Activity Expression and Property Analysis of Codon-Optimized Polyphenol Oxidase from Camellia sinensis in Pichia pastoris KM71
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Genomic DNA Extraction
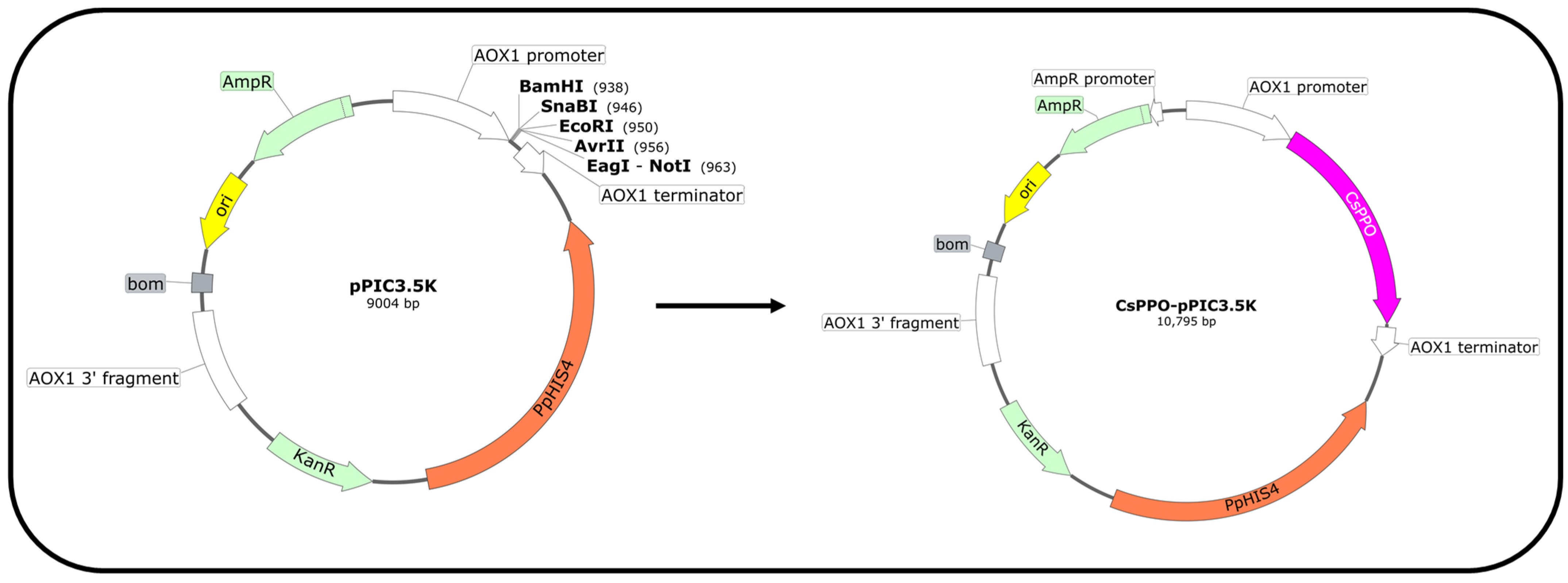
2.3. Construction of CsPPO–pPIC3.5K and Codon-Optimized CsPPO–pPIC3.5K
2.4. Heterologous Expression in P. pastoris
2.5. Extraction and Purification of Recombinant CsPPO
2.6. Detection of Protein Concentration
2.7. CsPPO Activity Assay
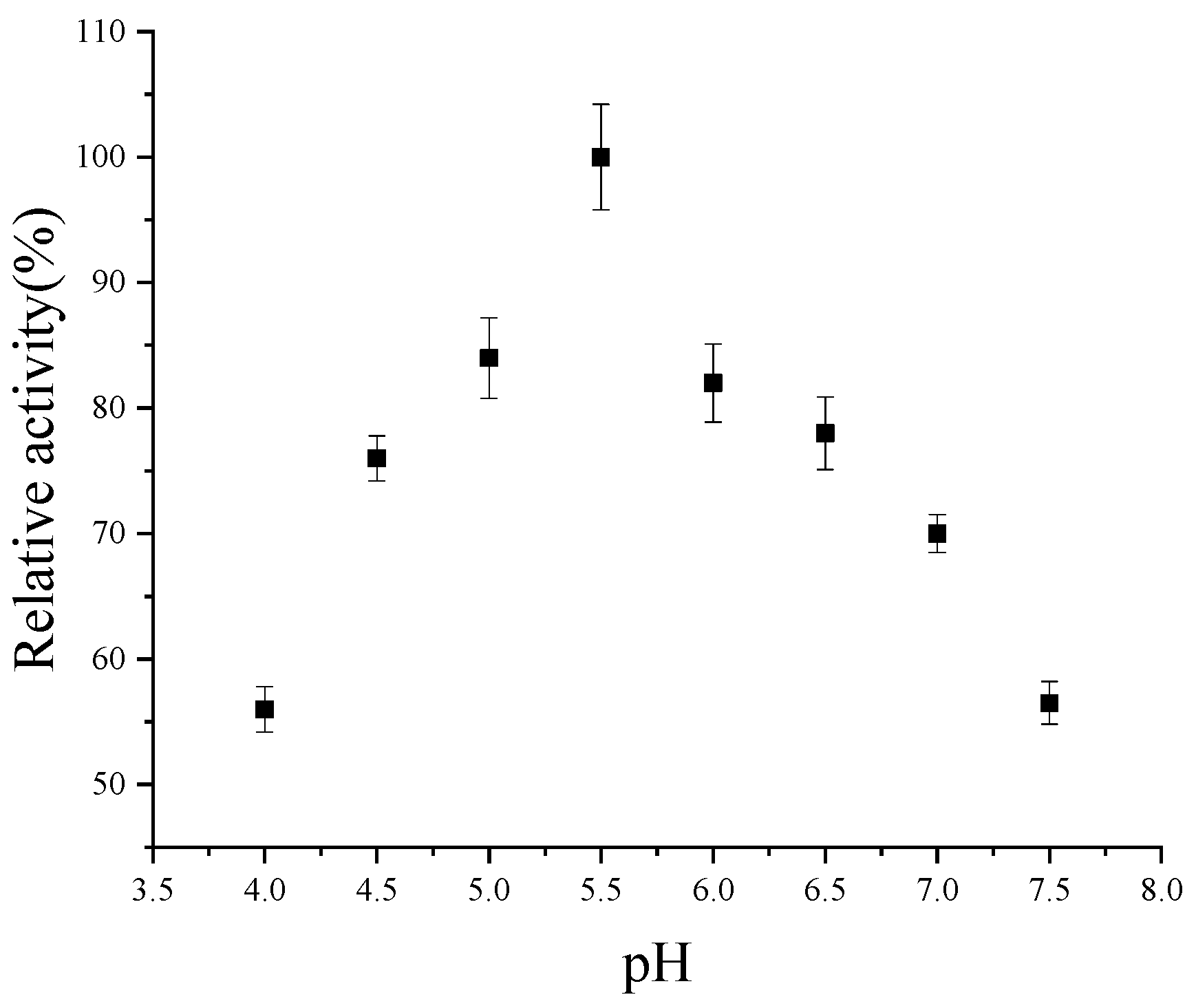
2.8. Determination of Optimal Temperature and Optimal pH
2.9. Statistical Analysis
3. Results and Discussion
3.1. Cloning of Wild-Type CsPPO
3.2. Codon Optimization of CsPPO
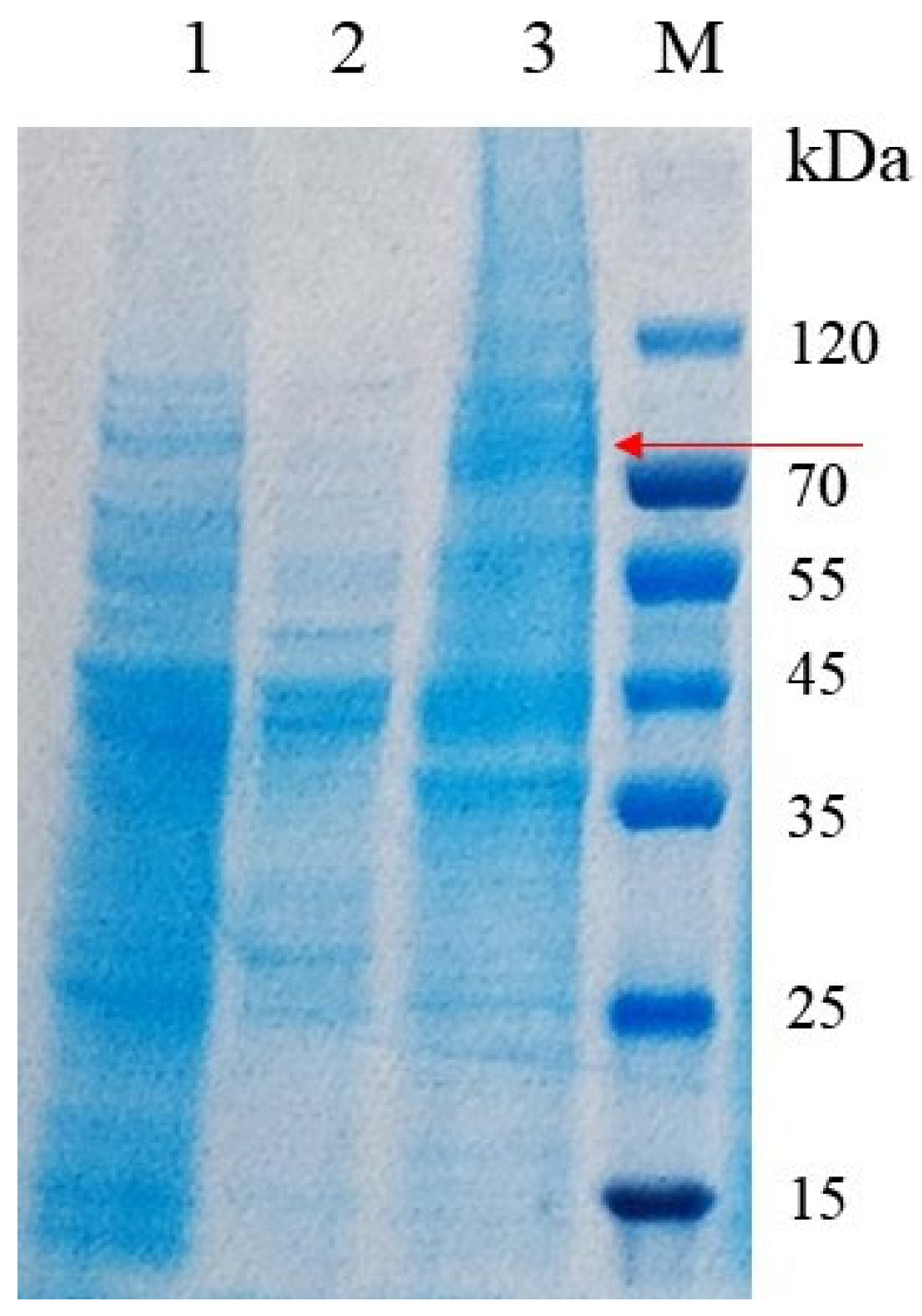
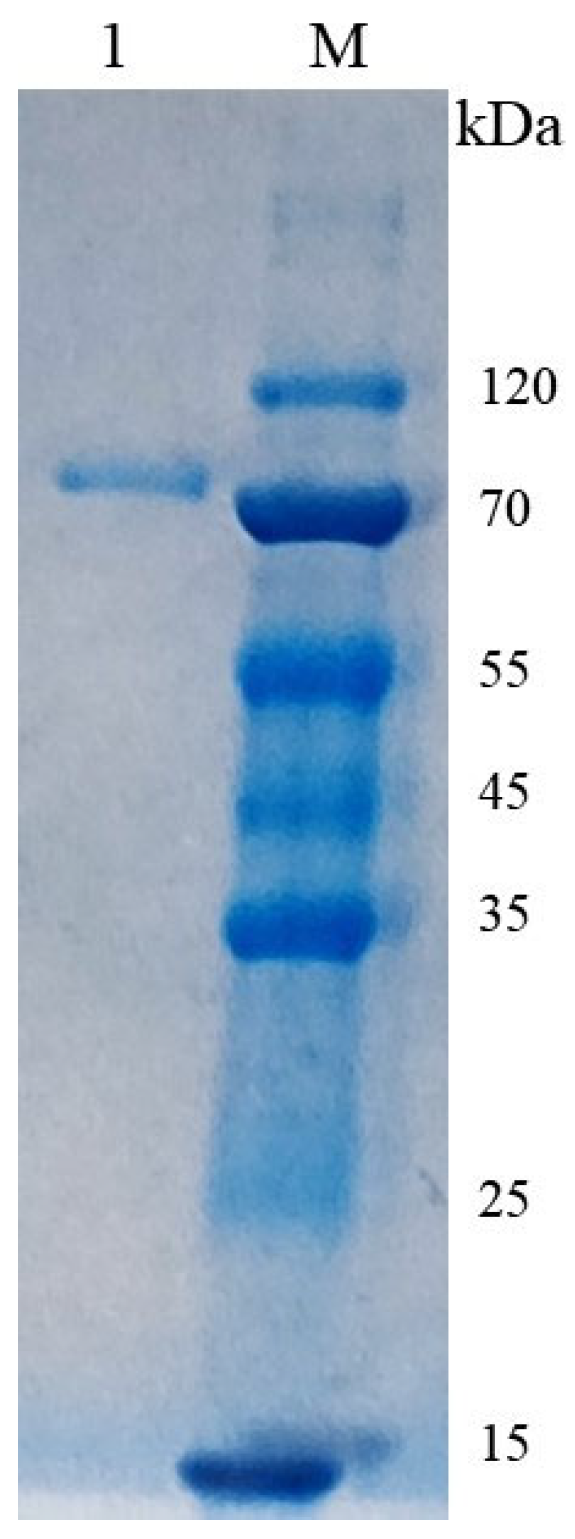
3.3. Activity Expression of CsPPO
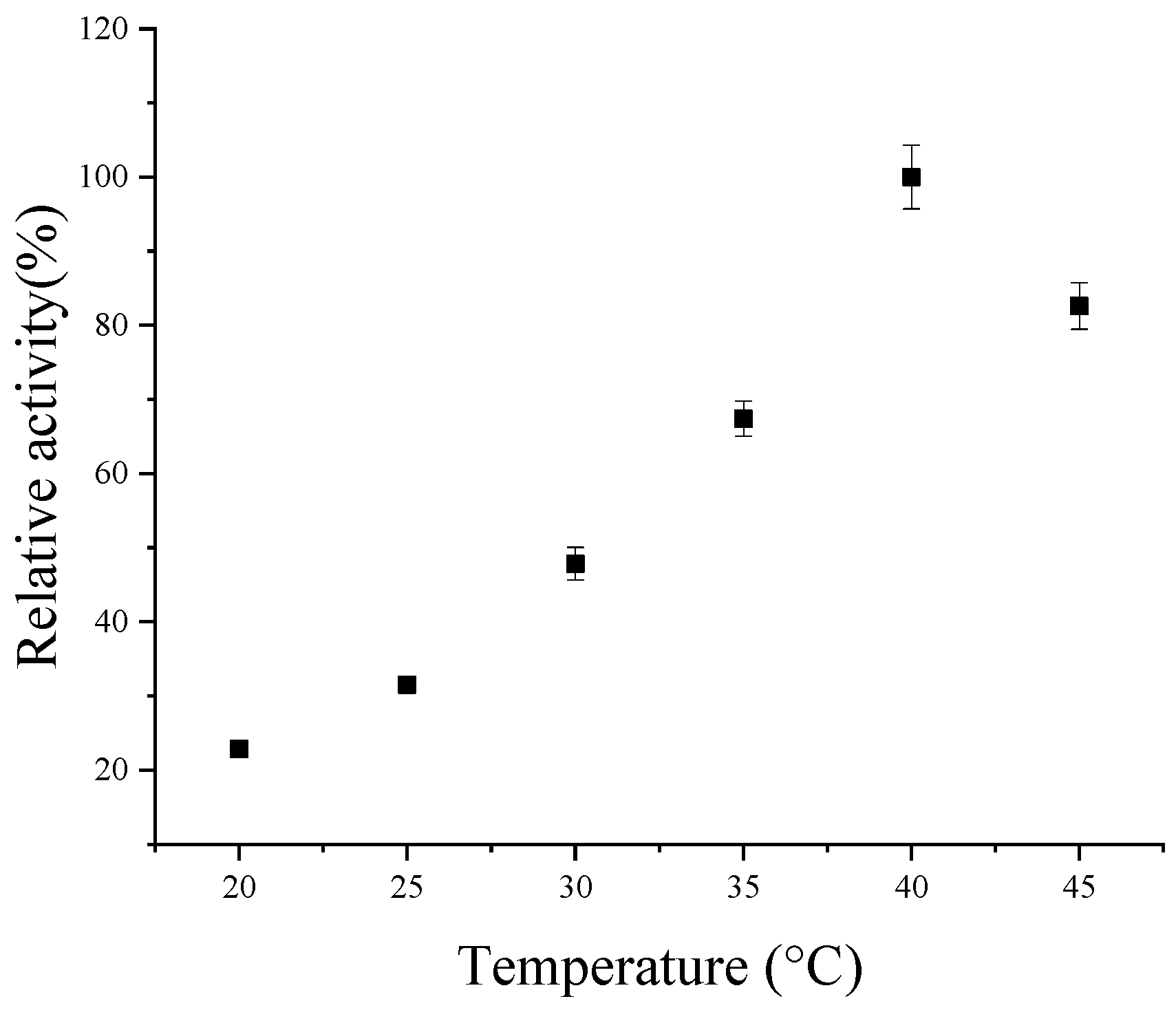
3.4. Property Analysis of Codon-Optimized CsPPO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Derardja, A.E.; Pretzler, M.; Barkat, M.; Rompel, A. Extraction, Purification, and Characterization of Olive (Olea europaea L., cv. Chemlal) Polyphenol Oxidase. J. Agr. Food Chem. 2024, 72, 3099–3112. [Google Scholar] [CrossRef]
- Hong, Q.; Chen, Y.L.; Lin, D.Q.; Yang, R.Q.; Cao, K.Y.; Zhang, L.J.; Liu, Y.M.; Sun, L.C.; Cao, M.J. Expression of polyphenol oxidase of Litopenaeus vannamei and its characterization. Food Chem. 2024, 432, 137258. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Elliot, M.; Zheng, Y.H.; Chen, J.; Chen, D.Z.; Deng, S.G. Aggregation and conformational change of mushroom (Agaricus bisporus) polyphenol oxidase subjected to atmospheric cold plasma treatment. Food Chem. 2022, 386, 132707. [Google Scholar] [CrossRef]
- Liang, S.; Granato, D.; Zou, C.; Gao, Y.; Zhu, Y.; Zhang, L.; Yin, J.F.; Zhou, W.B.; Xu, Y.Q. Processing technologies for manufacturing tea beverages: From traditional to advanced hybrid processes. Trends Food Sci. Tech. 2021, 118, 431–446. [Google Scholar] [CrossRef]
