Freeze-Dried Probiotic Fermented Camel Milk Enriched with Ajwa Date Pulp: Evaluation of Functional Properties, Probiotic Viability, and In Vitro Antidiabetic and Anticancer Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
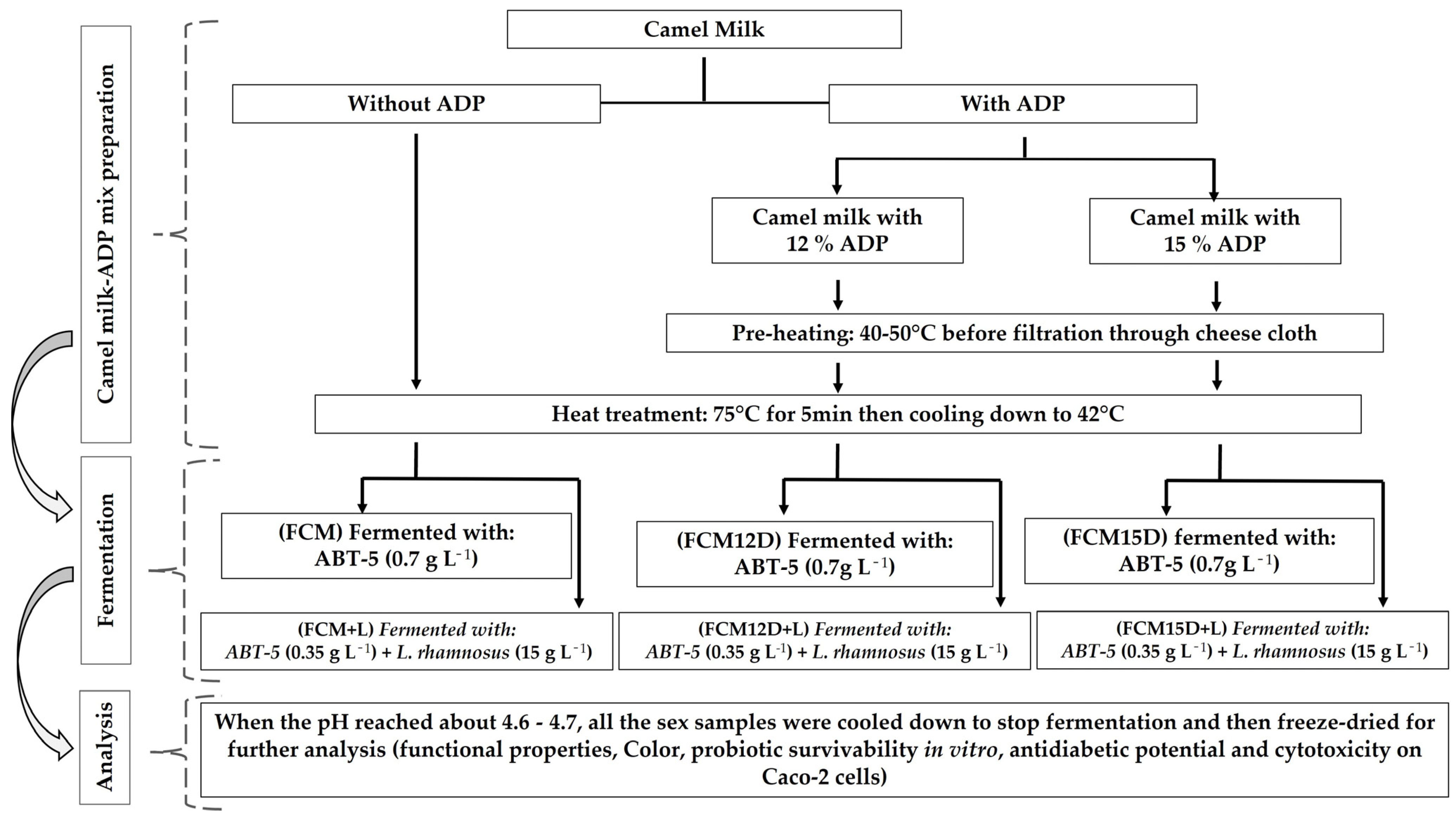
2.2. Ajwa Date and Milk Preparation
2.3. Propagation of L. rhamnosus B-1937
2.4. Preparation of Fermented Probiotic Camel Milk
2.5. Functional and Physical Properties Analyses
2.5.1. Analysis of Functional Properties of Different Freeze-Dried FCM Powders
2.5.2. Reconstitution Properties of Different FCM Freeze-Dried FCM Powders
2.5.3. Color Analysis
2.6. Survivability of Different Bacterial Strains in Simulated Gastrointestinal Conditions
2.7. Bio-Functionality of Probiotic-Camel Milk Supplemented with ADP
2.7.1. Antidiabetic Potential
2.7.2. Anticancer Potential
2.8. Statistical Analysis
3. Results and Discussion
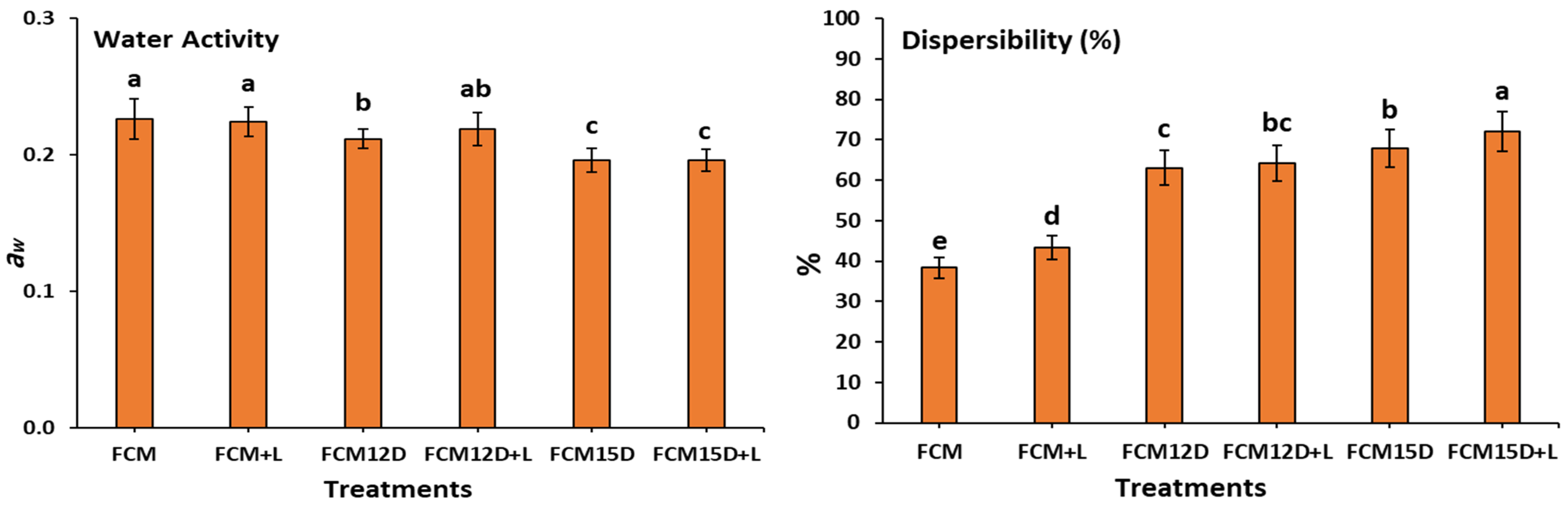
3.1. Functional Properties of Different FCM Powders
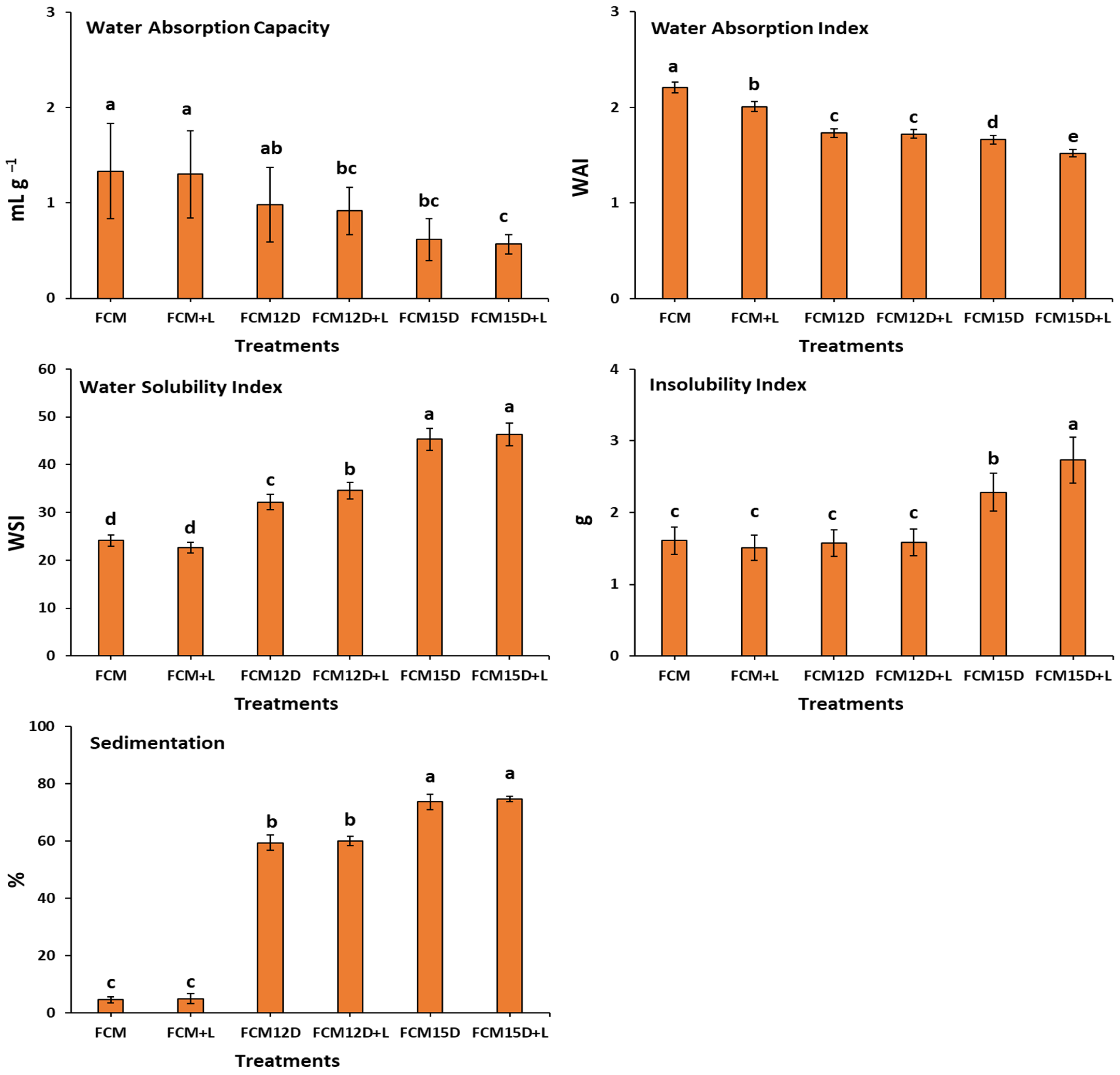
3.2. Reconstitution Properties of Different FCM Powders
3.3. Color Parameters of FCM
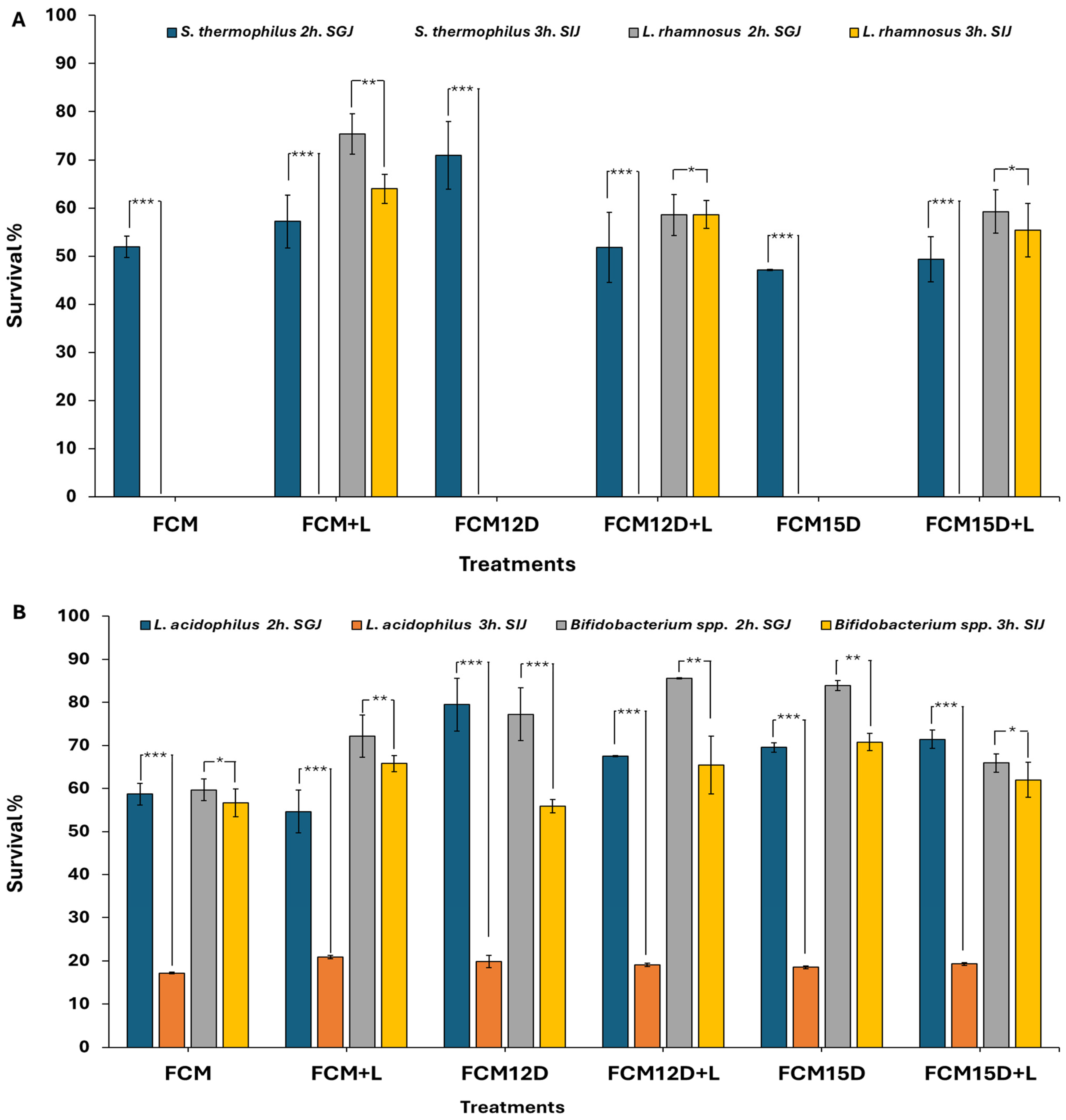
3.4. Survivability of Different Bacterial Strains in Simulated Gastrointestinal Environments
3.5. Antidiabetic and Anticancer Bio-Functionality of FCM
3.5.1. Antidiabetic Properties
3.5.2. Cytotoxicity Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, R. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Khosravi–Darani, K.; Jahadi, M.; Tripathi, A.D.; Nandan, A.; Paul, V.; Chakraborty, A.; Verma, T.; Agarwal, A. Probiotic dairy dessert from camel milk—A review. Indian J. Dairy Sci. 2023, 76, 203–215. [Google Scholar] [CrossRef]
- Mbye, M.; Sobti, B.; Al Nuami, M.K.; Al Shamsi, Y.; Al Khateri, L.; Al Saedi, R.; Saeed, M.; Ramachandran, T.; Hamed, F.; Kamal-Eldin, A. Physicochemical properties, sensory quality, and coagulation behavior of camel versus bovine milk soft unripened cheeses. NFS J. 2020, 20, 28–36. [Google Scholar] [CrossRef]
- Rakhmatulina, A.; Dikhanbayeva, F.; Tlevlessova, D.; Zagorska, J.; Aralbayev, N.; Majore, K.; Yessenova, A. Advancements in camel milk drying technology: A comprehensive review of methods, chemical composition, and nutritional preservation. Dairy 2024, 5, 360–371. [Google Scholar] [CrossRef]
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.S.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E.; et al. Nutritional, antimicrobial and medicinal properties of Camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef]
- Alharbi, Y.M.; Sakr, S.S.; Albarrak, S.M.; Almundarij, T.I.; Barakat, H.; Hassan, M.F.Y. Antioxidative, Antidiabetic, and Hypolipidemic Properties of Probiotic-Enriched Fermented Camel Milk Combined with Salvia officinalis Leaves Hydroalcoholic Extract in Streptozotocin-Induced Diabetes in Rats. Antioxidants 2022, 11, 668. [Google Scholar] [CrossRef]
- Homayouni-Tabrizi, M.; Asoodeh, A.; Soltani, M. Cytotoxic and antioxidant capacity of camel milk peptides: Effects of isolated peptide on superoxide dismutase and catalase gene expression. J. Food Drug Anal. 2017, 25, 567–575. [Google Scholar] [CrossRef]
- AlFaris, N.A.; AlTamim, J.Z.; AlMousa, L.A.; Albarid, N.A.; AlGhamidi, F.A. Nutritional values, Nutraceutical properties, and health benefits of Arabian Date Palm fruit. Emir. J. Food Agric. 2023, 6, 488–510. [Google Scholar] [CrossRef]
