Dietary Fiber as Prebiotics: A Mitigation Strategy for Metabolic Diseases
Abstract
1. Introduction
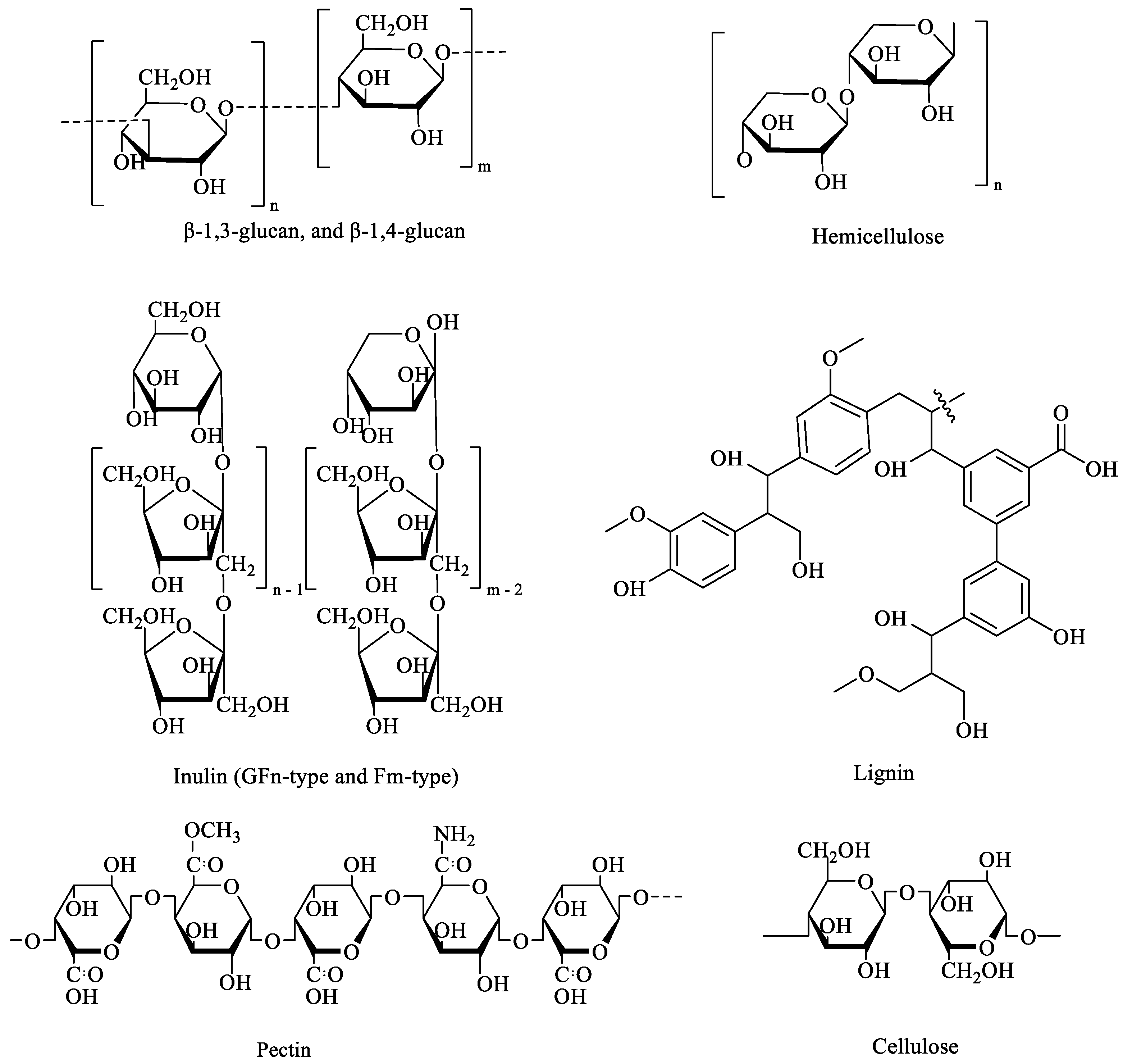
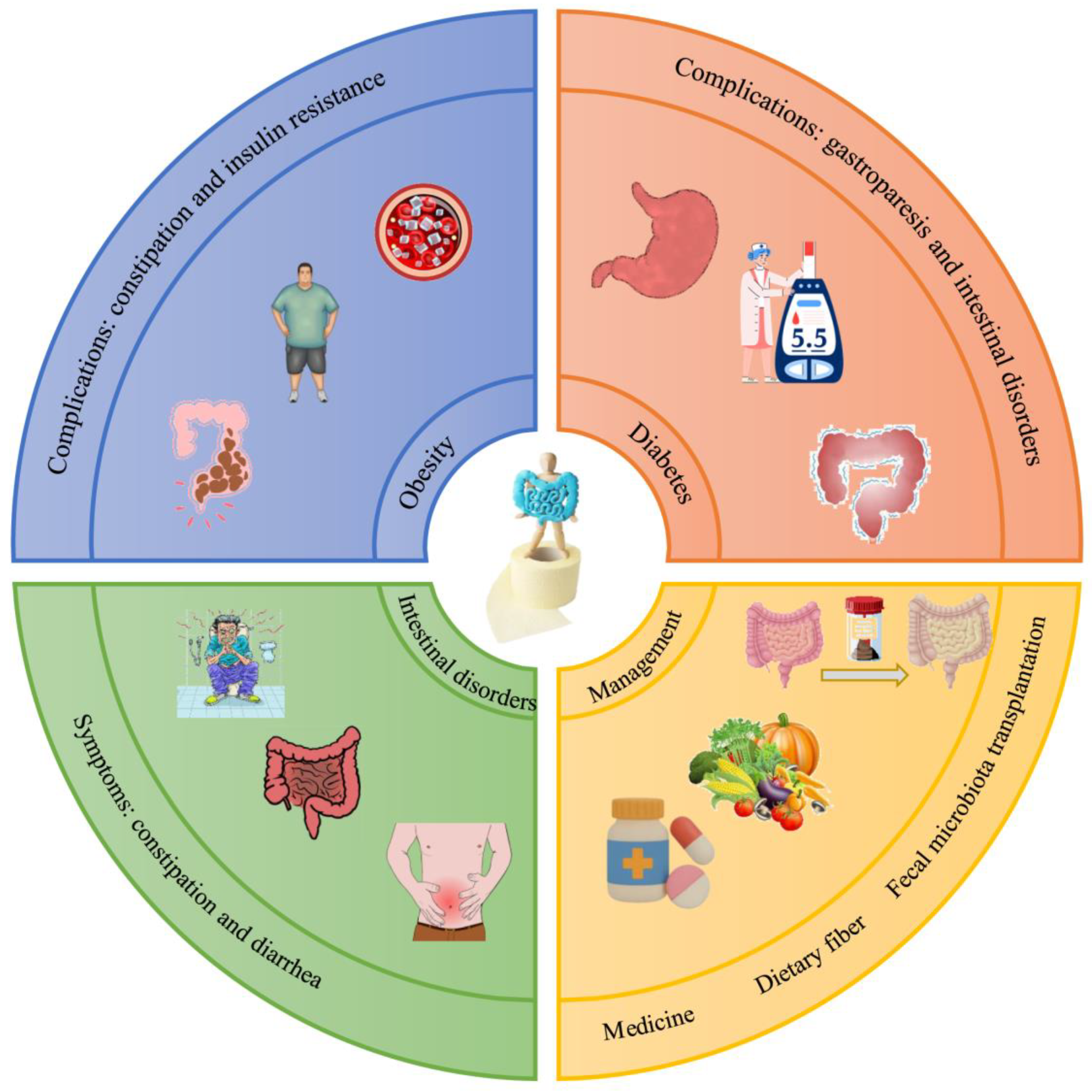
2. The Health Benefits of DF in Alleviating Metabolic Diseases
2.1. Prevention of Overweight and Obesity
2.2. Prevention of Diabetes and Reduction of Blood Glucose Levels
2.3. Prevention of CVD and Improvement of Dyslipidemia
2.4. Bowel-Function-Related Metabolic Diseases
3. DF in the Management of Intestinal Health
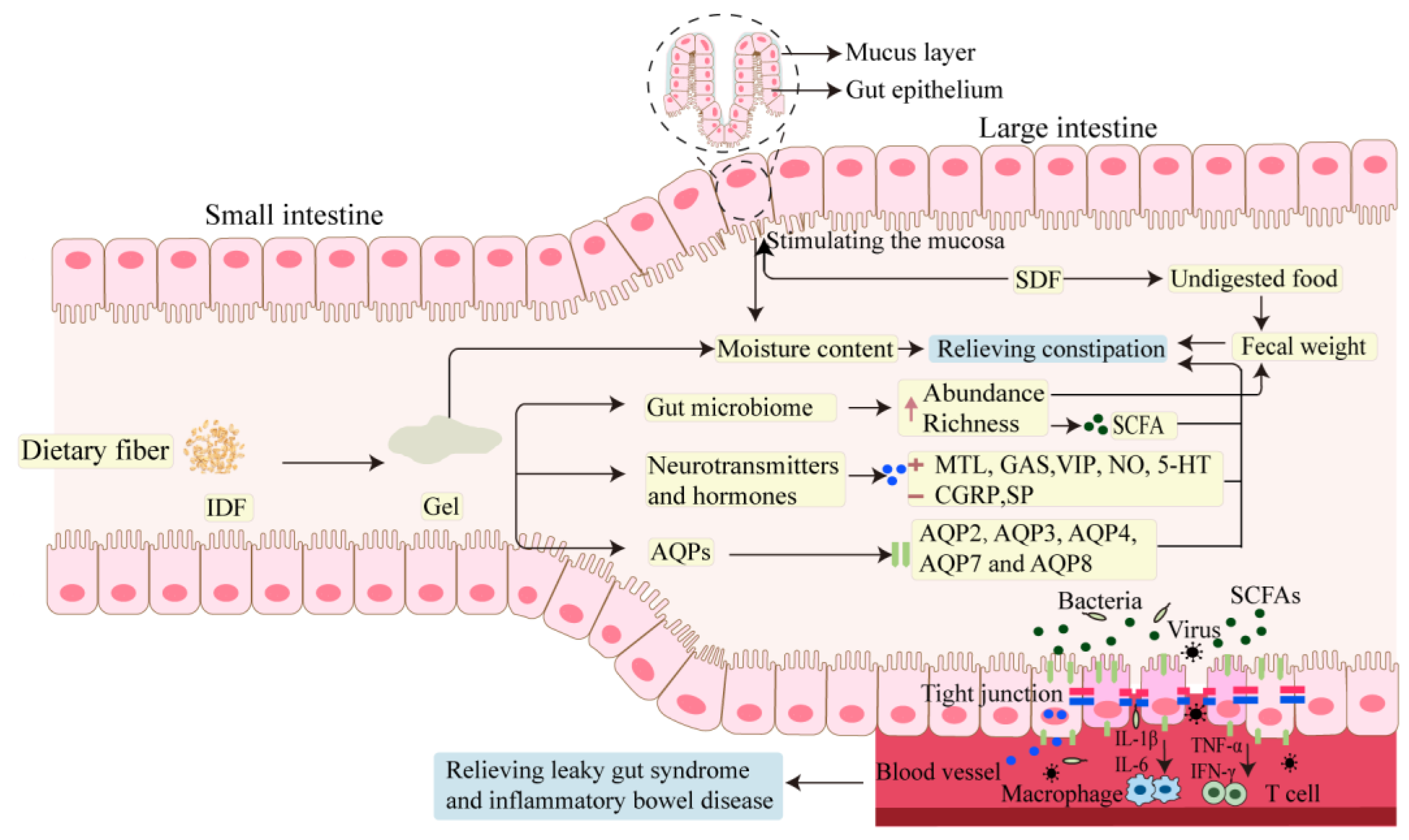
3.1. Effect of DF in Relieving Intestinal Disorder
3.2. Molecular Mechanism of DF in Relieving Intestinal Disorder
3.2.1. The Effect of DF on Gastrointestinal Neurotransmitters and Hormones
3.2.2. The Effect of DF on AQPs
3.2.3. The Effect of DF on SCFAs
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 101–116. [Google Scholar] [CrossRef]
- Tang, W.; Lin, X.Y.; Walayat, N.; Liu, J.; Zhao, P. Dietary fiber modification: Structure, physicochemical properties, bioactivities, and application—A review. Crit. Rev. Food Sci. 2023, 64, 7895–7915. [Google Scholar] [CrossRef]
- Kumar, R.; Butreddy, A.; Kommineni, N.; Reddy, P.G.; Bunekar, N.; Sarkar, C.; Dutt, S.; Mishra, V.K.; Aadil, K.R.; Mishra, Y.K.; et al. Lignin: Drug/Gene Delivery and Tissue Engineering Applications. Int. J. Nanomed. 2021, 16, 2419–2441. [Google Scholar] [CrossRef]
- Ye, S.X.; Shah, B.R.; Li, J.; Liang, H.S.; Zhan, F.C.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Guan, Z.W.; Yu, E.Z.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef]
- Han, X.B.; Ma, Y.; Ding, S.J.; Fang, J.; Liu, G. Regulation of dietary fiber on intestinal microorganisms and its effects on animal health. Anim. Nutr. 2023, 14, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Cui, L.; Qi, J.; Ojo, O.; Du, X.; Liu, Y.; Wang, X. The effect of dietary fiber (oat bran) supplement on blood pressure in patients with essential hypertension: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2458–2470. [Google Scholar] [CrossRef] [PubMed]
- Bakr, A.F.; Farag, M.A. Soluble Dietary Fibers as Antihyperlipidemic Agents: A Comprehensive Review to Maximize Their Health Benefits. ACS Omega 2023, 8, 24680–24694. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Olivares, M.; Neyrinck, A.M.; Beaumont, M.; Kjolbaek, L.; Larsen, T.M.; Benitez-Paez, A.; Romani-Perez, M.; Garcia-Campayo, V.; Bosscher, D.; et al. Nutritional interest of dietary fiber and prebiotics in obesity: Lessons from the MyNewGut consortium. Clin. Nutr. 2020, 39, 414–424. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymanska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F.J. Dietary Fiber: An Opportunity for a Global Control of Hyperlipidemia. Oxid. Med. Cell Longev. 2021, 2021, 5542342. [Google Scholar] [CrossRef]
- Beccard, S.; Bernard, J.; Wouters, R.; Gehrich, K.; Zielbauer, B.; Mezger, M.; Vilgis, T.A. Alteration of the structural properties of inulin gels. Food Hydrocolloid. 2019, 89, 302–310. [Google Scholar] [CrossRef]
- Kadyan, S.; Sharma, A.; Arjmandi, B.H.; Singh, P.; Nagpal, R. Prebiotic Potential of Dietary Beans and Pulses and Their Resistant Starch for Aging-Associated Gut and Metabolic Health. Nutrients 2022, 14, 1726. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, I.A.; Allen, A.; Pearson, J.P.; Dettmar, P.W.; Havler, M.E.; Atherton, M.R.; Onsoyen, E. Alginate as a source of dietary fiber. Crit. Rev. Food Sci. Nutr. 2005, 45, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Sun, Y.; Nie, L.; Cui, A.; Zhao, P.; Leung, W.K.; Wang, Q. Metabolic memory: Mechanisms and diseases. Signal Transduct. Target. Ther. 2024, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Ioniță-Mîndrican, C.-B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.-E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Impact of a short-term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients: A randomized placebo-controlled clinical trial. Eur. J. Nutr. 2021, 60, 655–663. [Google Scholar] [CrossRef]
