Postharvest Application of Myo-Inositol Extends the Shelf-Life of Banana Fruit by Delaying Ethylene Biosynthesis and Improving Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. Screening for the Optimum Concentration of MI and Storage Treatment
2.3. Measurement of the Pulp Firmness and Peel Color
2.4. Determination of Respiration Rate and Ethylene Production
2.5. Measurement of Antioxidant Enzyme Activities and Oxidative Damage
2.6. Measurement of Soluble Sugar Alcohol Content
2.7. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.8. Statistical Analysis
3. Results
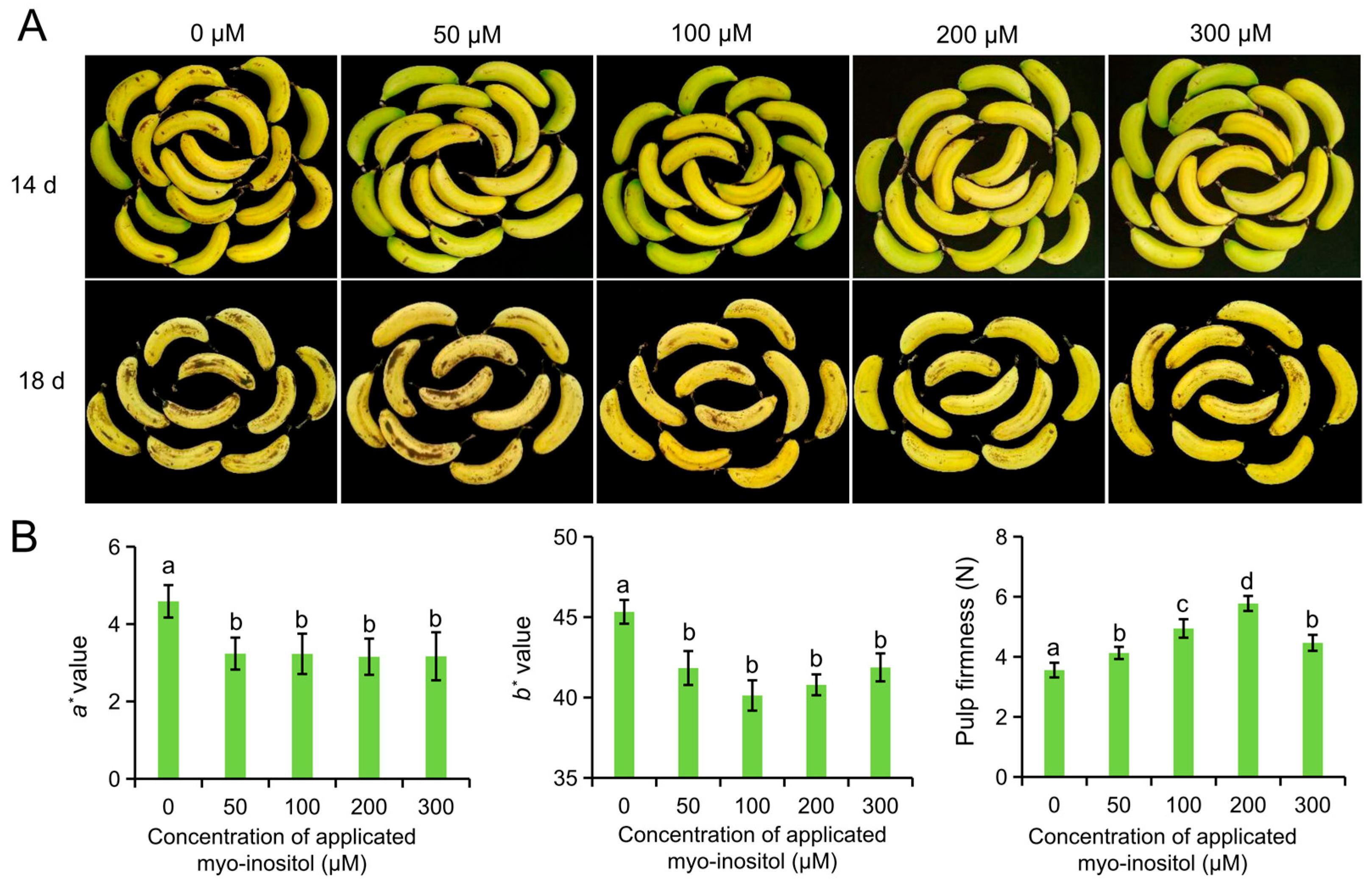
3.1. Screening of Exogenous MI Concentrations
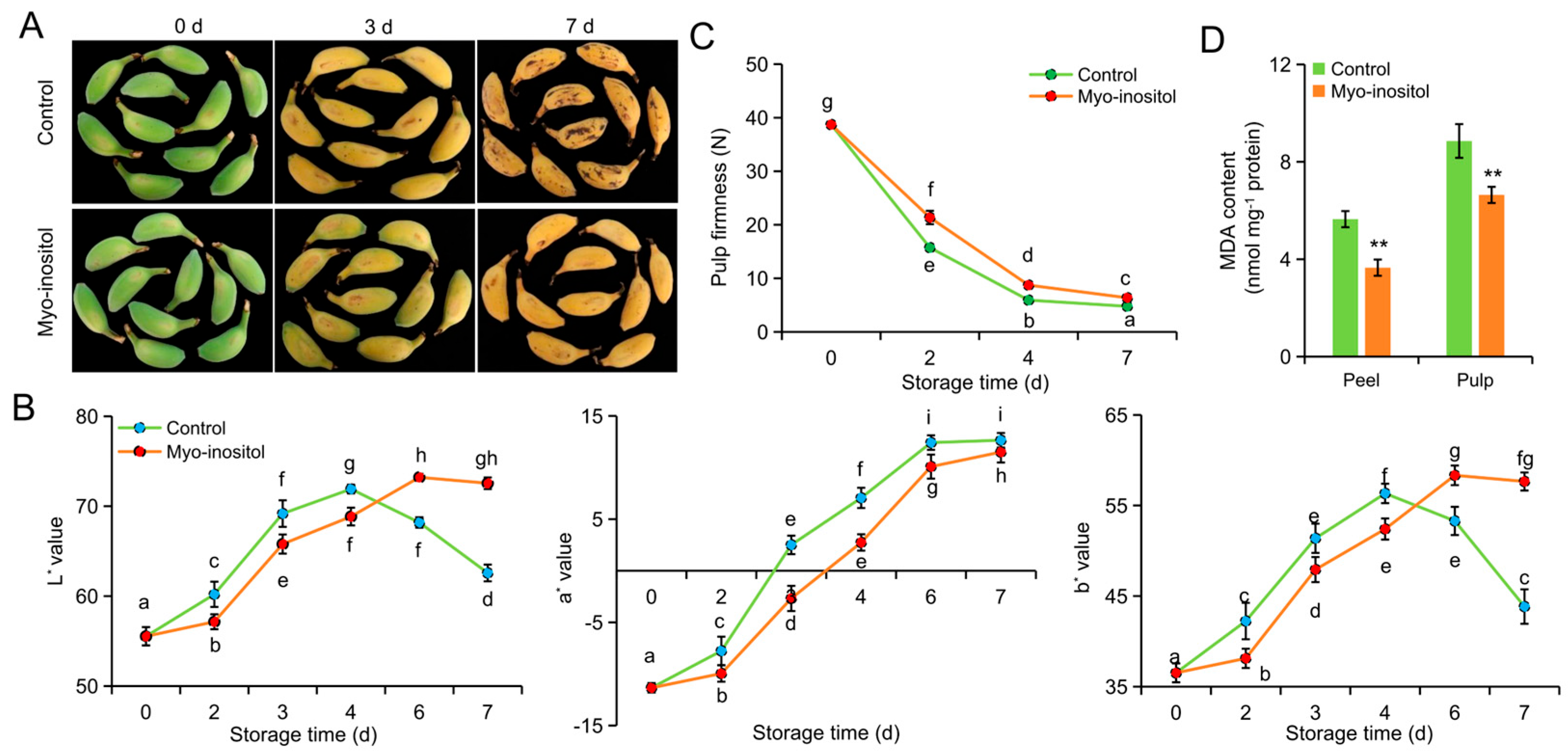
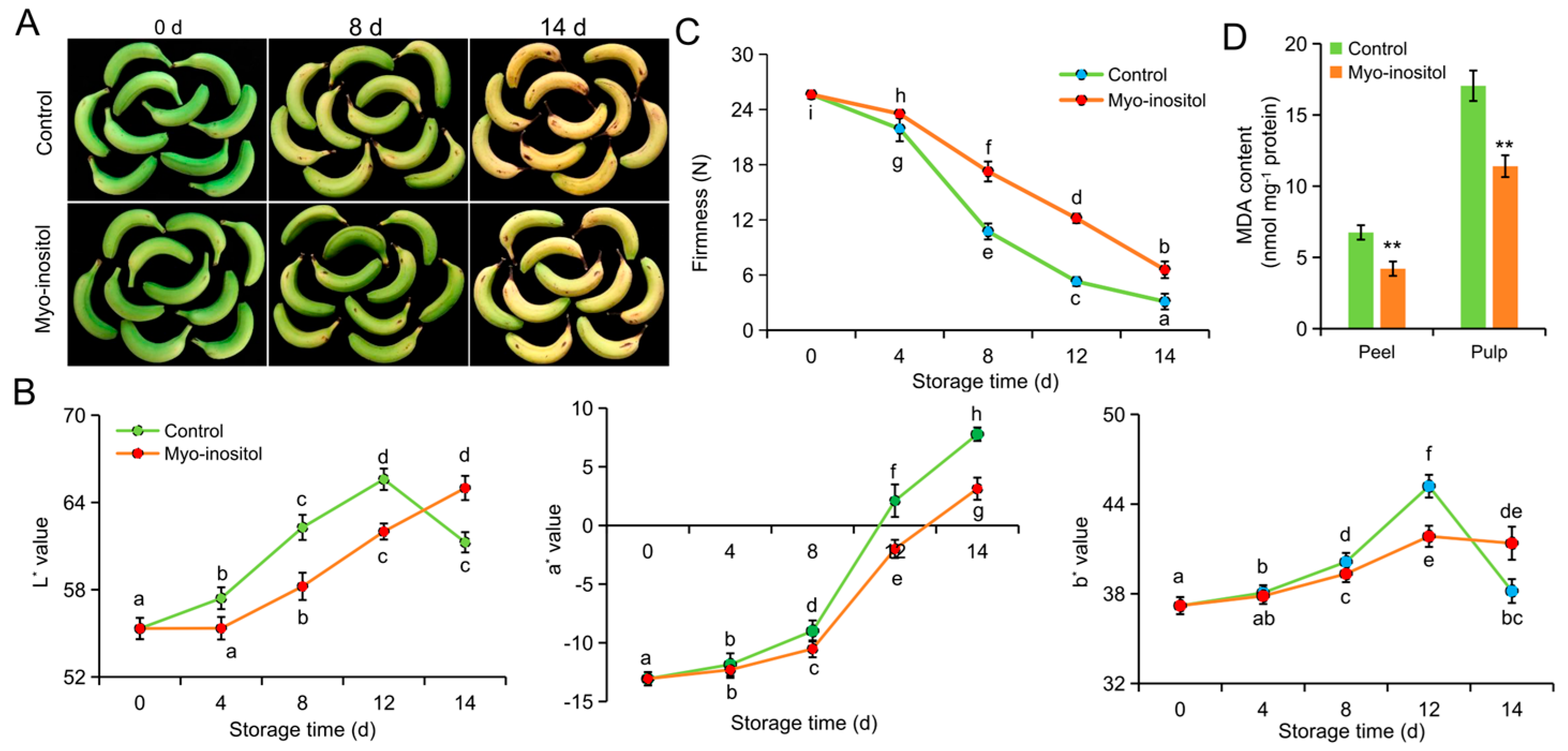
3.2. Effects of MI Application on Postharvest Fruit Ripening and Senescence
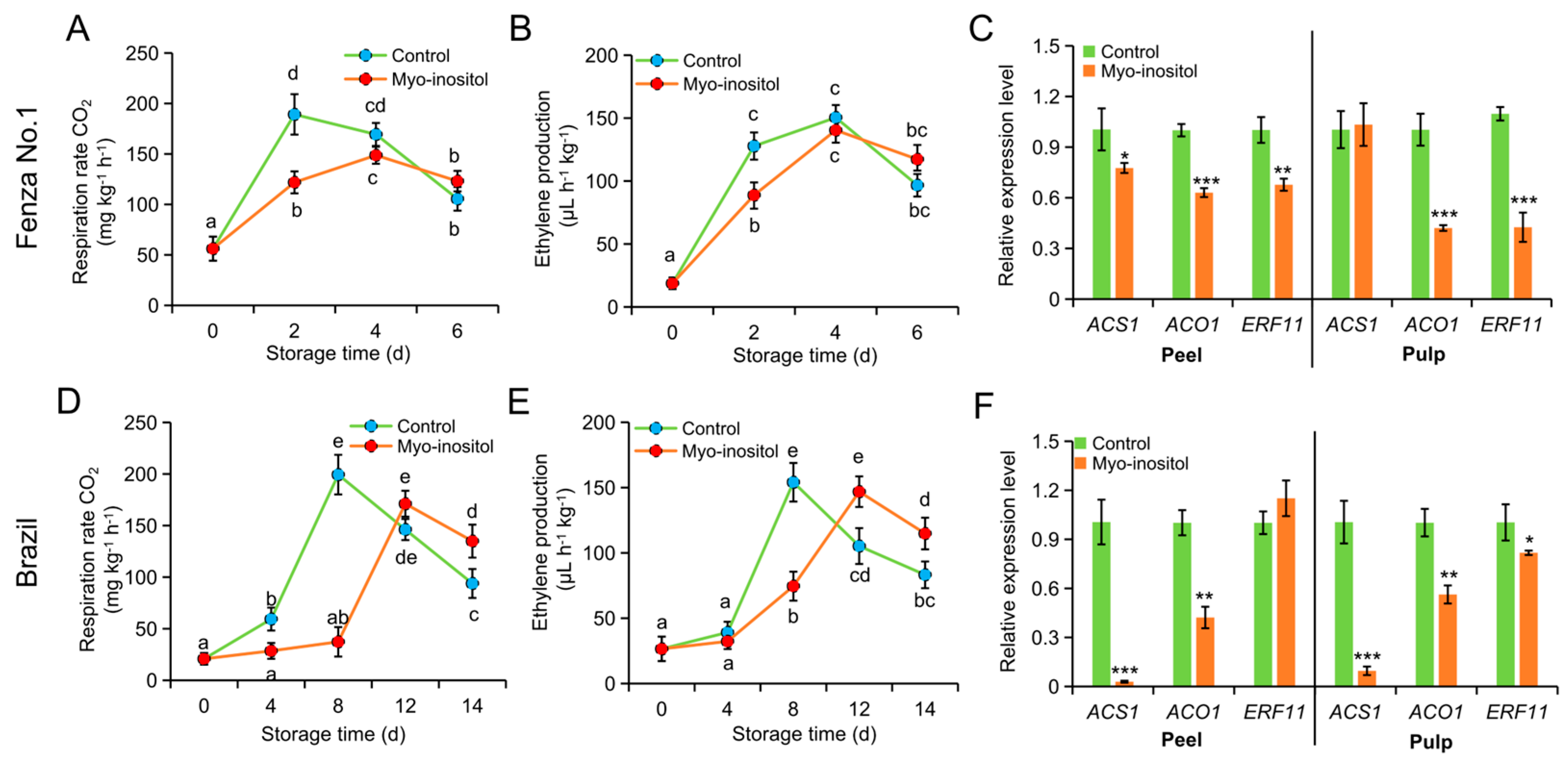
3.3. Effects of MI Application on Respiration Rate and Ethylene Production
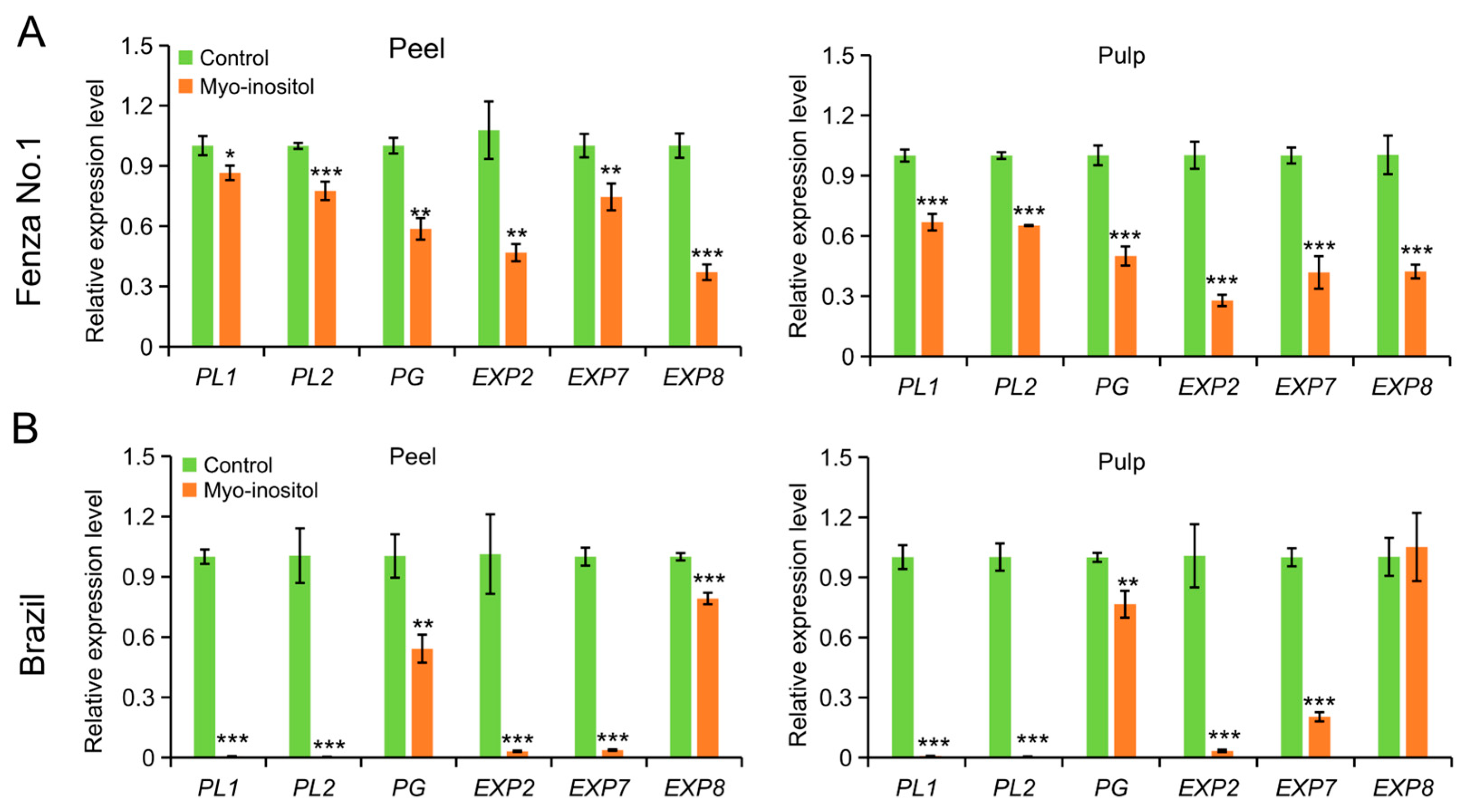
3.4. Effects of MI Application on the Accumulation of Soluble Sugars and Cell Wall Modification
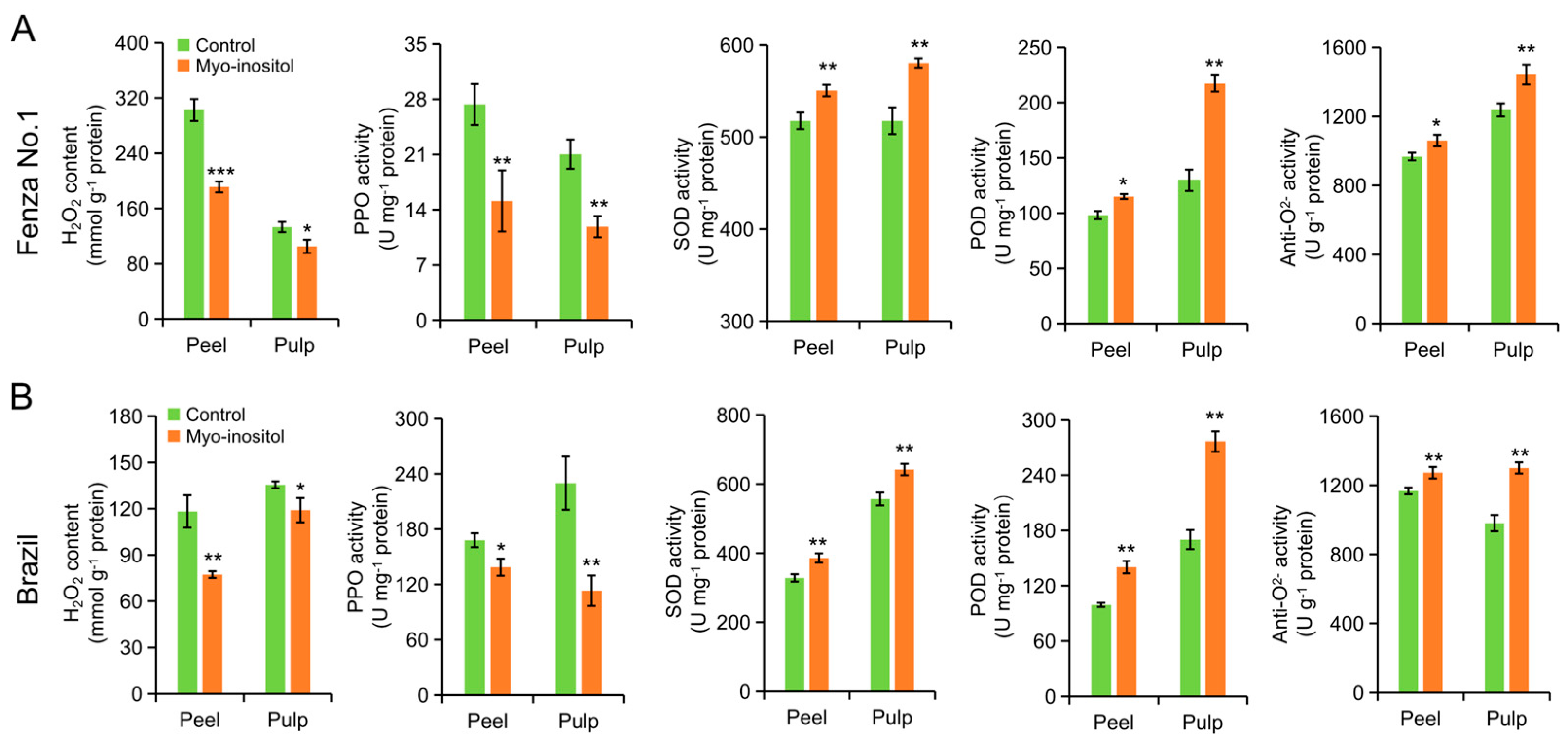
3.5. Effects of MI Application on H2O2 Accumulation and Activity of Antioxidant Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| ACS1 | ACAAGTTCAAGATCACCCAAGC | AGTGCATCCTTTTCTCGTTGAC |
| ACO1 | CTGCCCCTCCTGCTCTGT | CTCTGACTGCCTCAAATCTCG |
| EXP2 | GCAGAGCAACGCGTACCTCACT | GAGAAGAGGTCGAACAGCGCGA |
| EXP8 | TCCTCAAGATCGCCGAGTACC | CAGTTCTGGCCCCAGTTGC |
| ERF11 | TGGGTTCAAGGGAATCGGG | TGTTCGTGGGTTCTGTCAAG |
| EXP7 | CGTCACCGCCACCAACTTCT | TGGACCCCTTGATCGACACC |