- Anwar, S.; Raut, R.; Alsahli, M.A.; Almatroudi, A.; Alfheeaid, H.; Alzahrani, F.M.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Role of Ajwa date fruit pulp and seed in the management of diseases through in vitro and in silico analysis. Biology 2022, 11, 78. [Google Scholar] [CrossRef]
- Khalid, S.; Khalid, N.; Khan, R.S.; Ahmed, H.; Ahmad, A. A review on chemistry and pharmacology of Ajwa date fruit and pit. Trends Food Sci. Technol. 2017, 63, 60–69. [Google Scholar] [CrossRef]
- Zihad, S.N.K.; Uddin, S.J.; Sifat, N.; Lovely, F.; Rouf, R.; Shilpi, J.A.; Sheikh, B.Y.; Göransson, U. Antioxidant properties and phenolic profiling by UPLC-QTOF-MS of Ajwah, Safawy and Sukkari cultivars of date palm. Biochem. Biophys. Rep. 2021, 25, 100909. [Google Scholar] [CrossRef] [PubMed]
- Shahein, M.R.; Atwaa, E.S.H.; Elkot, W.F.; Hijazy, H.H.A.; Kassab, R.B.; Alblihed, M.A.; Elmahallawy, E.K. The impact of date syrup on the physicochemical, microbiological, and sensory properties, and antioxidant activity of bio-fermented camel milk. Fermentation 2022, 8, 192. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Sakadani, E.; Kandylis, P. Enhancing Probiotic Viability in Yogurt: The Role of Apple Fibers in Supporting Lacticaseibacillus casei ATCC 393 During Storage and Gastrointestinal Transit. Foods 2025, 14, 376. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.; Ibrahim, H.I.M.; Sheikh, A.; Khalil, H.E. Probiotic-Fermented Camel Milk Attenuates Neurodegenerative Symptoms via SOX5/miR-218 Axis Orchestration in Mouse Models. Pharmaceuticals 2023, 16, 357. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Samakkhah, S.A.; Mirzakhani, E. An overview of probiotic camel milk as a nutritional beverage: Challenges and perspectives. Food Sci. Nutr. 2024, 12, 6123–6141. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Z.; Shen, F.; Zhou, W.; Manaer, T.; Jiaerken, D.; Nabi, X. Exploring the immunomodulatory effects and mechanisms of Xinjiang fermented camel milk-derived bioactive peptides based on network pharmacology and molecular docking. Front. Pharmacol. 2023, 13, 1038812. [Google Scholar] [CrossRef]
- Nawaz, Z.; Zahoor, M.K.; Shafique, M.; Athar, R.; Yasmin, A.; Zahoor, M.A. In vitro assessment of probiotic properties of lactic acid bacteria isolated from camel milk: Enhancing sustainable foods. Front. Sustain. Food Syst. 2024, 8, 1437201. [Google Scholar] [CrossRef]
- Hamed, N.S.; Mbye, M.; Ayyash, M.; Ulusoy, B.H.; Kamal-Eldin, A. Camel Milk: Antimicrobial Agents, Fermented Products, and Shelf Life. Foods 2024, 13, 381. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb. Cell Factories 2014, 13, S7. [Google Scholar] [CrossRef] [PubMed]
- Subrota Hati, D.S. Fermented camel milk: A Review on its bio-functional properties. Emir. J. Food Agric. 2018, 30, 268–274. [Google Scholar] [CrossRef]
- Alhaj, O.A. Identification of potential ACE-inhibitory peptides from dromedary fermented camel milk. CyTA J. Food 2016, 15, 191–195. [Google Scholar] [CrossRef]
- Ayyash, M.; Abdalla, A.; Alhammadi, A.; Ranadheera, S.; Baig, M.A.; Al-Ramadi, B.; Chen, G.; Kamal-Eldin, A.; Huppertz, T. Probiotic survival, biological functionality and untargeted metabolomics of the bioaccessible compounds in fermented camel and bovine milk after in vitro digestion. Food Chem. 2021, 363, 130243. [Google Scholar] [CrossRef] [PubMed]
- Boontun, C.; Vatanyoopaisarn, S.; Phalakornkule, C.; Domrongpokkaphan, V.; Thitisak, P.; Thaveetheptaikul, P.; Bamrungchue, N. Influence of protectant for encapsulation by freeze-drying and spray-drying techniques, and packaging environments on the stability of the probiotic Bifidobacterium animalis subsp. lactis strain KMP-H9-01 during storage. Dry. Technol. 2024, 42, 762–774. [Google Scholar] [CrossRef]
