Exploring Sorghum Flour as a Sustainable Ingredient in Gluten-Free Cookie Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Cookie Formulations
2.2. Physical Properties and Color of the Cookies
- eccentricity—the ratio of the diameters, serving as a measure of deviation from an ideal circular shape.
- spread factor—the ratio between the average diameter and the height of the cookies.
2.3. Textural Properties of Cookies
2.4. Proximate Analysis
2.5. Determination of Water Activity (aw) of Biscuits
2.6. Polyphenolic Analysis
2.7. Amino Acid Analysis
2.8. Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physical and Color Characteristics
3.2. Textural Properties
3.3. Proximate Composition
3.4. Polyphenolic Profile
3.5. Amino Acid Profile
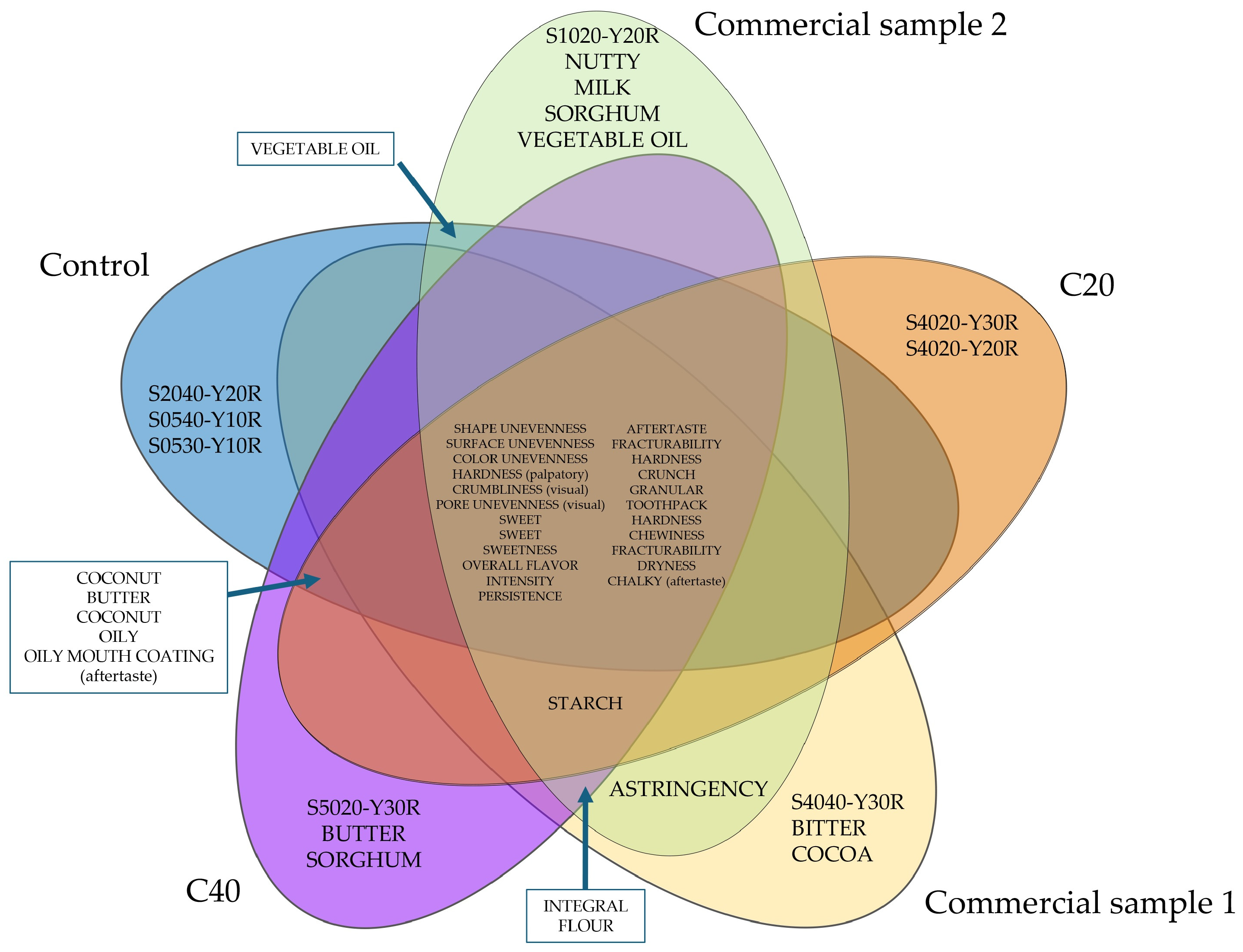
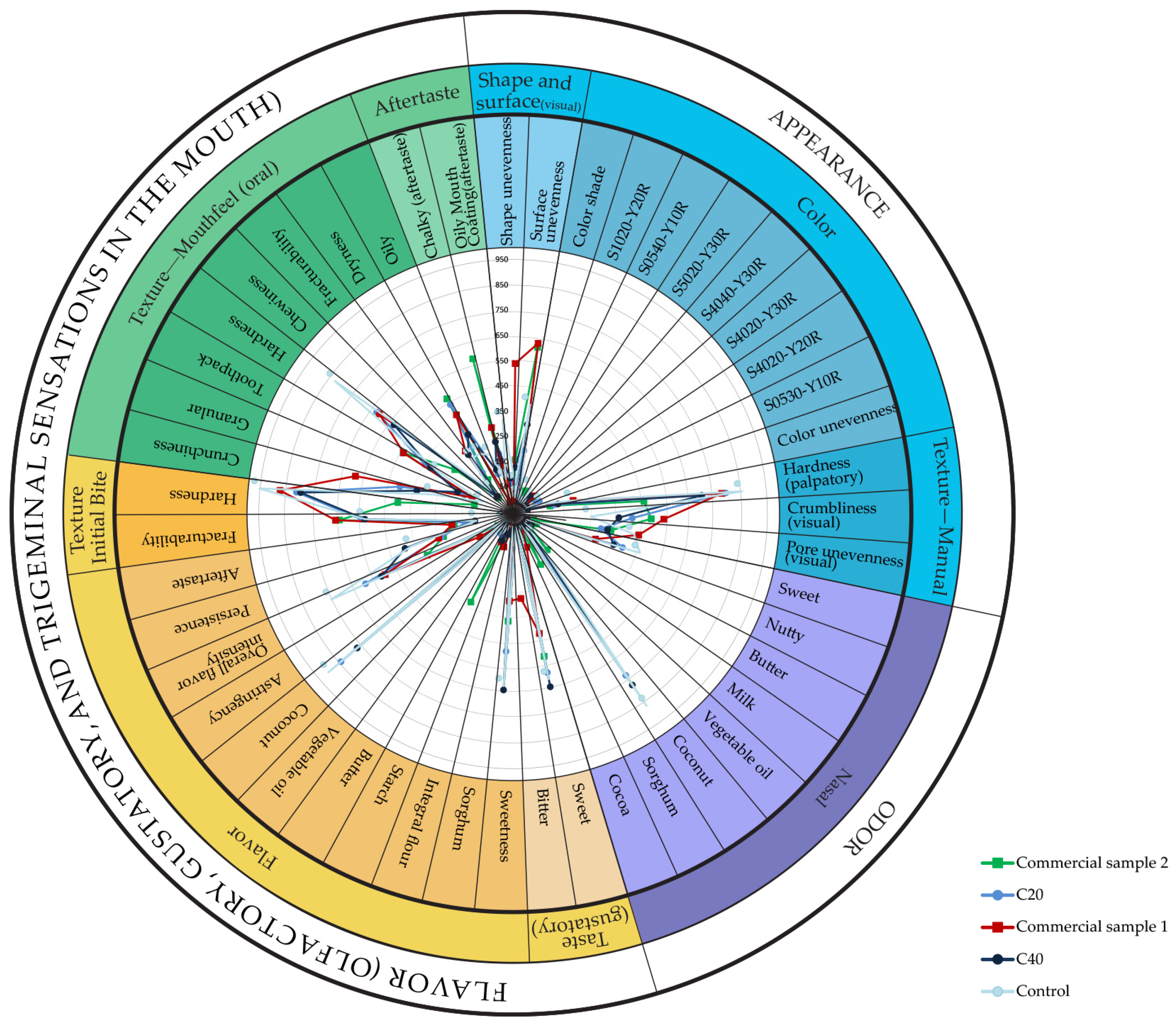
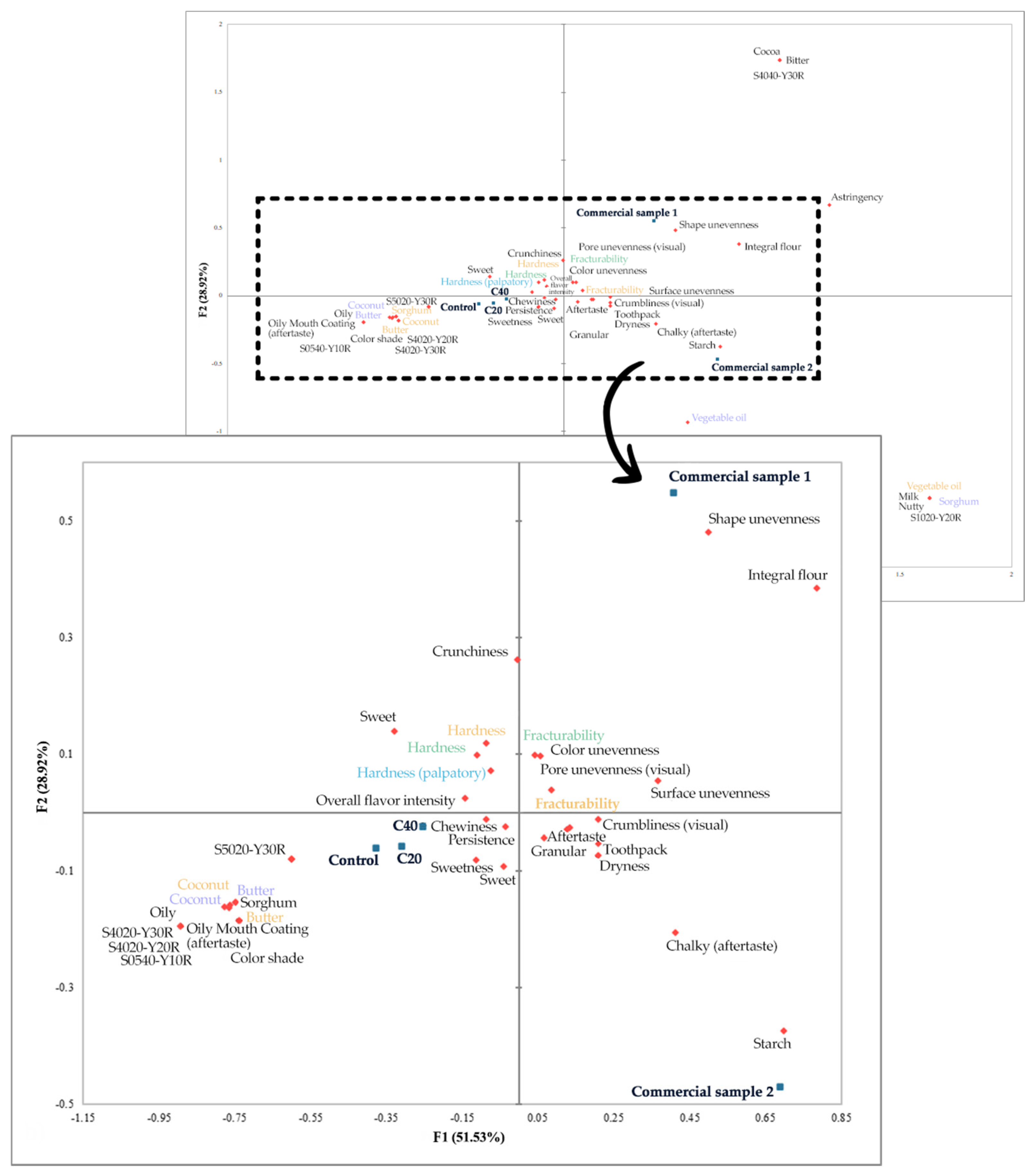
3.6. Sensory Profile of Cookies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medeiros, R.J.; dos Santos, L.M.G.; Freire, A.S.; Santelli, R.E.; Braga, A.M.C.B.; Krauss, T.M.; Jacob, S.C. Determination of inorganic trace elements in edible marine fish from Rio de Janeiro State, Brazil. Food Control 2012, 23, 535–541. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Schober, T.J.; Bean, S.R. Novel food and non-food uses for sorghum and millets. J. Cereal. Sci. 2006, 44, 252–271. [Google Scholar] [CrossRef]
