Interaction Between Lactic Acid Bacteria and Acetic Acid Bacteria in Sichuan Bran Vinegar: Impact on Their Growth and Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Interaction Between Lactic Acid Bacteria and Acetic Acid Bacteria
2.2.1. Solid-State Fermentation and Sampling
2.2.2. Enumeration of Strains
2.2.3. Determination of Total Acid, Lactic Acid, and Acetic Acid Content
2.2.4. Volatile Compounds
2.2.5. Non-Volatile Compounds
2.3. Statistical Analysis
3. Results and Discussion
3.1. Enumeration of Strains in Pure Culture and Co-Culture
3.2. Changes in Total Acid Content in Pure Culture and Co-Culture
3.3. Changes in Lactic Acid and Acetic Acid Contents in Pure Culture and Co-Culture
3.4. Volatile Compounds in Pure Culture and Co-Culture
3.5. Non-Volatile Compounds in Pure Culture and Co-Culture
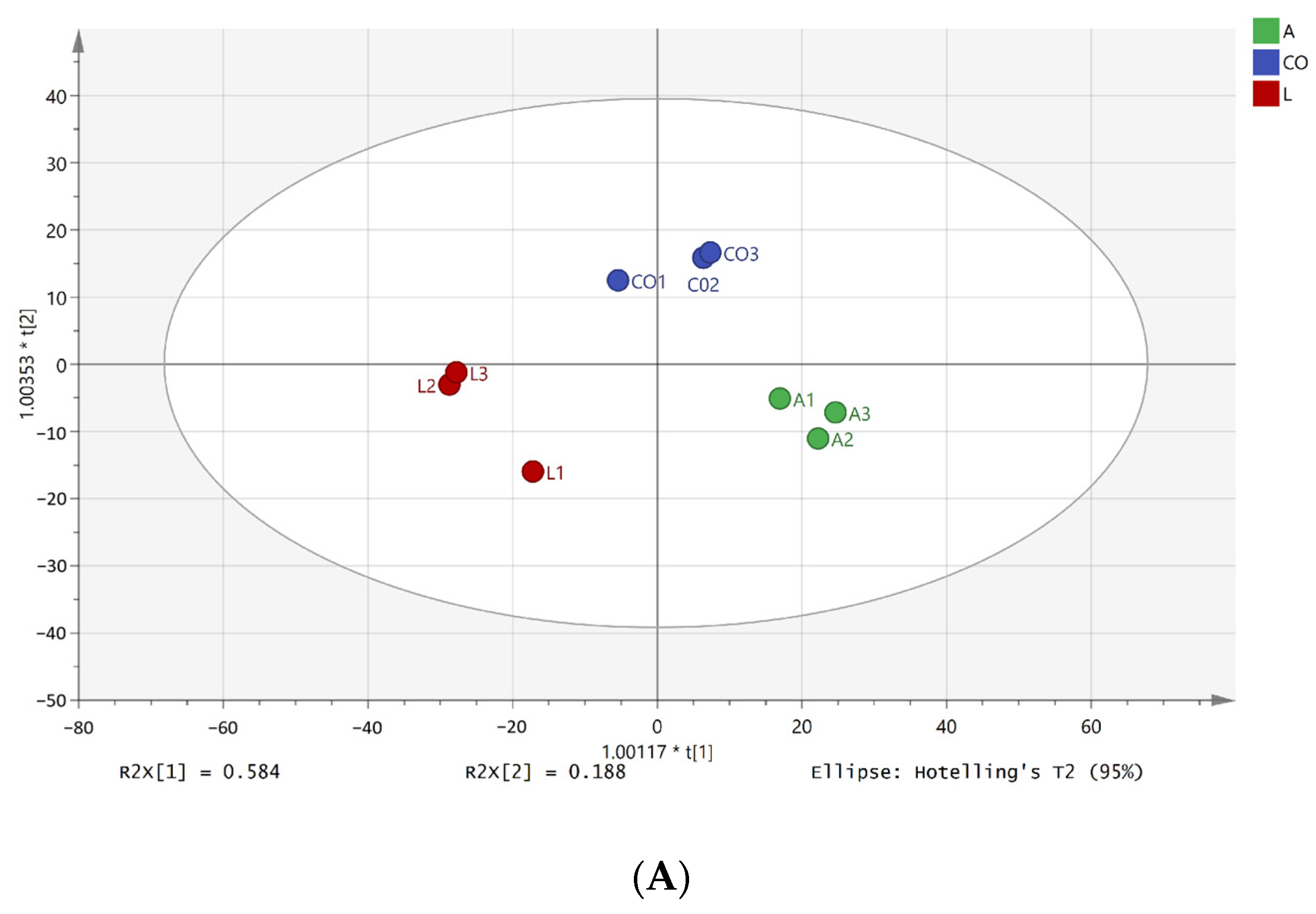
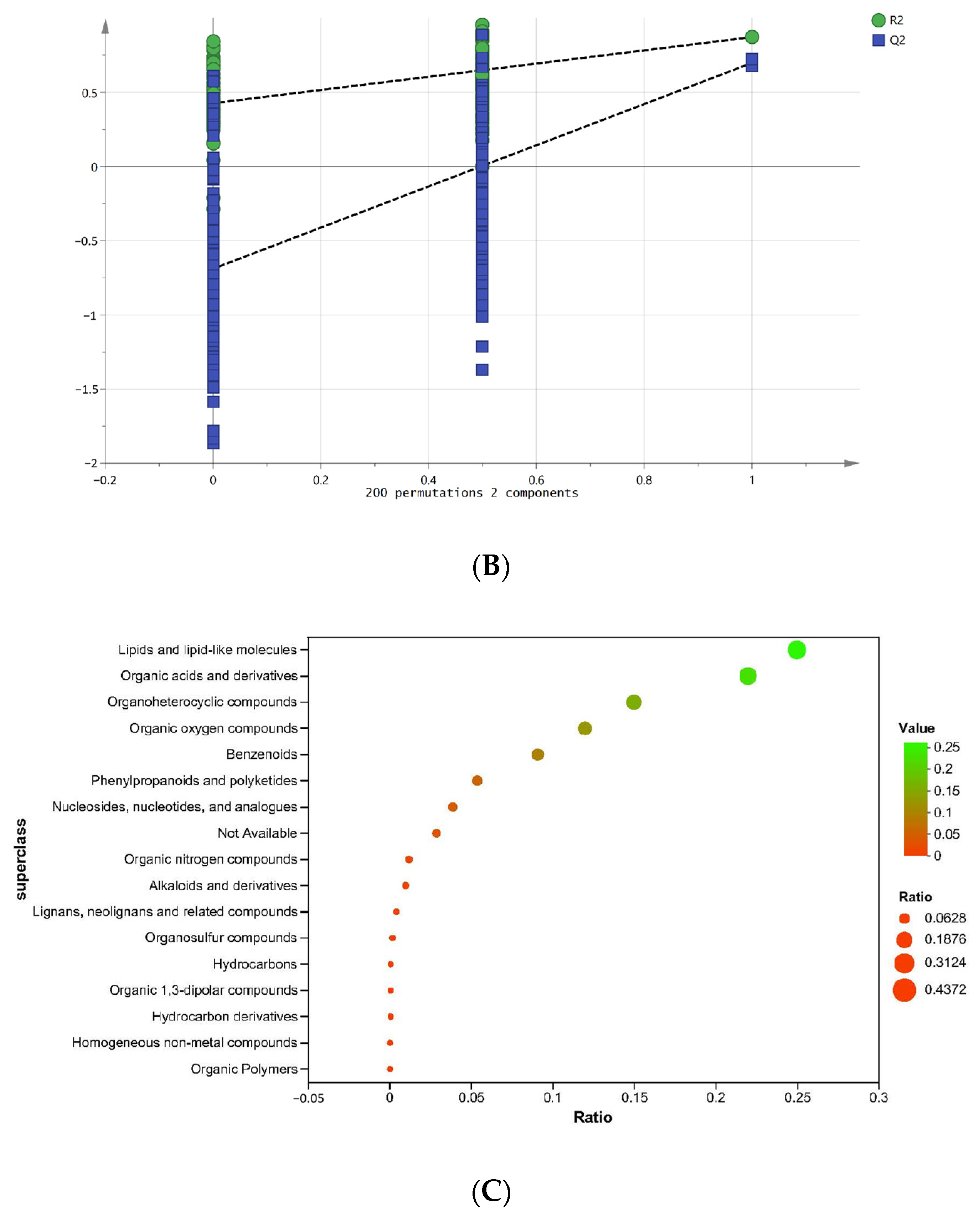
3.5.1. Metabolite Profile
3.5.2. Differential Metabolite Analysis
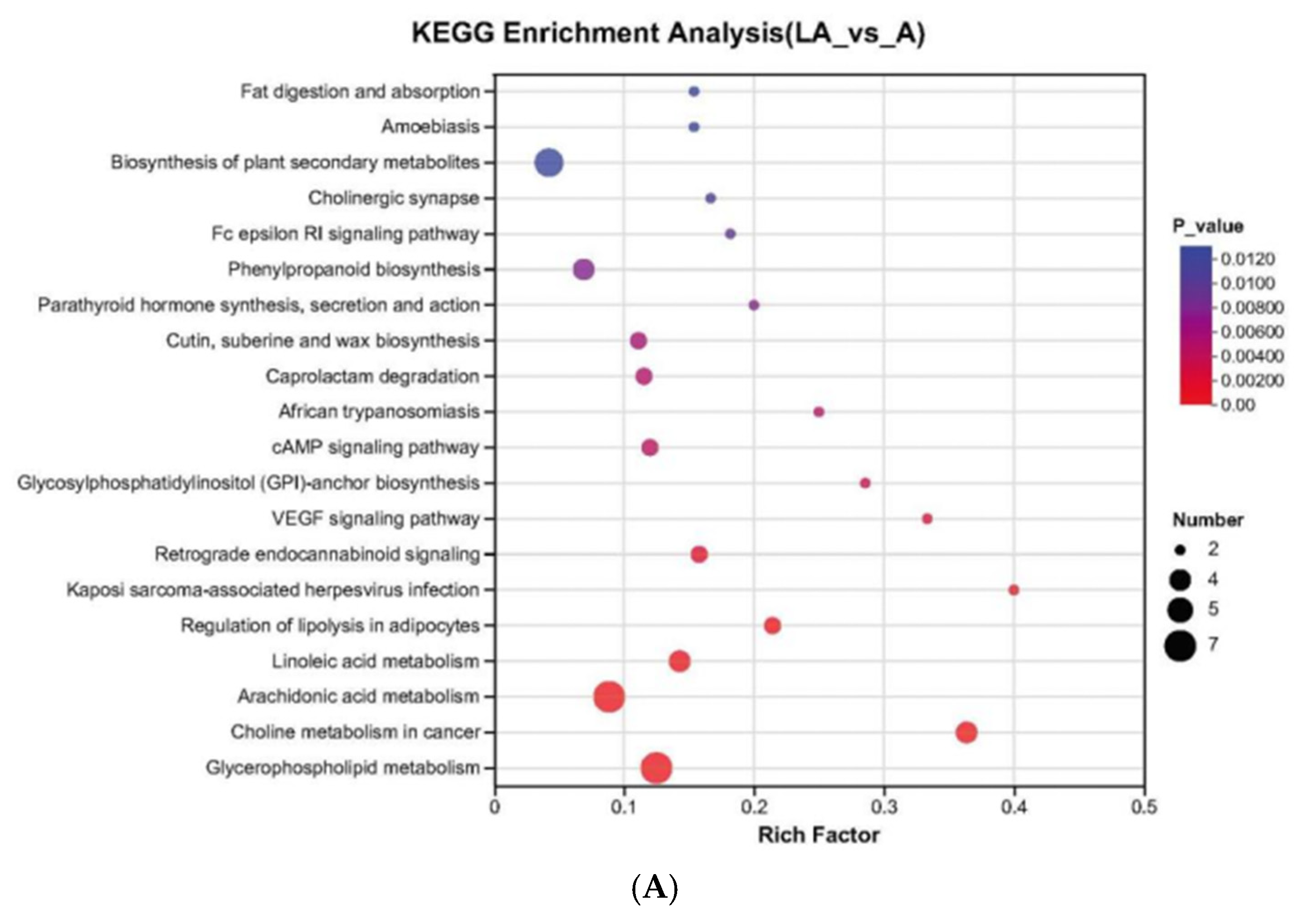
3.5.3. KEGG Metabolic Pathway Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Zheng, F.; Xu, B.; Abughoush, M.; Li, L.; Sun, B. Processing Technologies and Flavor Analysis of Chinese Cereal Vinegar: A Comprehensive Review. Food Anal. Methods. 2023, 16, 1–28. [Google Scholar] [CrossRef]
- Chen, H.; Chen, T.; Giudici, P.; Chen, F. Vinegar Functions on Health: Constituents, Sources, and Formation Mechanisms. Compr. Rev. Food. Sci. Food Saf. 2016, 15, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Min, Y.; Xiao, J.; Feng, N.; Chen, Y.; Luo, Q.; Zhou, M.; Li, D.; Hu, Z.; Wang, C. Liquid state fermentation vinegar enriched with catechin as an antiglycative food product. Food Funct. 2019, 10, 4877–4887. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Zhou, R.; Wu, C. Evaluating the feasibility of fermentation starter inoculated with Bacillus amyloliquefaciens for improving acetoin and tetramethylpyrazine in Baoning bran vinegar. Int. J. Food Microbiol. 2017, 255, 42–50. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, F.; Zhang, C.; Yang, L.; Fan, G.; Xu, Y.; Sun, B.; Li, X. Dynamic microbial succession of Shanxi aged vinegar and its correlation with flavor metabolites during different stages of acetic acid fermentation. Sci. Rep. 2018, 8, 8612. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, C.; Li, X.; Xia, M.; Wang, X.; Zhang, Q.; Yan, Y.; Lang, F.; Song, J.; Wang, M. Kinetics of predominant microorganisms in the multi-microorganism solid-state fermentation of cereal vinegar. Lwt-Food Sci. Technol. 2022, 159, 113209. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, G.; Wu, C.; Hu, Z.; Xu, X.; Xie, G.; Wu, D.; Lu, J. Profiling the key metabolites produced during the modern brewing process of Chinese rice wine. Food Res. Int. 2021, 139, 109955. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, C.; Zhao, C.; Yang, S.; Zheng, Y.; Xia, M.; Yan, Y.; Lang, F.; Wang, M. Monitoring microbial succession and metabolic activity during manual and mechanical solid-state fermentation of Chinese cereal vinegar. Lwt-Food Sci. Technol. 2020, 133, 109868. [Google Scholar] [CrossRef]
- Huang, T.; Lu, Z.; Peng, M.; Liu, Z.; Chai, L.; Zhang, X.; Shi, J.; Li, Q.; Xu, Z. Combined effects of fermentation starters and environmental factors on the microbial community assembly and flavor formation of Zhenjiang aromatic vinegar. Food Res. Int. 2022, 152, 110900. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Fan, J. Acetic acid in aged vinegar affects molecular targets for thrombus disease management. Food Funct. 2015, 6, 2845–2853. [Google Scholar] [CrossRef]
- Zheng, X.; Yan, Z.; Nout, M.R.; Smid, E.J.; Zwietering, M.H.; Boekhout, T.; Han, J.; Han, B. Microbiota dynamics related to environmental conditions during the fermentative production of Fen-Daqu, a Chinese industrial fermentation starter. Int. J. Food Microbiol. 2014, 182, 57–62. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, X.; Xiao, Y.; Sheng, Q.; Tu, L.; Chen, F.; Yan, Y.; Zheng, Y.; Wang, M. Interaction of acetic acid bacteria and lactic acid bacteria in multispecies solid-state fermentation of traditional Chinese cereal vinegar. Front. Microbiol. 2022, 13, 964855. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, N.; Li, Q.; Zhao, K.; Tu, M.; Li, J.; Hu, K.; Chen, S.; Liu, S.; Liu, A. Metagenomics and Metagenome-Assembled Genomes: Analysis of Cupei from Sichuan Baoning Vinegar, One of the Four Traditional Renowned Vinegars in China. Foods 2025, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Hu, K.; Li, J.; Duran-Guerrero, E.; Liu, S.; Guo, M.; Liu, A. Microbial characterization of Sichuan Baoning vinegar: Lactic acid bacteria, acetic acid bacteria and yeasts. Arch. Microbiol. 2024, 206, 59. [Google Scholar] [CrossRef]
- Liu, A.; Peng, Y.; Ao, X.; Pan, W.; Chen, S.; He, L.; Yang, Y.; Chen, F.; Du, D.; Liu, S. Effects of Aspergillus niger biofortification on the microbial community and quality of Baoning vinegar. Lwt-Food Sci. Technol. 2020, 131, 109728. [Google Scholar] [CrossRef]
- Du Toit, W.D.; Pretorius, I.S.; Lonvaud Funel, A. The effect of sulphur dioxide and oxygen on the viability and culturability of a strain of Acetobacter pasteurianus and a strain of Brettanomyces bruxellensis isolated from wine. J. Appl. Microbiol. 2005, 98, 862–871. [Google Scholar] [CrossRef]
- Kang, H.; Ao, X.; Tang, Q.; Li, H.; Fan, Y.; Liu, A.; Zou, L.; Liu, S.; Yang, Y.; Zhao, N.; et al. Effects of yeast screened from traditional fermented milk on commercial fermented milk as adjunct flavor culture. Food Biosci. 2024, 57, 103551. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.; Zhang, X.; Wang, Z.; Yu, Y.; Shi, J.; Xu, Z. Metagenomics reveals flavour metabolic network of cereal vinegar microbiota. Food Microbiol. 2017, 62, 23–31. [Google Scholar] [CrossRef]
- Li, W.; Tong, S.; Yang, Z.; Xiao, Y.; Lv, X.; Weng, Q.; Yu, K.; Liu, G.; Luo, X.; Wei, T.; et al. The dynamics of microbial community and flavor metabolites during the acetic acid fermentation of Hongqu aromatic vinegar. Curr. Res. Food Sci. 2022, 5, 1720–1731. [Google Scholar] [CrossRef]
- Li, K.; Tang, J.; Zhang, Z.; Wu, Z.; Zhong, A.; Li, Z.; Wang, Y. Correlation between flavor compounds and microorganisms of Chaling natural fermented red sufu. Lwt-Food Sci. Technol. 2022, 154, 112873. [Google Scholar] [CrossRef]