- Singh, R.; Zogg, H.; Wei, L.; Bartlett, A.; Ghoshal, U.C.; Rajender, S.; Ro, S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J. Neurogastroenterol. Motil. 2021, 27, 19–34. [Google Scholar] [CrossRef]
- Wei, L.; Ji, L.; Miao, Y.; Han, X.; Li, Y.; Wang, Z.; Fu, J.; Guo, L.; Su, Y.; Zhang, Y. Constipation in DM are associated with both poor glycemic control and diabetic complications: Current status and future directions. Biomed. Pharmacother. 2023, 165, 115202. [Google Scholar] [CrossRef]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Olqi, N.A.; Al Uraimi, T. Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Veluvali, A.; Snyder, M. Dietary fiber deficiency in individuals with metabolic syndrome: A review. Curr. Opin. Clin. Nutr. 2023, 26, 564–569. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.L.; Wu, Y. Bioactive Components and Health Beneficial Properties of Whole Wheat Foods. J. Agric. Food. Chem. 2020, 68, 12904–12915. [Google Scholar] [CrossRef]
- Gona, P.N.; Kimokoti, R.W.; Gona, C.M.; Ballout, S.; Rao, S.R.; Mapoma, C.C.; Lo, J.; Mokdad, A.H. Changes in body mass index, obesity, and overweight in Southern Africa development countries, 1990 to 2019: Findings from the Global Burden of Disease, Injuries, and Risk Factors Study. Obes. Sci. Pr. 2021, 7, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, Prebiotics, Synbiotics, Postbiotics, and Obesity: Current Evidence, Controversies, and Perspectives. Curr. Obes. Rep. 2020, 9, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Huwiler, V.V.; Schönenberger, K.A.; von Brunegg, A.S.; Reber, E.; Mühlebach, S.; Stanga, Z.; Balmer, M.L. Prolonged Isolated Soluble Dietary Fibre Supplementation in Overweight and Obese Patients: A Systematic Review with Meta-Analysis of Randomised Controlled Trials. Nutrients 2022, 14, 2627. [Google Scholar] [CrossRef]
- Li, H.T.; Zhang, L.; Li, J.; Wu, Q.; Qian, L.L.; He, J.S.; Ni, Y.Q.; Kovatcheva-Datchary, P.; Yuan, R.; Liu, S.B.; et al. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat. Metab. 2024, 6, 578–579. [Google Scholar] [CrossRef]
- Jovanovski, E.; Mazhar, N.; Komishon, A.; Khayyat, R.; Li, D.; Blanco Mejia, S.; Khan, T.; L Jenkins, A.; Smircic-Duvnjak, L.; Sievenpiper, J.L.; et al. Can dietary viscous fiber affect body weight independently of an energy-restrictive diet? A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 111, 471–485. [Google Scholar] [CrossRef]
- Deehan, E.C.; Zhang, Z.X.; Riva, A.; Armet, A.M.; Perez-Muñoz, M.E.; Nguyen, N.K.; Krysa, J.A.; Seethaler, B.; Zhao, Y.Y.; Cole, J.; et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome 2022, 10, 77. [Google Scholar] [CrossRef]
- Freire, R.H.; Alvarez-Leite, J.I. Appetite control: Hormones or diet strategies? Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 328–335. [Google Scholar] [CrossRef]
- Akhlaghi, M. The role of dietary fibers in regulating appetite, an overview of mechanisms and weight consequences. Crit. Rev. Food Sci. Nutr. 2024, 64, 3139–3150. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pr. 2023, 204, 109119. [Google Scholar] [CrossRef]
