Recent Advances in Technologies for Preserving Fresh-Cut Fruits and Vegetables
Abstract
1. Introduction
2. Reasons for Quality Deterioration of Fresh-Cut Fruits and Vegetables
2.1. Enhanced Ethylene Production
2.2. Enhanced Respiration Rate
2.3. Increased Transpiration
2.4. Increased Color Loss
2.4.1. Cut Surface Browning
2.4.2. Green Color Loss
2.5. Increased Texture Loss
2.6. Microbial Spoilage
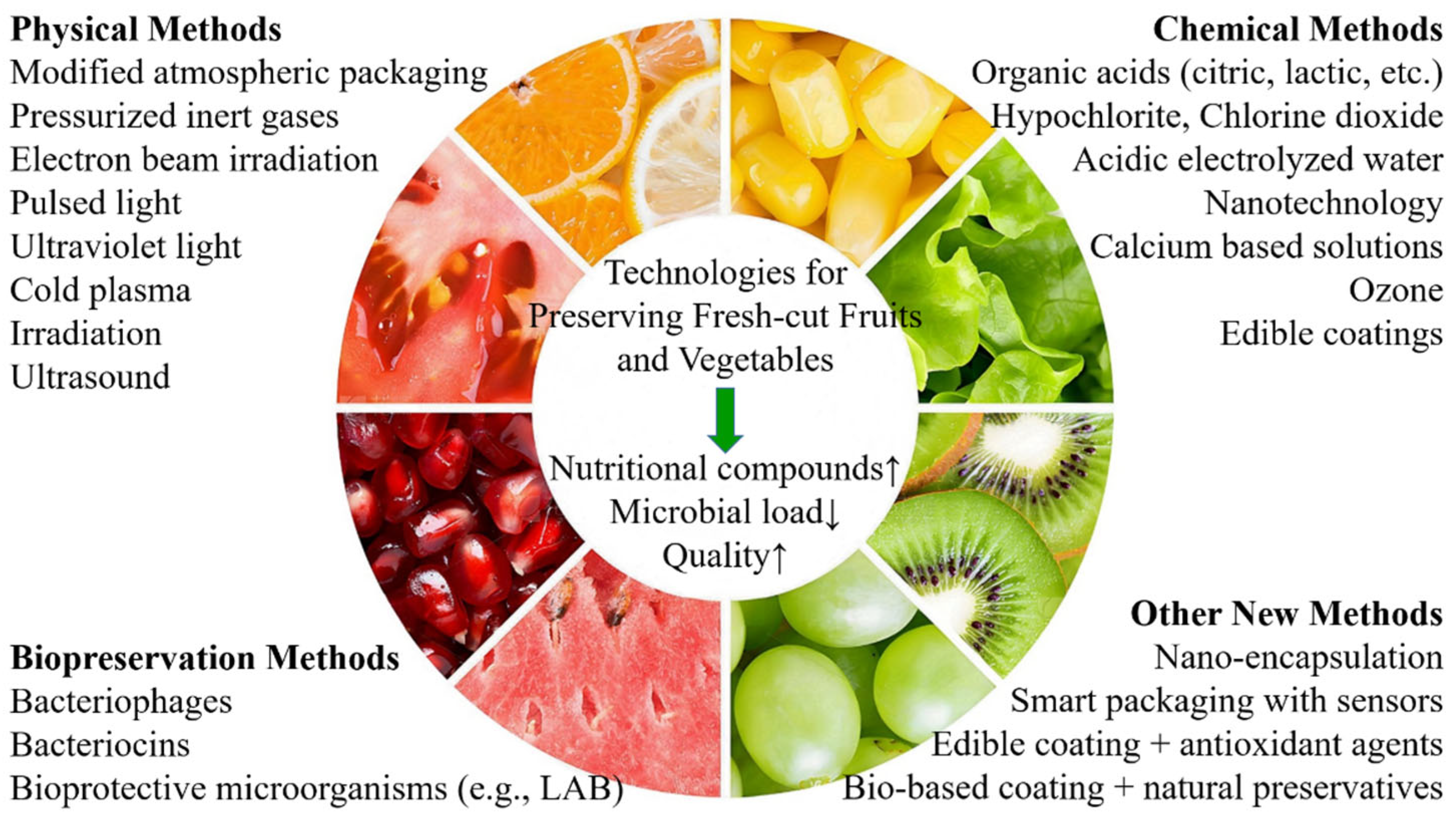
3. Novel Methods for Preserving Fresh-Cut Fruits and Vegetables
3.1. Physical Methods
3.1.1. Modified Atmosphere Packaging (MAP)
3.1.2. Pressurized Inert Gases
3.1.3. Electron Beam Irradiation (EBI)
3.1.4. Pulsed Light (PL)
3.1.5. Ultraviolet Light (UV)
3.1.6. Cold Plasma (CP)
3.2. Chemical Methods
3.2.1. Acidic Electrolyzed Water (AEW)
3.2.2. Nanotechnology
3.2.3. Ozone
3.2.4. Chlorine Dioxide
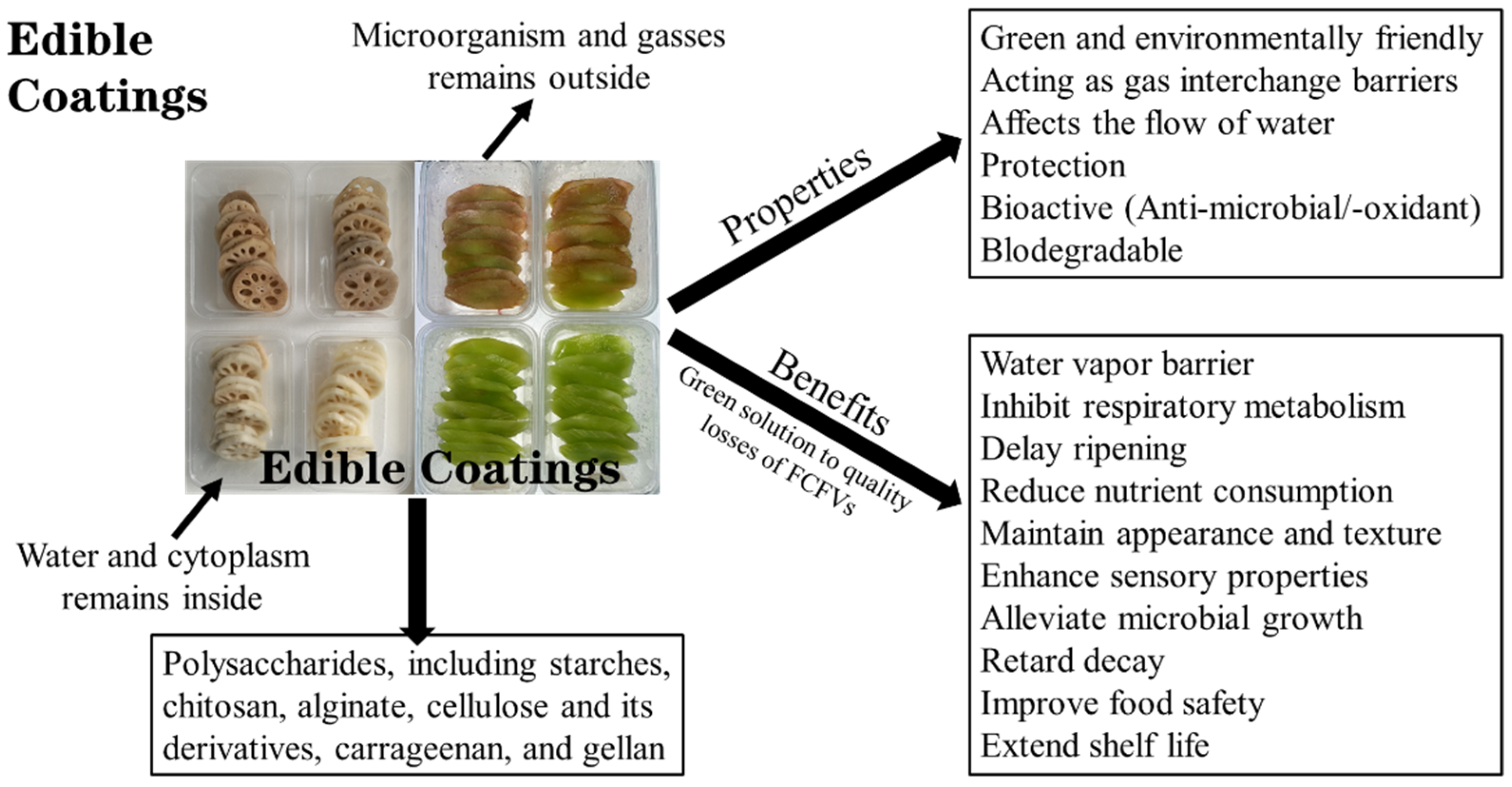
3.2.5. Edible Coatings
3.3. Biopreservation Methods
3.3.1. Bacteriocins
3.3.2. Bioprotective Microorganisms
3.3.3. Bacteriophages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cannata, C.; Rutigliano, C.A.C.; Restuccia, C.; Muratore, G.; Sabatino, L.; Geoffriau, E.; Leonardi, C.; Mauro, R.P. Effects of polysaccharide-based edible coatings on the shelf life of fresh-cut carrots with different pigmentations. J. Agric. Food Res. 2025, 20, 101765. [Google Scholar] [CrossRef]
- Song, C.; Wang, J.; Wu, L.; Liu, J.; Liu, G.; Gong, D.; Zhang, W.; Wei, J.; Zhang, Z. Quality and physiological changes in fresh-cut mango fruit as affected by cold plasma-activated water. Postharvest Biol. Technol. 2025, 225, 113524. [Google Scholar] [CrossRef]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Technol. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z.; Song, Y.; Du, T.; Han, F.; Wang, S.; Feng, J.; Wang, J.; Zhang, W. Photodynamic inactivation mediated by natural alizarin on bacteria for the safety of fresh-cut apples. Food Res. Int. 2025, 200, 115441. [Google Scholar] [CrossRef] [PubMed]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohame, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- de Chiara, M.L.V.; Castagnini, J.M.; Capozzi, V. Cutting-edge physical techniques in postharvest for fruits and vegetables: Unveiling their power, inclusion in ‘hurdle’ approach, and latest applications. Trends Food Sci. Technol. 2024, 151, 104619. [Google Scholar] [CrossRef]
- Luo, H. Studies on the Mechanism of Quality Deterioration and Preservation Technology of Fresh-Cut Zizania latifolia. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2011. [Google Scholar] [CrossRef]
- Chen, H.; Yang, C.; Zhuansun, X.; Li, Y.; Han, R.; Wang, L.; Ding, S.; Liu, Q. Zein film loaded with Fructus Ligustri Lucidi essential oil: Preparation, characterization and application in fresh-cut apple preservation. Food Control 2025, 167, 110811. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, M.; Jiang, Y.; Hu, B.; Guo, X.; Gong, D.; Zhang, Y. Compositional shifts in fresh-cut apples microbiome in response to application of Lactiplantibacillus plantarum assessed by next-generation sequencing. LWT Food Sci. Technol. 2024, 191, 115627. [Google Scholar] [CrossRef]
- Zhou, F.; Ma, R.; Xu, D.; Jiang, A. Ascorbic acid treatment inhibits early wound healing in the fresh-cut potato by relegating jasmonic acid biosynthesis and signal transduction. Food Chem. 2025, 464, 141885. [Google Scholar] [CrossRef]
- Gouda, M.H.B.; Zhang, C.; Peng, S.; Kong, X.; Chen, Y.; Li, H.; Li, X.; Luo, H.; Yu, L. Combination of sodium alginate-based coating with L-cysteine and citric acid extends the shelf-life of fresh-cut lotus root slices by inhibiting browning and microbial growth. Postharvest Biol. Technol. 2021, 175, 111502. [Google Scholar] [CrossRef]
- Peng, H.; Lavelle, D.O.; Truco, M.J.; Michelmore, R.W.; Simko, I. Differential low oxygen response and transcriptomic shifts drive fresh-cut lettuce deterioration in modified atmosphere packaging. Postharvest Biol. Technol. 2025, 227, 113571. [Google Scholar] [CrossRef]
- Li, X.; Peng, S.; Yu, R.; Li, P.; Zhou, C.; Qu, Y.; Li, H.; Luo, H.; Yu, L. Co-application of 1-MCP and laser microporous plastic bag packaging maintains postharvest quality and extends the shelf-life of honey peach fruit. Foods 2022, 11, 1733. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, L.; Zhang, L.; Jiang, J.; Yu, Z. Quality changes of whole and fresh-cut Zizania latifolia during refrigerated (1 °C) storage. Food Bioprocess Technol. 2012, 5, 1411–1415. [Google Scholar] [CrossRef]
- Pang, X.; Lin, Z.; Wang, M.; Liang, H.; Zhao, Y.; Li, Y.; Yan, B.; He, Y.; Wu, X.; Wang, Q.; et al. Mechanisms underlying the effect of high-temperature curing treatments on the browning response of fresh-cut yams. Food Chem. 2025, 476, 143317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, W.; Fang, X.; Li, W.; Sun, Y. Carbon dots-based reinforced hydrogen-rich water nanocomposite coating for storage quality of fresh-cut pear. Food Biosci. 2023, 53, 102837. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, X.; Wang, D.; Wang, P.; Zhao, S.; Chen, H.; Han, Y.; Zhao, W. Different packaging films regulate textural quality of fresh-cut chili peppers by regulating reactive oxygen species and membrane lipid metabolisms. LWT Food Sci. Technol. 2025, 220, 117596. [Google Scholar] [CrossRef]
