Abstract
Acetate may act as a signaling molecule, regulating Paracin 1.7 production via quorum sensing (QS) in Lacticaseibacillus paracasei HD1.7. The “acetate switch” phenomenon requires mechanistic exploration to optimize Paracin 1.7 production. The “acetate switch” phenomenon delays with higher glucose levels (30 h, 36 h, and 96 h). Before the occurrence of the “acetate switch”, the ATP content increases and peaks at the “acetate switch” point and the NAD+/NADH ratio decreases, indicating energy changes. Moreover, the QS genes used for the pre-regulation of bacteriocin, such as prcKR, comCDE, were highly expressed. After the “acetate switch”, the ATP content decreased and the QS genes for the post-regulation of bacteriocin were highly expressed, such as rggs234 and sigma70-1/70-2. The “acetate switch” could act as an energy switch, regulating bacterial growth and QS genes. Before and after the “acetate switch”, some metabolic pathways were significantly altered according to the transcriptomic analysis by HD1.7 and HD1.7-Δpta. In this study, acetate was used as an input signal to regulate the two-component system, significantly influencing the bacteriocin expression system. And this study clarifies the roles of acetate, energy, and quorum sensing in promoting Paracin 1.7 production, providing a theoretical basis for optimizing the bacteriocin fermentation process of HD1.7.
1. Introduction
Quorum sensing (QS) is an intercellular communication mechanism among bacteria mediated by quorum signaling molecules (QSM). QS represents a cell-to-cell communication mechanism in bacteria, mediated through the synthesis, detection, and response to extracellular signaling molecules. This system enables bacterial populations to synchronize collective behaviors based on shifts in population density and vicinal community composition [1]. The phenomenon of QS was first discovered in the 1970s through studies of bioluminescence in Vibrio fischeri [2]. This groundbreaking finding revealed that bacteria engage in chemical communication by secreting signaling molecules into their environment. When the concentration of these signaling molecules reaches a critical threshold, bacteria can sense their population density and subsequently activate the expression of specific genes, thereby coordinating collective behaviors. Since this initial discovery, research on QS has advanced rapidly. It has now been conclusively demonstrated that QS plays pivotal roles in diverse biological processes, including antibiotic production, biofilm formation, virulence factor synthesis, and bioluminescence regulation [3]. The widely used QSM include autoinducing peptides, N-acyl-homoserine lactones (AHLs), and autoinducer-2 (AI-2) [4,5,6]. In 2003, Wolfe et al. demonstrated that acetyl phosphate, an intermediate of acetate metabolism, was involved in Escherichia coli biofilm formation as a global signal molecule [7]. Previous studies on Lacticaseibacillus paracasei HD1.7 reported a high expression of QS-related genes (QSRGs) prcK, prcR, comR, and sigma upon adding exogenous acetate. Also, the production of bacteriocin Paracin1.7 was improved. At the same time, some metabolic pathways such as histidine metabolism, pyrimidine metabolism, and amino acid biosynthesis were disturbed [8,9,10,11]. These results suggested that acetate and its related metabolites might act as signaling molecules involved in QS.
Acetate content is often under the “on-off” status due to carbon metabolism and energy variation in the cell. That is, the rapid growth of cells under aerobic conditions leads to the production of organic acids via overflow metabolism in bacteria, fungi, and higher organisms, such as acetate overflow in bacteria [12], Crabtree effect in yeast [13,14], and Warburg effect in mammals [15]. This is termed as “acetate on”. Among these processes, bacterial acetate overflow metabolism is considered to be an independent pathway beneficial to bacteria on the saturation of the enzymes in glycolysis and Krebs cycle. It can provide the cell with a potent tool to profit from ecological stress in population competition [16].
Acetyl-CoA can be oxidized to acetate with phosphotransacetylase and acetate kinase. High concentrations of acetate also serve as a back-up carbon source for conversion into acetyl-CoA, a process that helps to slow human aging [17,18]. Such a decrease in acetate concentration is “acetate off” [19]. During the conversion between acetate and acetyl-CoA, many intermediates become the basis for intracellular matter cycling and energy variation owing to the presence of acetyl and phosphate groups [20].
QS depends on cell density (matter cycle) and ATP consumption (energy flow), and the “acetate switch” is related to not only acetate content but also energy conversion in the cell. Hence, finding the cellular “acetate switch” points implies unlocking the secret of QS regulated by acetate overflow and energy metabolism.
Previous studies have shown that exogenously added acetate could regulate QS; however, the role of intracellular acetate remains unclear. The objectives of this study are as follows: to analyze the regulatory rules of glucose concentration on the “acetate switch” point in HD1.7, clarify the dynamic associations between acetate, energy metabolism, and quorum sensing before and after the “acetate switch”, and to provide a theoretical basis for optimizing the bioprocesses of bacteriocin production from the perspective of the “acetate switch”. Based on these objectives, the following aspects of exploration were conducted: (1) the effect of glucose concentration on the time point of the “acetate switch” of HD1.7; (2) the changes in growth, energy, and acetate metabolism of HD1.7 before and after the “acetate switch”, and the association between the “acetate switch” and QS; (3) the correlation between intracellular acetate content, bacterial physicochemical indicators, and acetate metabolism-related genes (AMRGs); and (4) the changes in differentially expressed genes and metabolic pathways in HD1.7 before and after the “acetate switch”. This study enriched our understanding of the role of the “acetate switch” mechanism in bacterial metabolic processes, defined the dual regulation of bacterial metabolism and community sensing by the “acetate switch”, and laid the foundation for further research on bacteriocins for promoting the industrialization of lactic acid bacteria.
2. Materials and Methods
2.1. Strain and Medium
HD1.7 (CCTCCM 205015) was provided by the Key Laboratory of Microbiology of Heilongjiang University (Harbin, China) [9,10,21]. The MRS medium required for the growth of HD1.7 included 10 g/L soy peptone, 0.2 g/L MgSO4·7H2O, 0.05 g/L MnSO4, 10 g/L beef extract, 5 g/L yeast extract, 20 g/L glucose, 2 g/L K2HPO4, 0.1 g/L Na2SO3, 5 g/L sodium acetate, 0.4 g/L ammonium citrate, and 1 mL Tween-80 (Polyoxyethylene sorbitan monooleate). MRS was adjusted to pH 5.5 and sterilized at 108 °C for 20 min. The reagents required for the above media were purchased from Beijing Auboxing Biotechnology, China. The influence of glucose concentration on the “acetate switch” points was investigated using 2, 5, and 20 g/L glucose in an MRS liquid medium. Three treatments were set up according to glucose concentration: Glu2 (2 g/L), Glu5 (5 g/L), and Glu20 (20 g/L), respectively.