| PL1 | CTGGAGGTCGGAAGGGGA | CGGCAGAGACGGTGATGG |
| PL2 | AAGACCTGGTTCAGAGGATGCCAA | TGGCTGTTTATAGTGGGAGCAGCA |
| PG | CGGATGAGCAATGTTTCCAACCCA | ACATGGAGAACTGTCGCTGCAAGA |
| Actin | GAGAAGATACAGTGTCTGGA | ATTACCATCGAAATATTAAAAG |
References
- Mohapatra, D.; Mishra, S.; Singh, C.B.; Jayas, D.S. Post-harvest processing of banana: Opportunities and challenges. Food Bioprocess Tech. 2011, 4, 327–339. [Google Scholar] [CrossRef]
- Ma, H.; Hu, L.M.; Zhao, J.Y.; He, J.; Wen, A.Q.; Lv, D.Z.; Xu, Z.; Lan, W.J.; Pan, L.Q. Comparative analysis of chilling injury in banana fruit during storage: Physicochemical and microstructural changes, and early optical-based nondestructive identification. Foods 2025, 14, 1319. [Google Scholar] [CrossRef] [PubMed]
- Golly, M.K.; Ma, H.L.; Sarpong, F.; Dotse, B.P.; Oteng-Darko, P.; Dong, Y.T. Shelf-life extension of grape (Pinot noir) by xanthan gum enriched with ascorbic and citric acid during cold temperature storage. J. Food Sci. Technol. 2019, 56, 4867–4878. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Zou, X.B.; Shi, J.Y.; Mahunu, G.K.; Zhai, X.D.; Mariod, A.A. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J. Food Biochem. 2018, 42, e12527. [Google Scholar] [CrossRef]
- Cordenunsi-Lysenko, B.R.; Nascimento, J.R.O.; Castro-Alves, V.C.; Purgatto, E.; Fabi, J.P.; Peroni-Okyta, F.H.G. The starch is (not) just another brick in the wall: The primary metabolism of sugars during banana ripening. Front. Plant Sci. 2019, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Kuang, J.F.; Wei, W.; Fan, Z.Q.; Deng, W.; Li, Z.G.; Bouzayen, M.; Pirrello, J.; Lu, W.J.; Chen, J.Y. MaXB3 modulates MaNAC2, MaACS1, and MaACO1 stability to repress ethylene biosynthesis during banana fruit ripening. Plant Physiol. 