- Hao, Z.; Feng, J.; Chen, Q.; Lin, H.; Zhou, X.; Zhuang, J.; Wang, J.; Tan, Y.; Sun, Z.; Wang, Y.; et al. Comparative volatiles profiling in milk-flavored white tea and traditional white tea Shoumei via HS-SPME-GC-TOFMS and OAV analyses. Food Chem. X 2023, 18, 100710. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Zhu, W.; Zhang, C.; Yin, L.; Li, L.; Liu, J. Effect of temperature on chemical compounds of Cupei (precursor of bran vinegar) during in-situ aging and revelation of functional microorganisms in the process. Lwt-Food Sci. Technol. 2023, 182, 114912. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Dong, L.; Huan, Y.; Yoshimoto, M.; Zhu, Y.; Tada, I.; Wang, X.; Zhao, S.; Zhang, F.; et al. Comprehensive Metabolomic Comparison of Five Cereal Vinegars Using Non-Targeted and Chemical Isotope Labeling LC-MS Analysis. Metabolites 2022, 12, 427. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Z.; Fang, B.; Hung, W.; Gao, H.; Zhao, W.; Lan, H.; Liu, M.; Zhao, L.; Zhang, M. Effect of Thermal Inactivation on Antioxidant, Anti-Inflammatory Activities and Chemical Profile of Postbiotics. Foods 2023, 12, 3579. [Google Scholar] [CrossRef]
- Langos, D.; Granvogl, M. Studies on the Simultaneous Formation of Aroma-Active and Toxicologically Relevant Vinyl Aromatics from Free Phenolic Acids During Wheat Beer Brewing. J. Agric. Food. Chem. 2016, 64, 2325–2332. [Google Scholar] [CrossRef]
- He, B.; Liu, Z.; Li, B.; Yuan, Y. Advances in biosynthesis of scopoletin. Microb. Cell. Fact. 2022, 21, 152. [Google Scholar] [CrossRef]
- Lian, B.; Gu, J.; Zhang, C.; Zou, Z.; Yu, M.; Li, F.; Wu, X.; Zhao, A.Z. Protective effects of isofraxidin against scopolamine-induced cognitive and memory impairments in mice involve modulation of the BDNF-CREB-ERK signaling pathway. Metab. Brain Dis. 2022, 37, 2751–2762. [Google Scholar] [CrossRef]
- Muratore, G.; Licciardello, F.; Restuccia, C.; Puglisi, M.L.; Giudici, P. Role of different factors affecting the formation of 5-hydroxymethyl-2-furancarboxaldehyde in heated grape must. J. Agric. Food. Chem. 2006, 54, 860–863. [Google Scholar] [CrossRef]