2.2. Construction of the Pta-Deficient Strain of HD1.7
The acetate content of HD1.7 was reduced after “acetate switch”. Therefore, the pta-deficient strain was used in this study as a negative control. Both flanks of pta were amplified with primers EcoR I-pta-up-F, Xba I-pta-up-R, Xba I-pta-down-F, and Sal I-pta-down-R to harvest pta-up (500 bp) and pta-down (500 bp) fragments with polymerase chain reaction (PCR). A cloning vector pGEM-T-∆pta was constructed by ligating pre-digested pGEM-T, pta-up, and pta-down with EcoRI and XbaI. pKR6K is a conjugative plasmid, on which sacB can encode fructokinase to hydrolyze sucrose to fructan. When sacB is transferred to the recipient cell, it can cause cell death because fructan is toxic to the bacterium. The suicide plasmid pKR6K-∆pta was prepared after digesting pKR6K and pGEM-T-∆pta with EcoRI and SalI and then ligating with T4 ligase.
Further, 0.5 mL of HD1.7 and 1 mL of ZW01 (strain obtained by introducing pKR6K-Δpta into the recipient cell E. coli S17-1 λpir using heat shock) were centrifuged at 12,000 rpm for 2 min. The pellet was resuspended in 500 μL of 10 mM MgSO4, and the suspension was centrifuged again at 12,000 rpm for 2 min. The pellet thus obtained was resuspended in 20 μL of 10 mM MgSO4, dropped onto a solid MRS medium, and incubated at 37 °C for 24–48 h to facilitate conjugation between the donor and the receptor. The mixture was diluted 100-fold. Then, 100 μL of it was spread on an MRS solid medium containing 100 μg/mL Kan and further incubated for 16–20 h to finish homologous recombination. HD1.7-Δpta could grow due to the presence of Kan, whereas the strain without the introduced plasmid did not survive (Figure S1).
2.3. Fermentation Parameters of HD1.7
The seed cultures of HD1.7 were inoculated into an MRS medium at a concentration of 2% (v/v) at 140 rpm and 37 °C for a certain time. For Glu2 and Glu5, the samples were taken at 1 h intervals for 12 h, followed by 18, 24, 30, 36, 48, 60, and 72 h. For Glu20, the samples were taken at 2 h intervals for 12 h, followed by 18, 24, 30, 36, 48, 60, 72, 84, 96, 108, and 120 h [10].
The cells in the collected fermentation broth were centrifuged at 8000 rpm and room temperature for 10 min to harvest the cell pellet. And then cell disruption was performed using an ultrasonic processor (SCIENTZ-IID, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) at 200 W power with ice-bath cooling. The treatment consisted of 30 cycles of ultrasonication (3 s on/10 s off per cycle) to ensure complete cell lysis, thereby releasing intracellular components for the accurate analysis of membrane-barrier-isolated substances [22]. This procedure was repeated 30 times in an ice bath at 200 W. The intracellular pH was determined with an FE20 pH meter. High-performance liquid chromatography was employed to determine the acetate (intracellular and extracellular) and residual glucose contents [23,24,25]. The number of viable HD1.7 was measured by the plate colony counting method. The pyruvate content assay kit (Shanghai Jingkang Biotechnology Co., Ltd., Shanghai, China), coenzyme I NAD(H) content assay kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), and ATP content assay kit (Jiangsu Suke Biotechnology Co., Ltd., Nantong, China) were used to detect pyruvate content, NAD+/NADH ratio, and ATP content, respectively.
2.4. Determination of Gene Expression Levels Using qRT-PCR
RNA of HD1.7 was extracted using the total RNA extraction kit (Tiangen Biotech Co., Ltd., Tiangen, China), and its purity and concentration were determined using a Nanodrop 2000 spectrophotometer (Thermo Scientific Co., Ltd., Waltham, MA, USA). The first strand of cDNA was amplified by reverse transcription using the BioRT cDNA first-strand synthesis kit (TransGen Biotech Co., Ltd., Beijing, China). A real-time PCR system was established, including a cDNA template, primers, SYBR Mastermix (Vazyme Biotech Co., Ltd., Nanjing, China), and deionized water, to measure the expression of AMRGs. These AMRGs included acetate kinase genes (ackA1 and ackA2), pyruvate oxidase genes (poxB1, poxB2, and poxB3), and phosphotransacetylase gene (pta). QS-related genes were also detected. These included competence-stimulating peptide genes (comC, comD, comE, and comS), AI-2 synthase gene (luxS), sensor kinase gene (prcK), response regulator gene (prcR), Rgg-family regulator genes (rgg2, rgg3, and rgg4), sigma factor genes (sigma X, sigma70-1, and sigma70-2), and bacteriocin gene. The values normalized by 16s rDNA were used to quantify relative gene expression by the 2−ΔΔCT method [24]. The primer sequences used in the study are shown in Table S1.
2.5. Transcriptomic Analysis
The transcriptomic analysis was performed to verify the effect of the “acetate switch” on the gene expression and physiological activities of HD1.7 and HD1.7-Δpta. Glu5 was fermented for 8 h (named as a) and 42 h (named as b), whereas HD1.7-Δpta was cultured for 42 h (named as c). The three treatment samples were selected to perform transcriptomic analysis at Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The process was repeated three times for each sample. b and a were contrast before and after the “acetate switch”, whereas b and c had low acetate concentrations.
2.6. Statistical Analysis
The results were presented in the form of three parallel samples along with their standard deviations. Statistical and graphical analyses were conducted using Origin 2024 software (OriginLab Corp., Northampton, MA, USA). And the statistical significance was set as p < 0.05. All data were reported as means ± standard deviation (three biological replicates were set up) and analyzed by ANOVA (one-way ANOVA). OmicStudio online platform (https://www.omicstudio.cn/analysis, accessed on 23 December 2023), lingbo Microclass (http://cloud.biomicroclass.com/CloudPlatform/home, accessed on 25 December 2023), Oebiotech platform (https://cloud.oebiotech.com/#/home, accessed on 2 January 2024), and Bioinformatics platform (https://www.bioinformatics.com.cn/, accessed on 6 January 2024) were chosen to perform heat maps, gene-pathway enrichment network diagrams, volcano plots, correlation analysis, Venn diagrams, and WGCNA analysis. The false discovery rate (FDR) was used to determine the threshold for p values in multiple tests (Table S2). A threshold of FDR ≤ 0.05 and an absolute value of fold change (FC) ≥ 1.2 or FC ≤ 1/1.2 were used in the analysis as the criteria for determining the significance of differences in gene expression. Then, differentially expressed genes (DEGs) were subjected to the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. Structural equation modeling (SEM) was performed using IBM SPSS AMOS (version 23.0).
3. Results and Discussion
3.1. Determination of the “Acetate Switch” Points Under Different Concentrations of Glucose
The intracellular acetate content under all the three treatments showed a trend of increase and then decrease (Figure 1A), with an obvious “acetate switch” characteristic. Therefore, the inflection point of acetate content was identified as the “acetate switch” point [26]. This phenomenon was not uncommon, some halophilic archaea, such as Halococcus saccharolyticus and Haloferax volcanii, underwent the “acetate switch” during their growth on glucose [27].