- Xanthopoulos, G.T.; Athanasiou, A.A.; Lentzou, D.I.; Boudouvis, A.G.; Lambrinos, G.P. Modelling of transpiration rate of grape tomatoes. Semi-empirical and analytical approach. Biosyst. Eng. 2014, 124, 16–23. [Google Scholar] [CrossRef]
- Madonna, M.; Caleb, O.J.; Sivakumar, D.; Mahajan, P.V. Understanding the physiological response of fresh-cut cauliflower for developing a suitable packaging system. Food Packag. Shelf Life 2018, 17, 179–186. [Google Scholar] [CrossRef]
- Sharma, M.; Bains, A.; Goksen, G.; Ali, N.; Khan, M.R.; Karabulut, G.; Chawla, P. Optimization of ultrasonication assisted extraction of Aegle marmelos fruit shell nano polysaccharide and evaluation of photocatalytic dye reduction and edible coating for fresh-cut fruits. Food Chem. X 2024, 24, 101895. [Google Scholar] [CrossRef]
- Song, L.; Zhao, R.; Hu, W.; Wang, Q.; Wang, Y.; Li, X.; Zhang, Y.; Luo, H. Gibberellic acid and 6-benzylaminopurine mitigate the yellowing of pak choi (Brassica rapa subsp. Chinensis) during storage by regulating sugar scarcity-induced chlorophagy. J. Agric. Food Chem. 2025, 73, 7584–7595. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Song, H.; Chen, Y.; Shi, N.; Shen, H.; Shi, P.; Shu, H.; Kong, X.; Yu, L.; Luo, H. Incorporation of ascorbic acid and L-cysteine in sodium carboxymethyl cellulose coating delays color deterioration and extends the shelf-life of fresh-cut asparagus lettuce (Lactuca sativa var. angustata). Postharvest Biol. Technol. 2023, 204, 112419. [Google Scholar] [CrossRef]
- Yamauchi, N. Postharvest Chlorophyll Degradation and Oxidative Stress. In Abiotic Stress Biology in Horticultural Plants; Springer: Tokyo, Japan, 2015; pp. 101–113. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Han, C.; Chen, Q.; Sun, F.; Fu, M.; Zhao, H.; Xiao, Z.; Tang, Z. ε-Polylysine treatment maintains sensory quality, texture, flavor, and inhibits aerobic bacteria growth in vacuum-packed fresh-cut lettuce. Postharvest Biol. Technol. 2024, 214, 113011. [Google Scholar] [CrossRef]
- Widjaja, F.; Steensma, P.; Annala, L.; Klami, A.; Kangasjärvi, S.; Lehtonen, M.; Mikkonen, K.S. Non-targeted LC-MS metabolomics reveal shifts from wound-induced enzymatic browning to lignification during extended storage of fresh-cut lettuce in modified atmosphere packaging. Curr. Res. Food Sci. 2025, 10, 100959. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Meng, J.; Zhang, Y.; Fan, G.; Pan, C.; Shen, C.; Long, Y. Effect of alternating current electric fields on the preservation of fresh-cut Chinese cabbage and spinach. Innov. Food Sci. Emerg. 2025, 102, 104005. [Google Scholar] [CrossRef]
- Cuggino, S.G.; Posada-Izquierdo, G.; Villegas, I.B.; Theumer, M.G.; Pérez-Rodríguez, F. Effects of chlorine and peroxyacetic acid wash treatments on growth kinetics of Salmonella in fresh-cut lettuce. Food Res. Int. 2023, 167, 112451. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables—A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Cortellino, G.; Gobbi, S.; Bianchi, G.; Rizzolo, A. Modified atmosphere packaging for shelf life extension of fresh-cut apples. Trends Food Sci. Technol. 2015, 46, 320–330. [Google Scholar] [CrossRef]
- Brown, A.L.; Brooks, J.C.; Karunasena, E.; Echeverry, A.; Laury, A.; Brashears, M.M. Inhibition of Escherichia coli O157: H7 and Clostridium sporogenes in spinach packaged in modified atmospheres after treatment combined with chlorine and lactic acid bacteria. J. Food Sci. 2011, 76, M427–M432. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Jiang, F. Ultrasound treatment to modified atmospheric packaged fresh-cut cucumber: Influence on microbial inhibition and storage quality. Ultrason. Sonochemistry 2019, 54, 162–170. [Google Scholar] [CrossRef]
- Paulsen, E.; Moreno, D.A.; Lema, P. Modified atmosphere packaging using cellulose-based film (NatureFlex™) preserved quality and bioactive compounds of fresh-cut broccolis. Postharvest Biol. Technol. 2024, 217, 113092. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Rhee, M.S. Growth of Listeria monocytogenes in fresh vegetables and vegetable salad products: An update on influencing intrinsic and extrinsic factors. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13423. [Google Scholar] [CrossRef]
- Moreira, J.; Mera, E.; Singh Chhetri, V.; King, J.M.; Gentimis, T.; Adhikari, A. Effect of storage temperature and produce type on the survival or growth of Listeria monocytogenes on peeled rinds and fresh-cut produce. Front. Microbiol. 2023, 14, 1151819. [Google Scholar] [CrossRef]
- Kroft, B.; Gu, G.; Bolten, S.; Micallef, S.A.; Luo, Y.; Millner, P.; Nou, X. Effects of temperature abuse on the growth and survival of Listeria monocytogenes on a wide variety of whole and fresh-cut fruits and vegetables during storage. Food Control 2022, 137, 108919. [Google Scholar] [CrossRef]
- Tong, X.; Song, H.; Chen, Y.; Shi, N.; Shi, P.; Yu, L.; Kong, X.; Luo, H. 1-MCP combined with laser microporous film packaging maintains the quality and prolongs the storage period of Xiahei grapes. Int. J. Food Sci. Technol. 2024, 59, 1550–1559. [Google Scholar] [CrossRef]
