Techno-Functional and Nutraceutical Assessment of Unprocessed and Germinated Amaranth Flours and Hydrolysates: Impact of the Reduction of Hydrolysis Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Amaranth Germination
2.3. Enzymatic Hydrolysis
2.4. Degree of Hydrolysis (DH)
2.5. Soluble Protein Quantification
2.6. SDS-PAGE
2.7. Total Phenolic Compounds (TPCs)
2.8. ORAC
2.9. Anti-Inflammatory Activity
2.10. Techno-Functional Properties
2.10.1. Water Absorption Index (WAI) and Water Solubility Index (WSI)
2.10.2. Oil Absorption Index (OAI)
2.10.3. Emulsion Activity Index (EAI)
2.10.4. pH
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Germination and Enzymatic Hydrolysis on the Degree of Hydrolysis and Amaranth Protein Profile
3.2. Effect of Germination and Enzymatic Hydrolysis on Bioactive Content and Nutraceutical Potential in Amaranth Flours/Hydrolysates
3.3. Effect of Germination and Enzymatic Hydrolysis on the Techno-Functional Properties in Amaranth Flours/Hydrolysates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montoya-Rodríguez, A.; Gómez-Favela, M.A.; Reyes-Moreno, C.; Milán-Carrillo, J.; González de Mejía, E. Identification of Bioactive Peptide Sequences from Amaranth (Amaranthus hypochondriacus) Seed Proteins and Their Potential Role in the Prevention of Chronic Diseases. Compr. Rev. Food Sci. Food Saf. 2015, 14, 139–158. [Google Scholar] [CrossRef]
- Sandoval-Sicairos, E.S.; Milán-Noris, A.K.; Luna-Vital, D.A.; Milán-Carrillo, J.; Montoya-Rodríguez, A. Anti-inflammatory and antioxidant effects of peptides released from germinated amaranth during in vitro simulated gastrointestinal digestion. Food Chem. 2021, 343, 128394. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, G.; Schneider, R.G. The Role of Amaranth, Quinoa, and Millets for the Development of Healthy, Sustainable Food Products–A Concise Review. Foods 2022, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Tye-Din, J.A.; Galipeau, H.J.; Agardh, D. Celiac Disease: A Review of Current Concepts in Pathogenesis, Prevention, and Novel Therapies. Front. Pediatr. 2018, 6, 350. [Google Scholar] [CrossRef]