| Class | No. | Name | Relative Contents (μg/kg) | ||
|---|---|---|---|---|---|
| Pure Culture of L. amylovorus LL34 | Pure Culture of A. pasteurianus LA10 | Co-Culture of L. amylovorus LL34 and A. pasteurianus LA10 | |||
| Acids | 1 | Acetic acid | 262.26 ± 66.25 b | 810.72 ± 235.26 a | 607.97 ± 205.27 ab |
| 2 | Hexanoic acid | / | 62.84 ± 5.85 a | 58.88 ± 12.67 a | |
| 3 | Pentanoic acid | 31.63 ± 0.02 a | / | / | |
| 4 | 2-Methylbutanoic acid | / | 3.72 ± 2.89 a | / | |
| 5 | 3-Methylbutanoic acid | / | 3.31 ± 1.04 a | / | |
| 6 | 2-Methylpropanoic acid | / | 2.28 ± 0.49 a | / | |
| Alcohols | 7 | Ethanol | 506.91 ± 56.92 a | 193.91 ± 30.37 b | 393.39 ± 90.21 a |
| 8 | 1-Hexanol | 10.56 ± 0.75 a | 7.27 ± 0.25 c | 9.39 ± 0.03 b | |
| 9 | Cyclohexanemethanol, 4-methyl-, cis- | / | 4.13 ± 0.39 a | / | |
| 10 | α-Methyl-benzenemethanol | / | 2.2 ± 0.16 a | / | |
| 11 | Benzenemethanol, 4-methyl-.alpha.-(1-methyl-2-propenyl)-, (R,R)- | / | 0.21 ± 0.02 a | / | |
| 12 | 1-Octen-3-ol | / | / | 9.02 ± 0.78 a | |
| 13 | 1-Pentanol | 0.99 ± 0.2 a | / | 1.05 ± 0.02 a | |
| 14 | 2-Methyl-1,8-octanediol | 1.09 ± 0.33 a | / | / | |
| Aldehydes | 15 | Benzaldehyde | 115.46 ± 18.99 a | 10.5 ± 0.65 b | 9 ± 0.84 b |
| 16 | 3-Furaldehyde | 61.55 ± 6.4 a | / | / | |
| 17 | Methylal | / | / | 39.88 ± 32.55 a | |
| 18 | 5-Ethylcyclopent-1-enecarboxaldehyde | 37.74 ± 2.99 a | / | / | |
| 19 | Benzeneacetaldehyde | 15.59 ± 2.45 a | / | / | |
| 20 | Nonanal | 8 ± 1.12 a | 1.27 ± 0.1 b | 2.02 ± 0.16 b | |
| 21 | Heptanal | 7.68 ± 0.36 a | / | / | |
| 22 | Hexanal | 6.69 ± 5.41 a | / | / | |
| 23 | 2-Butenal | / | 2.54 ± 1.52 a | 4.8 ± 3.54 a | |
| 24 | Decanal | 0.94 ± 0.18 a | / | / | |
| 25 | Isophthalaldehyde | 0.41 ± 0.02 a | / | / | |
| Esters | 26 | Ethyl acetate | / | 167.98 ± 15.94 a | 158.19 ± 26.88 a |
| 27 | Methyl 2-hydroxypropanoate | / | 66.86 ± 19.81 a | 55.08 ± 45.24 a | |
| 28 | Propyl 2-hydroxypropanoate | / | / | 52.79 ± 59.97 a | |
| 29 | Ethyl 2-(methylamino)acetate | / | 28.21 ± 10.76 a | 35.5 ± 3.45 a | |