- Kong, X.; Su, H.; Shen, H.; Chen, Y.; Yang, F.; Tong, X.; Guo, Y.; Luo, H.; Yu, L. The combined use of 1-MCP and laser microporous film packaging maintains the quality of Shine Muscat grapes by inhibiting oxidative stress and cell wall catabolism. LWT Food Sci. Technol. 2025, 225, 117958. [Google Scholar] [CrossRef]
- Tabassum, N.; Aftab, R.A.; Yousuf, O.; Ahmad, S.; Zaidi, S. Application of nanoemulsion based edible coating on fresh-cut papaya. J. Food Eng. 2023, 355, 111579. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, M.; Devahastin, S.; Guo, Z. Effects of pressurized argon and nitrogen treatments in combination with modified atmosphere on quality characteristics of fresh-cut potatoes. Postharvest Biol. Technol. 2019, 149, 159–165. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Adhikari, B.; Gao, Z. Recent advances in pressure modification-based preservation technologies applied to fresh fruits and vegetables. Food Rev. Int. 2016, 33, 538–559. [Google Scholar] [CrossRef]
- Lu, S.; Xia, Q. Effects of Combined Treatments with Modified-Atmosphere Packaging on Shelf-Life Improvement of Food Products. In Progress in Food Preservation; Wiley: Hoboken, NJ, USA, 2012; pp. 67–109. [Google Scholar] [CrossRef]
- Yang, X.; Bai, J.; Xie, Y.; Geng, Z.; Zhang, Q.; Wang, J. Evaluation of electron beam irradiation on the microbiological, phytochemicals, and aromatic profiles of three paprika (Capsicum annuum L.) varieties. Innov. Food Sci Emerg. 2024, 98, 103869. [Google Scholar] [CrossRef]
- Woodside, J.V. Nutritional aspects of irradiated food. Stewart Postharvest Rev. 2015, 11, 1–6. [Google Scholar] [CrossRef]
- Palekar, M.; Cabrera-Diaz, E.; Kalbasi-Ashtari, A.; Maxim, J.E.; Miller, R.; Cisneros-Zevallos, L.; Castillo, A. Effect of electron beam irradiation on the bacterial load and sensorial quality of sliced cantaloupe. J. Food Sci. 2004, 69, M267–M273. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Kumar, V.; Singh, B.; Dhiman, A. Irradiation: A tool for the sustainability of fruit and vegetable supply chain-Advancements and future trends. Radiat. Phys. Chem. 2024, 217, 111511. [Google Scholar] [CrossRef]
- de Sousa, A.E.D.; Ribeiro, L.B.; da Silveira, M.R.S.; de Oliveira Silva, E.; Germano, T.A.; Aziz, S.; de Miranda, M.R.A.; Gallão, M.I.; Fonseca, K.S.; Puschmann, R. Effect of pulsed light fluences on quality, biochemistry and physiology of fresh-cut mangoes during refrigerated storage. Sci. Hortic. 2023, 321, 112328. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, M.; Mujumdar, A.S. Phototreatment (below 1100 nm) improving quality attributes of fresh-cut fruits and vegetables: A review. Trends Food Sci. Technol. 2023, 163, 112252. [Google Scholar] [CrossRef]
- Jaiswal, M.; Srivastava, B. Optimization of plasma-activated water generation, antimicrobial efficacy assessment, and sequential application with pulsed light for enhancing microbial safety of fresh-cut pineapple. Postharvest Biol. Technol. 2025, 229, 113681. [Google Scholar] [CrossRef]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Hanani, Z.A.N. Repetitive pulsed light treatment at certain interval on fresh-cut cantaloupe (Cucumis melo L. reticulatus cv. Glamour). Innov. Food Sci. Emerg. Technol. 2016, 36, 92–103. [Google Scholar] [CrossRef]
- Aguilo-Aguayo, I.; Oms-Oliu, G.; Martin-Belloso, O.; Soliva-Fortuny, R. Impact of pulsed light treatments on quality characteristics and oxidative stability of fresh-cut avocado. LWT Food Sci. Technol. 2014, 59, 320–326. [Google Scholar] [CrossRef]
- Ramos-Villarroel, A.Y.; Aron-Maftei, N.; Martín-Belloso, O.; Soliva-Fortuny, R. Influence of spectral distribution on bacterial inactivation and quality changes of fresh-cut watermelon treated with intense light pulses. Postharvest Biol. Technol. 2012, 69, 32–39. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Zhang, L.; Ge, Z.; Li, G.; Wang, L.; Zong, W. Combination of intense pulsed light and modified atmosphere packaging delays the texture softening of apricot fruit by inhibiting the degradation of pectin components. Sci. Hortic. 2025, 340, 113918. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, X.; Zhang, Y.; Shang, J.; Lv, M.; Li, X.; Li, F. Characterization of carbon quantum dots doped with various heteroatoms and their influence on inhibiting the browning of fresh-cut potatoes. Food Packag. Shelf Life 2025, 47, 101440. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Rushing, J.W. Effect of ultraviolet-C light on quality and microbial population of fresh-cut watermelon. Postharvest Biol. Technol. 2006, 40, 256–261. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, H.W.; Bak, J.-Y.J.; Min, S.C. Microbial decontamination of fresh-cut celery using simultaneous ultrasound and plasma-activated water treatment. Int. J. Food Microbiol. 2025, 432, 110912. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, R.; Chen, H. Application of water-assisted ultraviolet light in combination of chlorine and hydrogen peroxide to inactivate Salmonella on fresh produce. Int. J. Food Microbiol. 2017, 257, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, L.; Ma, Y.; Zhang, M.; Zhao, Y.; Zhao, X. Effect of UV-C treatment on the quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root. Food Chem. 2019, 278, 659–664. [Google Scholar] [CrossRef]