- Luo, S.K.; Hou, Y.; Hu, S.Q. Proteolytic activation and characterization of recombinant polyphenol oxidase from Rosa chinensis for efficient synthesis of theaflavins. Ind. Crop Prod. 2023, 200, 116810. [Google Scholar] [CrossRef]
- Liu, L.; Guan, H.; Jiao, M.; Ma, Z.; Bao, Y.; Xie, X.; Ma, Y.; Zhou, J.; Bao, L.; Yu, Y.; et al. Identification of two antagonistic fungi and antifungal activity analysis against anthracnose in tea plant (Camellia sinensis). Beverage Plant Res. 2024, 4, e032. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zeng, B.; Xiao, S.L.; Wang, D.; Liu, Y.; Chen, S.C.; Teng, J. Purification of polyphenol oxidase from tea (Camellia sinensis) using three-phase partitioning with a green deep eutectic solvent. Food Chem. X 2024, 23, 101720. [Google Scholar] [CrossRef]
- Ravichandran, R.; Parthiban, R. Changes in enzyme activities (polyphenol oxidase and phenylalanine ammonia lyase) with type of tea leaf and during black tea manufacture and the effect of enzyme supplementation of dhool on black tea quality. Food Chem. 1998, 62, 277–281. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.Q.; Xu, X.Y.; Chen, S.H.; Yao, Y.N.; Huang, Y.Y. Optimization of the extraction method of polyphenol oxidase from Camellia sinensis var. Longjing. J. Food Saf. Qual. 2015, 6, 1237–1242. [Google Scholar]
- Öztürk, C.; Aksoy, M.; Küfrevioglu, Ö.I. Purification of tea leaf (Camellia sinensis) polyphenol oxidase by using affinity chromatography and investigation of its kinetic properties. J. Food Meas. Charact. 2020, 14, 31–38. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, X.; Xu, Y.Q.; Yin, J.F. Recent Advances Regarding Polyphenol Oxidase in Camellia sinensis: Extraction, Purification, Characterization, and Application. Foods 2024, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Zhou, J.H.; Huang, J.A.; Xu, W.; Liu, Z.H. Study on the Synthesis of Theaflavin-3,3’-Digallate Catalyzed by Expressing Tea Tree Polyphenol Oxidase Isozymes and Its Enzymatic Solution. Fermentation 2023, 9, 770. [Google Scholar] [CrossRef]
- Gan, Y.D.; Sun, K.; Li, H.J.; Du, Z.Y.; Zhao, Z.; Pang, X.; Li, X.H.; Chen, X. Effect of two prokaryotic expressed vectors on the activity of PPO from Camellia sinensis. J. Tea Sci. 2018, 38, 396–405. [Google Scholar]
- Huang, C.; Zhang, J.; Zhang, X.; Yu, Y.C.; Bian, W.B.; Zeng, Z.P.; Sun, X.L.; Li, X.H. Two New Polyphenol Oxidase Genes of Tea Plant (Camellia sinensis) Respond Differentially to the Regurgitant of Tea Geometrid, Ectropis obliqua. Int. J. Mol. Sci. 2018, 19, 2414. [Google Scholar] [CrossRef]
- Liu, J.W.; Huang, Y.Y.; Ding, J.; Liu, C.; Xiao, X.D.; Ni, D.J. Prokaryotic expression of polyphenol oxidase mature protein from Camellia sinensis. J. Tea Sci. 2010, 30, 136–140. [Google Scholar]
- Wu, Y.L.; Pan, L.P.; Yu, S.L.; Li, H.H. Cloning, microbial expression and structure-activity relationship of polyphenol oxidases from Camellia sinensis. J. Biotechnol. 2010, 145, 66–72. [Google Scholar] [CrossRef]
- Xu, Z.X.; Xia, L.Q.; Sun, M.S.; Huang, P.; Zeng, J.G. Effects of codon optimization, N-terminal truncation and gene dose on the heterologous expression of berberine bridge enzyme. World J. Microbiol. Biotechnol. 2022, 38, 77. [Google Scholar] [CrossRef]
- Zou, C.; Yin, J.F. The Properties of Polyphenol Oxidase and Its Research Progress in Tea Processing. China Tea 2021, 43, 41–47. [Google Scholar]
- Liu, J.W.; Huang, Y.Y.; Ding, J.A.; Liu, C.; Xiao, X.D.; Ni, D.J. Prokaryotic expression and purification of Camellia sinensis polyphenol oxidase. J. Sci. Food Agr. 2010, 90, 2490–2494. [Google Scholar] [CrossRef]