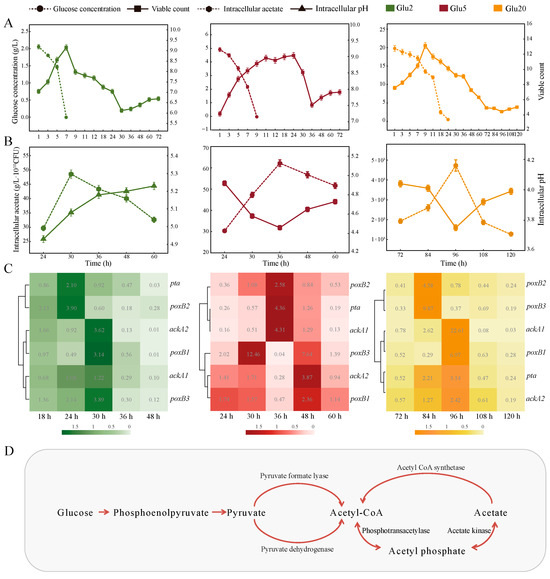
Figure 1.
Determination of the “acetate switch” points under different concentration of glucose. (A) Changes in intracellular acetate content as well as intracellular pH in the three treatments (Glu2, Glu5, Glu20) as time progressed; (B) Glucose consumption as well as bacterial growth in the three treatments as time progressed; (C) Heat map of the expression of AMRGs in the three treatments as time progressed; (D) Metabolic pathway map utilizing glucose and acetate.
The “acetate switch” points occurred at 30, 36, and 96 h, respectively, in the Glu2, Glu5, and Glu20 groups (Figure 1A). The intracellular acetate content in all three groups reached its peak at these time points, with Glu20 displaying higher acetate levels than Glu5 and Glu2, and Glu5 exhibiting higher levels than Glu2. The “acetate switch” in HD1.7 was delayed and the intracellular acetate content inducing this phenomenon was improved with the increase in exogenous glucose concentration (Figure 1A). HD1.7 metabolized glucose to acetate, which could be used as a backup carbon source for its growth and metabolic activities under stress (Figure 1D) [28]. In this study, the glucose concentration decreased over the fermentation period and it was completely consumed before the “acetate switch” points, which were 7, 9, and 24 h, respectively (Figure 1B).
The number of viable HD1.7 in Glu2, Glu5, and Glu20 increased and then decreased (up to a maximum of 1.43 × 109 ± 3.68 × 108, 1.08 × 109 ± 6.45 × 107, and 1.39 × 1013 ± 1.18 × 1012), showing an increase again in 30, 36, and 96 h (6.35 × 105 ± 3.81 × 104, 1.54 × 106 ± 9.24 × 104, and 2.75 × 104 ± 1.65 × 103), respectively. This secondary growth proved the backup carbon source role of acetate (Figure 1B). The intracellular pH also increased to 5.23 ± 0.02 (60 h in Glu2), 4.72 ± 0.03 (60 h in Glu5), and 3.99 ± 0.02 (120 h in Glu20) with the depletion of intracellular acetate (Figure 1A).
The expression of AMRGs could further explain the reason and genetic basis of “acetate switch” points (Figure 1C). Also, pta, ackA1, ackA2, poxB1, poxB2, and poxB3 in Glu2, Glu5, and Glu20 were more highly expressed before the “acetate switch”. They were highly expressed in the early stage of fermentation, reached the maximum expression level when the “acetate switch” occurred, and then decreased compared with the previous stage over time after the “acetate switch”. The fold change of these genes in Glu2 could be up to 5.24 ± 0.05, 5.17 ± 0.05, 28.38 ± 0.28, 5.44 ± 0.05, 9.66 ± 0.10, and 11.50 ± 0.11 (p < 0.05), respectively. pta had the largest fold change of 2.38 ± 0.05 in Glu5; a 100-fold change was found in ackA1 in Glu20 (p < 0.05).
3.2. Energy Variation Before and After “Acetate Switch” Points
Both the pyruvate content and NAD+/NADH ratio decreased (Figure 2A,B) before the “acetate switch” point, accompanied by ATP production in ATP, to 0.245 ± 0.003, 0.384 ± 0.01, and 0.366 ± 0.01 in Glu2, Glu5, and Glu20, respectively. This suggested glucose consumption by the bacteria to produce energy via pyruvate oxidation and electron transport (Figure 2C). However, the pyruvate utilization speed and ATP production decreased and the NAD+/NADH ratio increased after the “acetate switch” points (Figure 2A,B), resulting in lower energy production ability. The energy generation and intracellular acetate content were found to be consistent: before “acetate switch” points, the energy produced was conducive to cell growth and served as the energy pool for QS functionality. However, after “acetate switch” points, energy production was inhibited with the decline in cell growth (Figure 2C).
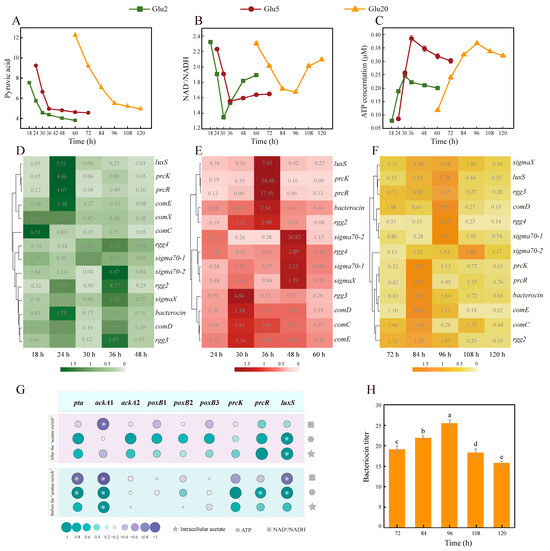
Figure 2.
Variation in energy, AMRGs, and QSRGs abundance before and after “acetate switch” points. (A) Changes in pyruvate in the three treatments over time; (B) Changes in NAD+/NADH in the three treatments over time; (C) Changes in ATP in the three treatments over time; (D–F) Heat map of the expression of QSRGs in the three treatments over time; (G) Heat map of correlation analysis of QSRGs with acetate and energy before and after the “acetate switch”; (H) Bacteriocin titer of HD1.7 in Glu 20. “*” indicates “p < 0.05”; Different lowercase letters indicate significant differences among groups (p < 0.05).
Pyruvate dehydrogenase (PDH) and pyruvate formate lyase could oxidate decarboxylated pyruvate to generate acetyl-CoA under aerobic and anaerobic conditions, which was converted into acetate via the pta-ackA operon [29,30]. This was a reversible low-affinity pathway, wherein the bacteria could also use acetate when its concentrations were high in the environment. This cycle resulted in a constant change in energy (Figure 1D).