- Fan, X.; Wang, W. Quality of fresh and fresh-cut produce impacted by nonthermal physical technologies intended to enhance microbial safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 362–382. [Google Scholar] [CrossRef]
- Zhou, D.; Li, T.; Cong, K.; Suo, A.; Wu, C. Influence of cold plasma on quality attributes and aroma compounds in fresh-cut cantaloupe during low temperature storage. LWT Food Sci. Technol. 2022, 154, 112893. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Wang, S. Study on the bactericidal mechanism of atmospheric-pressure low-temperature plasma against escherichia coli and its application in fresh-cut cucumbers. Molecules 2018, 23, 975. [Google Scholar] [CrossRef]
- Tappi, S.; Gozzi, G.; Vannini, L.; Berardinelli, A.; Romani, R.L.; Pietro, R. Cold plasma treatment for fresh-cut melon stabilization. Innov. Food Sci. Emerg. 2016, 33, 225–233. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Maimaitiyiming, R.; Aihaiti, A. Comparison of plasma-activated water and free chlorine in disinfecting Escherichia coli O157:H7- and Salmonella Typhimurium-inoculated blueberry, cherry tomato, fresh-cut lettuce, and baby spinach. LWT Food Sci. Technol. 2023, 187, 115384. [Google Scholar] [CrossRef]
- Min, S.C.; Roh, S.H.; Boyd, G.; Sites, J.E.; Uknalis, J.; Fan, X.; Niemira, B.A. Inactivation of Escherichia coli O157: H7 and aerobic microorganisms in romaine lettuce packaged in a commercial polyethylene terephthalate container using atmospheric cold plasma. J. Food Prot. 2017, 80, 35–43. [Google Scholar] [CrossRef]
- Min, S.C.; Roh, S.H.; Niemira, B.A.; Boyd, G.; Sites, J.E.; Uknalis, J.; Fan, X. In-package inhibition of E. coli O157: H7 on bulk Romaine lettuce using cold plasma. Food Microbiol. 2017, 65, 1–6. [Google Scholar] [CrossRef]
- Shirazi, S.; Ramezan, Y.; Moslehishad, M.; Marzdashti, H.G.; Mirsaeedghazi, H. Effects of cold atmospheric plasma on patulin degradation, polyphenol oxidase inactivation and other physicochemical properties of fresh-cut apple slices during storage. Food Chem. 2025, 465, 142017. [Google Scholar] [CrossRef] [PubMed]
- Denoya, G.I.; Polenta, G.A.; Apóstolo, N.M.; Cejas, E.; Fina, B.; Chamorro, J.C.; Ferreyra, M.; Prevosto, L.; Vaudagna, S.R. Effect of in-package cold plasma treatments on the quality of minimally processed apples. Int. J. Food Sci. Technol. 2023, 58, 2465–2475. [Google Scholar] [CrossRef]
- Yi, F.; Wang, J.; Xiang, Y.; Yun, Z.; Pan, Y.; Jiang, Y.; Zhang, Z. Physiological and quality changes in fresh-cut mango fruit as influenced by cold plasma. Postharvest Biol. Technol. 2022, 194, 112105. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Han, C.; Ji, N.; Jin, P.; Zheng, Y. Physiological and metabolomic analysis of cold plasma treated fresh-cut strawberries. J. Agric. Food Chem. 2019, 67, 4043–4053. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, X.; Li, F. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT Food Sci. Technol. 2019, 115, 108447. [Google Scholar] [CrossRef]
- Sudarsan, A.; Keener, K. Inactivation of spoilage organisms on baby spinach leaves using high voltage atmospheric cold plasma (HVACP) and assessment of quality. Innov. Food Sci. Emerg. 2022, 79, 103023. [Google Scholar] [CrossRef]
- Liu, D.; Wang, F.; Xiao, G.; Brennan, C.; Ying, X.; Bu, Z.; Ma, L. Effects of cold plasma treatment on maintaining the quality of fresh-cut bamboo shoots during shelf-life storage. LWT Food Sci. Technol. 2023, 185, 115151. [Google Scholar] [CrossRef]
- You, W.; Meng, X.; Zhang, J.; Ru, X.; Xu, F.; Wu, Z.; Jin, P.; Zheng, Y.; Cao, S. Effects of dielectric barrier discharge cold plasma treatment on quality maintenance, energy and γ-aminobutyric acid accumulation of fresh-cut carrots. Food Control 2024, 165, 110649. [Google Scholar] [CrossRef]
- Laika, J.; Tatasciore, S.; De Flaviis, R.; Valbonetti, L.; Molina-Hernandez, J.B.; Laurita, R.; Ricci, A.; López, C.C.; Neri, L. Impact of surface dielectric barrier discharge cold atmospheric plasma on quality and stability of fresh-cut iceberg lettuce. LWT Food Sci. Technol. 2024, 211, 116941. [Google Scholar] [CrossRef]
- Bußler, S.; Ehlbeck, J.; Schlüter, O.K. Pre-drying treatment of plant related tissues using plasma processed air: Impact on enzyme activity and quality attributes of cut apple and potato. Innov. Food Sci. Emerg. 2017, 40, 78–86. [Google Scholar] [CrossRef]
- Du, Y.; Huang, X.; Yuan, S.; Yu, H.; Guo, Y.; Cheng, Y.; Yao, W. Cold plasma and honey synergistically inhibit polyphenol oxidase to enhance fresh-cut apple preservation. Food Chem. 2025, 468, 142490. [Google Scholar] [CrossRef] [PubMed]
- Nyamende, N.E.; Belay, Z.A.; Caleb, O.J. Recent advances in electrolyzed water treatments: Mechanisms of action and its effect on browning, bioactive compounds, and disinfection of fresh-cut fruit and vegetables—A review. Food Chem. Adv. 2023, 3, 100569. [Google Scholar] [CrossRef]