- Wang, N.D.; Zhang, L.X.; Huang, X.Q.; Han, X.Y.; Li, Z. Construction and expression of fusion protein eukaryotic expression vector of polyphenol oxidase gene of tea plant (Camellia sinensis). J. Tea Sci. 2012, 32, 269–275. [Google Scholar]
- Liu, K.S.; Ouyang, Y.Q.; Lin, R.; Ge, C.Y.; Zhou, M.A. Strong negative correlation between codon usage bias and protein structural disorder impedes protein expression after codon optimization. J. Biotechnol. 2022, 343, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, D.; Kumar, S. Expression and biochemical analysis of codon-optimized polyphenol oxidase from Camellia sinensis (L.) O. Kuntze E. coli. Process Biochem. 2017, 59, 180–186. [Google Scholar] [CrossRef]
- Zhao, S.J.; Wang, K.B.; Fu, D.H.; Li, Y.L.; Lei, Y.G.; Li, Q. On the enzymological feature of polyphenol oxidase from tea. J. Hunan Agric. Univ. 2008, 34, 84–86. [Google Scholar]
- Liu, Y.; Chen, Q.C.; Liu, D.C.; Yang, L.; Hu, W.; Kuang, L.Q.; Teng, J.; Liu, Y. Comparison of the biochemical properties and enzymatic synthesis of theaflavins by soluble and membrane-bound polyphenol oxidases from tea (Camellia sinensis) leaves. Food Sci. Technol. 2022, 42, e117321. [Google Scholar] [CrossRef]
- Teng, J.; Gong, Z.H.; Deng, Y.L.; Chen, L.; Li, Q.; Shao, Y.Y.; Lin, L.; Xiao, W.J. Purification, characterization and enzymatic synthesis of theaflavins of polyphenol oxidase isozymes from tea leaf (Camellia sinensis). LWT 2017, 84, 263–270. [Google Scholar] [CrossRef]
- Halder, J.; Tamuli, P.; Bhaduri, A.N. Isolation and characterization of polyphenol oxidase from Indian tea leaf (Camellia sinensis). J. Nutr. Biochem. 1998, 9, 75–80. [Google Scholar] [CrossRef]
- Ke, L.J.; Xu, W.; Gao, J.N.; Gao, G.Z.; Wang, H.Q.; Zhou, J.W.; Liu, J.; Rao, P.F.; Xu, Y.Q. Isolation and characterization of thermo-tolerant polyphenol oxidases in a black tea infusion. Food Control 2021, 119, 107465. [Google Scholar] [CrossRef]