3.3. Variation of QSRGs
QSRGs can all participate in QS to regulate bacteriocin synthesis and play different roles, for example, stress responses, bacteriocin production, biofilm formation, and virulence development, among others. The interspecies signaling molecule AI-2, autoinducing peptide Paracin 1.7, and acetate could all have been found to be able to regulate bacteriocin production using QS in HD1.7 [31,32,33]. In our previous research, the production of Paracin 1.7 was induced by pre-signal peptide CSP and the post-signal peptide XIP, respectively [31]. CSPs encoded by comC could regulate prcK, prcR, luxS, comD, and comE to promote bacteriocin gene expression, which is referred to as the pre-regulation system. The signaling peptide XIP encoded by comS activated the family of transcriptional regulators rgg (rgg2, rgg3, and rgg4), which could regulate stress responses, nutrient metabolism, bacteriocin production, biofilm formation, QS, and virulence formation [34,35]. rgg also regulated the expression of the global transcriptional regulator sigma (sigmaX, sigma70-1, and sigma70-2) to control biofilm formation and rapid adaptation of bacteria to global metabolic changes [24]. CSP-prcK/R could activate the expression of XIP-rgg-sigma, and hence XIP-rgg-sigma was also known as the post-regulation system.
Under all three treatments, QS genes (prcK, prcR, and luxS) and pre-regulation system genes, such as comC, comD, and comE were highly expressed before the “acetate switch” points, with the smallest fold change in comC expression (3.3 ± 0.03) and the largest one in luxS expression (9.48 ± 0.19) in Glu2 (p < 0.05) (Figure 2D). However, prcK (66 ± 1.32) and comE (29.3 ± 0.58) showed the largest fold changes in Glu5 and Glu20, respectively (p < 0.05) (Figure 2E,F). The expression of ackA1 was significantly correlated with intracellular acetate and ATP levels and NAD+/NADH ratio before the “acetate switch” point. These correlations decreased after the “acetate switch”, suggesting the dependence of ackA1 expression on intracellular acetate concentration. QS genes, such as luxS, were positively correlated with ATP both before and after the “acetate switch” point (p < 0.05) (Figure 2G), implying the involvement of acetate in intracellular energy flow as a common signaling molecule between species. Acetate can influence the expression levels of QS-related protein kinases in bacteria. These kinases can regulate downstream gene expression through phosphorylation processes. Additionally, the regulatory effect of acetate on protein kinase expression can enhance the stress response capability of bacteria to external environments. As observed in Saccharomyces cerevisiae SPSC01 fermentation studies, acetate can enhance cellular stress response capacity via the following mechanism: signaling molecules bind to the receptor PYR/PYL/RCAR, triggering phosphorylation to regulate the expression of genes such as srk2e/ost1/snrk2.6, thereby enhancing cellular stress response capacity [36].
Combined with the intracellular acetate content before the “acetate switch” points (Figure 1A), it was inferred that acetate might act as a signaling molecule to activate QS in HD1.7, resulting in high expression of signal transduction genes prcK and prcR and interspecific signaling molecule luxS, which was not only beneficial to the production of Paracin 1.7 but also consumed energy (Figure 2C). Our previous study showed that the exogenous addition of acetate could improve the concentration of Paracin 1.7 by 133.92%, which was accompanied by the high expression of prcK and prcR [9].
Around or after the “acetate switch” points, the post-regulation system genes, such as sigmaX, sigma70-1, sigma70-2, rgg2, rgg3, and rgg4, were highly expressed in Glu2, Glu5, and Glu20, with the highest fold change of 16.69 ± 0.33 (p < 0.05). After the “acetate switch” points, the extracellular acetate contents were 4.34 g/L (30 h in Glu2), 4.72 g/L (36 h in Glu5), and 5.80 g/L (96 h in Glu20) whereas the intracellular acetate content reached 48.55 g/L·1010 (30 h in Glu2), 62.16 g/L·1010 (36 h in Glu5), and 4700 g/L·1010 CFU (96 h in Glu20) [32].
Our research team previously found that low concentrations of acetate (2 g/L and 6 g/L) were more favorable to upregulate the pre-regulation system. In contrast, high concentrations of acetate (10 g/L) were inclined to upregulate the post-regulation system, which was consistent with the findings of this study [9]. In brief, intracellular acetate served as a switch for energy flow and gene expression, whereas extracellular acetate concentration helped to regulate gene expression.
Obviously, HD1.7 entered into the late stationary stage after the “acetate switch” points, characterized by the accumulation of various organic acids and secondary metabolites. The cells experienced stress under each treatment to increase the expression of sigma70-1, sigma70-2, and sigmaX. Moreover, the cell populations could also form biofilms to deal with such a situation; rgg2, rgg3, and rgg4 were overexpressed. In fact, Meng et al. also found that acetate could enhance the resistance of Lactobacillus plantarum, Lactobacillus sakei, and Lactobacillus rhamnosus to Staphylococcus aureus [37].
3.4. SEM Analysis
Acetate (intracellular/extracellular) concentrations were more directly related to energy. The correlation between intracellular acetate and ATP changed from −0.834 (p < 0.001) (Glu2) to 0.598 (p < 0.001) (Glu5) and 1.174 (p < 0.001) (Glu20) upon adding glucose. A similar situation occurred in the relationship between intracellular acetate and NAD+/NADH (Figure 3). In Glu2, low concentrations of acetate (intracellular/extracellular) directly influenced both QSRGs and AMRGs with correlation coefficients of −1.052 (p < 0.001) and −0.793 (p < 0.001), and also indirectly affected QSRGs and AMRGs via ATP (Figure 3A,D).
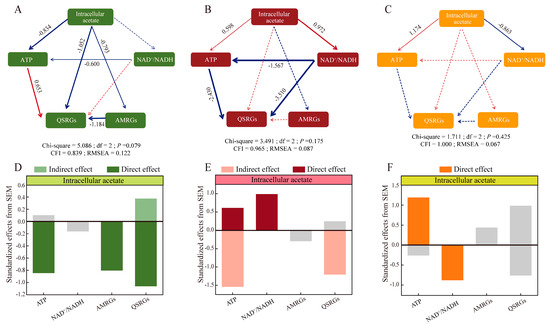
Figure 3.
SEM analysis of the effects of intracellular acetate on energy as well as quorum sensing. (A,D) SEM analysis of Glu2, direct and indirect effects of Lacticaseibacillus paracasei HD1.7 intracellular acetate on ATP, reducing power, AMRGs, and QSRGs. (B,E) SEM analysis of Glu5, direct and indirect effects of Lacticaseibacillus paracasei HD1.7 intracellular acetate on ATP, reducing power, AMRGs, and QSRGs. (C,F) SEM analysis of Glu20, direct and indirect effects of Lacticaseibacillus paracasei HD1.7 intracellular acetate on ATP, reducing power, AMRGs, and QSRGs. Differently colored bars represent different groups; gray bars indicate statistically tested p > 0.05. Red symbolizes a positive correlation, and blue symbolizes a negative correlation; solid lines indicate a significant effect between variables, while dashed lines indicate a non-significant effect.