| 30 | Ethyl 2-hydroxypropanoate | / | 10.58 ± 0.31 b | 33.94 ± 2.26 a | |
| 31 | Allyl acetate | / | 4.49 ± 1.28 a | / | |
| 32 | Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl-, methylcarbamate | 3.02 ± 0.66 a | / | / | |
| 33 | Hexyl acetate | / | 2.07 ± 0.07 a | 1.77 ± 0.05 b | |
| 34 | 2-Ethylhexyl hexyl sulfite | 1.52 ± 0.24 a | 0.8 ± 0.78 a | 3.24 ± 2.31 a | |
| 35 | Methyl acetate | / | 1.03 ± 0.38 a | 1.11 ± 0.3 a | |
| 36 | Sulfurous acid, isobutyl pentyl ester | / | 0.45 ± 0.22 a | / | |
| Ketones | 37 | Acetyl methyl carbinol | / | 145.4 ± 31.4 a | 107.75 ± 20.93 a |
| 38 | Gamma-nonanoic lactone | 15.45 ± 2.34 a | 20.3 ± 0.4 a | 19.12 ± 3.95 a | |
| 39 | 2-Heptanone | 1.84 ± 0.47 b | 24.53 ± 1.48 a | / | |
| 40 | 5-Methyl-2-hexanone | 25.03 ± 1.57 a | 2.29 ± 0.21 b | 13.63 ± 15.89 ab | |
| 41 | 2-Nonanone | 1.04 ± 0.1 b | 3.96 ± 0.1 a | 3.55 ± 0.38 a | |
| 42 | Acetone | / | / | 6.97 ± 2.54 a | |
| 43 | 5-Methyl-4-hexen-3-one | / | 2.96 ± 0.32 a | / | |
| 44 | 3-Ethylcyclopentanone | / | / | 2.94 ± 0.15 a | |
| 45 | Acetophenone | 2.56 ± 0.34 a | / | 2.87 ± 0.26 a | |
| 46 | 3-Octen-2-one | 2.53 ± 0.15 a | / | 1.55 ± 0.18 b | |
| 47 | 4-Nonanone | / | 1.54 ± 0.04 a | 1.55 ± 0.24 a | |
| 48 | 2,5-Dimethyl-3-hexanone | 0.36 ± 0.2 a | 0.34 ± 0.1 a | / | |
| 49 | 2,2,5-Trimethylhexane-3,4-dione | 0.88 ± 0.16 a | / | / | |
| Heterocycles | 50 | 2-Pentylfuran | 817.05 ± 95.09 b | 833.04 ± 37.86 b | 1024.09 ± 37.76 a |
| 51 | 2-(1-Pentenyl)furan | 2.89 ± 0.58 a | 3.33 ± 0.21 a | 3.82 ± 0.95 a | |
| 52 | 2-Hexylfuran | 1.85 ± 0.22 a | / | / | |
| Terpenes | 53 | 3-Methyl-1-hexene | / | 0.83 ± 0.01 a | |
| 54 | 9-Methyl-1-undecene | 1.03 ± 0.12 a | / | / | |
| Others | 55 | 1H-Indene, octahydro-2,2,4,4,7,7-hexamethyl-, trans- | 9.48 ± 1.25 a | / | 9.33 ± 3.35 a |
| 56 | Naphthalene | 3.46 ± 0.54 a | 3.01 ± 0.14 a | 3.3 ± 1 a | |
| 57 | 1,2,4,5-Tetramethylbenzene | 2.32 ± 0.19 b | / | 3.17 ± 0.24 a | |
| 58 | 1H-Tetrazol-5-amine | / | 0.24 ± 0.08 a | 0.27 ± 0.03 a | |
| 59 | Semicarbazide | / | / | 2.89 ± 2.25 a | |
| 60 | (1-Ethylpropyl)benzene | / | / | 2.69 ± 0.35 a | |