2020, 184, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.L.; Luo, S.F.; An, R.H.; Li, P.X. Endogenous melatonin delays sepal senescence and extends the storage life of broccoli florets by decreasing ethylene biosynthesis. Postharvest Biol. Tec. 2022, 188, 111894. [Google Scholar] [CrossRef]
- Lelièvre, J.M.; Latché, A.; Jones, B.; Bouzayen, M.; Pech, J.C. Ethylene and fruit ripening. Physiol. Plant. 1997, 101, 727–739. [Google Scholar] [CrossRef]
- Mustapha, A.T.; Zhou, C.S. Novel assisted/unassisted ultrasound treatment: Effect on respiration rate, ethylene production, enzymes activity, volatile composition, and odor of cherry tomato. LWT-Food Sci. Technol. 2021, 149, 111779. [Google Scholar] [CrossRef]
- Li, X.X.; Yu, S.; Cheng, Z.H.; Chang, X.J.; Yun, Y.Z.; Jiang, M.W.; Chen, X.Q.; Wen, X.H.; Li, H.; Zhu, W.J.; et al. Origin and evolution of the triploid cultivated banana genome. Nat. Genet. 2024, 56, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Netlak, P.; Buensanteai, N.; Allan, A.C.; Imsabai, W. Rate of banana fruit ripening depends on genome composition and gene expression of ethylene signaling and ethylene biosynthesis. Sci. Hortic. 2021, 290, 110552. [Google Scholar] [CrossRef]
