Quality Status and Skin-Related Functional Properties of Traditional Korean Fermented Vinegars
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Korean Fermented Vinegar Samples
2.2. Physicochemical Analysis
2.2.1. pH
2.2.2. Titratable Acidity
2.2.3. Brix
2.2.4. Total Nitrogen
2.3. Free Sugar Content
2.4. Organic Acid Content
2.5. Free Amino Acid Content
2.6. Antioxidant Capacity
2.6.1. Total Polyphenol Content
2.6.2. Total Flavonoid Content
2.6.3. DPPH (1,1-Diphenyl-2-Picrylhydrazyl) Assay
2.6.4. ABTS [2,2′-Azinobis(3-Ethylbenzothiazoline-6-Sulphonic Acid)] Assay
2.6.5. FRAP (Ferric Reducing Antioxidant Power) Assay
2.7. Enzyme Activity Assay
2.7.1. Collagenase Inhibitory Activity
2.7.2. Tyrosinase Inhibitory Activity
2.8. Statistical Analysis
3. Results and Discussion
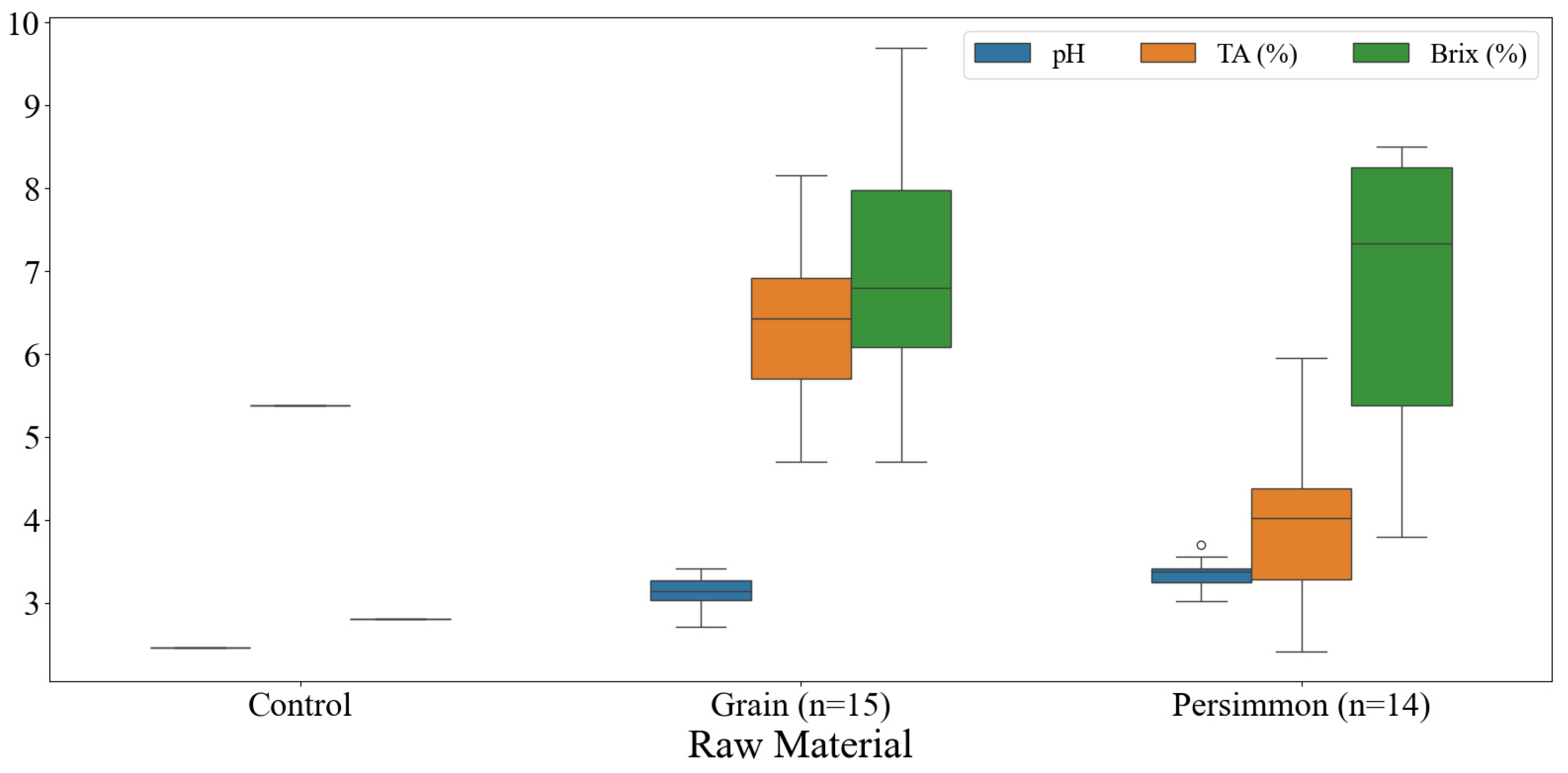