In Glu5, acetate (intracellular/extracellular) did not directly influence QSRGs, but indirectly influenced QSRGs via ATP and NAD+/NADH (−2.430 and −3.510, p < 0.001); energy served as an intermediate bridge (Figure 3B,E).
However, in Glu20, the effects of ATP and NAD+/NADH on QSRGs and AMRGs were not significant, probably due to the high concentration of acetate (intracellular/extracellular) (Figure 3C,F).
3.5. Changes of Genes in HD1.7 with Different Acetate-Producing Capacity Before and After the “Acetate Switch”
Under the same fermentation conditions, the intracellular acetate content of strain HD1.7-Δpta was lower than that of the normal strain HD1.7 (Figure 4A). The visual analysis using volcano plots revealed that 1218 and 127 DEGs were significantly upregulated in the b vs a (samples were before and after “acetate switch”, AOF) and c vs b (samples all had a low content of acetate, LCA) (p < 0.05), respectively, whereas 1219 and 260 DEGs were significantly downregulated in the two groups (Figure 4B,C)
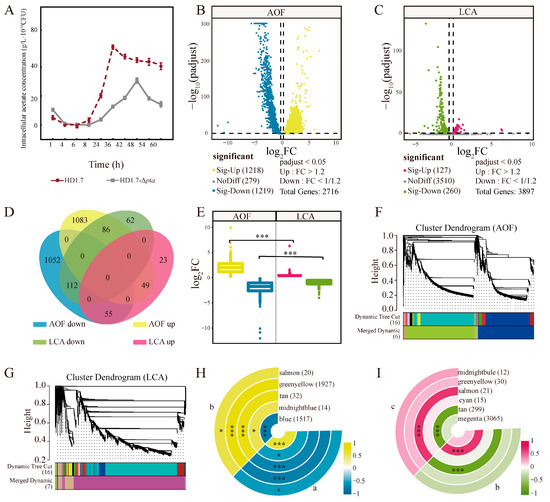
Figure 4.
Changes of genes in HD1.7 with different acetate-producing capacity before and after the “acetate switch”. (A) Intracellular acetate content of HD1.7 and strain HD1.7-Δpta under 5 g/L glucose conditions; (B) Volcano gram of DEGs in the AOF group; (C) Volcano gram of DEGs in the LCA group; (D) Venn diagram of DEGs in AOF vs. LCA; (E) Variance analysis of DEG in LCA and LCA, the color labeling involved in this figure is consistent with that in 4D.; (F) WGCNA of DEGs in the AOF group; (G) WGCNA of DEGs in the LCA group; (H) Ringed heat map of modular trait in WGCNA associations of AOF, where each ring represents a module, the letters in the figure indicate the treatment groups; (I) Ring heatmap of module trait associations in WGCNA of LCA, where each ring represents a module, the letters in the figure indicate the treatment groups. “*” indicates “p < 0.05”; “***” indicates “p < 0.001”.
Venn diagram analysis showed 49 and 112 commonly significantly upregulated and downregulated DEGs, respectively, among the DEGs (Figure 4D). The number of more significant DEGs was higher in the AOF than in the LCA, which might be due to the more obvious changes before and after the “acetate switch” in the same strain. Significant differences were found between up- and downregulated genes in both groups (Figure 4E) (p < 0.001).
WGCNA analysis was performed to understand the functional module classification of the DEGs (all DEGs with expression levels lower than 1 were deleted, the threshold for module merging was 0.25, and the minimum number of genes in the module was 10). Five functional modules (blue, midnight blue, tan, yellow-green, and light pink) in AOF were clustered, each comprising 1517, 14, 32, 1927, and 20 DEGs, respectively. Moreover, 3065, 299, 15, 21, 30, and 12 DEGs in magenta, sepia, cyan, light pink, yellow-green, and midnight blue modules, respectively, were classified in LCA (Figure 4F,G). Blue, midnight blue, tan, yellowish green, and light pink functional modules were significantly correlated with the AOF. In contrast, LCA had a strong relationship with dark brown and light pink functional modules (Figure 4H,I).
3.6. Level in Metabolic Pathways of HD1.7 with Different Acetate-Producing Capacity Before and After the “Acetate Switch”
3.6.1. KEGG Enrichment
Five metabolic pathways were found to be significantly enriched in AOF (p < 0.05), namely, ribosomal pathway, QS, biosynthesis of nucleotide sugars, two-component system, and mismatch repair pathway (Figure 5A). Each of these metabolic pathways influenced a wide range of life activities in HD1.7. The ribosomal pathway, biosynthesis of nucleotide sugars, and mismatch repair pathway were all related to the most basic bacterial DNA replication, transcription, and translation; all of these impacted bacterial growth, metabolism, and stress responses [38]. These metabolic pathways changed significantly before and after the “acetate switch” (p < 0.05), indicating the importance of “acetate switch” points. The expression of QS and the two-component system further confirmed the close relationship between “acetate switch” points and these systems.
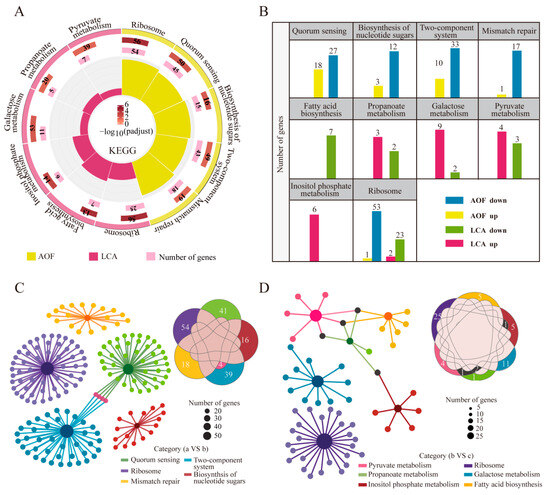
Figure 5.
Changes in metabolic pathways of HD1.7 with different acetate-producing capacity before and after the “acetate switch”. (A) KEGG enrichment in AOF and LCA to analyze differential metabolic pathways and their enriched genes; (B) histogram of the number of up- and downregulated genes in the differential metabolic pathways of AOF and LCA; (C) dynamic Venn diagram of enriched genes in the differential metabolic pathways of AOF; (D) dynamic Venn diagram of enriched genes in the differential metabolic pathways of LCA.
In the LCA, the ribosomal pathway, pyruvate metabolism, propionate metabolism, galactose metabolism, inositol phosphate metabolism, and fatty acid biosynthesis pathway were significantly enriched (p < 0.05), which were all related to acetate metabolism (Figure 5A). Both b and c groups had low acetate content, due to the consumption of acetate as the backup carbon source when HD1.7 grew to the late stationary stage in the former case, and the change in acetate metabolism in the latter case. This also explained the cellular response to acetate metabolism before and after the “acetate switch” point. Overall, both AOF and LCA were co-enriched in the ribosome pathway, with 54 and 25 DEGs, respectively, suggesting that this pathway was jointly influenced by acetate content. The number of downregulated genes in each pathway was higher in AOF, whereas the number of upregulated genes in each pathway was higher in LCA, except for the inositol phosphate metabolism (Figure 5B).