- Plesoianu, A.M.; Nour, V.; Tutulescu, F.; Ionica, M.E. Quality of fresh-cut apples as affected by dip wash treatments with organic acids and acidic electrolyzed water. Food Sci. Technol. 2021, 42, e62620. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, X.; Chen, Y.; Lin, M.; Tang, J.; Lin, Q.; Fang, L.; Li, M.; Hung, Y.; Lin, H. Recent trends and applications of electrolyzed oxidizing water in fresh foodstuff preservation and safety control. Food Chem. 2022, 369, 130873. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, H.; Tang, J.; Lin, M.; Hung, Y.-C.; Lin, H. Effects of acidic electrolyzed water treatment on storability, quality attributes and nutritive properties of longan fruit during storage. Food Chem. 2020, 320, 126641. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Li, H.; Wan, Y.; Liu, H. Effect of slightly acidic electrolyzed water (SAEW) treatment on the microbial reduction and storage quality of fresh-cut cilantro. J. Food Process. Preserv. 2015, 39, 559–566. [Google Scholar] [CrossRef]
- Luo, Z.; Li, G.; Du, Y.; Yi, J.; Hu, X.; Jiang, Y. Enhancing fresh-cut apple preservation: Impact of slightly acidic electrolyzed water and chitosan–apple essence microencapsulation coating on browning and flavor. Foods 2024, 13, 1585. [Google Scholar] [CrossRef]
- Filho, J.G.D.O.; Miranda, M.; Ferreira, M.D.; Plotto, A. Nanoemulsions as edible coatings: A potential strategy for fresh fruits and vegetables preservation. Foods 2021, 10, 2438. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. 2019, 59, 411–422. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Ren, R.; Yao, L.; Tong, L.; Yuan, J.; Wang, D. Effect of chitosan and hyperbranched poly-l-lysine treatment on quality of cucumber (Cucumis sativus L.) during storage. Foods 2024, 13, 1354. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, X.; Zhang, T.; Zhang, F.; Zhang, S.; Li, Y.; Gao, W.; You, C.; Wang, X.; et al. Cellulose nanofibers extracted from natural wood improve the postharvest appearance quality of apples. Front. Nutr. 2022, 9, 881783. [Google Scholar] [CrossRef]
- Nehme, R.; Abdennebi-Najar, L.; Rault, L.; Maillard, M.-B.; Even, S.; Andrćs, S.; Hamon, P.; Pereira, D.M.; Ceciliani, F.; Falleh, H.; et al. Antibacterial effect of free, nanoemulsified and protein-complexed carvacrol. Food Chem. Adv. 2025, 6, 100881. [Google Scholar] [CrossRef]
- Rathod, N.B.; Smaoui, S.; Agrawal, R.; Bhagwat, P.; Amobonye, A.; Pillai, S.; Yilmaz, N.; Ozogul, F. Sustainable processing technologies (pulsed light, electrolysed water and ozonation) for microbial decontamination of muscle foods. Innov. Food Sci. Emerg. 2024, 96, 103778. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yu, Y.; Wu, Z.; Wang, H. Combination of ozone and ultrasonic-assisted aerosolization sanitizer as a sanitizing process to disinfect fresh-cut lettuce. Ultrason. Sonochemistry 2021, 76, 105622. [Google Scholar] [CrossRef]
- Maryam, A.; Anwar, R.; Malik, A.U.; Muhammad, K.A.S.; Hasan, M.U.; Hussain, Z.; Siddique, Z. Combined aqueous ozone and ultrasound application inhibits microbial spoilage, reduces pesticide residues and maintains storage quality of strawberry fruits. J. Food Meas. Charact. 2020, 15, 1437–1451. [Google Scholar] [CrossRef]
- Barthwa, R.; Negi, A.; Kathuria, D.; Singh, N. Ozonation: Post-harvest processing of different fruits and vegetables enhancing and preserving the quality. Food Chem. 2025, 463, 141489. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.; Chrysargyris, A. Postharvest ozone application for the preservation of fruits and vegetables. Food Rev. Int. 2017, 33, 270–315. [Google Scholar] [CrossRef]
- Zhao, S.; Han, X.; Liu, B.; Wang, S.; Guan, W.; Wu, Z.; Theodorakis, P.E. Shelf-life prediction model of fresh-cut potato at different storage temperatures. J. Food Eng. 2022, 317, 110867. [Google Scholar] [CrossRef]
- Banach, J.L.; van Overbeek, L.S.; Groot, M.N.N.; van der Zouwen, P.S.; van der Fels-Klerx, H.J. Efficacy of chlorine dioxide on Escherichia coli inactivation during pilot-scale fresh-cut lettuce processing. Int. J. Food Microbiol. 2018, 269, 128–136. [Google Scholar] [CrossRef]
- Dong, L.; Wall, M.; Li, Y. Behaviors of Salmonella enterica serovar Typhimurium and Listeria monocytogenes on whole avocado during storage at 21 or 7 °C and their reduction by aqueous chlorine dioxide and peroxyacetic acid. LWT Food Sci. Technol. 2023, 173, 114359. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Seididamyeh, M.; Mantilla, S.M.O.; Netzel, M.E.; Mereddy, R.; Sultanbawa, Y. Gum Arabic edible coating embedded aqueous plant extracts: Interactive effects of partaking components and its effectiveness on cold storage of fresh-cut capsicum. Food Control 2024, 159, 110267. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, J.; Hu, H.; Wang, H.; Liu, Z.; Zhou, W.; Zhang, X. Impacts of tannin inclusion on fabrication, characterization and antioxidant activity of sodium alginate-silk fibroin-tannin films and their application on fresh-cut apple packaging. Food Biosci. 2023, 54, 102897. [Google Scholar] [CrossRef]
- Wong, C.H.; Mak, I.E.K.; Li, D. Bilayer edible coating with stabilized Lactobacillus plantarum 299v improved the shelf life and safety quality of fresh-cut apple slices. Food Packag. Shelf Life 2021, 30, 100746. [Google Scholar] [CrossRef]
- Sarengaowa, H.W.; Feng, K.; Xiu, Z.; Jiang, A.; Lao, Y. Thyme oil alginate-based edible coatings inhibit growth of pathogenic microorganisms spoiling fresh-cut cantaloupe. Food Biosci. 2019, 32, 100467. [Google Scholar] [CrossRef]