- Chauhan, A.; Saxena, D.C.; Singh, S. Total dietary fibre and antioxidant activity of gluten free cookies made from raw and germinated amaranth (Amaranthus spp.) flour. LWT-Food Sci. Technol. 2015, 63, 939–945. [Google Scholar] [CrossRef]
- Carmona-Garcia, R.; Agama-Acevedo, E.; Pacheco-Vargas, G.; Bello-Perez, L.A.; Tovar, J.; Alvarez-Ramirez, J. Pregelatinised amaranth flour as an ingredient for low-fat gluten-free cakes. Int. J. Food Sci. Technol. 2022, 57, 2346–2355. [Google Scholar] [CrossRef]
- Yeşil, S.; Levent, H. The influence of fermented buckwheat, quinoa and amaranth flour on gluten-free bread quality. LWT 2022, 160, 113301. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, A.; de Mejía, E.G.; Dia, V.P.; Reyes-Moreno, C.; Milán-Carrillo, J. Extrusion improved the anti-inflammatory effect of amaranth (Amaranthus hypochondriacus) hydrolysates in LPS-induced human THP-1 macrophage-like and mouse RAW 264.7 macrophages by preventing activation of NF-κB signaling. Mol. Nutr. Food Res. 2014, 58, 1028–1041. [Google Scholar] [CrossRef]
- Milan-Carrillo, J.; Montoya-Rodríguez, A.; Reyes Moreno, C. High-Antioxidant Capacity Beverages Based on Extruded and Roasted Amaranth (Amaranthus hypochondriacus) Flour. ACS Symp. Ser. 2012, 1109, 199–216. [Google Scholar] [CrossRef]
- Sandoval-Sicairos, E.S.; Domínguez-Rodríguez, M.; Montoya-Rodríguez, A.; Milán-Noris, A.K.; Reyes-Moreno, C.; Milán-Carrillo, J. Phytochemical Compounds and Antioxidant Activity Modified by Germination and Hydrolysis in Mexican Amaranth. Plant Foods Hum. Nutr. 2020, 75, 192–199. [Google Scholar] [CrossRef]
- Pilco-Quesada, S.; Tian, Y.; Yang, B.; Repo-Carrasco-Valencia, R.; Suomela, J.-P. Effects of germination and kilning on the phenolic compounds and nutritional properties of quinoa (Chenopodium quinoa) and kiwicha (Amaranthus caudatus). J. Cereal Sci. 2020, 94, 102996. [Google Scholar] [CrossRef]
- Gupta, A.; Sharama, S.; Singh, B. Influence of Germination Conditions on the Techno-functional Properties of Amaranth flour. In Proceedings of the International Conference of Food Propierties (ICFP 2018), Sharjah, United Arab Emirates, 22–24 January 2018. [Google Scholar]