- Cai, H.L.; Zhong, Z.H.; Chen, Y.R.; Zhang, S.Y.; Ling, H.; Fu, H.W.; Zhang, L. Genes cloning, sequencing and function identification of recombinant polyphenol oxidase isozymes for production of monomeric theaflavins from Camellia sinensis. Int. J. Biol. Macromol. 2023, 240, 124353. [Google Scholar] [CrossRef]
| Primers | Primer Sequence (5′–3′) |
|---|---|
| WT-CsPPO-F | aactaattattcgaaggatccATGGCTTCCATTCTCCCTCC |
| WT-CsPPO-R | cgcggccgccctagggaattcTTAAGAATCAAACTCAATCTTGACACC |
| CO-CsPPO-F | aactaattattcgaaggatccATGGCTTCCATCTTGCCACC |
| CO-CsPPO-R | cgcggccgccctagggaattcTTAGGAGTCGAACTCAATCTTGACACC |
| R 1-60 | ATG GCT TCC ATT CTC CCT CCA ACC ACC ACC AAG ACT ACC ACC ACC TCC TCC ACC CTC TAT ATG GCT TCC ATC TTG CCA CCA ACT ACT ACC AAG ACT ACT ACT ACC TCC TCC ACC TTG TAC |
| P 1-60 | |
| R 61-120 | TCT TAT CCC ATT TTC AAG AAC ACC TCT AAA ATT CCC ACA ATT AGA AAG CAT AAC CAT TCC TCT TAC CCA ATC TTC AAG AAC ACC TCC AAG ATT CCA ACC ATC AGA AAG CAC AAC CAT TCC |
| P 61-120 | |
| R 121-180 | TTC AAT GTG TCA TGC AGC AAA GCC AAA GAT AGT GAC CCA AAC CTT ACT CCC CCC TCC CAA TTC AAC GTG TCC TGT TCC AAG GCT AAG GAT TCT GAC CCA AAC TTG ACT CCA CCA TCT CAG |
| P 121-180 | |
| R 181-240 | AAT ACA CAA ACC TCC CTA GGG AAG TTT GAT AGG AGA AAC ATG GTC ATT GGC TTA GGA GGC AAC ACT CAA ACT TCC CTG GGA AAG TTC GAC AGA CGT AAC ATG GTT ATT GGT CTT GGT GGT |
| P 181-240 | |
| R 241-300 | CTC TAC GGA GCA GCC GGA CTC ACC GAC ACT GAC CCA CCA GCC TTG GCC GCT CCG GTC ACC CTG TAC GGT GCT GCT GGT TTG ACT GAT ACT GAT CCA CCA GCT TTG GCT GCT CCA GTT ACT |
| P 241-300 | |
| R 301-360 | GCC CCG GAT TTA TCC AAA TGC GGG GCG GCG GAT TTA CCC GCG GAT GCA AAA CCG ACC AAC GCT CCA GAT TTG TCT AAG TGT GGT GCT GCA GAT TTG CCA GCT GAT GCT AAG CCA ACT AAC |
| P 301-360 | |