- Chavez, D.; Ascheri, J.; Martins, A.; Carvalho, C.; Bernardo, C.; Teles, A. Sorghum, an alternative cereal for GF product. Rev. Chil. Nutr. 2018, 45, 169–177. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.M.; Yeon, S.J.; Ham, H.J.; Kim, J.H.; Oh, D.H. Flavonoids in decorticated sorghum grains exert antioxidant, antidiabetic and antiobesity activities. Molecules 2020, 25, 2854. [Google Scholar] [CrossRef] [PubMed]
- Pontieri, P.; Mamone, G.; De Caro, S.; Tuinstra, M.R.; Roemer, E.; Okot, J.; De Vita, P.; Ficco, D.B.; Alifano, P.; Pignone, D. Sorghum, a healthy and gluten-free food for celiac patients as demonstrated by genome, biochemical and immunochemical analyses. J. Agric. Food Chem. 2013, 61, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Palavecino, P.M.; Ribotta, P.D.; León, A.E.; Bustos, M.C. Gluten-free sorghum pasta: Starch digestibility and antioxidant capacity compared with commercial products. J. Sci. Food Agric. 2019, 99, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, H.E.; Peiris, K.H.S.; Bean, S.R. Assessment of Grain Protein in Tropical Sorghum Accessions from the NPGS Germplasm Collection. Agronomy 2023, 13, 1330. [Google Scholar] [CrossRef]
- Queiroz, V.A.V.; Dizlek, H.; de Barros, F.A.R.; Tardin, F.D.; Figueiredo, J.E.F.; Awika, J.M. Baking Process Effects and Combined Cowpea Flour and Sorghum Bran on Functional Properties of Gluten-Free Cookies. Plant Foods Hum. Nutr. 2022, 77, 552–559. [Google Scholar] [CrossRef]