- Wu, S.M.; Jia, W.; He, H.M.; Yin, J.; Xu, H.L.; He, C.Y.; Zhang, Q.Q.; Peng, Y.; Cheng, R.Y. A New Dietary Fiber Can Enhance Satiety and Reduce Postprandial Blood Glucose in Healthy Adults: A Randomized Cross-Over Trial. Nutrients 2023, 15, 4569. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, E.; Morita, S.; Nagashima, H.; Oshio, K.; Iwamoto, H.; Miyaji, K. Blood Glucose Response of a Low-Carbohydrate Oral Nutritional Supplement with Isomaltulose and Soluble Dietary Fiber in Individuals with Prediabetes: A Randomized, Single-Blind Crossover Trial. Nutrients 2022, 14, 2386. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Liu, B.; Ren, L.X.; Du, H.; Fei, C.H.; Qian, C.; Li, B.; Zhang, R.X.; Liu, H.X.; Li, Z.J.; et al. High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front. Cell Infect. Mi. 2023, 13, 1069954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.P.; Zhang, F.; Ding, X.Y.; Wu, G.J.; Lam, Y.Y.; Wang, X.J.; Fu, H.Q.; Xue, X.H.; Lu, C.H.; Ma, J.L.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151. [Google Scholar] [CrossRef]
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D.; Su, X.; Zhang, C. Effects of dietary fiber on human health. Food Sci. Hum. Wellness 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Wu, H.C.; Chiou, J.C. Potential Benefits of Probiotics and Prebiotics for Coronary Heart Disease and Stroke. Nutrients 2021, 13, 2878. [Google Scholar] [CrossRef]
- Ribas, S.A.; Cunha, D.B.; Sichieri, R.; da Silva, L.C.S. Effects of psyllium on LDL-cholesterol concentrations in Brazilian children and adolescents: A randomised, placebo-controlled, parallel clinical trial. Br. J. Nutr. 2015, 113, 134–141. [Google Scholar] [CrossRef]
- González, A.P.; Flores-Ramírez, A.; Gutiérrez-Castro, K.P.; Luévano-Contreras, C.; Gómez-Ojeda, A.; Sosa-Bustamante, G.P.; Caccavello, R.; Barrera-de León, J.C.; Garay-Sevilla, M.E.; Gugliucci, A. Reduction of small dense LDL and Il-6 after intervention with Plantago psyllium in adolescents with obesity: A parallel, double blind, randomized clinical trial. Eur. J. Pediatr. 2021, 180, 2493–2503. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Z.; Ji, X.; Zhang, W.; Luan, J.; Zahr, T.; Qiang, L. Adipokines, adiposity, and atherosclerosis. Cell Mol. Life Sci. 2022, 79, 272. [Google Scholar] [CrossRef]
- Zou, X.; Xu, X.; Chao, Z.; Jiang, X.; Zheng, L.; Jiang, B. Properties of plant-derived soluble dietary fibers for fiber-enriched foods: A comparative evaluation. Int. J. Biol. Macromol. 2022, 223, 1196–1207. [Google Scholar] [CrossRef]
- Ranaivo, H.; Thirion, F.; Bèra-Maillet, C.; Guilly, S.; Simon, C.; Sothier, M.; Van den Berghe, L.; Feugier-Favier, N.; Lambert-Porcheron, S.; Dussouse, I.; et al. Increasing the diversity of dietary fibers in a daily-consumed bread modifies gut microbiota and metabolic profile in subjects at cardiometabolic risk. Gut Microbes 2022, 14, 2044722. [Google Scholar] [CrossRef] [PubMed]
- Sabbione, A.C.; Anon, M.C.; Scilingo, A. Characterization and Bile Acid Binding Capacity of Dietary Fiber Obtained from Three Different Amaranth Products. Plant Foods Hum. Nutr. 2024, 79, 38–47. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; López-Marcos, M.C.; Fernández-López, J.; Sendra, E.; López-Vargas, J.H.; Pérez-Alvarez, J.A. Role of Fiber in Cardiovascular Diseases: A Review. Compr. Rev. Food. Sci. F 2010, 9, 240–258. [Google Scholar] [CrossRef]
- Mare, R.; Sporea, I. Gastrointestinal and Liver Complications in Patients with Diabetes Mellitus-A Review of the Literature. J. Clin. Med. 2022, 11, 5223. [Google Scholar] [CrossRef]
- Camilleri, M. Gastrointestinal motility disorders in neurologic disease. J. Clin. Investig. 2021, 131, e143771. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef]
- Gudzune, K.A.; Kushner, R.F. Medications for Obesity: A Review. JAMA 2024, 332, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Daliri, E.B.; Baltriukiene, D.; Burokas, A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Battigaglia, M.S.; Murone, E.; Dozio, E.; Pensabene, L.; Agosti, M. Dietary Fibers in Healthy Children and in Pediatric Gastrointestinal Disorders: A Practical Guide. Nutrients 2023, 15, 2208. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Gong, L.; Liu, F.; Liu, J.; Wang, J. Dietary fiber (oligosaccharide and non-starch polysaccharide) in preventing and treating functional gastrointestinal disorders—Challenges and controversies: A review. Int. J. Biol. Macromol. 2024, 258, 128835. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Cani, P.D. Interaction between obesity and the gut microbiota: Relevance in nutrition. Annu. Rev. Nutr. 2011, 31, 15–31. [Google Scholar] [CrossRef]