| R 361-420 | TGT TGT CCT CCA AAA ACC AAC AAA ATC ATC GAA TTC AAG CTC CCT CCT CCC TCC AAC ATT TGT TGT CCA CCA AAG ACT AAC AAG ATC ATC GAG TTC AAG CTG CCA CCA CCA TCC AAC ATC |
| P 361-420 | |
| R 421-480 | TTA CGA GTC CGA CCC GCG GCC CAT TTA GCC GAC GAA AAA TAC ATA GCC AAG TTT TCT AAA TTG AGA GTT AGA CCA GCT GCT CAC TTG GCT GAC GAG AAG TAC ATT GCT AAG TTC TCC AAG |
| P 421-480 | |
| R 481-540 | GCC CTC CAA CTC ATG AAA TCG CTC CCC GAT GAC GAC CCA CGA AGC TTC AAG CAA CAA TCC GCT CTG CAG CTG ATG AAG TCT TTG CCT GAT GAT GAC CCA AGA TCC TTC AAG CAG CAG TCC |
| P 481-540 | |
| R 541-600 | AAT ATT CAC TGC GCT TAT TGC GAA GGC GCT TAT CAC CAA GTC GGT TTT CCA AGC ACA GAA AAC ATT CAC TGT GCT TAC TGT GAA GGT GCC TAC CAC CAA GTT GGT TTC CCA TCT ACT GAG |
| P 541-600 | |
| R 601-660 | CTC CAA GTT CAC AAC TCA TGG CTC TTC TTT CCC TTC CAT AGA TTC TAT CTC TAC TTC TTC TTG CAG GTT CAC AAC TCC TGG CTG TTT TTC CCA TTC CAC AGA TTC TAC CTG TAC TTC TTC |
| P 601-660 | |
| R 661-720 | GAA AAG ATT TTG GGA ATG CTG CTC GAT GAT CCA GCG TTT GCA ATT CCT TTT TGG AAT TGG GAG AAG ATC CTG GGT ATG TTG TTG GAC GAT CCA GCT TTC GCT ATC CCA TTC TGG AAC TGG |
| P 661-720 | |
| R 721-780 | GAT TCT CCG GCG GGC ATG AAA ATA CCC GCC ATG TAT GCG GAC ATA AAT TCG CCA CTC TAT GAT TCT CCA GCT GGT ATG AAG ATC CCA GCT ATG TAC GCT GAC ATT AAC TCC CCA CTG TAC |
| P 721-780 | |
| R 781-840 | AAC CGT CTC CGT GAC GCC AAA CAC CAG CCA CCG ACA TTA ATT GAC CTT GAC TAC AAT TTG AAC AGA TTG AGA GAT GCC AAG CAC CAG CCA CCA ACC TTG ATT GAT TTG GAC TAC AAC CTG |
| P 781-840 | |
| R 841-900 | ACT GAT CCG AAA AAT GTC GAT GAG GAG AAG CAG AAG TTG AGA AAT CTA ACT ATA ATG TAC ACT GAC CCA AAG AAC GTT GAC GAA GAG AAG CAG AAG TTG AGG AAC CTG ACC ATC ATG TAC |
| P 841-900 | |
| R 901-960 | CGA CAA GTG GTG TCG GGT GGG AAA ACG CCT CGG CTT TTC CTT GGA AGC TCG TAC CGT GCG AGA CAG GTT GTT TCC GGT GGT AAG ACC CCT AGA CTG TTC TTG GGT TCT TCT TAC AGA GCT |
| P 901-960 | |