- Célia, J.A.; Resende, O.; de Lima, M.S.; Correia, J.S.; de Oliveira, K.B.; Takeuchi, K.P. Technological properties of gluten-free biscuits from sorghum flour granifero (Sorghum bicolor (L.) Moench). Food Sci. Technol. 2022, 42, e29222. [Google Scholar] [CrossRef]
- Zhu, L.; Snider, L.; Vu, T.H.; Desam, G.P.; Herald, T.J.; Dogan, H.; Khaled, A.Y.; Adedeji, A.A.; Alavi, S. Effect of Whey Protein Concentrate on Rheological Properties of Gluten-Free Doughs and Their Performance in Cookie Applications. Foods 2023, 15, 10170. [Google Scholar] [CrossRef]
- Meena, K.; Visarada, K.B.R.S.; Meena, D.K. Sorghum bicolor (L.) Moench: A multifarious crop—Fodder to therapeutic potential and biotechnological applications: A future food for the millennium. Future Foods 2022, 6, 100188. [Google Scholar] [CrossRef]
- Oliveira, L.L.L.; Figueiredo, L.F.A. Sorghum phytonutrients and their health benefits: A systematic review from cell to clinical trials. J. Food Sci. 2024, 89, 5–29. [Google Scholar] [CrossRef]
- Collins, A.; Santhakumar, A.B.; Latif, S.; Chinkwo, K.; Francis, N.; Blanchard, C.L. Impact of Processing on the Phenolic Content and Antioxidant Activity of Sorghum bicolor L. Moench. Molecules 2024, 29, 3626. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Hussain, M.E. Obesity and diabetes: An update. Diabetol. Metab. Syndr. 2017, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef]