- Nascimento, J.I.G.; Stamford, T.C.M.; Melo, N.F.C.B.; Nunes, I.D.S.; Lima, M.A.B.; Pintado, M.M.E.; Stamford-Arnaud, T.M.; Stamford, N.P.; Stamford, T.L.M. Chitosan–citric acid edible coating to control Colletotrichum gloeosporioides and maintain quality parameters of fresh-cut guava. Int. J. Biol. Macromol. 2020, 163, 1127–1135. [Google Scholar] [CrossRef]
- Ortiz-Duarte, G.; Pérez-Cabrera, L.E.; Artés-Hernández, F.; Martínez-Hernández, G.B. Ag-chitosan nanocomposites in edible coatings affect the quality of fresh-cut melon. Postharvest Biol. Technol. 2019, 147, 174–184. [Google Scholar] [CrossRef]
- Rasool, F.; Zahoor, I.; Ayoub, W.S.; Ganaie, T.A.; Dar, A.H.; Farooq, S.; Mir, T.A. Formulation and characterization of natural almond gum as an edible coating source for enhancing the shelf life of fresh cut pineapple slices. Food Chem. Adv. 2023, 3, 100366. [Google Scholar] [CrossRef]
- Hu, X.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M.-H. Chitosan nanoparticles as edible surface coating agent to preserve the fresh-cut bell pepper (Capsicum annuum L. var. grossum L. Sendt). Int. J. Biol. Macromol. 2020, 165, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Nikhanj, P. Investigating potential of edible coatings for microbial safety, quality and shelf life extension of fresh cut cucumbers: A statistical optimization approach. J. Stored Prod. Res. 2024, 108, 102393. [Google Scholar] [CrossRef]
- Wu, S. Extending shelf-life of fresh-cut potato with cactus Opuntia dillenii polysaccharide-based edible coatings. Int. J. Biol. Macromol. 2019, 130, 640–644. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Ramachandran, C.; Hu, X.; Oh, D.-H.; Wang, M.-H. Chitosan-tea tree oil nanoemulsion and calcium chloride tailored edible coating increase the shelf life of fresh cut red bell pepper. Prog. Org. Coat. 2021, 151, 106010. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Jin, L.; Abdollahi, M.; Zhao, G.; Venkatachalam, K.; Ban, Z. Controlled release and stability enhancement of cinnamon essential oil in glutathione-modified soy protein particles: Its antimicrobial application for fresh-cut cantaloupe. Food Res. Int. 2025, 211, 116523. [Google Scholar] [CrossRef]
- Liu, L.; Jin, L.; Yang, S.; Li, H.; Chen, C.; Farouk, A.; Ban, Z.; Liang, H.; Huang, J. pH-driven formation of soy protein isolate-thymol nanoparticles for improved the shelf life of fresh-cut lettuce. Food Control 2024, 160, 110306. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, Z.; Yang, C.; Dong, C.; Chen, F.; Liu, K. Smart antimicrobial system based on enzyme-responsive high methoxyl pectin-whey protein isolate nanocomplex for fresh-cut apple preservation. Int. J. Biol. Macromol. 2023, 253, 127064. [Google Scholar] [CrossRef]
- Azevedoa, V.M.; Dias, M.V.; Elias, H.H.D.S.; Fukushima, K.L.; Silva, E.K.; Carneiro, J.D.D.S.; Soares, N.D.F.F.; Borges, S.V. Effect of whey protein isolate films incorporated with montmorillonite and citric acid on the preservation of fresh-cut apples. Food Res. Int. 2018, 107, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Vega, W.R.; Pizato, S.; Souza, J.T.A.D.; Prentice, C. Using edible coatings from Whitemouth croaker (Micropogonias furnieri) protein isolate and organo-clay nanocomposite for improve the conservation properties of fresh-cut ‘Formosa’ papaya. Innov. Food Sci. Emerg. 2014, 22, 197–202. [Google Scholar] [CrossRef]
- Cheruvari, A.; Kammara, R. Bacteriocins future perspectives: Substitutes to antibiotics. Food Control 2025, 168, 110834. [Google Scholar] [CrossRef]
- Silva, S.P.M.; Teixeira, J.A.; Silva, C.C.G. Recent advances in the use of edible films and coatings with probiotic and bacteriocin-producing lactic acid bacteria. Food Biosci. 2023, 56, 103196. [Google Scholar] [CrossRef]
- Garcia, A.; Bonilla, F.; Villasmil, E.; Reyes, V.; Sathivel, S. Antilisterial activity of freeze-dried bacteriocin-containing powders produced by lactic acid bacteria against Listeria innocua NRRL B-33016 on cantaloupe (Cucumis melo) surface. LWT Food Sci. Technol. 2022, 154, 112440. [Google Scholar] [CrossRef]
- Yi, L.; Chen, S.; Li, G.; Ren, J.; Zhou, R.; Zeng, K. Prevalence of antibiotic resistance pathogens in online fresh-cut fruit from Chongqing, China and controlling Enterococcus faecalis by bacteriocin GF-15. LWT Food Sci. Technol. 2022, 165, 113678. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Marcelli, V.; Osimani, A.; Aquilanti, L. Research progress in the use of lactic acid bacteria as natural biopreservatives against Pseudomonas spp. in meat and meat products: A review. Trends Food Sci. Technol. 2024, 196, 115129. [Google Scholar] [CrossRef] [PubMed]
- Allende, A.; Martínez, B.; Selma, V.; Gil, M.I.; Suárez, J.E.; Rodríguez, A. Growth and bacteriocin production by lactic acid bacteria in vegetable broth and their effectiveness at reducing Listeria monocytogenes in vitro and in fresh-cut lettuce. Food Microbiol. 2007, 24, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Bhaskar, R.; Han, S.S. Bacteriophages: Natural antimicrobial bioadditives for food preservation in active packaging. Int. J. Biol. Macromol. 2024, 276, 133945. [Google Scholar] [CrossRef] [PubMed]