- Cornejo, F.; Novillo, G.; Villacrés, E.; Rosell, C.M. Evaluation of the physicochemical and nutritional changes in two amaranth species (Amaranthus quitensis and Amaranthus caudatus) after germination. Food Res. Int. 2019, 121, 933–939. [Google Scholar] [CrossRef]
- Nisov, A.; Ercili-Cura, D.; Nordlund, E. Limited hydrolysis of rice endosperm protein for improved techno-functional properties. Food Chem. 2020, 302, 125274. [Google Scholar] [CrossRef]
- Alarcón-García, M.A.; Perez-Alvarez, J.A.; López-Vargas, J.H.; Pagán-Moreno, M.J. Techno-Functional Properties of New Andean Ingredients: Maca (Lepidium meyenii) and Amaranth (Amaranthus caudatus). Proceedings 2021, 70, 74. [Google Scholar] [CrossRef]
- Badia-Olmos, C.; Laguna, L.; Haros, C.M.; Tárrega, A. Techno-Functional and Rheological Properties of Alternative Plant-Based Flours. Foods 2023, 12, 1411. [Google Scholar] [CrossRef] [PubMed]
- Gamel, T.H.; Linssen, J.P.; Mesallam, A.S.; Damir, A.A.; Shekib, L.A. Seed treatments affect functional and antinutritional properties of amaranth flours. J. Sci. Food Agric. 2006, 86, 1095–1102. [Google Scholar] [CrossRef]
- Soria-Hernández, C.; Serna-Saldívar, S.; Chuck-Hernández, C. Physicochemical and Functional Properties of Vegetable and Cereal Proteins as Potential Sources of Novel Food Ingredients. Food Technol. Biotechnol. 2015, 53, 269–277. [Google Scholar] [CrossRef]
- Shannon, E.; Hayes, M. Alaria esculenta, Ulva lactuca, and Palmaria palmata as Potential Functional Food Ingredients for the Management of Metabolic Syndrome. Foods 2025, 14, 284. [Google Scholar] [CrossRef] [PubMed]
- Du, S.-K.; Jiang, H.; Yu, X.; Jane, J.-L. Physicochemical and functional properties of whole legume flour. LWT-Food Sci. Technol. 2014, 55, 308–313. [Google Scholar] [CrossRef]
- Paredes-López, O.; Ordorica-Falomir, C.; Olivares-Vázquez, M.R. Chickpea Protein Isolates: Physicochemical, Functional and Nutritional Characterization. J. Food Sci. 1991, 56, 726–729. [Google Scholar] [CrossRef]