- Tian, S.P.; Qin, G.Z.; Li, B.Q. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol. Biol. 2013, 82, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Guo, L.N.; Hong, C.; Wu, P.; Tuly, J.; Ma, H.L. Accumulation of phenolic in fresh-cut lotus roots induced by thermosonication: Regulation of phenylpropanoid pathway and reactive oxygen species metabolism. Food Chem. 2025, 467, 142206. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Y.; Yu, H.D.; Chen, K.J.; Cui, R.; Cao, J.X.; Wang, Z.X.; Zhang, Z.H.; Soteyome, T. Effects of chitosan/eugenol-loaded IRMOF-3 nanoparticles composite films on reactive oxygen species metabolism and microbial community dynamics in postharvest strawberries. Food Biosci. 2024, 63, 105652. [Google Scholar] [CrossRef]
- Wu, X.; Wu, C.N.; Bian, Z.; Ye, Z.Y.; Meng, L.L.; Xia, L.R.; Bao, E.C.; Cao, K. Abscisic acid and reactive oxygen species were involved in slightly acidic electrolyzed water-promoted seed germination in watermelon. Sci. Hortic. 2021, 291, 110581. [Google Scholar] [CrossRef]
- Cheng, G.; Duan, X.; Shi, J.; Lu, W.J.; Luo, Y.B.; Jiang, W.B.; Jiang, Y.M. Effects of reactive oxygen species on cellular wall disassembly of banana fruit during ripening. Food Chem. 2008, 109, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.W.; Yuan, B.; Qiao, H.S.; Gao, L.L.; Zhao, B.B.; Chen, W.; Cao, C.J. LED light-triggered release of nitric oxide from NTC to delay the ripening of banana. LWT-Food Sci. Tech. 2020, 134, 110129. [Google Scholar] [CrossRef]
- Xiao, L.; Li, T.T.; Jiang, G.X.; Jiang, Y.M.; Duan, X.W. Cell wall proteome analysis of banana fruit softening using iTRAQ technology. J. Proteom. 2019, 209, 103506. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Seymour, G.B. Molecular and biochemical basis of softening in tomato. Mol. Hortic. 