- Sillankorva, S.M.; Oliveira, H.; Azeredo, J. Bacteriophages and their role in food safety. Int. J. Microbiol. 2012, 1, 863945. [Google Scholar] [CrossRef]
- Safarirad, M.; Shahdadi, M.; Berizi, E.; Hosseinzadeh, S.; Majlesi, M. A systematic review and meta-analysis of the efficacy of specific bacteriophages on E. coli O157:H7 reduction in vegetables. Food Control 2025, 175, 111304. [Google Scholar] [CrossRef]
- Oliveira, M.; Viñas, I.; Colàs, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef]
- López-Cuevas, O.; Medrano-Félix, J.; Castro-Del Campo, N.; Chaidez, C. Bacteriophage applications for fresh produce food safety. Int. J. Environ. Health Res. 2021, 31, 687–702. [Google Scholar] [CrossRef] [PubMed]


| Treatment Conditions | Fruits and Vegetables | Effect on FCFVs | References |
|---|---|---|---|
| Water activated by CP (a plasma treatment performed on 2.5 L of distilled water at 2.6 kV/20 min). | Mango | Inhibited the growth and reproduction of microbes, retarded the membrane lipid peroxidation, and delayed quality loss such as color, weight, firmness, titratable acidity, and soluble solids contents. | [2] |
| Plasma-activated water (0.5 L of water was subjected to plasma at 51.7 W, 14.4 kHz, 8 kV/61.5 min). | Celery | The inoculation of L. monocytogenes and E. coli O157:H7 on celery resulted in inactivation levels of 1.1 ± 0.1 and 1.2 ± 0.1 log (CFU g−1), respectively. | [55] |
| CP at 40 kV/90 s. | Cantaloupe | Reduced the development and reproduction of mold and bacteria, enhanced firmness, quality, and sensory characteristics; aromas with floral and fruity notes were significantly improved. | [59] |
| Dielectric-barrier discharge (DBD) cold atmospheric plasma at 23 kV/2.5 or 8.5 min. | Apple | Eliminated the total viable count and patulin, reduced the PPO activity and total phenolic content, and increased the quality and lifespan. | [65] |
| In-package CP product; storage time: 1, 4, and 7 days; exposure time: 0, 1, and 3 min. | Apple | Improved the fruit’s quality and maximum antioxidant concentration and decreased PPO activity, but not enough to maintain the product’s antioxidant qualities. | [66] |
| DBD CP at 75 kV/3 min. | Mango | Prevented microbiological growth and preserved physicochemical characteristics, delaying the loss of organoleptic and nutritional properties. | [67] |
| DBD CP at 45 kV/1 min. | Strawberries | Maintained the textural qualities, inhibited microbiological growth, and increased the flavonoids, anthocyanins, and phenolic compounds. | [68] |
| CP at 60 kV/5 min. | Pitaya | Enhanced antioxidant activity, accelerated phenolic accumulation, improved energy status, promoted the consumption of primary sugars, and inhibited the growth of aerobic microorganisms. | [69] |
| High voltage cold atmospheric plasma (HVCAP) for 2 and 5 min. | Baby spinach leaves | Eliminated microbiota by 2.6 and 3.5 log CFU per sample, respectively, until 7 days in the refrigerator. The 5 min indirect, 80 kV HVCAP treatment did not affect the leaves’ moisture content, color, or texture. | [70] |
| CP at 50 kV/30 s. | Bamboo shoots | Exhibited higher firmness and reduced yellowing. Six key flavoring chemicals were identified (odor activity value (OAV) > 1), reduced alkenes and alcohols, while keeping ester, aldehyde, and ketone flavor compounds, and increased microbiological diversity. | [71] |
| CP at 60 kV/5 min. | Carrot | Reduced microbiological development and color changes, enhanced the accumulation of γ-aminobutyric acid, and improved the energy status and reducing power. | [72] |
| Dielectric-barrier discharge cold atmospheric plasma at 42.54 ± 2.58 W, 23 kHz, 6 kV/15, 30, or 60 min. | Iceberg lettuce | Inhibited PPO and POD activity, reduced chlorophylls, polyphenols, ascorbic acid contents, and antioxidant activity, decreased mesophilic and psychrotrophic bacteria, while it had no effect on yeast. | [73] |
| 2.45 GHz, 1.2 kW for 10 min of plasma-processed air. | Potato and apple | In fresh sliced apple and potato tissue, PPO and POD activity decreased, the pH of the tissue surface decreased, and cell integrity and dry matter content remained unchanged. | [74] |
| Edible Coating Material | Fruits and Vegetables | Additives | Effect on FCFVs | References |
|---|---|---|---|---|
| Chitosan and locust bean gum (LBG) | Carrot | Glycerol and potassium sorbate | In Dordogne, chitosan reduced browning, improved functional and microbiological properties; however, in Purple Sun, its poor O2/CO2 permeability led to browning and hastened degradation; LBG induced deterioration in microbiological quality in both cultivars. | [1] |
| Sodium alginate | Lotus root | L-cysteine and citric acid | Inhibited the browning and microbial proliferation, maintained the quality, and extended the lifespan of lotus root slices. | [11] |
| Aegle marmelose fruit shell polysaccharide (AMFSP) | Apple | Glycerol and glacial acetic acid | Shelf life increased by 1% AMFSP coating, maintaining nutrient content and sensory appeal, reducing oxidative degradation, microbial growth, moisture loss, and enzymatic browning. | [20] |