- Chen, B.; Pangloli, P.; Dia, V.P. Comparative Physicochemical and Functional Analyses of Protein Ingredients and Their Enzymatic Hydrolysates from Industrial Hempseed (Cannabis sativa L.) Hearts. ACS Food Sci. Technol. 2024, 4, 1480–1489. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Serna-Saldívar, S.O. Functionality and characterization of kafirin-rich protein extracts from different whole and decorticated sorghum genotypes. J. Cereal Sci. 2016, 70, 57–65. [Google Scholar] [CrossRef]
- Perales-Sánchez, J.X.K.; Reyes-Moreno, C.; Gómez-Favela, M.A.; Milán-Carrillo, J.; Cuevas-Rodríguez, E.O.; Valdez-Ortiz, A.; Gutiérrez-Dorado, R. Increasing the Antioxidant Activity, Total Phenolic and Flavonoid Contents by Optimizing the Germination Conditions of Amaranth Seeds. Plant Foods Hum. Nutr. 2014, 69, 196–202. [Google Scholar] [CrossRef]
- Guardado-Félix, D.; Serna-Saldivar, S.O.; Cuevas-Rodríguez, E.O.; Jacobo-Velázquez, D.A.; Gutiérrez-Uribe, J.A. Effect of sodium selenite on isoflavonoid contents and antioxidant capacity of chickpea (Cicer arietinum L.) sprouts. Food Chem. 2017, 226, 69–74. [Google Scholar] [CrossRef] [PubMed]
- de Souza Rocha, T.; Hernandez, L.M.R.; Chang, Y.K.; de Mejía, E.G. Impact of germination and enzymatic hydrolysis of cowpea bean (Vigna unguiculata) on the generation of peptides capable of inhibiting dipeptidyl peptidase IV. Food Res. Int. 2014, 64, 799–809. [Google Scholar] [CrossRef]
- Conforti, F.; Menichini, F. Phenolic Compounds from Plants as Nitric Oxide Production Inhibitors. Curr. Med. Chem. 2011, 18, 1137–1145. [Google Scholar] [CrossRef]
- de la Barca, A.M.C.; Rojas-Martínez, M.E.; Islas-Rubio, A.R.; Cabrera-Chávez, F. Gluten-Free Breads and Cookies of Raw and Popped Amaranth Flours with Attractive Technological and Nutritional Qualities. Plant Foods Hum. Nutr. 2010, 65, 241–246. [Google Scholar] [CrossRef]
- Cornejo, F.; Rosell, C.M. Influence of germination time of brown rice in relation to flour and gluten free bread quality. J. Food Sci. Technol. 2015, 52, 6591–6598. [Google Scholar] [CrossRef]
- Jan, R.; Saxena, D.C.; Singh, S. Effect of Germination on Nutritional, Functional, Pasting, and Microstructural Properties of Chenopodium (Chenopodium album) Flour. J. Food Process. Preserv. 2017, 41, e12959. [Google Scholar] [CrossRef]