- Miyazaki, H.; Nagae, M.; Uchida, H.; Shimizu, K. Effect of sorghum intake on postprandial blood glucose levels: A randomized, double-blind, crossover study. Funct. Foods Health Dis. 2024, 14, 87–95. [Google Scholar] [CrossRef]
- Acosta, D.; Pérez, M.; Peña, M. Sensory evaluation of sorghum-based products: A review. Food Qual. 2003, 14, 621–630. [Google Scholar]
- Hryhorenko, N.; Krupa-Kozak, U.; Bączek, N.; Wróblewska, B. Gluten-free bread enriched with whole-grain red sorghum flour gains favourable technological and functional properties and consumers acceptance. J. Cereal Sci. 2023, 110, 103646. [Google Scholar] [CrossRef]
- Mba, J.C.; Paes, L.T.; Viana, L.M.; Ferreira, A.J.C.; Queiroz, V.A.V.; Martino, H.S.D.; Azevedo, L.; de Carvalho, C.W.P.; Felisberto, M.H.F.; de Barros, F.A.R. Evaluation of the Physical, Chemical, Technological, and Sensorial Properties of Extrudates and Cookies from Composite Sorghum and Cowpea Flours. Foods 2023, 12, 3261. [Google Scholar] [CrossRef]
- Aljobair, M.O. Physicochemical Properties and Sensory Attributes of Cookies Prepared from Sorghum and Millet Composite Flour. Food Sci. Nutr. 2022, 10, 3415–3423. [Google Scholar] [CrossRef]