- Gao, X.; Kong, J.; Zhu, H.; Mao, B.; Cui, S.; Zhao, J. Lactobacillus, Bifidobacterium and Lactococcus response to environmental stress: Mechanisms and application of cross-protection to improve resistance against freeze-drying. J. Appl. Microbiol. 2022, 132, 802–821. [Google Scholar] [CrossRef]
- Keivani, F.; Mokarram, R.; Benis, K.; Gholian, M.; Zendeboodi, F.; Zadeh, S. External and internal factors affecting survival of probiotic living cells during desiccation. Int. J. Pharm. 2014, 3, 309–316. [Google Scholar]
- Sakr, S.; Barakat, H. Biofunctional and Nutritional Characteristics of Freeze-Dried Fermented Camel Milk Fortified with Probiotics and Ajwa Dates. Int. J. Dairy Sci. 2025, 20, 20–31. [Google Scholar] [CrossRef]
- El-Sayed, M.I.; Awad, S.; Abou-Soliman, N.H.I. Improving the antioxidant properties of fermented camel milk using some strains of Lactobacillus. Food Nutr. Sci. 2021, 12, 352. [Google Scholar]
- Malik, M.; Sharma, A. Physicochemical and functional properties of yoghurt powder. Int. J. Chem. Stud. 2021, 9, 298–303. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Singh, A.K.; Kumar, D.; Sharma, H. Effect of spray and freeze drying on physico-chemical, functional, moisture sorption and morphological characteristics of camel milk powder. LWT 2020, 134, 110117. [Google Scholar] [CrossRef]
- Schuck, P.; Jeantet, R.; Dolivet, A. Analytical Methods for Food and Dairy Powders; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Reddy, R.S.; Ramachandra, C.; Hiregoudar, S.; Nidoni, U.; Ram, J.; Kammar, M. Influence of processing conditions on functional and reconstitution properties of milk powder made from Osmanabadi goat milk by spray drying. Small Rumin. Res. 2014, 119, 130–137. [Google Scholar] [CrossRef]
- Meena, G.S.; Singh, A.K.; Arora, S.; Borad, S.; Sharma, R.; Gupta, V.K. Physico-chemical, functional and rheological properties of milk protein concentrate 60 as affected by disodium phosphate addition, diafiltration and homogenization. J. Food Sci. Technol. 2017, 54, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Afolayan, A.J.; Muchenje, V. Phytochemical Constituents and Antioxidant Activity of Sweet Basil (Ocimum basilicum L.) Essential Oil on Ground Beef from Boran and Nguni Cattle. Int. J. Food Sci. 2019, 2019, 2628747. [Google Scholar] [CrossRef] [PubMed]
- Aljutaily, T.; Barakat, H.; Moustafa, M.M.; Rehan, M. Incorporation of Sukkari Date in Probiotic-Enriched Fermented Camel Milk Improves the Nutritional, Physicochemical, and Organoleptical Characteristics. Fermentation 2021, 8, 5. [Google Scholar] [CrossRef]
- Buahom, J.; Siripornadulsil, S.; Sukon, P.; Sooksawat, T.; Siripornadulsil, W. Survivability of freeze-and spray-dried probiotics and their effects on the growth and health performance of broilers. Vet. World 2023, 16, 1849. [Google Scholar] [CrossRef]
- Udo, T.; Mummaleti, G.; Qin, Z.; Chen, J.; Singh, R.K.; Jiao, Y.; Kong, F. Survival of Lactobacillus rhamnosus GG in Chitosan-Coated Alginate Beads: Effects of Food Matrices (Casein, Corn Starch, and Soybean Oil) and Dynamic Gastrointestinal Conditions. Foods 2025, 14, 2094. [Google Scholar] [CrossRef]
- Tharmaraj, N.; Shah, N.P. Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and propionibacteria. J. Dairy Sci. 2003, 86, 2288–2296. [Google Scholar] [CrossRef]