| R 961-1020 | GGA GAT GAC CCG GAC CCA GGT GCC GGG TCC CTG GAG AAC ATC CCG CAT GGT CCG GTT CAC GGT GAT GAC CCT GAT CCA GGT GCT GGT TCT TTG GAA AAC ATT CCA CAT GGT CCA GTC CAC |
| P 961-1020 | |
| R 1021-1080 | ATA TGG TGC GGG GAC CGC ACC CAG CCG AAT CTA GAA GAC ATG GGG AAC TTC TAC TCT GCG ATC TGG TGT GGT GAT AGA ACT CAG CCA AAC TTG GAG GAC ATG GGT AAC TTC TAC TCC GCT |
| P 1021-1080 | |
| R 1081-1140 | GGA CGA GAT CCG ATC TTC TAC GGT CAT CAC GCG AAC GTC GAT CGG ATC TGG ACG GTG TGG GGT AGA GAT CCA ATC TTC TAC GGT CAT CAC GCC AAC GTT GAC AGA ATC TGG ACT GTC TGG |
| P 1081-1140 | |
| R 1141-1200 | AAG ACA TTA GGA GGA AAA CGA AAC GAT TTC AAG GAT TCG GAT TGT TTG AAT TCA GAG TTC AAA ACC CTT GGT GGA AAG AGA AAC GAC TTC AAG GAC TCC GAC TGT CTG AAC TCC GAG TTT |
| P 1141-1200 | |
| R 1201-1260 | ACC TTT TAC GAC GAA AAT GCT CAG CTT GTG ACT GTA AAA GTA AAA GAG AGT TTG GAT CAT ACT TTC TAC GAC GAG AAC GCC CAG TTG GTT ACC GTT AAG GTC AAA GAA TCC CTG GAC CAC |
| P 1201-1260 | |
| R 1261-1320 | CGA AAA CTC GGC TAC GTC TAC CAA GAC GTG GAA ATT CCA TGG CTA AAC GCT CGA CCC AGT AGA AAG CTG GGT TAC GTT TAC CAG GAC GTT GAG ATC CCA TGG TTG AAC GCT AGA CCA TCT |
| P 1261-1320 | |
| R 1321-1380 | CCT CGT ATT TCA AAT TTT TTT CGA AAA ATA AAG AAC AAG GCC GGG ATA GCA ATG GCG ACA CCA AGA ATC TCC AAC TTC TTC AGA AAG ATC AAG AAC AAG GCC GGT ATC GCT ATG GCT ACT |
| P 1321-1380 | |
| R 1381-1440 | GAG ACA CTG GAT TCT GCT GCC ATT GTA TTC CCA AGA AAG CTT GAT GAG GTG GTG AAG GTG GAA ACT TTG GAT TCC GCC GCT ATC GTT TTC CCA AGA AAG TTG GAT GAG GTC GTC AAG GTT |
| P 1381-1440 | |
| R 1441-1500 | GTG GTG AAG CGG CCG ACG AAG TCG AGG AGT GAG AGA GAG AAG GAA GAA GAG GAG GAG GTG GTT GTC AAG AGG CCA ACT AAG TCC AGA TCC GAG AGA GAG AAA GAG GAA GAA GAA GAG GTT |
| P 1441-1500 | |
| R 1501-1560 | GTG GTA GTG GAG GGG ATA GAG ATG GAG AGA GAT GTG TCT GTG AAG TTT GAT GTG TTT ATT GTC GTC GTT GAG GGT ATC GAA ATG GAA AGG GAC GTT TCC GTT AAG TTC GAC GTG TTC ATT |