3.1. Physicochemical Analysis of Korean Fermented Vinegar
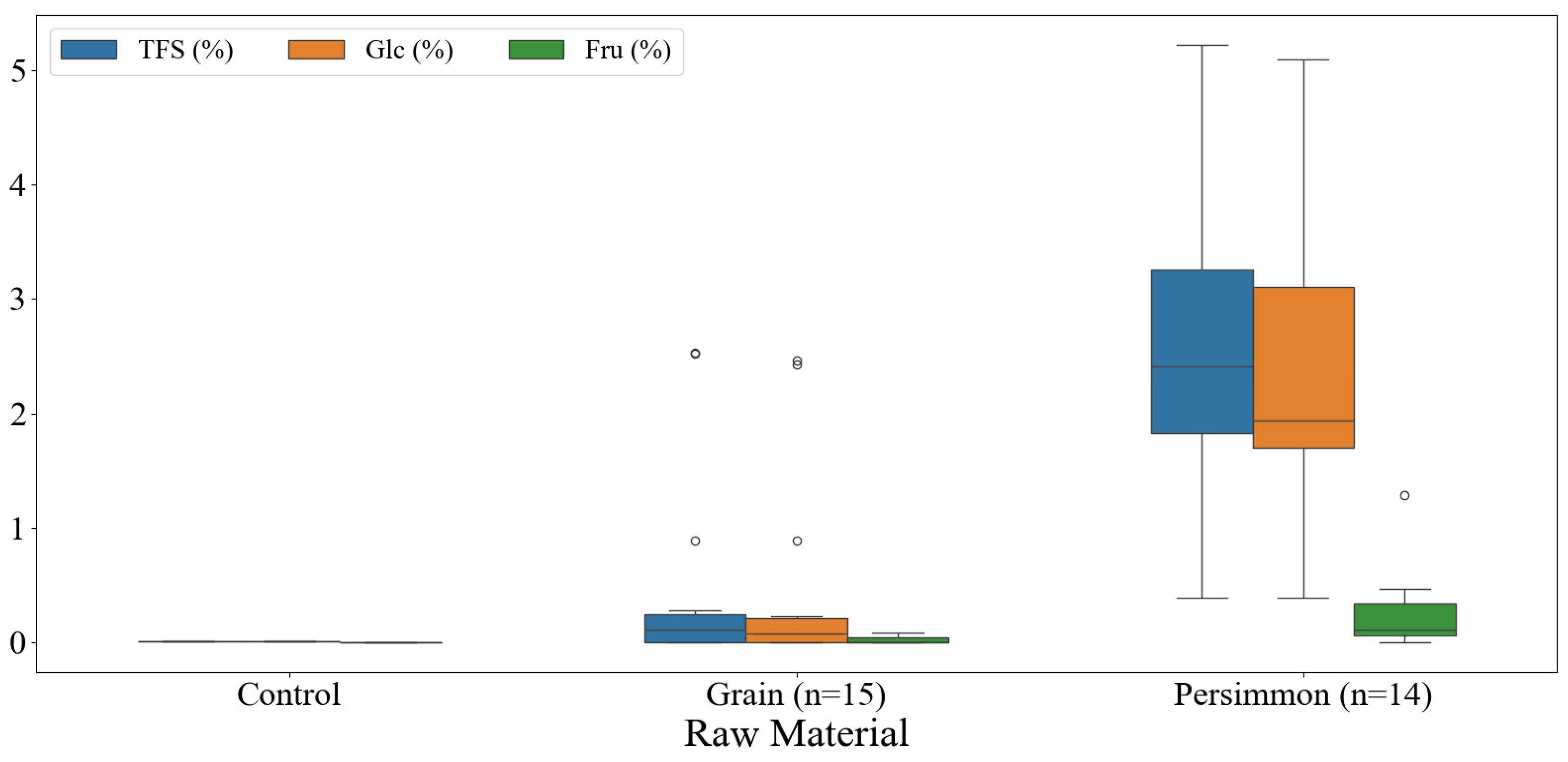
3.2. Free Sugar Contents of Korean Fermented Vinegar
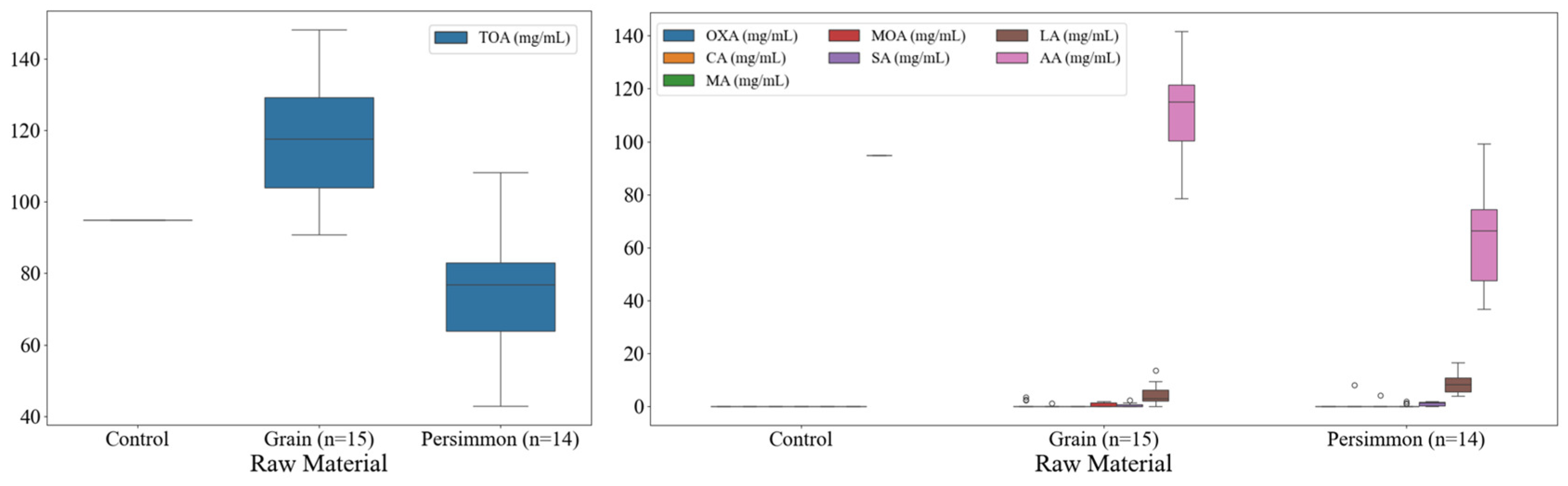
3.3. Organic Acid Contents of Korean Fermented Vinegar
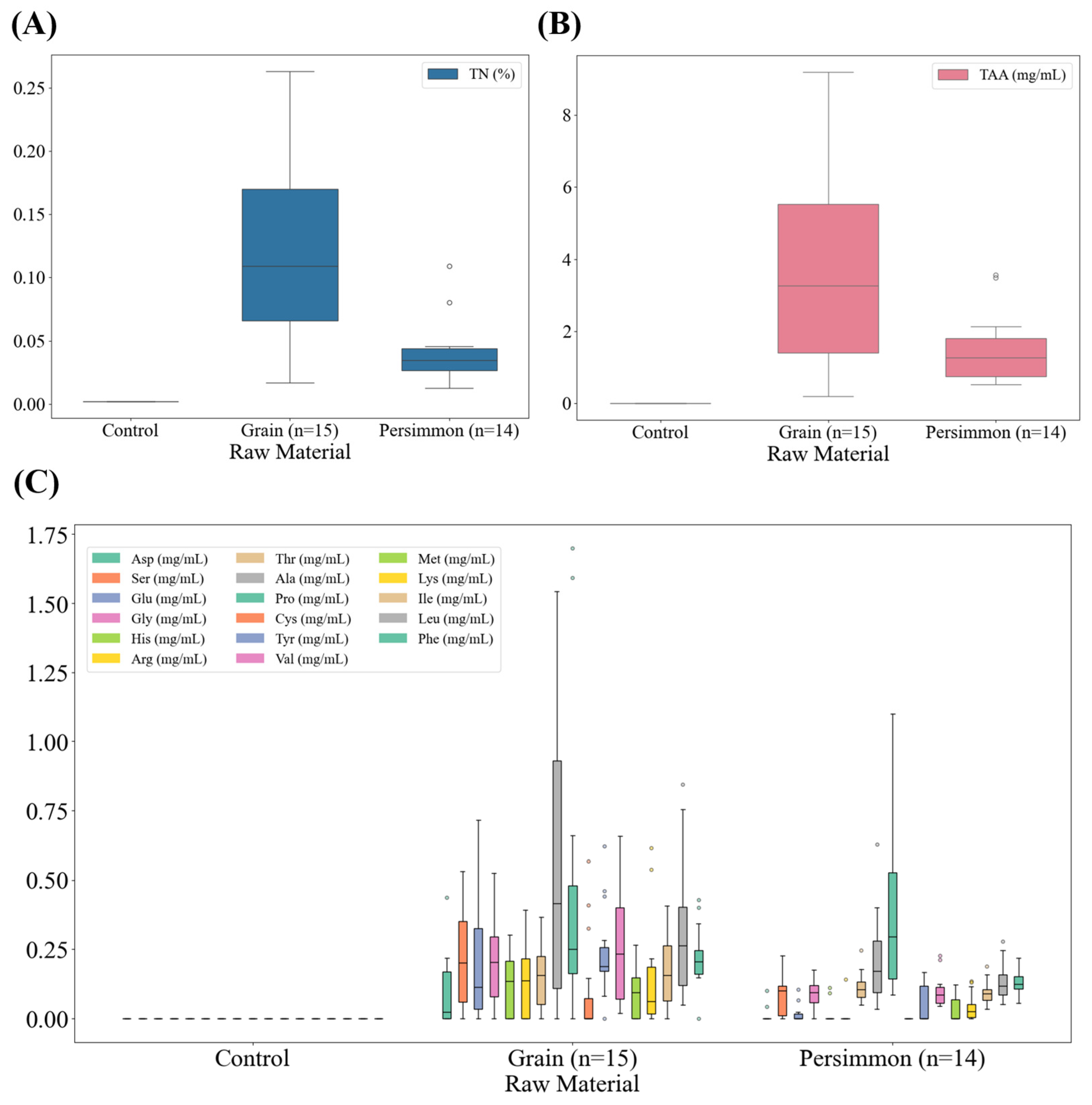
3.4. Total Nitrogen and Amino Acid Contents of Korean Fermented Vinegar
3.5. Antioxidant Capacities of Korean Fermented Vinegar
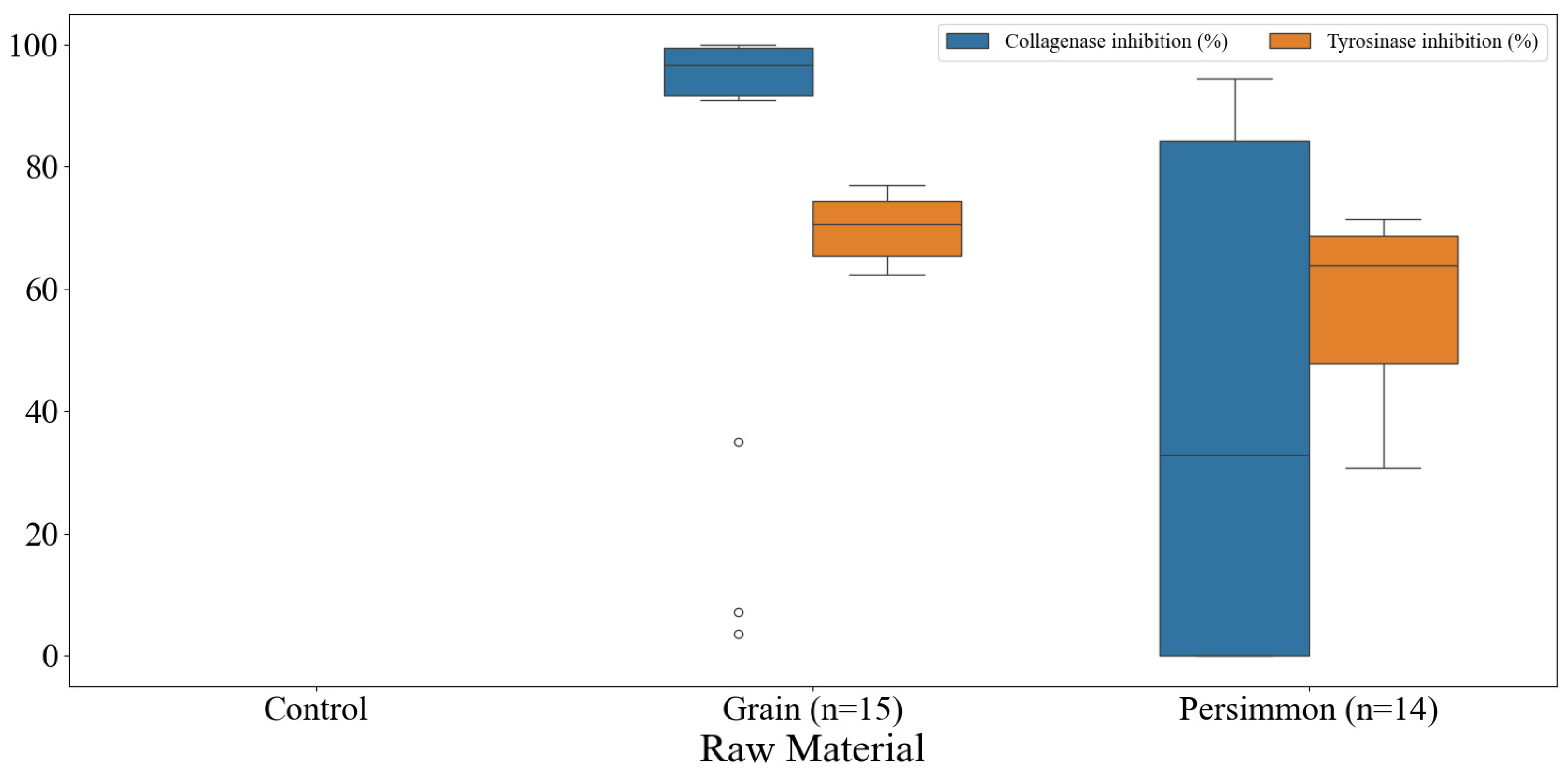
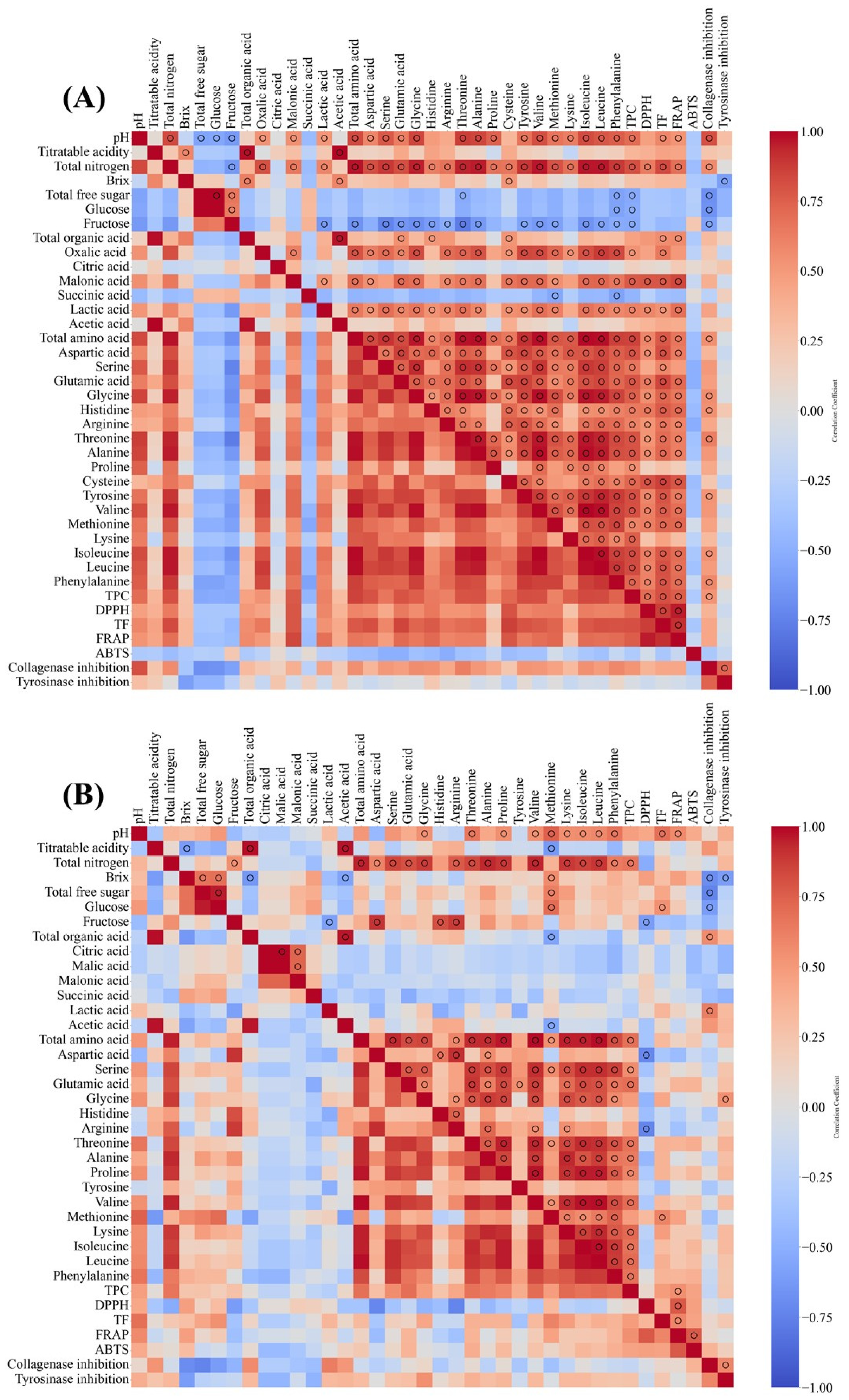
3.6. Skin-Related Functionalities of Korean Fermented Vinegar and Correlation with Qualities of Vinegar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional properties of vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Chung, N.; Jo, Y.; Gao, Y.; Gu, S.Y.; Jeong, Y.J.; Kwon, J.H. Comparison of physicochemical properties and antioxidant activities of naturally fermented commercial rice vinegars produced in Korea, China, and Japan. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1799–1805. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Luo, Q.; Xu, N.; Zhou, M.; Gao, B.; Wang, C.; Shi, Y. Fermenting liquid vinegar with higher taste, flavor and healthy value by using discarded Cordyceps militaris solid culture medium. LWT 2018, 98, 654–660. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Q.; Zhang, F.; Tang, Y.; Wang, J.; Sun, J. Changes in bioactive and volatile aroma compounds in vinegar fermented in a rotary drum bioreactor. J. Food Compos. Anal. 2023, 121, 105345. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Nagano, M.; Ishigo, S.; Ito, Y.; Hashiguchi, K.; Hishida, N.; Mita, M.; Lindner, W.; Hamase, K. Chiral amino acid analysis of Japanese traditional Kurozu and the developmental changes during earthenware jar fermentation processes. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 966, 187–192. [Google Scholar] [CrossRef]
- Shibayama, Y.; Kanouchi, H.; Fujii, A.; Nagano, M. A review of Kurozu, amber rice vinegar made in pottery jars. Funct. Foods Health Dis. 2020, 10, 254–264. [Google Scholar] [CrossRef]
- Bertelli, D.; Maietti, A.; Papotti, G.; Tedeschi, P.; Bonetti, G.; Graziosi, R.; Brandolini, V.; Plessi, M. Antioxidant activity, phenolic compounds, and NMR characterization of balsamic and traditional balsamic vinegar of Modena. Food Anal. Methods 2015, 8, 371–379. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Zheng, F.; Xu, B.; Abughoush, M.; Li, L.; Sun, B. Processing technologies and flavor analysis of Chinese cereal vinegar: A comprehensive review. Food Anal. Methods 2023, 16, 1–28. [Google Scholar] [CrossRef]