- Erhardt, R.; Harnett, J.E.; Steels, E.; Steadman, K.J. Functional constipation and the effect of prebiotics on the gut microbiota: A review. Br. J. Nutr. 2023, 130, 1015–1023. [Google Scholar] [CrossRef]
- Sangnes, D.A.; Lundervold, K.; Bekkelund, M.; von Volkmann, H.L.; Berentsen, B.; Gilja, O.H.; Dimcevski, G.; Softeland, E. Gastrointestinal transit and contractility in diabetic constipation: A wireless motility capsule study on diabetes patients and healthy controls. United Eur. Gastroenterol. J. 2021, 9, 1168–1177. [Google Scholar] [CrossRef]
- Ito, H.; Ito, K.; Tanaka, M.; Hokamura, M.; Tanaka, M.; Kusano, E.; Kondo, J.; Izutsu, T.; Matsumoto, S.; Inoue, H.; et al. Constipation Is a Frequent Problem Associated with Vascular Complications in Patients with Type 2 Diabetes: A Cross-sectional Study. Intern. Med. 2022, 61, 1309–1317. [Google Scholar] [CrossRef]
- Silveira, E.A.; Santos, A.S.e.A.d.C.; Ribeiro, J.N.; Noll, M.; dos Santos Rodrigues, A.P.; de Oliveira, C. Prevalence of constipation in adults with obesity class II and III and associated factors. BMC Gastroenterol. 2021, 21, 217. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, S.; Zhou, X. A causal association between obesity and constipation: A two-sample bidirectional Mendelian randomization study and meta-analysis. Front. Nutr. 2024, 11, 1430280. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Yamagata, K.; Kovesdy, C.P. Constipation in CKD. Kidney Int. Rep. 2020, 5, 121–134. [Google Scholar] [CrossRef]
- Erdur, B.; Ayar, M. The treatment of functional constipation significantly increased quality of life in children aged 4-17 years. Turk. J. Gastroenterol. 2020, 31, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Lacy, B.E. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020, 158, 1232–1249.e1233. [Google Scholar] [CrossRef]
- Caetano, A.C.; Costa, D.; Silva-Mendes, S.; Correia-Pinto, J.; Rolanda, C. Constipation: Prevalence in the Portuguese community using Rome IV-Associated factors, toilet behaviours and healthcare seeking. United Eur. Gastroent. 2022, 10, 376–384. [Google Scholar] [CrossRef]
- Milosavljevic, T.; Popovic, D.D.; Mijac, D.D.; Milovanovic, T.; Krstic, S.; Krstic, M.N. Chronic Constipation: Gastroenterohepatologist’s Approach. Dig. Dis. 2022, 40, 175–180. [Google Scholar] [CrossRef]
- Arco, S.; Saldaña, E.; Serra-Prat, M.; Palomera, E.; Ribas, Y.; Font, S.; Clavé, P.; Mundet, L. Functional Constipation in Older Adults: Prevalence, Clinical Symptoms and Subtypes, Association with Frailty, and Impact on Quality of Life. Gerontology 2022, 68, 397–406. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, S. Constipation: Pathophysiology and Current Therapeutic Approaches. In Gastrointestinal Pharmacology; Greenwood-Van Meerveld, B., Ed.; Springer: Cham, Switzerland, 2017; Volume 239, pp. 59–74. [Google Scholar]
- A M Stephen, J.H.C. The microbial contribution to human faecal mass. J. Med. Microbiol. 1980, 13, 45–56. [Google Scholar] [CrossRef]
- Monro, J.A. Quantitative management of human faecal bulking response to combinations of functionally distinct dietary fibers, using functional equivalents and a validated rat model. Int. J. Food Sci. Nutr. 2024, 75, 518–526. [Google Scholar] [CrossRef]
- Keller, E.; Laxalde, J.; Tranier, N.; von Kretschmann, P.B.; Jackson, A.; van Hoek, I. Psyllium husk powder increases defecation frequency and faecal score, bulk and moisture in healthy cats. J. Feline Med. Surg. 2024, 26, 1098612x241234151. [Google Scholar] [CrossRef]