- Jaćimović, S.; Kiprovski, B.; Sikora, V.; Pezo, L.; Pantelić, N. Diversity in Nutritional and Functional Quality of Sorghum Restorer Lines Collection. J. Food Nutr. Res. 2024, 63, 205–214. [Google Scholar]
- Jaćimović, S.; Kiprovski, B.; Ristivojević, P.; Dimić, D.; Nakarada, Đ.; Dojčinović, B.; Sikora, V.; Teslić, N.; Pantelić, N.Đ. Chemical Composition, Antioxidant Potential, and Nutritional Evaluation of Cultivated Sorghum Grains: A Combined Experimental, Theoretical, and Multivariate Analysis. Antioxidants 2023, 12, 1485. [Google Scholar] [CrossRef] [PubMed]
- CIE. Recommendations on Uniform Color Spaces, Color Difference Equations, Psychometric Color Terms; CIE Publication No. 15 (E-1.3.1); CIE: Paris, France, 1976. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- FSAI. Accuracy of Nutrition Labelling of Pre-Packaged Food in Ireland; FSAI: Dublin, Ireland, 2010. [Google Scholar]
- Spackman, D.H.; Stein, W.H.; Moore, S. Automatic recording apparatus for use in the chromatography of amino acids. Anal. Chem. 1958, 30, 1190–1206. [Google Scholar] [CrossRef]
- Tomičić, Z.; Pezo, L.; Spasevski, N.; Lazarević, J.; Čabarkapa, I.; Tomičić, R. Diversity of amino acids composition in cereals. Food Feed Res. 2022, 49, 11–22. [Google Scholar] [CrossRef]
- SRPS EN ISO 8589:2015; Sensory Analysis—General Guidance for the Design of Test Rooms. Serbian Institute for Standardization: Belgrade, Serbia, 2015.