- Shi, T.; Nishiyama, K.; Nakamata, K.; Aryantini, N.P.D.; Mikumo, D.; Oda, Y.; Yamamoto, Y.; Mukai, T.; Sujaya, I.N.; Urashima, T. Isolation of potential probiotic Lactobacillus rhamnosus strains from traditional fermented mare milk produced in Sumbawa Island of Indonesia. Biosci. Biotechnol. Biochem. 2012, 76, 1897–1903. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.; Chuah, P. The effects of fish collagen on the proteolysis of milk proteins, ACE inhibitory activity and sensory evaluation of plain-and Allium sativum-yogurt. J. Taiwan Inst. Chem. Eng. 2013, 44, 701–706. [Google Scholar] [CrossRef]
- Wickramaratne, M.N.; Punchihewa, J.C.; Wickramaratne, D.B. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef] [PubMed]
- Pistia-Brueggeman, G.; Hollingsworth, R.I. A preparation and screening strategy for glycosidase inhibitors. Tetrahedron 2001, 57, 8773–8778. [Google Scholar] [CrossRef]
- Sharma, A.; Lavania, M.; Singh, R.; Lal, B. Identification and probiotic potential of lactic acid bacteria from camel milk. Saudi J. Biol. Sci. 2021, 28, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Steel, R. Analysis of Variance I: The One-Way Classification; McGraw-Hill: New York, NY, USA, 1997; pp. 139–203. [Google Scholar]
- How, Y.H.; Teo, M.Y.M.; In, L.L.A.; Yeo, S.K.; Bhandari, B.; Pui, L.P. Powder characteristics, moisture sorption isotherm, and shelf-life prediction of freeze-dried recombinant Lactococcus lactis NZ3900-fermented milk powder. Dry. Technol. 2023, 41, 2501–2515. [Google Scholar] [CrossRef]
- Türker, D.A.; Saraç, M.G.; Doğan, M. Influence of particle size on powder flow behaviour, textural and viscoelastic properties of milk-based whippable emulsions. Int. Dairy J. 2024, 148, 105806. [Google Scholar] [CrossRef]
- Seth, D.; Mishra, H.N.; Deka, S.C. Functional and reconstitution properties of spray-dried sweetened yogurt powder as influenced by processing conditions. Int. J. Food Prop. 2016, 20, 1603–1611. [Google Scholar] [CrossRef]
- Ismail, E.A.; Aly, A.A.; Atallah, A.A. Quality and microstructure of freeze-dried yoghurt fortified with additives as protective agents. Heliyon 2020, 6, e05196. [Google Scholar] [CrossRef]
- Tontul, İ.; Ergin, F.; Eroğlu, E.; Küçükçetin, A.; Topuz, A. Physical and microbiological properties of yoghurt powder produced by refractance window drying. Int. Dairy J. 2018, 85, 169–176. [Google Scholar] [CrossRef]
- Hardy, Z.; Jideani, V.A. Functional characteristics and microbiological viability of foam-mat dried Bambara groundnut (Vigna subterranea) yogurt from reconstituted Bambara groundnut milk powder. Food Sci. Nutr. 2020, 8, 5238–5248. [Google Scholar] [CrossRef]
- Tunick, M.H.; Onwulata, C.I. Rheological Properties of Extruded Milk Powders. Int. J. Food Prop. 2006, 9, 835–844. [Google Scholar] [CrossRef]
- Suhag, R.; Kellil, A.; Razem, M. Factors influencing food powder flowability. Powders 2024, 3, 65–76. [Google Scholar] [CrossRef]
- Hazlett, R.; Schmidmeier, C.; O’Mahony, J.A. Approaches for improving the flowability of high-protein dairy powders post spray drying—A review. Powder Technol. 2021, 388, 26–40. [Google Scholar] [CrossRef]
- Arain, M.A.; Salman, H.M.; Ali, M.; Khaskheli, G.B.; Barham, G.S.; Marghazani, I.B.; Ahmed, S. A review on camel milk composition, techno-functional properties and processing constraints. Food Sci. Anim. Res. 2024, 44, 739. [Google Scholar] [CrossRef]
- Fang, Y.; Selomulya, C.; Chen, X.D. On measurement of food powder reconstitution properties. Dry. Technol. 2007, 26, 3–14. [Google Scholar] [CrossRef]