- Monro, J.A.; Paturi, G. Kiwifruit Skin and Flesh Contributions to Fecal Bulking and Bacterial Abundance in Rats. Plant Food Hum. Nutr. 2020, 75, 525–531. [Google Scholar] [CrossRef]
- Tomlin, J.R.N. Laxative properties of indigestible plastic particles. Br. Med. J. 1988, 297, 1175–1176. [Google Scholar] [CrossRef] [PubMed]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.M.; de Vos, W.M.; Spiller, R. The Effect of Psyllium Husk on Intestinal Microbiota in Constipated Patients and Healthy Controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef] [PubMed]
- Major, G.; Murray, K.; Singh, G.; Nowak, A.; Hoad, C.L.; Marciani, L.; Silos-Santiago, A.; Kurtz, C.B.; Johnston, J.M.; Gowland, P.; et al. Demonstration of differences in colonic volumes, transit, chyme consistency, and response to psyllium between healthy and constipated subjects using magnetic resonance imaging. Neurogastroenterol. Motil. 2018, 30, e13400. [Google Scholar] [CrossRef] [PubMed]
- de Wit, N.; Esser, D.; Siebelink, E.; Fischer, A.; Sieg, J.; Mes, J. Extrinsic wheat fibre consumption enhances faecal bulk and stool frequency; a randomized controlled trial. Food Funct. 2019, 10, 646–651. [Google Scholar] [CrossRef]
- Brandl, B.; Lee, Y.M.; Dunkel, A.; Hofmann, T.; Hauner, H.; Skurk, T. Effects of Extrinsic Wheat Fiber Supplementation on Fecal Weight; A Randomized Controlled Trial. Nutrients 2020, 12, 298. [Google Scholar] [CrossRef]
- Baer, D.J.; Stote, K.S.; Henderson, T.; Paul, D.R.; Okuma, K.; Tagami, H.; Kanahori, S.; Gordon, D.T.; Rumpler, W.V.; Ukhanova, M.; et al. The Metabolizable Energy of Dietary Resistant Maltodextrin Is Variable and Alters Fecal Microbiota Composition in Adult Men. J. Nutr. 2014, 144, 1023–1029. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Peleg-Evron, O.; Davidovich-Pinhas, M.; Bianco-Peled, H. Crosslinking konjac-glucomannan with kappa-carrageenan nanogels: A step toward the design of sacrificial materials. Int. J. Biol. Macromol. 2023, 227, 654–663. [Google Scholar] [CrossRef]
- McRorie, J.W.; Fahey, G.C.; Gibb, R.D.; Chey, W.D. Laxative effects of wheat bran and psyllium: Resolving enduring misconceptions about fiber in treatment guidelines for chronic idiopathic constipation. J. Am. Assoc. Nurse Pra. 2020, 32, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, A.J.M.; Groves, C. Effect of Bran Particle-Size on Stool Weight. Gut 1978, 19, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med.-Lausanne 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.Y.; Xu, D.Q.; Yue, S.J.; Fu, R.J.; Yang, J.; Xing, L.M.; Tang, Y.P. Action Mode of Gut Motility, Fluid and Electrolyte Transport in Chronic Constipation. Front. Pharmacol. 2021, 12, 630249. [Google Scholar] [CrossRef]
- Wang, H.L. Understanding the Pathogenesis of Slow-Transit Constipation: One Step Forward. Dig. Dis. Sci. 2015, 60, 2216–2218. [Google Scholar] [CrossRef]
- Ge, Z.Y.; Duan, Z.J.; Yang, H.; Zhang, S.G.; Zhang, S.; Wang, L.X.; Yang, D.; Sun, X.Y.; Zhang, Z.F.; Su, L.P.; et al. Home-Based Transcutaneous Neuromodulation Improved Constipation via Modulating Gastrointestinal Hormones and Bile Acids. Evid.-Based Complement. Altern. Med. 2018, 2018, 2086163. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.T.; Ding, X.F.; Li, F.; Gu, J.; Zeng, F.M.; Jiang, J.; Ji, L.J. Hemp seeds attenuate loperamide-induced constipation in mice. Front. Microbiol. 2024, 15, 1353015. [Google Scholar] [CrossRef]
- Lan, J.H.; Wang, K.L.; Chen, G.Y.; Cao, G.T.; Yang, C.M. Effects of inulin and isomalto-oligosaccharide on diphenoxylate-induced constipation, gastrointestinal motility-related hormones, short-chain fatty acids, and the intestinal flora in rats. Food Funct. 2020, 11, 9216–9225. [Google Scholar] [CrossRef]