2022, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Ling, J.; Zhou, H.S.; Tian, M.Y.; Huang, W.; Luo, S.F.; Hu, H.L.; Li, P.X. 1-Methylcyclopropene counteracts ethylene inhibition of anthocyanin accumulation in peach skin after harvest. Postharvest Biol. Tec. 2022, 183, 111737. [Google Scholar] [CrossRef]
- Yang, L.; Huang, W.; Xiong, F.; Xian, Z.; Su, D.; Ren, M.; Li, Z. Silencing of SlPL, which encodes a pectate lyase in tomato, confers enhanced fruit firmness, prolonged shelf-life and reduced susceptibility to grey mould. Plant Biotechnol. J. 2017, 15, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena-an overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, W.J.; Jiang, Y.M.; Luo, Y.B.; Jiang, W.B.; Daryl, J. Expression of ethylene-related expansin genes in cool-stored ripening banana fruit. Plant Sci. 2006, 170, 962–967. [Google Scholar] [CrossRef]
- Loewus, F.A.; Murthy, P.P.N. Myo-inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Valluru, R.; Van den Ende, W. Myo-inositol and beyond-emerging networks under stress. Plant Sci. 2011, 181, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Liang, D.; Wu, S.; Ma, F.W.; Lei, Y.S. Isolation and developmental expression analysis of L-myo-inositol-1-phosphate synthase in four Actinidia species. Plant Physiol. Bioch. 