- Sibian, M.S.; Saxena, D.C.; Riar, C.S. Effect of germination on chemical, functional and nutritional characteristics of wheat, brown rice and triticale: A comparative study. J. Sci. Food Agric. 2017, 97, 4643–4651. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Physicochemical, Pasting, and Functional Properties of Amaranth Seed Flours: Effects of Lipids Removal. J. Food Sci. 2014, 79, C1271–C1277. [Google Scholar] [CrossRef]
- Chavan, U.D.; McKenzie, D.B.; Shahidi, F. Functional properties of protein isolates from beach pea (Lathyrus maritimus L.). Food Chem. 2001, 74, 177–187. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; O’Brien, N.M. Protein Hydrolysates from Agricultural Crops—Bioactivity and Potential for Functional Food Development. Agriculture 2013, 3, 112–130. [Google Scholar] [CrossRef]
- Aluko, R.E.; Monu, E. Functional and Bioactive Properties of Quinoa Seed Protein Hydrolysates. J. Food Sci. 2003, 68, 1254–1258. [Google Scholar] [CrossRef]
- Horax, R.; Vallecios, M.S.; Hettiarachchy, N.; Osorio, L.F.; Chen, P. Solubility, functional properties, ACE-I inhibitory and DPPH scavenging activities of Alcalase hydrolysed soy protein hydrolysates. Int. J. Food Sci. Technol. 2017, 52, 196–204. [Google Scholar] [CrossRef]
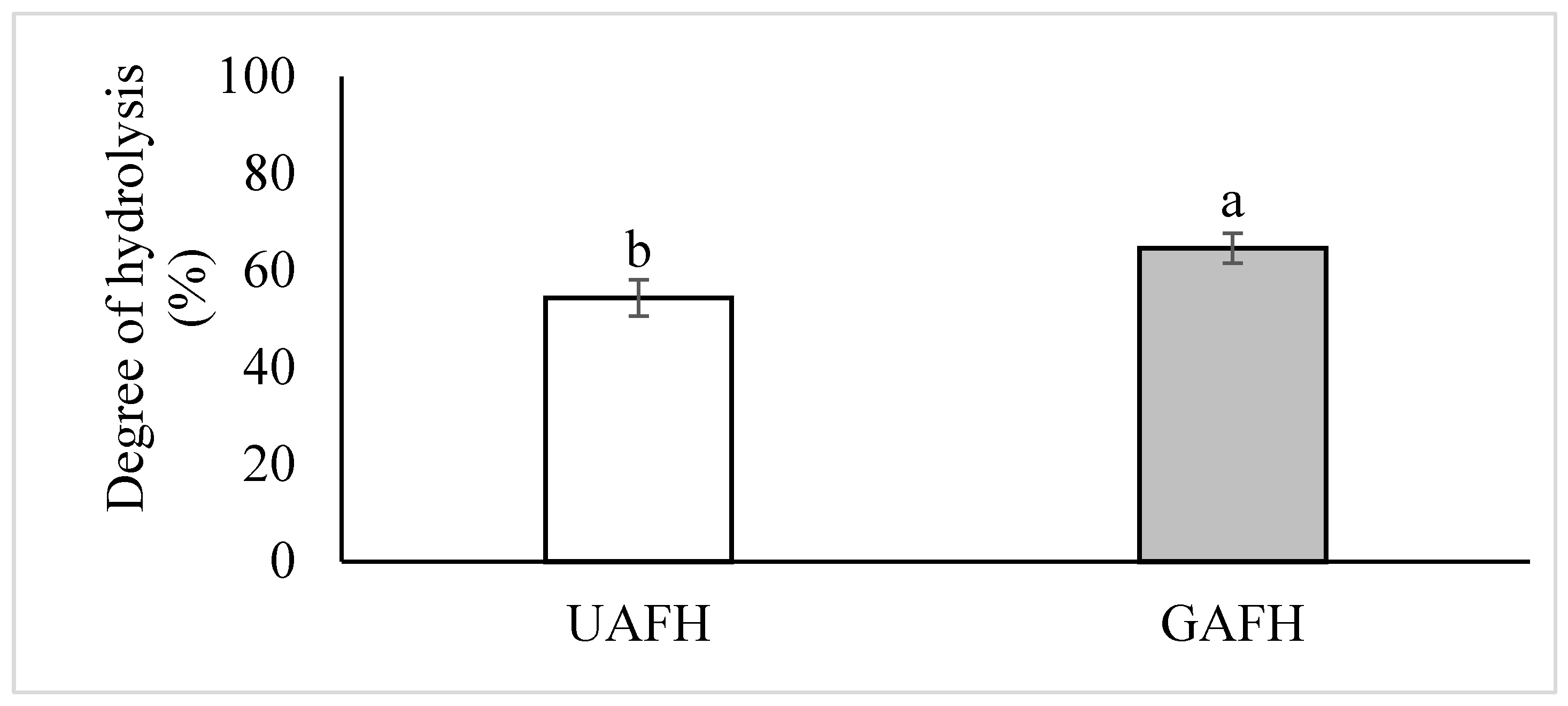
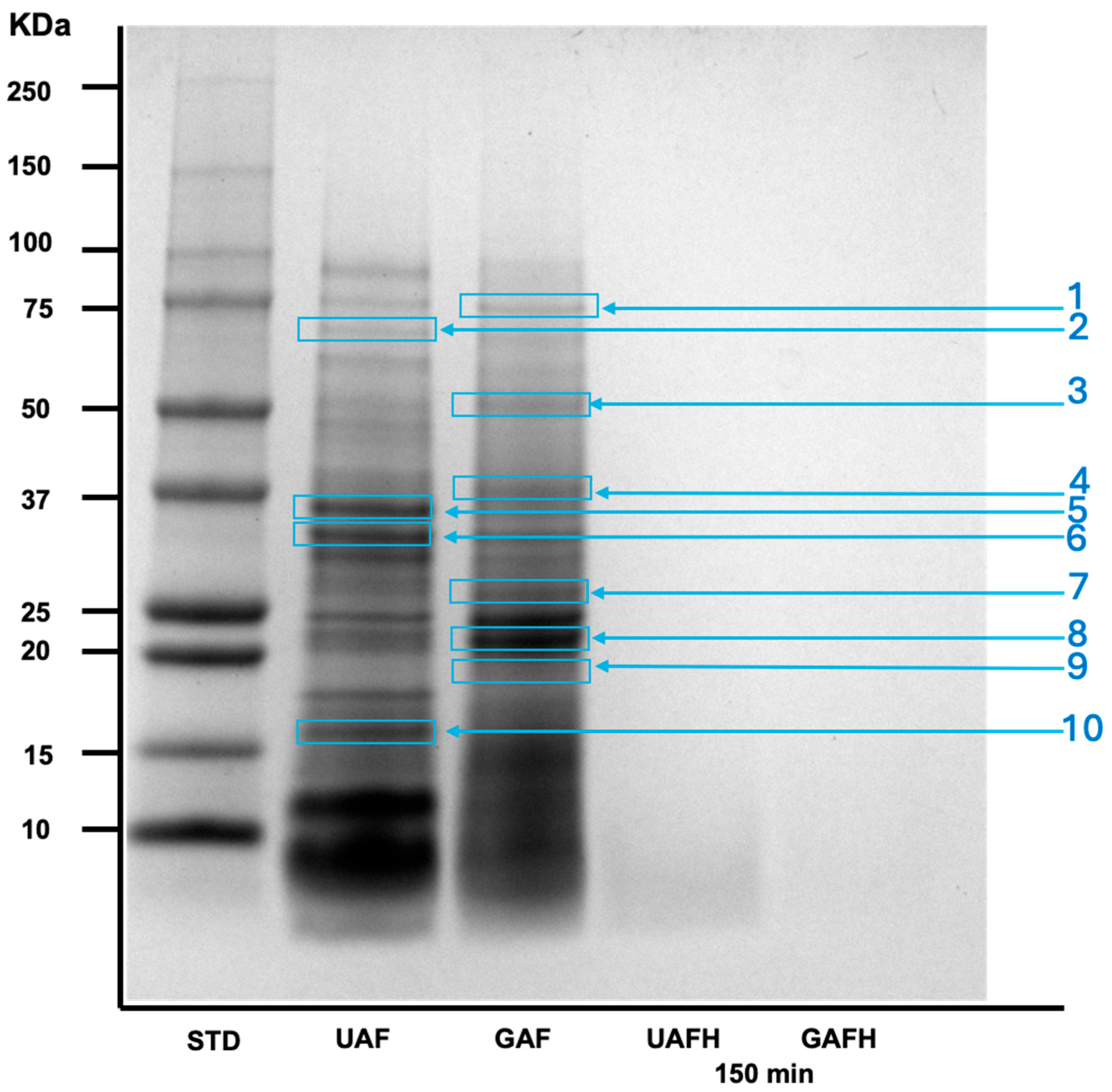
| Parameter | Flour (F) | Hydrolysate 1 (FH) | ||
|---|---|---|---|---|
| SP (mg/g) | UA | 82.47 ± 0.76 Bb | 131.52 ± 8.96 Aa | (p < 0.0007) |
| GA | 92.80 ± 1.32 Ba | 144.68 ± 3.40 Aa | (p < 0.0001) | |
| (p < 0.0003) | NS | |||
| TPC (mg GAE/100 g) | UA | 21.22 ± 1.14 Bb | 301.69 ± 1.23 Ab | (p < 0.0001) |
| GA | 26.25 ± 0.75 Ba | 388.64 ± 6.21 Aa | (p < 0.0001) | |
| (p < 0.0031) | (p < 0.0001) | |||
| Aox (mmol TE/100 g) | UA | 12.5 ± 0.2 Bb | 38.93 ± 0.58 Aa | (p < 0.0001) |
| GA | 34.6 ± 0.4 Ba | 43.20 ± 2.54 Aa | (p < 0.0092) | |
| (p < 0.0001) | NS | |||
| AIA (% NOI) | UA | ND | 41.63 ± 0.84 a | |
| GA | ND | 44.29 ± 1.69 a | ||
| NS |
| Parameter | Flour (F) | Hydrolysate 1 (FH) | ||
|---|---|---|---|---|
| WAI (g/g) | UA | 5.47 ± 0.05 Aa | 0.47 ± 0.02 Bb | (p < 0.0001) |
| GA | 3.77 ± 0.05 Ab | 0.70 ± 0.03 Ba | (p < 0.0001) | |
