oxygen species metabolism. Fungal Biol. 2023, 127, 949–957. [Google Scholar] [CrossRef]
- André, V.; Braga, D.; Grepioni, F.; Duarte, M.T. Crystal forms of the antibiotic 4-aminosalicylic acid: Solvates and molecular salts with dioxane, morpholine, and piperazine. Cryst. Growth Des. 2009, 9, 5108–5116. [Google Scholar] [CrossRef]
- Lin, Z.; Bing, X.I.A.; Hong, C.; Jin, L.I.; Mei, Y.E. Effect of 4-aminosalicylic acid on trinitrobenzene suffonic acid-induced colitis in rats. J. Fourth Mil. Med. Univ 2006, 27, 1108–1112. [Google Scholar]
- Hsu, Y.L.; Chen, C.Y.; Lin, I.P.; Tsai, E.M.; Kuo, P.L.; Hou, M.F. 4-shogaol, an active constituent of dietary ginger, inhibits metastasis of MDA-MB-231 human breast adenocarcinoma cells by decreasing the repression of NF-κB/Snail on RKIP. J. Agric. Food Chem. 2012, 60, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Park, C.M. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem. Toxicol. 2014, 65, 70–75. [Google Scholar] [CrossRef]
- Zhu, M.T.; Sun, Y.P.; Su, Y.; Guan, W.; Wang, Y.; Han, J.W.; Wang, S.; Yang, B.Y.; Wang, Q.H.; Kuang, H.X. Luteolin: A promising multifunctional natural flavonoid for human diseases. Phytother. Res. 2024, 38, 3417–3443. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Pinya, S.; Martorell, M.; Capó, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential anti-inflammatory effects of hesperidin from the genus Citrus. Curr. Med. Chem. 2018, 25, 4929–4945. [Google Scholar] [CrossRef]
- Kaviani, F.; Baratpour, I.; Ghasemi, S. The antidiabetic mechanisms of hesperidin: Hesperidin nanocarriers as promising therapeutic options for diabetes. Curr. Mol. Med. 2024, 24, 1483–1493. [Google Scholar] [CrossRef]
- Meng, C.Y.; Guo, Z.H.; Li, D.G.; Li, H.W.; He, J.B.; Wen, D.G.; Luo, B. Preventive effect of hesperidin modulates inflammatory responses and antioxidant status following acute myocardial infarction through the expression of PPAR- and Bcl-2 in model mice. Mol. Med. Rep. 2018, 17, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhai, Y.H.; Zhang, Y.Q.; Xu, S.; Yu, S.X.; Wei, Y.X.; Xiao, H.F.; Song, Y.D. Inhibition of α-glucosidase by trilobatin and its mechanism: Kinetics, interaction mechanism and molecular docking. Food Funct. 2022, 13, 857–866. [Google Scholar] [CrossRef]
- Zhong, H.; Hao, L.; Li, X.; Wang, C.J.; Wu, X. Anti-inflammatory Role of Trilobatin on Lipopolysaccharide-induced Acute Lung Injury through Activation of AMPK/GSK3β-Nrf2 Pathway. Signa Vitae 2020, 16, 160–166. [Google Scholar] [CrossRef]
- Kang, Y.S.; Chung, Y.C.; Lee, J.N.; Kim, B.S.; Hyun, C.G. Anti-inflammatory effects of 6,7-dihydroxy-4-methylcoumarin on LPS-stimulated macrophage phosphorylation in MAPK signaling pathways. Nat. Prod. Commun. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Si, R.; Tuyatsetseg, J.; Banzragch, M.; Ming, L.; He, J.; Ji, R. Analysis of differential muscle metabolites in Bactrian camels slaughtered at different ages by non-targeted metabolomics based on ultra-high performance liquid chromatography-mass spectrometry. Food Sci. 2024, 45, 154–163. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, X.X.; Li, L.; Guo, M.M.; He, Y.F.; Dong, Y.M.; Meng, H.; Yi, F. Study of the mechanism by gentiopicroside protects against skin fibroblast glycation damage via the RAGE pathway. Sci. Rep. 2024, 14, 4685. [Google Scholar] [CrossRef]
- Guan, G.Q.; Chen, Y.X.; Dong, Y.L. Unraveling the AMPK-SIRT1-FOXO Pathway: The In-Depth Analysis and Breakthrough Prospects of Oxidative Stress-Induced Diseases. Antioxidants 2025, 14, 70. [Google Scholar] [CrossRef]
- Ke, Z.L.; Tan, S.; Li, H.Y.; Jiang, S.S.; Li, Y.P.; Chen, R.; Li, M.X. Tangeretin improves hepatic steatosis and oxidative stress through the Nrf2 pathway in high fat diet-induced nonalcoholic fatty liver disease mice. Food Funct. 2022, 13, 2782–2790. [Google Scholar] [CrossRef]
- Gan, R.Y.; Liu, Y.; Li, H.; Xia, Y.; Guo, H.; Geng, F.; Zhuang, Q.G.; Li, H.B.; Wu, D.T. Natural sources, refined extraction, biosynthesis, metabolism, and bioactivities of dietary polymethoxyflavones (PMFs). Food Sci. Hum. Wellness 2024, 13, 27–49. [Google Scholar] [CrossRef]







| Resins | D101 | AB-8 | HPD-450A | NKA-9 | ADS-7 |
|---|---|---|---|---|---|
| Particle size (mm) | 0.3–1.25 | 0.3–1.25 | 0.3–1.25 | 0.3–1.25 | 0.3–1.25 |
| Surface area (m2/g) | None | 480–520 | 500–550 | 500–550 | ≥100 |
| Average pore diameter (nm) | None | 130–140 | 90–100 | 100–120 | 25–30 |
| Polarity | Non-polar | Weak polar | Middle polar | Polar | Strongly polar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Zhao, J.; Zhu, S.; Chen, M.; Wu, D.; Wu, Y.; Lin, J.; Miao, R.; Feng, R.; Li, X.; et al. Widely Targeted Metabolomic and Network Pharmacology Analyses of Active Compounds Enriched from Ethanolic Extract of Oudemansiella raphanipes. Foods 2025, 14, 2820. https://doi.org/10.3390/foods14162820
Wu Z, Zhao J, Zhu S, Chen M, Wu D, Wu Y, Lin J, Miao R, Feng R, Li X, et al. Widely Targeted Metabolomic and Network Pharmacology Analyses of Active Compounds Enriched from Ethanolic Extract of Oudemansiella raphanipes. Foods. 2025; 14(16):2820. https://doi.org/10.3390/foods14162820
Chicago/Turabian StyleWu, Zhi, Jin Zhao, Shuang Zhu, Mengxing Chen, Dan Wu, Yiyou Wu, Junbin Lin, Renyun Miao, Rencai Feng, Xiang Li, and et al. 2025. "Widely Targeted Metabolomic and Network Pharmacology Analyses of Active Compounds Enriched from Ethanolic Extract of Oudemansiella raphanipes" Foods 14, no. 16: 2820. https://doi.org/10.3390/foods14162820
APA StyleWu, Z., Zhao, J., Zhu, S., Chen, M., Wu, D., Wu, Y., Lin, J., Miao, R., Feng, R., Li, X., Gan, B., & Wang, T. (2025). Widely Targeted Metabolomic and Network Pharmacology Analyses of Active Compounds Enriched from Ethanolic Extract of Oudemansiella raphanipes. Foods, 14(16), 2820. https://doi.org/10.3390/foods14162820