2013, 73, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Chevilly, S.; Dolz-Edo, L.; Blanca, J.; Yenush, L.; Mulet, J.M. Identification of distinctive primary metabolites influencing broccoli (Brassica oleracea, var. Italica) taste. Foods 2023, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Zhou, K.; Li, Y.T.S.; Chen, X.F.; Liu, B.B.; Li, C.Y.; Gong, X.Q.; Ma, F.W. Exogenous myo-inositol alleviates salinity-induced stress in Malus hupehensis Rehd. Plant Physiol. Bioch. 2018, 133, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.X.; Ren, W.B.; Kong, L.Q.; Zhang, W.J.; Wang, T. Transcriptomic profiling analysis of Arabidopsis thaliana treated with exogenous myo-inositol. PLoS ONE 2016, 11, e0161949. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Zhou, K.; Liu, Y.; Yang, S.L.; Zhang, J.Y.; Gong, X.Q.; Ma, F.W. Overexpression of MdMIPS1 enhances salt tolerance by improving osmosis, ion balance, and antioxidant activity in transgenic apple. Plant Sci. 2020, 301, 110654. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Zhou, K.; Ren, G.J.; Yang, S.L.; Liu, Y.; Zhang, Z.J.; Li, Y.T.S.; Gong, X.Q.; Ma, F.W. Myo-inositol mediates reactive oxygen species-induced programmed cell death via salicylic acid-dependent and ethylene-dependent pathways in apple. Hortic. Res. 2020, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.G.; Zhang, Z.K.; Rao, J.P.; Huber, D.J.; Lv, J.Y.; Hou, Y.L.; Song, K.H. Identification of xyloglucan endotransglucosylase/hydrolase genes (XTHs) and their expression in persimmon fruit as influenced by 1-methylcyclopropene and gibberellic acid during storage at ambient temperature. Food Chem. 2013, 138, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Hu, L.Y.; Li, P.M.; Gong, X.Q.; Ma, F.W. Genome-wide identification of glycosyltransferases converting