- Hachani, S.; Hamia, C.; Boukhalkhal, S.; Silva, A.M.; Djeridane, A.; Yousfi, M. Morphological, physico-chemical characteristics and effects of extraction solvents on UHPLC-DAD-ESI-MSn profiling of phenolic contents and antioxidant activities of five date cultivars (Phoenix dactylifera L.) growing in Algeria. NFS J. 2018, 13, 10–22. [Google Scholar] [CrossRef]
- Gross, J.; Haber, O.; Ikan, R. The carotenoid pigments of the date. Sci. Hortic. 1983, 20, 251–257. [Google Scholar] [CrossRef]
- Serratosa, M.P.; Lopez-Toledano, A.; Merida, J.; Medina, M. Changes in color and phenolic compounds during the raisining of grape cv. Pedro Ximenez. J. Agric. Food Chem. 2008, 56, 2810–2816. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Schwember, A.R. Phenolic-driven sensory changes in functional foods. J. Food Bioact. 2019, 5, 6–7. [Google Scholar] [CrossRef]
- Szopa, K.; Szajnar, K.; Pawlos, M.; Znamirowska-Piotrowska, A. Probiotic Fermented Goat’s and Sheep’s Milk: Effect of Type and Dose of Collagen on Survival of Four Strains of Probiotic Bacteria during Simulated In Vitro Digestion Conditions. Nutrients 2023, 15, 3241. [Google Scholar] [CrossRef]
- de Oliveira, I.K.C.P.; Oliveira, M.M.; Oliveira, A.T.; da Silva, G.M.; de Oliveira, T.A.; dos Santos, K.M.O.; do Egito, A.S.; Buriti, F.C.A. Fermentative behavior of native lactobacilli in goat milk and their survival under in vitro simulated gastrointestinal conditions. LWT 2021, 135, 109905. [Google Scholar] [CrossRef]
- Moumita, S.; Goderska, K.; Johnson, E.M.; Das, B.; Indira, D.; Yadav, R.; Kumari, S.; Jayabalan, R. Evaluation of the viability of free and encapsulated lactic acid bacteria using in-vitro gastro intestinal model and survivability studies of synbiotic microcapsules in dry food matrix during storage. LWT 2017, 77, 460–467. [Google Scholar] [CrossRef]
- Varga, L.; Süle, J.; Nagy, P. Survival of the characteristic microbiota in probiotic fermented camel, cow, goat, and sheep milks during refrigerated storage. J. Dairy Sci. 2014, 97, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhong, Q. Drying of probiotics to enhance the viability during preparation, storage, food application, and digestion: A review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13287. [Google Scholar] [CrossRef]
- Devarajan, A.; Mudgil, P.; Aldhaheri, F.; Hamed, F.; Dhital, S.; Maqsood, S. Camel milk-derived probiotic strains encapsulated in camel casein and gelatin complex microcapsules: Stability against thermal challenge and simulated gastrointestinal digestion conditions. J. Dairy Sci. 2022, 105, 1862–1877. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, I.; Ben Moussa, O.; Boulares, M.; Chouaibi, M.; Hassouna, M. Novel probiotic camel milk yoghurt supplemented with inulin: Antibacterial, antioxidant and antidiabetic effects. Mljekarstvo Časopis Unaprjeđenje Proizv. Prerade Mlijeka 2022, 72, 201–212. [Google Scholar] [CrossRef]
- Kajaria, D.; Ranjana; Tripathi, J.; Tripathi, Y.B.; Tiwari, S. In-vitro α amylase and glycosidase inhibitory effect of ethanolic extract of antiasthmatic drug—Shirishadi. J. Adv. Pharm. Technol. Res. 2013, 4, 206–209. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Nuaimi, A.K.; Al-Mahadin, S.; Liu, S.-Q. In vitro investigation of anticancer and ACE-inhibiting activity, α-amylase and α-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. Food Chem. 2018, 239, 588–597. [Google Scholar] [CrossRef]