| 61 | N-Isobutyl(phenyl)methanesulfonamide | / | / | 2.74 ± 0.56 a | |
| 62 | 2,4,5-Trimethyl-1,3-dioxolane | / | 1.72 ± 0 a | / | |
| 63 | 1,1,4a,5,6-Pentamethyldecahydronaphthalene | / | 0.96 ± 0 a | / | |
| 64 | 2-Acetylthiazole | 0.81 ± 0.05 a | / | / | |
| 65 | 2-Methoxy phenol | 0.59 ± 0.13 a | / | / | |
| 66 | Dihexyverine | / | 0.23 ± 0.05 a | / | |
| 67 | Ethoxyethene | / | 0.2 ± 0.04 a | / | |
| Metabolite | Fold Change | Regulate | VIP Value |
|---|---|---|---|
| 8-Hydroxyguanine | 0.7166 | down | 8.2403 |
| 3-Hydroxy-4-methoxyphenyllactic acid | 1.2953 | up | 6.8915 |
| Phenyllactic acid | 1.1295 | up | 6.3087 |
| Xanthine amine | 0.8554 | down | 6.2165 |
| Franguloside | 1.1626 | up | 6.0155 |
| S-Adenosylhomocysteine | 1.1013 | up | 4.7833 |
| Phenylpyruvic acid | 0.9111 | down | 4.4769 |
| 5′-Methylthioadenosine | 0.9299 | down | 4.1068 |
| (E)-10-Hydroxy-8-decenoic acid | 1.0675 | up | 4.0472 |
| Ketoleucine | 0.9479 | down | 3.9116 |
| Enniatin B | 1.0441 | up | 3.8112 |
| 4-Vinylphenol | 1.0659 | up | 3.7345 |
| Ethyl 3-hydroxydodecanoate | 1.0993 | up | 3.7238 |
| 1,4,7,10,13,16-Hexaoxacyclooctadecane | 1.0604 | up | 3.4713 |
| 6-Hydroxyhexanoic acid | 1.0352 | up | 3.1451 |
| Uric acid | 0.9635 | down | 3.134 |
| 5-Chloro-2′-deoxyuridine | 1.0459 | up | 3.1175 |
| (2S,3R,4S,5R,6R)-6-Ethyloxane-2,3,4,5-tetrol | 1.0401 | up | 3.0507 |
| PC(18:2(9Z,12Z)/18:2(9Z,12Z)) | 0.9675 | down | 3.016 |
| 5-Methyl-2-furancarboxaldehyde | 0.9615 | down | 2.9399 |
| Enniatin B1 | 1.0264 | up | 2.9028 |
| Epitiostanol | 1.0326 | up | 2.8945 |
| 1-Hexanol | 1.0388 | up | 2.7946 |
| Metabolite | Fold Change | Regulate | VIP Value |
|---|---|---|---|
| L-Asparagine | 0.5834 | down | 8.7843 |
| Scopoletin | 1.7944 | up | 8.6923 |
| Iminodiacetic acid | 0.6175 | down | 8.0915 |
| 3a,7a-Dihydroxy-5b-cholestane | 1.4394 | up | 7.2836 |
| 4-Oxo-2-azetidinecarboxylic acid | 0.7232 | down | 7.162 |
| L-Aspartic acid | 0.7397 | down | 6.8104 |
| Ureidopropionic acid | 0.6903 | down | 6.8023 |
| Aspartic acid | 0.7887 | down | 6.5172 |
| Isofraxidin | 1.3725 | up | 6.3634 |