phloretin to phloridzin in Malus species. Plant Sci. 2017, 265, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Luo, Z.Y.; Zhao, X.Y.; Li, S.C.; Wu, F.; Chen, J.Y.; Xiang, M.L. Exogenously applied methyl jasmonate increased the resistance of postharvest pear fruit to blue mold. Fruit Res. 2022, 2, 11. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Q.; Deng, S.F.; Zeng, K.F.; Ming, J.; Hou, D.J.; Deng, L.L. Influence of myoinositol on post-ripening and softening of Prunus salicina ‘Wushan plum’. Postharvest Biol. Tec. 2024, 210, 112772. [Google Scholar] [CrossRef]
- Dominguez, M.; Vendrell, M. Ethylene biosynthesis in banana fruit: Evolution of EFE activity and ACC levels in peel and pulp during ripening. J. Hort. Sci. 1993, 68, 63–70. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Xiao, H.; Han, Q.Y.; Tran, D.T.; Crouch, E.; Hertog, M.L.A.T.M.; Verboven, P. Spatio-temporal dynamics of the metabolome of climacteric fruit during ripening and post-harvest storage. J. Exp. Bot. 2023, 74, 6321–6330. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Zabihzadeh Khajavi, M.; Ahmadi, S.; Mortazavian, A.M.; Abdolshahi, A.; Rafiee, S.; Farhoodi, M. Novel strategies to control ethylene in fruit and vegetables for extending their shelf life: A review. Int. J. Environ. Sci. Technol. 2022, 19, 4599–4610. [Google Scholar] [CrossRef]