- Khakhariya, R.; Basaiawmoit, B.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Padhi, S.; Rai, A.K.; Liu, Z.; Hati, S. Production and Characterization of ACE Inhibitory and Anti-Diabetic Peptides from Buffalo and Camel Milk Fermented with Lactobacillus and Yeast: A Comparative Analysis with In Vitro, In Silico, and Molecular Interaction Study. Foods 2023, 12, 2006. [Google Scholar] [CrossRef]
- Mudgil, P.; Al Dhaheri, M.K.O.; Alsubousi, M.S.M.; Khan, H.; Redha, A.A.; Yap, P.-G.; Gan, C.-Y.; Maqsood, S. Molecular docking studies on α-amylase inhibitory peptides from milk of different farm animals. J. Dairy Sci. 2024, 107, 2633–2652. [Google Scholar] [CrossRef]
- Yusuf, D.; Nuraida, L.; Dewanti-Hariyadi, R.; Hunaefi, D. In vitro Antioxidant and α-Glucosidase Inhibitory Activities of Lactobacillus spp. Isolated from Indonesian Kefir Grains. Appl. Food Biotechnol. 2020, 8, 39–46. [Google Scholar] [CrossRef]
- Huligere, S.S.; Chandana Kumari, V.B.; Alqadi, T.; Kumar, S.; Cull, C.A.; Amachawadi, R.G.; Ramu, R. Isolation and characterization of lactic acid bacteria with potential probiotic activity and further investigation of their activity by α-amylase and α-glucosidase inhibitions of fermented batters. Front. Microbiol. 2023, 13, 1042263. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-B.; Yu, L.-Y.; Zeng, X.; Zheng, J.-W.; Wang, B.; Pan, L. Screening of probiotics with efficient α-glucosidase inhibitory ability and study on the structure and function of its extracellular polysaccharide. Food Biosci. 2022, 45, 101452. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Dhaheri, A.S.; Al Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. J. Dairy Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Çakmakoğlu, S.K.; Dere, S.; Bekiroğlu, H.; Bozkurt, F.; Karasu, S.; Dertli, E.; Türker, M.; Sagdic, O. Production of bioactive peptides during yogurt fermentation, their extraction and functional characterization. Food Biosci. 2024, 61, 104805. [Google Scholar] [CrossRef]
- Castañeda-Pérez, E.; Jiménez-Morales, K.; Quintal-Novelo, C.; Moo-Puc, R.; Chel-Guerrero, L.; Betancur-Ancona, D. Enzymatic protein hydrolysates and ultrafiltered peptide fractions from Cowpea Vigna unguiculata L bean with in vitro antidiabetic potential. J. Iran. Chem. Soc. 2019, 16, 1773–1781. [Google Scholar] [CrossRef]
- Siow, H.-L.; Gan, C.-Y. Extraction, identification, and structure–activity relationship of antioxidative and α-amylase inhibitory peptides from cumin seeds (Cuminum cyminum). J. Funct. Foods 2016, 22, 1–12. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.; Gaspar, A.R. Structural properties of bioactive peptides with α-glucosidase inhibitory activity. Chem. Biol. Drug Des. 2018, 91, 370–379. [Google Scholar] [CrossRef]
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- Elkashef, H.; Awad, A.A.; Hassan, A.A.M. Development of Functional Dairy Byproducts-based Beverages Using Rutab Date and Probiotic Apilactobacillus Kunkeei EABW06. Probiotics Antimicrob. Proteins 2025, 1–16. [Google Scholar] [CrossRef]
- Begunova, A.V.; Savinova, O.S.; Glazunova, O.A.; Moiseenko, K.V.; Rozhkova, I.V.; Fedorova, T.V. Development of Antioxidant and Antihypertensive Properties during Growth of Lactobacillus helveticus, Lactobacillus rhamnosus and Lactobacillus reuteri on Cow’s Milk: Fermentation and Peptidomics Study. Foods 2021, 10, 17. [Google Scholar] [CrossRef]
- Moslehishad, M.; Ehsani, M.R.; Salami, M.; Mirdamadi, S.; Ezzatpanah, H.; Naslaji, A.N.; Moosavi-Movahedi, A.A. The comparative assessment of ACE-inhibitory and antioxidant activities of peptide fractions obtained from fermented camel and bovine milk by Lactobacillus rhamnosus PTCC 1637. Int. Dairy J. 2013, 29, 82–87. [Google Scholar] [CrossRef]