- Khoddami, A.; Messina, V.; Vadabalija Venkata, K.; Farahnaky, A.; Blanchard, C.L.; Roberts, T.H. Sorghum in foods: Functionality and potential in innovative products. Crit. Rev. Food Sci. Nutr. 2024, 64, 1170–1186. [Google Scholar] [CrossRef]
- Chauhan, A.; Saxena, D.C.; Singh, S. Physical, textural, and sensory characteristics of wheat and amaranth flour blend cookies. Cogent Food Agr. 2016, 2, 1125773. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Brousse, M.M.; Linares, R.A. Variation of Color with Baking Time in Snacks Made with Pregelatinized Cassava. J. Food Sci. 2021, 86, 4100–4109. [Google Scholar] [CrossRef]
- Badi, S.M.; Hoseney, R.C. Use of sorghum and pearl millet flours in cookies. Cereal Chem. 1975, 53, 733–738. [Google Scholar]
- Rai, S.; Kaur, A.; Singh, B. Quality characteristics of gluten free cookies prepared from different flour combinations. J. Food Sci. Tech. 2014, 51, 785–789. [Google Scholar] [CrossRef]
- Arslan Unal, N.; Ozkaya, B. Nutritional and physical properties of cookies enriched with whole wheat flour of ancient wheats. Cereal Chem. 2024, 101, 356–366. [Google Scholar] [CrossRef]
- Sudha, M.L.; Vetrimani, R.; Leelavathi, K. Influence of fibre from different cereals on the rheological characteristics of wheat flour dough and on biscuit quality. Food Chem. 2007, 100, 1365–1370. [Google Scholar] [CrossRef]
- Mitevski, J.; Pantelić, Đ.N.; Dodevska, S.M.; Kojić, J.; Vulić, J.; Zlatanović, S.; Gorjanović, S.; Laličić-Petronijević, J.; Marjanović, S.; Antić, V. Effect of Beetroot Powder Incorporation on Functional Properties and Shelf Life of Biscuits. Foods 2023, 12, 322. [Google Scholar] [CrossRef] [PubMed]
- Yousif, A.M.; Nhepera, D.; Johnson, S.K.; Gamlath, S. Influence of sorghum flour addition on in vitro starch digestibility, antioxidant capacity and consumer acceptability of durum wheat pasta. Food Chem. 2012, 134, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Renzella, E.; Arendt, E.K.; Gallagher, E. Sorghum (Sorghum bicolor L. Moench) Gluten Free Bread: The Effect of Milling Conditions on the Technological Properties and In Vitro Bioaccessibility of Polyphenols and Minerals. Foods 2023, 12, 3030. [Google Scholar] [CrossRef]
- Ruiz-Hernández, A.A.; Rouzaud-Sández, O.; Valenzuela-González, M.; Domínguez-Avila, J.A.; González-Aguilar, G.A.; Robles-Sánchez, M. Antioxidant/Anti-Inflammatory Potential and Sensory Acceptance of Granola Bars Developed with Sorghum Sprout Flour Irradiated with UV-A LED Light. Foods 2025, 14, 1787. [Google Scholar] [CrossRef]
- Ares, G.; Bruzzone, F.; Vidal, L.; Cadena, R.S.; Giménez, A.; Pineau, B.; Hunter, D.C.; Paisley, A.G.; Jaeger, S.R. Evaluation of a Rating-Based Variant of Check-All-That-Apply Questions: Rate-All-That-Apply (RATA). Food Qual. Prefer. 2014, 36, 87–95. [Google Scholar] [CrossRef]