| (p < 0.0001) | (p < 0.0004) | |||
| WSI (%) | UA | 13.60 ± 0.46 Bb | 97.47 ± 0.60 Aa | (p < 0.0001) |
| GA | 48.90 ± 2.15 Ba | 98.28 ± 0.39 Aa | (p < 0.0001) | |
| (p < 0.0001) | NS | |||
| OAI (mL/g) | UA | 1.23 ± 0.03 Bb | 1.41 ± 0.01 Aa | (p < 0.0006) |
| GA | 1.55 ± 0.06 Aa | 0.86 ± 0.08 Bb | (p < 0.0003) | |
| (p < 0.0012) | (p < 0.0003) | |||
| EAI (m2/g) | UA | 2.24 ± 0.08 Aa | 1.62 ± 0.04 Ba | (p < 0.0003) |
| GA | 2.38 ± 0.21 Aa | 1.69 ± 0.04 Ba | (p < 0.005) | |
| NS | NS | |||
| pH | UA | 6.56 ± 0.00 Aa | 4.03 ± 0.00 Bb | (p < 0.0001) |
| GA | 5.72 ± 0.06 Ab | 4.06 ± 0.00 Ba | (p < 0.0001) | |
| (p < 0.0001) | (p < 0.0001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Rodríguez, A.; Domínguez-Rodríguez, M.; Sandoval-Sicairos, E.S.; Milán-Noris, E.M.; Milán-Carrillo, J.; Milán-Noris, A.K. Techno-Functional and Nutraceutical Assessment of Unprocessed and Germinated Amaranth Flours and Hydrolysates: Impact of the Reduction of Hydrolysis Time. Foods 2025, 14, 2666. https://doi.org/10.3390/foods14152666
Montoya-Rodríguez A, Domínguez-Rodríguez M, Sandoval-Sicairos ES, Milán-Noris EM, Milán-Carrillo J, Milán-Noris AK. Techno-Functional and Nutraceutical Assessment of Unprocessed and Germinated Amaranth Flours and Hydrolysates: Impact of the Reduction of Hydrolysis Time. Foods. 2025; 14(15):2666. https://doi.org/10.3390/foods14152666
Chicago/Turabian StyleMontoya-Rodríguez, Alvaro, Maribel Domínguez-Rodríguez, Eslim Sugey Sandoval-Sicairos, Evelia Maria Milán-Noris, Jorge Milán-Carrillo, and Ada Keila Milán-Noris. 2025. "Techno-Functional and Nutraceutical Assessment of Unprocessed and Germinated Amaranth Flours and Hydrolysates: Impact of the Reduction of Hydrolysis Time" Foods 14, no. 15: 2666. https://doi.org/10.3390/foods14152666
APA StyleMontoya-Rodríguez, A., Domínguez-Rodríguez, M., Sandoval-Sicairos, E. S., Milán-Noris, E. M., Milán-Carrillo, J., & Milán-Noris, A. K. (2025). Techno-Functional and Nutraceutical Assessment of Unprocessed and Germinated Amaranth Flours and Hydrolysates: Impact of the Reduction of Hydrolysis Time. Foods, 14(15), 2666. https://doi.org/10.3390/foods14152666