- Lu, D.D.; Pi, Y.; Ye, H.; Wu, Y.J.; Bai, Y.; Lian, S.; Han, D.D.; Ni, D.J.; Zou, X.H.; Zhao, J.B.; et al. Consumption of Dietary Fiber with Different Physicochemical Properties during Late Pregnancy Alters the Gut Microbiota and Relieves Constipation in Sow Model. Nutrients 2022, 14, 2511. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zu, Q.X.; Zhang, J.C.; Liu, S.W.; Zhang, G.H.; Chang, X.D.; Li, X.J. Soluble Dietary Fiber of Hawthorn Relieves Constipation Induced by Loperamide Hydrochloride by Improving Intestinal Flora and Inflammation, Thereby Regulating the Aquaporin Ion Pathway in Mice. Foods 2024, 13, 2220. [Google Scholar] [CrossRef]
- Guo, Y.A.; Song, L.Q.; Huang, Y.M.; Li, X.P.; Xiao, Y.C.; Wang, Z.H.; Ren, Z.H. Furu2019 and stachyose as probiotics, prebiotics, and synbiotics alleviate constipation in mice. Front Nutr. 2023, 9, 1039403. [Google Scholar] [CrossRef]
- Yang, Z.D.; Ye, S.M.; Xu, Z.M.; Su, H.H.; Tian, X.; Han, B.; Shen, B.C.; Liao, Q.F.; Xie, Z.Y.; Hong, Y.J. Dietary synbiotic ameliorates constipation through the modulation of gut microbiota and its metabolic function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef]
- Lin, C.H.; He, H.Q.; Kim, J.J.; Zheng, X.; Huang, Z.H.; Dai, N. Osmotic pressure induces translocation of aquaporin-8 by P38 and JNK MAPK signaling pathways in patients with functional constipation. Dig. Liver Dis. 2023, 55, 1049–1059. [Google Scholar] [CrossRef]
- Cai, T.; Dong, Y.; Feng, Z.Y.; Cai, B. Ameliorative effects of the mixed aqueous extract of Aurantii Fructus Immaturus and Magnoliae Officinalis Cortex on loperamide-induced STC mice. Heliyon 2024, 10, e33705. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.K.; Peng, P.; Zhang, J.; Du, M.Y.; Lan, L.X.; Qian, Y.; Zhou, J.; Zhao, X. CQPC02-Fermented Soybean Milk Improves Loperamide-Induced Constipation in Mice. J. Med. Food 2019, 22, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Jiang, H.; Wang, L.J.; Gan, H.P.; Xiao, X.C.; Huang, L.W.; Li, W.X.; Li, Z.R. Luteolin ameliorates loperamide-induced functional constipation in mice. Braz. J. Med. Biol. Res. 2023, 56, e12466. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Youguang, X.; Kai, H.; Weiwei, Q.; Qing, L.H.; Qing, Z.; Jingbo, X. Hetong decoction relieves loperamide-induced constipation in rats by regulating expression of aquaporins. J. Tradit. Chin. Med. 2023, 43, 1160–1167. [Google Scholar]
- Kon, R.; Ikarashi, N.; Onuma, K.; Yasukawa, Z.; Ozeki, M.; Sakai, H.; Kamei, J. Effect of partially hydrolyzed guar gum on the expression of aquaporin-3 in the colon. Food Sci. Nutr. 2023, 11, 1127–1133. [Google Scholar] [CrossRef]
- Zhai, X.C.; Lin, D.H.; Zhao, Y.; Yang, X.B. Bacterial Cellulose Relieves Diphenoxylate-Induced Constipation in Rats. J. Agric. Food Chem. 2018, 66, 4106–4117. [Google Scholar] [CrossRef]
- Cong, L.; Duan, L.W.; Su, W.P.; Hao, S.H.; Li, D.F. Efficacy of High Specific Volume Polysaccharide—A New Type of Dietary Fiber—On Molecular Mechanism of Intestinal Water Metabolism in Rats With Constipation. Med. Sci. Monit. 2019, 25, 5028–5035. [Google Scholar] [CrossRef]
- Wang, J.K.; Yao, S.K. Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation. Evid.-Based Complement. Altern. Med. 2021, 2021, 5560310. [Google Scholar] [CrossRef]
- Deehan, E.C.; Mocanu, V.; Madsen, K.L. Effects of dietary fibre on metabolic health and obesity. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 301–318. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, D.; Gao, X.Y.; Jiang, Y.; Yu, T.; Jiang, L.Q.; Tang, Y.R. Association between fecal short-chain fatty acid levels and constipation severity in subjects with slow transit constipation. Eur. J. Gastroen. Hepat. 2024, 36, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, E.; Theunissen, R.; Roux, S. Short chain fatty acids and monocarboxylate transporters in irritable bowel syndrome. Turk. J. Gastroenterol. 