- Shirkhan, F.; Mirdamadi, S.; Mirzaei, M.; Akbari-adergani, B.; Nasoohi, N. The role of lactic acid bacteria in production of bioactive peptides in fermented milk with antioxidant and antidiabetic properties. J. Food Meas. Charact. 2023, 17, 4727–4738. [Google Scholar] [CrossRef]
- Anwar, I.; Khan, F.B.; Baby, B.; Antony, P.; Mudgil, P.; Gan, C.-Y.; Maqsood, S.; Vijayan, R.; Muhammad, K.; Ayoub, M.A. Functional profiling of synthetic camel milk-derived peptides with implication in glucose transport and diabetes. PLoS ONE 2025, 20, e0320812. [Google Scholar] [CrossRef]
- Ramezan, M.; Arzhang, P.; Shin, A.C. Milk-derived bioactive peptides in insulin resistance and type 2 diabetes. J. Nutr. Biochem. 2025, 138, 109849. [Google Scholar] [CrossRef]
- Rosa, L.S.; Santos, M.L.; Abreu, J.P.; Balthazar, C.F.; Rocha, R.S.; Silva, H.L.A.; Esmerino, E.A.; Duarte, M.C.K.H.; Pimentel, T.C.; Freitas, M.Q.; et al. Antiproliferative and apoptotic effects of probiotic whey dairy beverages in human prostate cell lines. Food Res. Int. 2020, 137, 109450. [Google Scholar] [CrossRef]
- Dharmisthaben, P.; Basaiawmoit, B.; Sakure, A.; Das, S.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Hati, S. Exploring potentials of antioxidative, anti-inflammatory activities and production of bioactive peptides in lactic fermented camel milk. Food Biosci. 2021, 44, 101404. [Google Scholar] [CrossRef]
- Solanki, D.; Hati, S. Considering the potential of Lactobacillus rhamnosus for producing Angiotensin I-Converting Enzyme (ACE) inhibitory peptides in fermented camel milk (Indian breed). Food Biosci. 2018, 23, 16–22. [Google Scholar] [CrossRef]
- Bidram, M.; Ganjalikhany, M.R. Bioactive peptides from food science to pharmaceutical industries: Their mechanism of action, potential role in cancer treatment and available resources. Heliyon 2024, 10, e40563. [Google Scholar] [CrossRef]
- Hamdi, M.; Kilari, B.P.; Mudgil, P.; Nirmal, N.P.; Ojha, S.; Ayoub, M.A.; Amin, A.; Maqsood, S. Bioactive peptides with potential anticancer properties from various food protein sources: Status of recent research, production technologies, and developments. Crit. Rev. Biotechnol. 2025, 45, 1076–1097. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Zhang, W.; Li, X. Bioactive peptides for anticancer therapies. Biomater. Transl. 2023, 4, 5–17. [Google Scholar] [CrossRef]
- Ghadiri, N.; Javidan, M.; Sheikhi, S.; Taştan, Ö.; Parodi, A.; Liao, Z.; Tayybi Azar, M.; Ganjalıkhani-Hakemi, M. Bioactive peptides: An alternative therapeutic approach for cancer management. Front. Immunol. 2024, 15, 1310443. [Google Scholar] [CrossRef] [PubMed]
- Paull, K.D.; Shoemaker, R.H.; Hodes, L.; Monks, A.; Scudiero, D.A.; Rubinstein, L.; Plowman, J.; Boyd, M.R. Display and Analysis of Patterns of Differential Activity of Drugs Against Human Tumor Cell Lines: Development of Mean Graph and COMPARE Algorithm. JNCI J. Natl. Cancer Inst. 1989, 81, 1088–1092. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
| Properties | FCM Incorporating ADP ** | |||||
|---|---|---|---|---|---|---|
| FCM | FCM+L | FCM12D | FCM12D+L | FCM15D | FCM15D+L | |
| Density properties (g mL−1) | ||||||
| Loose bulk density (LBD) | 0.22 ± 0.00 d | 0.24 ± 0.01 c | 0.32 ± 0.01 b | 0.32 ± 0.01 b | 0.36 ± 0.01 a | 0.35 ± 0.01 a |
| Packed bulk density (PBD) | 1.24 ± 0.02 b | 1.26 ± 0.02 b | 1.41 ± 0.02 a | 1.41 ± 0.02 a | 1.47 ± 0.02 a | 1.44 ± 0.02 a |