In AOF, four genes, ciaH, ciaR, nisK, and agrA, were shared between the QS system and the two-component system. During the “acetate switch” took place, bacteria might respond to stress via QS (Figure 5C). In LCA, accB, pflB, and accC, which are involved in propionate metabolism and pyruvate metabolism, were chosen. The “acetate switch” modified the central carbon metabolism of the bacteria, aiming to enhance the bacterial resilience and their ability to adapt to environmental changes (Figure 5D).
3.6.2. QS and Two-Component System
ComC, comD, and comE belonged to the pre-regulation system and influenced the production of Paracin 1.7, their phosphorylated protein gene, ciaR, was upregulated in AOF, indicating that the production of bacteriocins increased with the progression of fermentation (Figure 6A and Figure 7). CiaR belongs to the phosphorylated protein genes and promotes bacteriocin production through a phosphorylation process. ciaR was also associated with biofilm formation, contributing to bacterial survival and competition [39]. Also, its expression was downregulated in LCA (Figure 6A), suggesting the engagement of cells in resistance to stressful environments by producing biofilms when the acetate metabolic pathway was blocked.
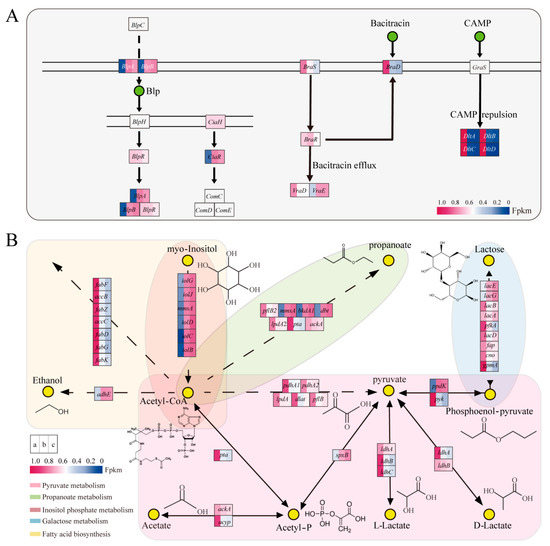
Figure 6.
Analysis of differential metabolic pathways in AOF and LCA. (A) QS and two-component system; (B) The other metabolic pathway. Solid arrows represent a direct reaction from the previous substance to the next, dotted arrows indicate that the reaction requires a complex process. Created with BioRender.com.
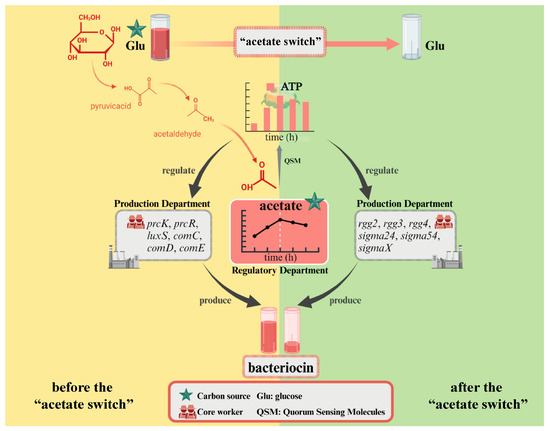
Figure 7.
Conceptual model of production of bacteriocin by L. paracasei before and after the “acetate switch”. Created with BioRender.com.
BlpA and blpB are ABC transporter protein genes, which can export QS signaling molecules, an important component involved in QS [40]. These proteins serve as critical components of the QS system, facilitating communication between bacteria. These two genes were upregulated in AOF and downregulated in LCA. This finding was consistent with the results of Kang et al., who found that either exogenous acetate addition or internal acetate content adjustment influenced ABC transporter proteins [9]. As ABC transporters were the stabilizers of comC, comD, and comE, the high expression of blpA and blpB contributed to the production of Paracin 1.7.
DltA, dltB, dltC, and dltD are two-component system genes that can modify cell wall composition and antibiotic resistance ability in Gram-positive bacteria [41]. Moreover, braD, braS, braR, and vraD are linked to bacitracin production [42]. DltA, dltB, dltC, dltD, braD, braS, braR, and vraD were downregulated in the AOF and LCA with the progression of fermentation. The resistance ability changed in the later growth stages or under the blocked acetate metabolic pathway.
3.6.3. The Other Metabolic Pathway
The expression levels of accB, accC, fabD, fabF, fabG, fabK, and fabZ were lower in AOF and LCA. They are involved in the synthesis of long-chain fatty acids and affect cellular resistance through cell membrane fluidity, as well as QS [43] (Figure 6B). Fatty acid metabolism was influenced by salinity, temperature, pH, and oxidative stress [44], in addition to intracellular acetate content [45,46], which was essential for bacterial stress and adaptation [47].
Similarly, not only pta, ackA, and acy, but also central carbon metabolism genes (ppdk, pyk, pdhA1, pdhA2, lpdA1, dlat, and pflB), galactose metabolism pathway genes (lacA, lacB, lacD, pfkA, fbp, eno, and gpmA), and propanoate metabolism pathway genes (pflB, lpdA) were downregulated with the change in acetate metabolism.
4. Conclusions
The higher the glucose concentration, the longer the “acetate switch” point (30 h in Glu2, 36 h in Glu5, and 96 h in Glu20), indicating that the “acetate switch” was related to the concentration of added glucose under the glucose concentration gradient set in this experiment. The viable count of HD1.7 initially increased, then decreased, and subsequently displayed secondary growth, suggesting that intracellular acetate could be used as a backup carbon source to induce the secondary growth of bacteria. Energy increased before the “acetate switch” and decreased afterward. The “acetate switch” could act as an energy switch, regulating bacterial growth and QS before the “acetate switch” and affecting the production of Paracin 1.7 due to energy loss after the “acetate switch”. The discussion of the results revealed that acetate and energy jointly influenced Paracin 1.7 production through different divisions of labor (Figure 7). Moreover, the impact of the “acetate switch” on metabolic pathways such as fatty acid synthesis and QS further highlighted its importance in optimizing bacteriocin production. This study laid a theoretical foundation for improving the bacteriocin yield of probiotics in industrial production in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14152691/s1, Figure S1: M: DNA Marker DL10000, Lane 1 is HD1.7-Δpta, Lane 2 is wild type, and Lane 3 is control; Table S1: Primers sequences in the experiment; Table S2: The abbreviations mentioned in this study.