2020, 31, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Li, M.Y.; Jian, C.; Wei, F.X.; Liu, H.L.; Li, K.; Qin, X.M. Polysaccharide Alleviates Constipation in the Elderly Via Modification of Gut Microbiota and Fecal Metabolism. Rejuv. Res. 2022, 25, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Guo, R.; Sun, X.B.; Kou, Y.X.; Ma, X.; Chen, Y.A.; Song, L.H.; Yuan, C.M.; Wu, Y. Xylooligosaccharides from corn cobs alleviate loperamide-induced constipation in mice modulation of gut microbiota and SCFA metabolism. Food Funct. 2023, 14, 8734–8746. [Google Scholar] [CrossRef]
- Wu, L.; Tang, C.H.; Chen, L.L.; Zhao, J.Y. Modified dietary fiber from soybean dregs by fermentation alleviated constipation in mice. Food Chem.-X 2023, 19, 100810. [Google Scholar] [CrossRef]
- Wang, L.L.; Cen, S.; Wang, G.; Lee, Y.K.; Zhao, J.X.; Zhan, H.; Chen, W. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Sinha, A.K.; Laursen, M.F.; Brinck, J.E.; Rybtke, M.L.; Hjorne, A.P.; Procházková, N.; Pedersen, M.; Roager, H.M.; Licht, T.R. Dietary fibre directs microbial tryptophan metabolism via metabolic interactions in the gut microbiota. Nat. Microbiol. 2024, 9, 1964–1978. [Google Scholar] [CrossRef]
- Duan, T.C.; Wang, X.Y.; Dong, X.Y.; Wang, C.N.; Wang, L.; Yang, X.B.; Li, T. Broccoli-Derived Exosome-like Nanoparticles Alleviate Loperamide-Induced Constipation, in Correlation with Regulation on Gut Microbiota and Tryptophan Metabolism. J. Agric. Food Chem. 2023, 71, 16568–16580. [Google Scholar] [CrossRef]
- Li, X.R.; Liu, C.J.; Tang, X.D.; Zhang, H.M.; Luo, Y.Y.; Zhang, L.; Yang, E. Gut Microbiota Alterations from Three-Strain Yogurt Formulation Treatments in Slow-Transit Constipation. Can. J. Infect. Dis. Med. 2020, 2020, 4583973. [Google Scholar] [CrossRef]
- Wang, L.; Lv, W.Q.; Yang, J.T.; Lin, X.; Liu, H.M.; Tan, H.J.; Quan, R.P.; Long, P.P.; Shen, H.; Shen, J.; et al. Enteric nervous system damage caused by abnormal intestinal butyrate metabolism may lead to functional constipation. Front. Microbiol. 2023, 14, 1117905. [Google Scholar] [CrossRef]
- Peng, A.W.; Juraschek, S.P.; Appel, L.J.; Miller, E.R., 3rd; Mueller, N.T. Effects of the DASH Diet and Sodium Intake on Bloating: Results From the DASH-Sodium Trial. Am. J. Gastroenterol. 2019, 114, 1109–1115. [Google Scholar] [CrossRef]
- Ho, K.S.; Tan, C.Y.; Mohd Daud, M.A.; Seow-Choen, F. Stopping or reducing dietary fiber intake reduces constipation and its associated symptoms. World J. Gastroenterol. 2012, 18, 4593–4596. [Google Scholar] [CrossRef]
| Diagnostic Criteria for FC | Diagnostic Criteria for IBS-C | Diagnostic Criteria for DD |
|---|---|---|
| 1. Must include ≥2 of the following: | 1. Recurrent abdominal pain at least 1 day/week with ≥2 of the following: | 1. The patient satisfies diagnostic criteria for FC and/or IBS-C. |
| a. >25% of defecations will be strained. | ||
| b. Lumpy or hard feces > 25% of defecations. | a. Related to defecation. | 2. During repeated attempts to defecate, the patient must have ≥2 of the following: |
| c. >25% of defecations feel like incomplete evacuation. | b. Related to change in frequency of stools. | |
| d. >25% of defecations feel anorectalobstruction/obstruclion. | c. Related to change in form of stools. | a. Abnormal balloon expulsion test. |
| e. Manual maneuvers to facilitate >25% of defecations. | 2. Lumpy or hard stools > 25% of defecations. | b. Abnormal anorectal evacuation pattern with manometry or anal surface electromiography. |
| f. Spontaneous defecations < 3/week. | ||
| 2. Loose stools are rarely present without the use of laxatives. | c. Impaired rectal evacuation by imaging. | |
| 3. Insufficient criteria for IBS. |
| Subjects | Number | Study Design | Intervention | Comparator | Duration | Outcomes | References |
|---|---|---|---|---|---|---|---|