| 3-Indolebutyric acid | 1.2467 | up | 6.0585 |
| 2-(Carbamoylamino)propanoic acid | 0.8051 | down | 5.9903 |
| 5-Hydroxymethyl-2-furancarboxaldehyde | 1.2321 | up | 5.7925 |
| MALEAMIC ACID | 0.7766 | down | 5.659 |
| Tolmetin | 1.1964 | up | 5.379 |
| 4-Hydroxyvalproic acid | 1.1994 | up | 5.2886 |
| Cyclopropyl–methoxycarbonyl metomidate | 1.1838 | up | 5.281 |
| DG(22:4(7Z,10Z,13Z,16Z)/15:0/0:0) | 1.1599 | up | 5.1935 |
| Franguloside | 1.1716 | up | 5.142 |
| 3-Ethyl-5-hydroxy-4,5-dimethyl-pyrrolin-2-one | 1.1865 | up | 5.0692 |
| 1,2-Dilinolenoyl-3-(4-aminobutyryl)propane-1,2,3-triol | 1.1516 | up | 4.8926 |
| 3-Methoxyphenol sulfate | 1.175 | up | 4.7342 |
| 2-Succinylbenzoate | 1.1424 | up | 4.3289 |
| N-lactoyl-Methionine | 1.127 | up | 4.3233 |
| 5-Hydroxyvalproic acid | 1.1097 | up | 4.245 |
| S-Adenosylhomocysteine | 1.1109 | up | 4.1535 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wu, J.; Tu, M.; Xiao, X.; Hu, K.; Li, Q.; Zhao, N.; Liu, A.; Ao, X.; Hu, X.; et al. Interaction Between Lactic Acid Bacteria and Acetic Acid Bacteria in Sichuan Bran Vinegar: Impact on Their Growth and Metabolites. Foods 2025, 14, 1471. https://doi.org/10.3390/foods14091471
Li J, Wu J, Tu M, Xiao X, Hu K, Li Q, Zhao N, Liu A, Ao X, Hu X, et al. Interaction Between Lactic Acid Bacteria and Acetic Acid Bacteria in Sichuan Bran Vinegar: Impact on Their Growth and Metabolites. Foods. 2025; 14(9):1471. https://doi.org/10.3390/foods14091471
Chicago/Turabian StyleLi, Jianlong, Jie Wu, Meiling Tu, Xue Xiao, Kaidi Hu, Qin Li, Ning Zhao, Aiping Liu, Xiaolin Ao, Xinjie Hu, and et al. 2025. "Interaction Between Lactic Acid Bacteria and Acetic Acid Bacteria in Sichuan Bran Vinegar: Impact on Their Growth and Metabolites" Foods 14, no. 9: 1471. https://doi.org/10.3390/foods14091471
APA StyleLi, J., Wu, J., Tu, M., Xiao, X., Hu, K., Li, Q., Zhao, N., Liu, A., Ao, X., Hu, X., & Liu, S. (2025). Interaction Between Lactic Acid Bacteria and Acetic Acid Bacteria in Sichuan Bran Vinegar: Impact on Their Growth and Metabolites. Foods, 14(9), 1471. https://doi.org/10.3390/foods14091471