Author Contributions
W.Y.: Data curation, Writing—original draft, Writing—review and editing. R.S.: Formal analysis. W.Z.: Investigation. J.K.: Investigation. Z.W.: Investigation. L.M.: Investigation. Y.Y.: Investigation. S.L.: Investigation. G.S.: Investigation. J.G.: Methodology, Conceptualization, Supervision, Resources. W.P.: Methodology, Conceptualization, Supervision, Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 32071519); Heilongjiang Provincial Natural Science Foundation of China (PL2024D015); Harbin Science and Technology Innovation Talent Project (RC2024DY192); Scientific Research Project of Ecological Environment Protection of Heilongjiang Provincial Department of Ecological Environment (HST2022TR004); Basic Research Operating Costs of Colleges and Universities in Heilongjiang Province (2023-KYYWF-1448); Key Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), Ministry of Education.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Jingping Ge’s group in the School of Life Sciences, Heilongjiang University for their support and help.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Nealson, K.H.; Platt, T.; Hastings, J.W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 1970, 104, 313–322. [Google Scholar] [CrossRef]
- Lydick, V.N.; Mass, S.; Pepin, R.; Podicheti, R.; Klempic, E.; Rusch, D.B.; Ushijima, B.; Brown, L.C.; Salomon, D.; Kessel, J.C. Quorum sensing regulates virulence factors in the coral pathogen Vibrio coralliilyticus. Appl. Env. Microbiol. 2025, 91, e0114324. [Google Scholar] [CrossRef]
- Gless, B.H.; Bejder, B.S.; Monda, F.; Bojer, M.S.; Ingmer, H.; Olsen, C.A. Rearrangement of thiodepsipeptides by S → N Acyl shift delivers homodetic autoinducing peptides. J. Am. Chem. Soc. 2021, 143, 10514–10518. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.; Jacquet, P.; Gaucher, F.; Chabriere, E.; Plener, L.; Daude, D. AHL-based quorum sensing regulates the biosynthesis of a variety of bioactive molecules in bacteria. J. Nat. Prod. 2024, 87, 1268–1284. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.S.; Valastyan, J.S.; Bassler, B.L. A host-produced autoinducer-2 mimic activates bacterial quorum sensing. Cell Host Microbe 2016, 19, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.J.; Chang, D.E.; Walker, J.D.; Seitz, J.E.; Vidaurri, M.D.; Lange, C.F.; Prüss, B.M.; Henk, M.C.; Larkin, J.C.; Conway, T. Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol. Microbiol. 2003, 48, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.P.; Ping, W.X.; Song, G.; Du, C.M.; Ling, H.Z.; Sun, X.; Gao, Y. Paracin 1.7, a bacteriocin produced by Lactobacillus paracasei HD1.7 isolated from Chinese cabbage sauerkraut, a traditional Chinese fermented vegetable food. Acta Microbiol. Sin. 2009, 49, 609–616. [Google Scholar]
- Kang, J.; Zhou, X.; Zhang, W.; Pei, F.Y.; Ge, J.P. Transcriptomic analysis of bacteriocin synthesis and stress response in Lactobacillus paracasei HD1.7 under acetic acid stress. LWT—Food Sci. Technol. 2022, 154, 112897. [Google Scholar] [CrossRef]
- Sun, R.; Kang, J.; Sun, Y.C.; Ji, X.L.; Ge, J.P.; Ping, W.X. Action mechanism of Bacillus subtilis driving the production of Paracin 1.7: Based on transcriptome-binding SPR analysis. LWT—Food Sci. Technol. 2024, 191, 115617. [Google Scholar] [CrossRef]
- Zhao, D.; Du, R.P.; Ping, W.X.; Ge, J.P. Lactobacillus paracasei HD1.7 used as a starter modulates the bacterial community and metabolome profile during fermentation of Chinese cabbage. Lett. Appl. Microbiol. 2018, 67, 411–419. [Google Scholar] [CrossRef]
- Ma, W.; Liu, Y.; Shin, H.D.; Li, J.; Chen, J.; Du, G.; Liu, L. Metabolic engineering of carbon overflow metabolism of Bacillus subtilis for improved N-acetyl-glucosamine production. Bioresour. Technol. 2018, 250, 642–649. [Google Scholar] [CrossRef]
- Crabtree, H.G. Observations on the carbohydrate metabolism of tumours. Biochem. J. 1929, 23, 536–545. [Google Scholar] [CrossRef]
- Dai, Z.; Huang, M.; Chen, Y.; Siewers, V.; Nielsen, J. Global rewiring of cellular metabolism renders Saccharomyces cerevisiae Crabtree negative. Nat. Commun. 2018, 9, 3059. [Google Scholar] [CrossRef]
- Liao, M.; Yao, D.H.; Wu, L.F.; Luo, C.D.; Wang, Z.W.; Zhang, J.; Liu, B. Targeting the Warburg effect: A revisited perspective from molecular mechanisms to traditional and innovative therapeutic strategies in cancer. Acta Pharm. Sin. B 2024, 14, 953–1008. [Google Scholar] [CrossRef]
- Anane, E.; Lopez, D.C.C.; Neubauer, P.; Bournazou, M.N.C. Modelling overflow metabolism in Escherichia coli by acetate cycling. Biochem. Eng. J. 2017, 125, 23–30. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yang, W.; Chen, H.; Liu, B.; Lin, B.X.; Tao, Y. Metabolic engineering for efficient supply of acetyl-CoA from different carbon sources in Escherichia coli. Microb. Cell Fact. 2019, 18, 130. [Google Scholar] [CrossRef]
- Bradshaw, P.C. Acetyl-CoA metabolism and histone acetylation in the regulation of aging and lifespan. Antioxidants 2021, 10, 572. [Google Scholar] [CrossRef]
- Regina, V.R.; Noorian, P.; Sim, C.B.W.; Constancias, F.; Kaliyamoorthy, E.; Booth, S.C.; Espinoza-Vergara, G.; Rice, S.A.; McDougald, D. Loss of the acetate switch in Vibrio vulnificus enhances predation defense against Tetrahymena pyriformis. Appl. Environ. Microbiol. 2022, 88, e0166521. [Google Scholar]
- Korobkina, E.D.; Calejman, C.M.; Haley, J.A.; Kelly, M.E.; Li, H.; Gaughan, M.; Pepper, H.L.; Ahmad, H.; Boucher, A.; Fluharty, S.M.; et al. Brown fat ATP-citrate lyase links carbohydrate availability to thermogenesis and guards against metabolic stress. Nat. Metab. 2024, 6, 2187–2202. [Google Scholar] [CrossRef]
- Ge, J.P.; Sun, Y.C.; Xin, X.; Wang, Y.; Ping, W.X. Purification and partial characterization of a novel bacteriocin synthesized by Lactobacillus paracasei HD1.7 isolated from chinese sauerkraut juice. Sci. Rep. 2016, 6, 19366. [Google Scholar]