- Park, E.H.; Choi, C.Y.; Kwon, H.J.; Kim, M.D. Literature review on type and manufacturing methods of Korean traditional vinegar. Food Sci. Ind. 2016, 49, 94–99. [Google Scholar]
- Hong, S.M.; Kang, M.J.; Lee, J.H.; Jeong, J.H.; Kwon, S.H.; Seo, K.I. Production of vinegar using Rubus coreanus and its antioxidant activities. Korean J. Food Preserv. 2012, 19, 594–603. [Google Scholar] [CrossRef]
- Jeon, S.H.; Park, H.J.; Kim, S.Y.; Yeo, S.H.; Gwon, H.M. Production of high-acidity peach vinegar by improving the manufacturing process of “Dochobang”. Korean J. Food Preserv. 2021, 28, 117–128. [Google Scholar] [CrossRef]
- Hwang, J.; Yun, J.K.; Kim, S.K.; Lee, S.H.; Han, K.H. Mass production of chaff-vinegar and its effect of anti-aging and whitening. Microbiol. Biotechnol. Lett. 2012, 40, 208–214. [Google Scholar] [CrossRef]
- Yun, Y.R.; Park, B.Y.; Kim, S.H.; Jung, J.H. Antioxidant, anti-obesity, and anti-aging activities of Jeju citrus blended vinegar. Foods 2021, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Cho, H.Y.; Pyo, Y.H. Effect of unpolished rice vinegar containing Monascus-fermented soybean on inhibitory activities of tyrosinase and elastase. J. Korean Soc. Food Sci. Nutr. 2016, 45, 149–154. [Google Scholar] [CrossRef]
- Takabe, W.; Yagi, M.; Ogura, M.; Yonei, Y. Effect of mangosteen pericarp extract-containing black vinegar drink on skin quality through anti-glycative actions. Glycative Stress Res. 2017, 4, 158–171. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety, Korea Food Code. 2021. Available online: https://various.foodsafetykorea.go.kr/fsd/ (accessed on 10 February 2025).