| P 1501-1560 | |
| R 1561-1620 | AAC GAC GAA GAC GAG GCG GCA AGT GGG CCG GAG AAG ACA GAG TTC GCC GGA AGC TTT GTG AAC GAC GAG GAC GAA GCT GCT TCT GGT CCA GAA AAG ACT GAA TTT GCC GGT TCC TTC GTG |
| P 1561-1620 | |
| R 1621-1680 | AAT GTG CCG CGT AAA CAT AAG CAT GAC AAG AAG ATA AGG ACT AGT TTG AGG TTG GGG ATA AAC GTG CCA AGA AAA CAT AAG CAC GAC AAG AAG ATC AGG ACC TCC TTG AGA TTG GGT ATC |
| P 1621-1680 | |
| R 1681-1740 | ACT GAG CTA TTG GAG GAC TTG GAA GCT GAA GAT GAT GAG AGC GTG CTG GTG ACT TTG GTC ACC GAG TTG TTG GAG GAC TTG GAA GCT GAA GAT GAC GAG TCC GTT TTG GTC ACT TTG GTC |
| P 1681-1740 | |
| R 1741-1800 | CCT AGA TAT GGG TCT GAT GCT GTC ACT ATT GGT GGT GTC AAG ATT GAG TTT GAT TCT TAA CCA AGA TAC GGT TCC GAC GCT GTT ACT ATT GGT GGT GTC AAG ATT GAG TTC GAC TCC TAA |
| P 1741-1800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xu, Y.-Q.; Yin, J.-F.; Zou, C. Activity Expression and Property Analysis of Codon-Optimized Polyphenol Oxidase from Camellia sinensis in Pichia pastoris KM71. Foods 2025, 14, 2749. https://doi.org/10.3390/foods14152749
Zhang X, Xu Y-Q, Yin J-F, Zou C. Activity Expression and Property Analysis of Codon-Optimized Polyphenol Oxidase from Camellia sinensis in Pichia pastoris KM71. Foods. 2025; 14(15):2749. https://doi.org/10.3390/foods14152749
Chicago/Turabian StyleZhang, Xin, Yong-Quan Xu, Jun-Feng Yin, and Chun Zou. 2025. "Activity Expression and Property Analysis of Codon-Optimized Polyphenol Oxidase from Camellia sinensis in Pichia pastoris KM71" Foods 14, no. 15: 2749. https://doi.org/10.3390/foods14152749
APA StyleZhang, X., Xu, Y.-Q., Yin, J.-F., & Zou, C. (2025). Activity Expression and Property Analysis of Codon-Optimized Polyphenol Oxidase from Camellia sinensis in Pichia pastoris KM71. Foods, 14(15), 2749. https://doi.org/10.3390/foods14152749