| Adult rats | n = 8, males | Crossover | Diet with 10% wheat bran, adding different doses of psyllium/psyllium/guar gum/raftilose | Diet with 10% wheat bran | 7 days | Increased fecal hydration capacity, increasing by 2.4 ± 0.29 g per gram of wheat bran ingested, and by 15.6 ± 1.52 g per g of psyllium | [71] |
| Healthy adult cats | Female (n = 6) and male (n = 3) | RCT | Dry extruded diet containing 6% psyllium | Dry extruded diet containing 6% cellulose | 10 days | The mean fecal score was higher (p < 0.0001) for the control vs. intervention group; the total fecal wet weight (p = 0.0003) and fecal moisture (%) were also higher (p = 0.0426) for the intervention group | [72] |
| Rats | n = 8 | Crossover RCT | Daily diet, adding skin or flesh of four kiwifruit cultivars/wheat bran | Daily diet | 7 days | Increasing the abundance of Lachnospiraceae and Lactobacillus spp. and three kiwifruit cultivars increased the fecal dry weight (p < 0.001) | [73] |
| Healthy adults | n = 10, males | Crossover | Normal diet + 37.5 g wheat bran | Normal diet | 10 days | Improved fecal weight (p < 0.05) and reduced gut transit time in intervention compared to normal diet (p < 0.05) | [74] |
| Healthy adults and constipatied patients | Healthy adults (n = 8), adults with chronic constipation (n = 16) | RCT | Diet with psyllium | Diet with maltodextrin | 7 days | Increased fecal water content in the control group of constipated patients and increased Lachnospira, Roseburia, and Faecalibacterium in healthy adults, with Veillonella and Subdoligranulum showing changes | [75] |
| Healthy adults and constipatied patients | Healthy adults (n = 9), constipated patients (n = 24) | Crossover RCT | Patients took maltodextrin (placebo) and psyllium 7 g | Controls group took three treatments in randomized order—placebo, psyllium 3.5 g, and 7 g | 6 days | Increased fasting colonic volumes (p < 0.05) and mean postprandial small bowel water in control and intervention groups after taking 7 g of psyllium | [76] |
| Healthy adults | n = 16, males | Crossover RCT | Normal diet with different doses of extrinsic wheat fiber | Normal diet | 10 days | Increased feces wet and dry weight compared to control (p < 0.01) and increased stool frequency from 1.1 ± 0.1 defecations per day to 1.3 ± 0.1 defecations per day (p < 0.05) | [77] |
| Healthy adults | Female (n = 5) and male (n = 5) | Crossover RCT | Normal diet with 10 g wheat fiber | Normal diet | 5 days | Increased fecal wet weight (p < 0.05) | [78] |
| Healthy adults | n = 14, males | Crossover RCT | 25 g/d RM + 25 g/d placebo and 50 g/d RM + 0 g/d placebo | 50 g/d placebo | 24 days | Increased fecal wet weight (p < 0.0001) and fecal dry weight (p < 0.0001) compared with the placebo group, and total counts of fecal bacteria increased by 12% (p = 0.17) and 18% (p = 0.019), respectively | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Hu, S.; Liu, Y.; De Alwis, S.A.S.S.; Yu, Y.; Li, Z.; Wang, Z.; Liu, J. Dietary Fiber as Prebiotics: A Mitigation Strategy for Metabolic Diseases. Foods 2025, 14, 2670. https://doi.org/10.3390/foods14152670
Gao X, Hu S, Liu Y, De Alwis SASS, Yu Y, Li Z, Wang Z, Liu J. Dietary Fiber as Prebiotics: A Mitigation Strategy for Metabolic Diseases. Foods. 2025; 14(15):2670. https://doi.org/10.3390/foods14152670
Chicago/Turabian StyleGao, Xinrui, Sumei Hu, Ying Liu, S. A. Sanduni Samudika De Alwis, Ying Yu, Zhaofeng Li, Ziyuan Wang, and Jie Liu. 2025. "Dietary Fiber as Prebiotics: A Mitigation Strategy for Metabolic Diseases" Foods 14, no. 15: 2670. https://doi.org/10.3390/foods14152670
APA StyleGao, X., Hu, S., Liu, Y., De Alwis, S. A. S. S., Yu, Y., Li, Z., Wang, Z., & Liu, J. (2025). Dietary Fiber as Prebiotics: A Mitigation Strategy for Metabolic Diseases. Foods, 14(15), 2670. https://doi.org/10.3390/foods14152670