- Maravić, N.; Pajin, B.; Hadnađev, M.; Pestorić, M.; Škrobot, D.; Tomić, J. Assessment of Whole Grain Ancient Wheat Sourdough in Lyophilised and Native Forms for Cookie Formulation. Foods 2023, 13, 3363. [Google Scholar] [CrossRef]

| Ingredients | Control | C20 | C40 |
|---|---|---|---|
| gluten-free mix | 150 | 120 | 90 |
| sorghum flour | / | 30 | 60 |
| cane sugar | 50 | 50 | 50 |
| coconut oil | 52.50 | 52.50 | 52.50 |
| sodium bicarbonate | 0.75 | 0.75 | 0.75 |
| water | 55 | 55 | 55 |
| Sample | Description | Ingredients |
|---|---|---|
| Commercial sample 1 | Cocoa biscuit PUSA (lactose-free, gluten-free, casein-free) | Millet flour, rice flour, corn flour, corn starch, vegetable fat, cocoa powder with reduced fat content (10%), sugar, emulsifier (soy lecithin), salt, raising agent (ammonium bicarbonate), thickener (guar gum). |
| Commercial sample 2 | Buckwheat cookies (lean, lactose-free, casein-free, preservative-free) | Rice flour, corn starch, sugar, millet flour, palm oil, buckwheat flour (6.5%), corn flour, guar bean flour, raw soy lecithin, leavening agent (ammonium bicarbonate), salt. |
| Sample | L* (D65) | a* (D65) | b* (D65) | Height (mm) | Weight (g) | Eccentricity | Spread Factor |
|---|---|---|---|---|---|---|---|
| Control | 73.18 ± 0.62 a | 0.31 ± 0.41 a | 34.59 ± 0.76 a | 5.54 ± 0.19 a | 7.55 ± 0.05 a | 1.01 ± 0.01 a | 7.74 ± 0.28 a |
| C20 | 51.78 ± 1.23 b | 8.38 ± 0.33 b | 17.52 ± 0.43 b | 5.57 ± 0.08 b | 7.49 ± 0.06 b | 1.00 ± 0.02 a | 7.56 ± 0.06 b |
| C40 | 46.78 ± 0.96 c | 9.91 ± 0.37 c | 14.92 ± 0.26 c | 5.55 ± 0.17 a | 7.52 ± 0.17 c | 1.00 ± 0.01 a | 7.83 ± 0.24 a |
| Comparison | ∆E Value |
|---|---|
| Control vs. C20 | 28.54 |
| Control vs. C40 | 34.29 |
| C20 vs. C40 | 5.84 |
| Sample | Hardness (g) | Fracturability (mm) |
|---|---|---|
| Control | 2145.76 ± 81.50 a | 36.52 ± 0.20 a |
| C20 | 2373.60 ± 157.95 a | 36.67 ± 0.20 a |
| C40 | 2340.32 ± 190.63 a | 36.56 ± 0.31 a |
| Sample | Moisture (%) | Ash (%) | Fat (%) | Protein (%) | Carbohydrates (%) | Energy Value (kcal 100 g−1) |
|---|---|---|---|---|---|---|
| Control | 4.21 ± 0.04 a | 0.44 ± 0.01 a | 20.02 ± 0.09 a | 3.52 ± 0.02 a | 71.81 ± 0.11 a | 481.47 |
| C20 | 4.12 ± 0.07 a | 0.57 ± 0.01 b | 20.23 ± 0.13 a | 4.41 ± 0.02 b | 70.68 ± 0.20 b | 482.37 |
| C40 | 4.02 ± 0.02 a | 0.70 ± 0.00 c | 20.96 ± 0.08 b | 4.24 ± 0.02 c | 70.08 ± 0.09 b | 485.94 |
| Compounds | Control | C20 | C40 |
|---|---|---|---|
| p-Hydroxybenzoic acid | 0.27 ± 0.00 a | 0.35 ± 0.00 b | 0.43 ± 0.00 c |
| Gallic acid | 1.01 ± 0.01 a | 1.33 ± 0.03 b | 1.53 ± 0.00 c |
| Protocatechuic acid | 0.20 ± 0.00 a | 0.23 ± 0.00 b | 0.33 ± 0.00 c |
| Chlorogenic acid | 0.23 ± 0.00 a | 0.23 ± 0.00 a | 0.23 ± 0.00 a |
| Caffeic acid | 0.07 ± 0.00 a | 0.59 ± 0.00 b | 1.33 ± 0.03 c |