- Das, P.R.; Kim, Y.; Hong, S.J.; Eun, J.B. Profiling of volatile and non-phenolic metabolites-amino acids, organic acids, and sugars of green tea extracts obtained by different extraction techniques. Food Chem. 2019, 296, 69–77. [Google Scholar] [CrossRef]
- Ge, L.; Lai, H.; Huang, Y.; Wang, Y.; Li, Y.; Zhu, S.; Shi, Q.; Li, H.; Zhu, Y.; Zhao, N. Comparative evaluation of package types in alleviating textural softening and package-swelling of Paocai during storage: Insight into microbial invasion, cell wall pectinolysis and alteration in sugar and organic acid profiles. Food Chem. 2021, 365, 130489. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.H.; Fayaz, M.; Kumar, A.; Dar, A.A.; Jain, A.K. Chromatographic method for determination of the amino acid content in Dioscorea bulbifera L. tubers by RP-HPLC. Pharm. Sci. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Dai, W.; Gu, S.; Xu, M.; Wang, W.; Yao, H.; Zhou, X.; Ding, Y. The effect of tea polyphenols on biogenic amines and free amino acids in bighead carp (Aristichthys nobilis) fillets during frozen storage. LWT 2021, 150, 111933. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Genisheva, Z.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M. Moderate electric fields as a potential tool for sustainable recovery of phenolic compounds from Pinus pinaster bark. ACS Sustain. Chem. Eng. 2019, 7, 8816–8826. [Google Scholar] [CrossRef]
- Ochieng, B.O.; Anyango, J.O.; Nduko, J.M.; Cheseto, X.; Mudalungu, C.M.; Khamis, F.M.; Ghemoh, C.J.; Egonyu, P.J.; Subramanian, S.; Nakimbugwe, D.; et al. Dynamics in nutrients, sterols and total flavonoid content during processing of the edible Long-Horned grasshopper (Ruspolia differens Serville) for food. Food Chem. 2022, 383, 132397. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Abuduaini, M.; Li, J.; Ruan, J.H.; Zhao, Y.X.; Maitinuer, M.; Aisa, H.A. Bioassay-guided preparation of antioxidant, anti-inflammatory active fraction from crabapples (Malus prunifolia (Willd.) Borkh.). Food Chem. 2023, 406, 135091. [Google Scholar] [CrossRef]
- Meneses, N.G.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Separation and purification technology. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Stoenescu, A.M.; Trandafir, I.; Tuțulescu, F. Comparison of chemical properties between traditional and commercial vinegar. Horticulturae 2022, 8, 225. [Google Scholar] [CrossRef]
- Ozturk, I.; Caliskan, O.Z.N.U.R.; Tornuk, F.; Ozcan, N.; Yalcin, H.; Baslar, M.; Sagdic, O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT 2015, 63, 144–151. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of acetic acid bacteria and their role in vinegar and fermented beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef]
- Leal Maske, B.; Murawski de Mello, A.F.; da Silva Vale, A.; Prado Martin, J.G.; de Oliveira Soares, D.L.; De Dea Lindner, J.; Soccol, C.R.; de Melo Pereira, G.V. Exploring diversity and functional traits of lactic acid bacteria in traditional vinegar fermentation: A review. Int. J. Food Microbiol. 2024, 412, 110550. [Google Scholar] [CrossRef]
- Roostita, R.; Fleet, G.H. The occurrence and growth of yeasts in Camembert and blue-veined cheeses. Int. J. Food Microbiol. 1996, 28, 393–404. [Google Scholar] [CrossRef]
- Viljoen, B.C. The interaction between yeasts and bacteria in dairy environments. Int. J. Food Microbiol. 2001, 69, 37–44. [Google Scholar] [CrossRef]
- Fang, G.Y.; Chai, L.J.; Zhong, X.Z.; Jiang, Y.J. Deciphering the succession patterns of bacterial community and their correlations with environmental factors and flavor compounds during the fermentation of Zhejiang rosy vinegar. Int. J. Food Microbiol. 2021, 341, 109070. [Google Scholar] [CrossRef]