- Li, W.; Deng, M.; Gong, J.; Hou, Y.; Zhao, L. Bidirectional regulation of sodium acetate on macrophage activity and its role in lipid metabolism of hepatocytes. Int. J. Mol. Sci. 2023, 24, 5536. [Google Scholar] [CrossRef]
- Guaragnella, N.; Bettiga, M. Acetic acid stress in budding yeast: From molecular mechanisms to applications. Yeast 2021, 38, 391–400. [Google Scholar] [CrossRef]
- Gottesman, S. Trouble is coming: Signaling pathways that regulate general stress responses in bacteria. J. Biol. Chem 2019, 294, 11685–11700. [Google Scholar] [CrossRef]
- Othman, M.; Ariff, A.B.; Wasoh, H.; Kapri, M.R.; Halim, M. Strategies for improving production performance of probiotic Pediococcus acidilactici viable cell by overcoming lactic acid inhibition. AMB Express 2017, 7, 215. [Google Scholar] [CrossRef]
- Wolfe, A.J. The acetate switch. Microbiol. Mol. Biol. R 2005, 69, 12–50. [Google Scholar] [CrossRef]
- Vo, C.H.; Goyal, N.; Karimi, I.A.; Kraft, M. First observation of an acetate switch in a methanogenic autotroph (Methanococcus maripaludis S2). Microbiol. Insights 2020, 13, 1178636120945300. [Google Scholar] [CrossRef]
- Da, Y.Y.; Liu, Z.H.; Zhu, R.; Li, Z.J. Coutilization of glucose and acetate for the production of pyruvate by engineered Escherichia coli. Biotechnol. Bioeng. 2021, 111, 1150–1160. [Google Scholar] [CrossRef]
- Moffett, J.R.; Puthillathu, N.; Vengilote, R.; Jaworski, D.M.; Namboodiri, A.M. Acetate revisited: A key biomolecule at the nexus of metabolism, epigenetics and oncogenesis-part 1: Acetyl-CoA, acetogenesis and Acyl-CoA short-chain synthetases. Front. Physiol. 2020, 11, 580167. [Google Scholar] [CrossRef]
- Zangari, J.; Petrelli, F.; Maillot, B.; Martinou, J.C. The multifaceted pyruvate metabolism: Role of the mitochondrial pyruvate carrier. Biomolecules 2020, 10, 1068. [Google Scholar] [CrossRef]
- Ge, J.P.; Kang, J.; Ping, W.X. Effect of acetic acid on bacteriocin production by Gram-positive bacteria. J. Microbiol. Biotechnol. 2019, 29, 1341–1348. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, W.; Sun, R.; Song, G.; Ping, W.X.; Ge, J.P. Acetate secretion induces bacteriocin synthesis and activates the transcriptional regulators rgg and rpod. Fermentation 2022, 8, 524. [Google Scholar] [CrossRef]
- Xia, K.; Han, C.C.; Xu, J.; Liang, X. Transcriptome response of Acetobacter pasteurianus Ab3 to high acetic acid stress during vinegar production. Appl. Microbiol. Biotechnol. 2020, 104, 10585–10599. [Google Scholar] [CrossRef]
- Kloska, A.; Cech, G.M.; Sadowska, M.; Krause, K.; Szalewska-Palasz, A.; Olszewski, P. Adaptation of the marine bacterium Shewanella baltica to low temperature stress. Int. J. Mol. Sci. 2020, 21, 4338. [Google Scholar] [CrossRef]
- Gogos, A.; Cristobal Jimenez, J.; Chang, J.C.; Wilkening, R.V.; Federle, M.J. A quorum sensing-regulated protein binds cell wall components and enhances lysozyme resistance in Streptococcus pyogenes. J. Bacteriol. 2018, 200, e00701–e00717. [Google Scholar] [CrossRef]
- Ye, P.L.; Wang, X.Q.; Yuan, B.; Liu, C.G.; Zhao, X.Q. Manipulating cell flocculation-associated protein kinases in Saccharomyces cerevisiae enables improved stress tolerance and efficient cellulosic ethanol production. Bioresour. Technol. 2022, 348, 126758. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, H.; Nie, T.; Lu, F.; Zhang, C.; Lu, Y.; Liu, L. Acetate activates Lactobacillus Bacteriocin synthesis by controlling quorum sensing. Appl. Environ. Microbiol. 2021, 87, e0072021. [Google Scholar] [CrossRef]
- Woodgate, J.; Zenkin, N. Transcription-translation coupling: Recent advances and future perspectives. Mol. Microbiol. 2023, 120, 539–546. [Google Scholar] [CrossRef]
- Zhu, B.; Ge, X.C.; Stone, V.; Kong, X.Z.; El Rami, F.; Liu, Y.; Kitten, T.; Xu, P. ciaR impacts biofilm formation by regulating an argintine biosynthesis pathway in Streptococcus sanguinis SK36. Sci. Rep. 2017, 7, 17183. [Google Scholar] [CrossRef]
- Rahman, S.; McHaourab, H.S. ATP-dependent interactions of a cargo protein with the transmembrane domain of a polypeptide processing and secretion ABC transporter. J. Biol. Chem. 2020, 295, 14678–14685. [Google Scholar] [CrossRef]
- Wood, B.M.; Santa Maria, J.P.; Matano, L.M.; Vickery, C.R.; Walker, S. A partial reconstitution implicates DltD in catalyzing lipoteichoic acid d-alanylation. J. Biol. Chem. 2018, 293, 17985–17996. [Google Scholar] [CrossRef] [PubMed]
- Hiron, A.; Falord, M.; Valle, J.; Debarbouille, M.; Msadek, T. Bacitracin and nisin resistance in Staphylococcus aureus: A novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol. Microbiol. 2011, 81, 602–622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.S.; Zhang, L.; Wang, Y.; Song, W.Y.; Huang, Y.Z.; Mu, Y.J.; Schmitz, W.; Zhang, S.Y.; Lin, H.; Chen, H.Z.; et al. The molecular basis of catalysis by SDR family members Ketoacyl-ACP reductase FabG and Enoyl-ACP reductase FabI in type-II fatty acid biosynthesis. Angew. Chem. Int. Ed. Engl. 2023, 62, e202313109. [Google Scholar] [CrossRef] [PubMed]
- Al-Beloshei, N.E.; Al-Awadhi, H.; Al-Khalaf, R.A.; Afzal, M. A comparative study of fatty acid profile and formation of biofilm in Geobacillus gargensis exposed to variable abiotic stress. Can. J. Microbiol. 2015, 61, 48–59. [Google Scholar] [CrossRef]
- James, E.S.; Cronan, J.E. Expression of two Escherichia coli acetyl-CoA carboxylase subunits is autoregulated. J. Biol. Chem. 2004, 279, 2520–2527. [Google Scholar] [CrossRef]
- Yu, X.; Liu, T.; Zhu, F.; Khosla, C. In vitro reconstitution and steady-state analysis of the fatty acid synthase from Escherichia coli. Proc. Natl. Acad. Sci. USA 2011, 108, 18643–18648. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.R.; Walker, A.R. Expanding the vocabulary of peptide signals in Streptococcus mutans. Front. Cell Infect. Microbiol. 2019, 9, 194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).