| Particle density (PD) | 3.03 ± 0.06 a | 2.36 ± 0.05 b | 2.17 ± 0.05 c | 1.75 ± 0.04 d | 1.61 ± 0.03 e | 1.64 ± 0.03 d,e |
| Density of powder solids | 1.31 ± 0.01 b | 1.31 ± 0.01 b | 1.36 ± 0.01 a | 1.36 ± 0.01 a | 1.38 ± 0.01 a | 1.38 ± 0.01 a |
| Flowability | ||||||
| Carrier index (CI%) | 82.13 ± 0.66 a | 80.63 ± 0.72 a | 77.24 ± 0.84 b | 77.33 ± 0.84 b | 75.17 ± 0.51 b | 75.50 ± 0.50 b |
| Hausner ratio (HR) | 5.61 ± 0.21 a | 5.18 ± 0.19 a | 4.41 ± 0.16 b | 4.42 ± 0.17 b | 4.03 ± 0.08 b | 4.09 ± 0.08 b |
| Air content (mL 100 g−1 powder) | ||||||
| Interstitial air content | 47.62 ± 1.76 a | 37.12 ± 1.92 b | 24.84 ± 1.88 c | 14.05 ± 2.10 d | 5.88 ± 0.720 e | 8.13 ± 0.68 e |
| Occluded air content | 31.78 ± 0.70 e | 41.15 ± 0.90 d | 44.77 ± 0.98 c | 55.76 ± 1.21 b | 60.92 ± 1.32 a | 59.74 ± 1.30 a |
| Porosity (%) | 58.97 ± 1.38 a | 46.60 ± 1.80 b | 34.94 ± 2.19 c | 19.68 ± 2.70 d | 8.63 ± 1.080 e | 11.75 ± 1.02 e |
| Treatments # | Instrumental Color Parameters | ||||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | C | H° | BI | ∆E | |
| FCM | 87.94 ± 0.04 a | −2.53 ± 0.10 b | 1.61 ± 0.08 c | 3.16 ± 0.17 c | 147.45 ± 0.26 a | −0.43 ± 0.19 e | 0 ± 0 e |
| FCM+L | 88.16 ± 0.09 a | −2.71 ± 0.19 b | 3.34 ± 0.16 b | 4.19 ± 0.14 b | 128.94 ± 0.68 b | 1.67 ± 0.20 d | 1.88 ± 0.18 d |
| FCM12D | 69.52 ± 0.09 b,c | −0.69 ± 0.21 a | 16.45 ± 0.31 a | 16.47 ± 0.32 a | 92.34 ± 0.69 c | 25.60 ± 0.34 b,c | 23.75 ± 0.17 b,c |
| FCM12D+L | 70.50 ± 0.18 b | −0.55 ± 0.13 a | 16.38 ± 0.15 a | 16.39 ± 0.15 a | 91.89 ± 0.45 c | 25.20 ± 0.38 c | 22.96 ± 0.22 c |
| FCM15D | 68.36 ± 1.23 c,d | −0.54 ± 0.16 a | 17.10 ± 0.17 a | 17.11 ± 0.16 a | 88.21 ± 0.57 d | 27.51 ± 0.95 a,b | 25.08 ± 1.02 a,b |
| FCM15D+L | 67.61 ± 0.53 d | −0.46 ± 0.05 a | 17.14 ± 0.52 a | 17.15 ± 0.52 a | 88.50 ± 0.14 d | 28.04 ± 1.21 a | 25.68 ± 0.73 a |
| Treatments | IC50 (μg mL−1) | ||
|---|---|---|---|
| α-Amylase | α-Glucosidase | Anticancer Activity | |
| FCM | 8.37 ± 0.003 e | 10.74 ± 0.01 e | 82.22 ± 0.3 f |
| FCM+L | 40.47 ± 1.17 c | 27.90 ± 1.1 c | 85.19 ± 0.72 e |
| FCM12D | 14.83 ± 0.01 d | 17.49 ± 1.01 d | 94.89 ± 0.53 d |
| FCM12D+L | 47.80 ± 1.12 b | 47.36 ± 2.21 b | 293.24 ± 8.71 b |
| FCM15D | 42.31 ± 1.31 c | 46.44 ± 0.75 b | 275.51 ± 1.1 c |
| FCM15D+L | 111.43 ± 2.10 a | 77.21 ± 1.21 a | 312.95 ± 1.4 a |
| Acarbose | 3.12 ± 0.03 f | 7.52 ± 0.01 f | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakr, S.S.; Barakat, H. Freeze-Dried Probiotic Fermented Camel Milk Enriched with Ajwa Date Pulp: Evaluation of Functional Properties, Probiotic Viability, and In Vitro Antidiabetic and Anticancer Activities. Foods 2025, 14, 2698. https://doi.org/10.3390/foods14152698
Sakr SS, Barakat H. Freeze-Dried Probiotic Fermented Camel Milk Enriched with Ajwa Date Pulp: Evaluation of Functional Properties, Probiotic Viability, and In Vitro Antidiabetic and Anticancer Activities. Foods. 2025; 14(15):2698. https://doi.org/10.3390/foods14152698
Chicago/Turabian StyleSakr, Sally S., and Hassan Barakat. 2025. "Freeze-Dried Probiotic Fermented Camel Milk Enriched with Ajwa Date Pulp: Evaluation of Functional Properties, Probiotic Viability, and In Vitro Antidiabetic and Anticancer Activities" Foods 14, no. 15: 2698. https://doi.org/10.3390/foods14152698
APA StyleSakr, S. S., & Barakat, H. (2025). Freeze-Dried Probiotic Fermented Camel Milk Enriched with Ajwa Date Pulp: Evaluation of Functional Properties, Probiotic Viability, and In Vitro Antidiabetic and Anticancer Activities. Foods, 14(15), 2698. https://doi.org/10.3390/foods14152698