- Shirzad, M.; Hamedi, J.; Motevaseli, E.; Modarressi, M.H. Anti-elastase and anti-collagenase potential of Lactobacilli exopolysaccharides on human fibroblast. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Marchellia, S.; Sutiono, A.; Lheman, J.; Tjandrawinata, R.R. Cosmeceutical potency of functional ripe buni cider. IOP Conf. Ser. Earth Environ. Sci. 2020, 591, 012023. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [PubMed]
- Carroll, E.; Trinh, T.N.; Son, H.; Lee, Y.W.; Seo, J.A. Comprehensive analysis of fungal diversity and enzyme activity in nuruk, a Korean fermenting starter, for acquiring useful fungi. J. Microbiol. 2017, 55, 357–365. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, Y.; Li, M.; Xiong, L.; Sun, Q. Characterization of Maillard reaction products micro/nano-particles present in fermented soybean sauce and vinegar. Sci. Rep. 2019, 9, 11285. [Google Scholar] [CrossRef]
- Xu, Q.; Tao, W.; Ao, Z. Antioxidant activity of vinegar melanoidins. Food Chem. 2007, 102, 841–849. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of organic acids, amino acids and phenolic compounds on antioxidant characteristic of Zhenjiang aromatic vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic acid contents of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef]
- Sakanaka, S.; Ishihara, Y. Comparison of antioxidant properties of persimmon vinegar and some other commercial vinegars in radical-scavenging assays and on lipid oxidation in tuna homogenates. Food Chem. 2008, 107, 739–744. [Google Scholar] [CrossRef]
- Yeerong, K.; Sriyab, S.; Somwongin, S.; Punyoyai, C.; Chantawannakul, P.; Anuchapreeda, S.; Prommaban, A.; Chaiyana, W. Skin irritation and potential antioxidant, anti-collagenase, and anti-elastase activities of edible insect extracts. Sci. Rep. 2021, 11, 22954. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jung, S.K.; Cho, Y.J.; Kim, B.O. Evaluation of the probiotic properties and physiological activities of novel lactic acid bacteria isolated from traditional fermented foods. J. Korean Soc. Food Sci. Nutr. 2024, 53, 519–528. [Google Scholar] [CrossRef]
- Marques, R.V.; Guillaumin, A.; Abdelwahab, A.B.; Salwinski, A.; Gotfredsen, C.H.; Bourgaud, F.; Enemark-Rasmussen, K.; Miguel, S.; Simonsen, H.T. Collagenase and tyrosinase inhibitory effect of isolated constituents from the moss Polytrichum formosum. Plants 2021, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, M.M.; Sun, Y.; Wang, L.F.; Wang, H.; Zhang, Y.J.; Li, H.Y.; Zhuang, P.W.; Yang, Z. Synergistic promotion on tyrosinase inhibition by antioxidants. Molecules 2018, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Widodo, W.S.; Widowati, W.; Ginting, C.N.; Lister, I.; Armansyah, A.; Girsang, E. Comparison of antioxidant and anti-collagenase activity of genistein and epicatechin. Pharm. Sci. Res. 2019, 6, 6. [Google Scholar] [CrossRef]
- Shin, E.J.; Kang, S.M. The effect of complex diet of collagen and vinegar on improvement of skin on the back of hands. Kor. J. Aesthet. Cosmetol. 2012, 10, 371–379. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.H.; Jang, S.-W.; Kim, E.; Kim, J.-C.; Jang, M. Quality Status and Skin-Related Functional Properties of Traditional Korean Fermented Vinegars. Foods 2025, 14, 2728. https://doi.org/10.3390/foods14152728
Yu HH, Jang S-W, Kim E, Kim J-C, Jang M. Quality Status and Skin-Related Functional Properties of Traditional Korean Fermented Vinegars. Foods. 2025; 14(15):2728. https://doi.org/10.3390/foods14152728
Chicago/Turabian StyleYu, Hwan Hee, So-Won Jang, Eungyeong Kim, Jong-Chan Kim, and Mi Jang. 2025. "Quality Status and Skin-Related Functional Properties of Traditional Korean Fermented Vinegars" Foods 14, no. 15: 2728. https://doi.org/10.3390/foods14152728
APA StyleYu, H. H., Jang, S.-W., Kim, E., Kim, J.-C., & Jang, M. (2025). Quality Status and Skin-Related Functional Properties of Traditional Korean Fermented Vinegars. Foods, 14(15), 2728. https://doi.org/10.3390/foods14152728