| Vanillic acid | 0.05 ± 0.00 a | 0.28 ± 0.00 b | 0.78 ± 0.00 c |
| Syringic acid | 0.33 ± 0.00 a | 0.32 ± 0.00 a | 0.36 ± 0.00 a |
| Sinapic acid | 0.15 ± 0.00 a | 0.16 ± 0.00 a | 0.15 ± 0.00 a |
| p-Coumaric acid | n.d. a | 0.04 ± 0.00 b | 0.28 ± 0.00 c |
| Cinamic acid | n.d. a | 0.05 ± 0.00 b | 0.21 ± 0.00 c |
| Ferulic acid | 0.43 ± 0.00 a | 0.47 ± 0.03 a | 0.49 ± 0.00 a |
| Luteolin | 0.05 ± 0.01 a | 0.16 ± 0.00 b | 0.31 ± 0.00 c |
| Naringin | n.d. a | 0.04 ± 0.00 b | 0.09 ± 0.00 c |
| Apigenin | n.d. a | 0.05 ± 0.00 b | 0.09 ± 0.01 c |
| Compounds | Control | C20 | C40 |
|---|---|---|---|
| Aspartic acid | 0.34 ± 0.01 a | 0.40 ± 0.01 b | 0.38 ± 0.00 b |
| Threonine | 0.13 ± 0.00 a | 0.15 ± 0.00 b | 0.15 ± 0.00 b |
| Serine | 0.20 ± 0.01 a | 0.24 ± 0.01 b | 0.26 ± 0.01 b |
| Glutamic acid | 0.69 ± 0.02 a | 0.85 ± 0.02 b | 0.86 ± 0.00 b |
| Proline | 0.17 ± 0.01 a | 0.22 ± 0.00 b | 0.25 ± 0.00 c |
| Glycine | 0.17 ± 0.00 a | 0.18 ± 0.00 a | 0.17 ± 0.00 a |
| Alanine | 0.25 ± 0.02 a | 0.32 ± 0.01 a | 0.32 ± 0.01 a |
| Cysteine | n.d. | n.d. | n.d. |
| Valine | 0.21 ± 0.00 a | 0.26 ± 0.00 b | 0.25 ± 0.01 b |
| Methionine | 0.06 ± 0.01 a | 0.08 ± 0.00 b | 0.09 ± 0.01 b |
| Isoleucine | 0.12 ± 0.00 a | 0.16 ± 0.00 b | 0.17 ± 0.00 b |
| Leucine | 0.30 ± 0.00 a | 0.41 ± 0.01 b | 0.45 ± 0.00 c |
| Tyrosine | 0.04 ± 0.01 a | 0.09 ± 0.00 b | 0.07 ± 0.00 c |
| Phenylalanine | 0.16 ± 0.00 a | 0.21 ± 0.00 b | 0.21 ± 0.00 b |
| Histidine | 0.06 ± 0.00 a | 0.08 ± 0.00 b | 0.08 ± 0.01 b |
| Lysine | 0.12 ± 0.01 a | 0.13 ± 0.00 a | 0.15 ± 0.00 b |
| Arginine | 0.28 ± 0.00 a | 0.37 ± 0.01 b | 0.39 ± 0.00 c |
| ∑ | 3.3 a | 4.15 b | 4.25 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukonja, S.; Tomić, J.; Pestorić, M.; Maravić, N.; Despotović, S.; Tomičić, Z.; Kiprovski, B.; Pantelić, N.Đ. Exploring Sorghum Flour as a Sustainable Ingredient in Gluten-Free Cookie Production. Foods 2025, 14, 2668. https://doi.org/10.3390/foods14152668
Bukonja S, Tomić J, Pestorić M, Maravić N, Despotović S, Tomičić Z, Kiprovski B, Pantelić NĐ. Exploring Sorghum Flour as a Sustainable Ingredient in Gluten-Free Cookie Production. Foods. 2025; 14(15):2668. https://doi.org/10.3390/foods14152668
Chicago/Turabian StyleBukonja, Simona, Jelena Tomić, Mladenka Pestorić, Nikola Maravić, Saša Despotović, Zorica Tomičić, Biljana Kiprovski, and Nebojša Đ. Pantelić. 2025. "Exploring Sorghum Flour as a Sustainable Ingredient in Gluten-Free Cookie Production" Foods 14, no. 15: 2668. https://doi.org/10.3390/foods14152668
APA StyleBukonja, S., Tomić, J., Pestorić, M., Maravić, N., Despotović, S., Tomičić, Z., Kiprovski, B., & Pantelić, N. Đ. (2025). Exploring Sorghum Flour as a Sustainable Ingredient in Gluten-Free Cookie Production. Foods, 14(15), 2668. https://doi.org/10.3390/foods14152668