| Sodium carboxymethyl cellulose | Asparagus lettuce | Ascorbic acid and L-cysteine | Inhibited browning and green color loss, reduced oxidative stress, sustained the quality, and enhanced the lifespan of lettuce slices. | [22] |
| Nanoemulsion-based alginate | Papaya | Oregano essential oil | Inhibited weight loss, moisture evaporation, microbial growth, respiration rate, color stability, and soluble solids, better antimicrobial efficacy, slight sensory changes, and prolonged shelf life. | [38] |
| Gum Arabic | Capsicum | Aqueous extracts of Syzygium aqueum, Tasmannia lanceolata, and Diploglottis bracteata | Inhibited microbial development and enhanced sensory attributes; however, it was insufficient in maintaining the retained moisture and firmness of fresh-cut capsicum. | [96] |
| Sodium alginate (SA) | Apple | Clove essential oil (CEO) | Decreased oxidative damage, moisture loss, and microbial growth, the shelf life was extended to 14 days at 4 °C; 1% CEO nanoemulsion had a more obvious effect for preventing quality loss. | [97] |
| Sodium carboxymethyl cellulose | Apple | Glycerol and zein | Prolonged shelf life, maintained probiotic viability, improved safety, sustained the microbial quality, and lowered the browning index. | [98] |
| Sodium alginate | Cantaloupe | Glycerine and thyme oil | Reduced weight loss, prevented S. aureus, S. Typhimurium, E. coli O157:H7, and L. monocytogenes growth, and retained color and flavor. | [99] |
| Chitosan | Guava | Chitosan nanoparticles and citric acid | Controlled postharvest fungal infections, preserved color, physicochemical and sensory qualities, reduced weight loss, delayed ripening, and maintained quality. | [100] |
| Chitosan | Melon | Ag–chitosan nanocomposites | Reduced respiration rate, prevented softening, maintained a relatively low translucency and high vitamin C content, sensory scored, no significant difference in color, pH, soluble solids, sucrose, glucose, fructose, titratable acidity, and citric and malic acid contents. | [101] |
| Almond gum from (prusnus domestica) | Pineapple | Glycerol monostearate | Extended shelf life compared to synthetic tragacanth gum, delayed color changes, weight loss, and microbial growth, maintained titratable acidity, ascorbic acid, firmness, and total soluble solids. | [102] |
| Chitosan nanoparticles | Bell pepper | Sodium tripolyphosphate pentabasic | Maintained the fresh-cut bell pepper for 12 days at 5 °C without loss of weight and sensory quality. | [103] |
| Chitosan, alginate, carboxymethyl cellulose, etc. | Cucumber | — | Maintained the physicochemical and sensory properties, reduced the microbe load, enhanced longevity up to 12 days at 5–7 °C. | [104] |
| Cactus Opuntia dillenii polysaccharide | Potato | — | Delayed browning, microbial proliferation, and respiration rate, reduced sugar accumulation and weight loss of fresh-cut potatoes stored at 5 °C for 5 days. | [105] |
| Low-molecular-weight chitosan | Red bell pepper | Calcium chloride and tea tree oil nanoemulsion | Retained texture, sensory quality, and overall integrity, suppressed L. monocytogenes, S. enterica, fungi, and microbial colonization of fresh-cut red bell peppers stored at 4 °C for 18 days. | [106] |
| Soy protein | Cantaloupe | Glutathione and cinnamon essential oil | Inhibited the growth of E. coli and S. aureus, reduced mass and firmness loss, maintained the ascorbic acid, total soluble solids, and titratable acidity contents, and extended the shelf life to 10 days at 4 °C. | [107] |
| Soy protein | Leaf lettuce | Thymol | Prevented microbial development, reduced mass losses, maintained ascorbic acid and chlorophyll levels, retained lettuce characteristic aroma and texture for 6 days at 4 °C. | [108] |
| High methoxyl pectin and whey protein isolate | Apple | — | Reduced total colony count, inhibited decay of fresh-cut apple stored at 5 °C for 6 days. | [109] |
| Whey protein isolate | Apple | Montmorillonite and citric acid | Maintained the color characteristics, reduced the loss of acidity, soluble solids, and water activity, inhibited PPO and POD activity. | [110] |
| Whitemouth croaker protein isolate | Papaya | Organo-clay | Reduced microbial growth and loss of weight, firmness, lightness, and pH of fresh-cut Formosa papaya stored at 5 °C for 12 days. | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, M.; Arshad, N.; Wang, H.; Li, C.; Ma, J.; Kong, X.; Luo, H.; Yu, L. Recent Advances in Technologies for Preserving Fresh-Cut Fruits and Vegetables. Foods 2025, 14, 2769. https://doi.org/10.3390/foods14162769
Faisal M, Arshad N, Wang H, Li C, Ma J, Kong X, Luo H, Yu L. Recent Advances in Technologies for Preserving Fresh-Cut Fruits and Vegetables. Foods. 2025; 14(16):2769. https://doi.org/10.3390/foods14162769
Chicago/Turabian StyleFaisal, Muhammad, Naeem Arshad, Hui Wang, Chengcheng Li, Jinju Ma, Xiaoxue Kong, Haibo Luo, and Lijuan Yu. 2025. "Recent Advances in Technologies for Preserving Fresh-Cut Fruits and Vegetables" Foods 14, no. 16: 2769. https://doi.org/10.3390/foods14162769
APA StyleFaisal, M., Arshad, N., Wang, H., Li, C., Ma, J., Kong, X., Luo, H., & Yu, L. (2025). Recent Advances in Technologies for Preserving Fresh-Cut Fruits and Vegetables. Foods, 14(16), 2769. https://doi.org/10.3390/foods14162769