- Wei, W.; Yang, Y.Y.; Wu, C.J.; Kuang, J.F.; Chen, J.Y.; Lu, W.J.; Shan, W. MaMADS1-MaNAC083 transcriptional regulatory cascade regulates ethylene biosynthesis during banana fruit ripening. Hortic. Res. 2023, 10, uhad177. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Kuan, C.; Chiu, C.H.; Chen, X.J.; Do, Y.Y.; Huang, P.L. Global transcriptomic analysis of targeted silencing of two paralogous ACC oxidase genes in banana. Int. J. Mol. Sci. 2016, 17, 1632. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shiomi, S.; Nakatsuka, A.; Kubo, Y.; Nakamura, R.; Inaba, A. Characterization of ethylene biosynthesis associated with ripening in banana fruit. Plant Physiol. 1999, 121, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Jourda, C.; Cardi, C.; Mbéguié-A-Mbéguié, D.; Bocs, S.; Garsmeur, O.; D’Hont, A.; Yahiaoui, N. Expansion of banana (Musa acuminata) gene families involved in ethylene biosynthesis and signalling after lineage-specific whole-genome duplications. New Phytol. 2014, 202, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, P.; Yu, X.F.; Cao, J.P.; Chen, X.; Gao, L.; Chen, K.S.; Grierson, D. Contrasting roles of ethylene response factors in pathogen response and ripening in fleshy fruit. Cells 2022, 11, 2484. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Leif, D.; Plunkett, B.; McGhie, T.; Henry-Kirk, R.; Hall, M.; Johnston, J.W.; Punter, M.P.; Boldingh, H.; Nardozza, S.; et al. Red to Brown: An Elevated Anthocyanic Response in Apple Drives Ethylene to Advance Maturity and Fruit Flesh Browning. Front. Plant Sci. 2019, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Chen, C.Y.; Zou, D.F.; Li, J.W.; Huang, Y.Y.; Zheng, X.B.; Tan, B.; Cheng, J.; Wang, W.; Zhang, L.L.; et al. Ethylene accelerates grape ripening via increasing VvERF75-induced ethylene synthesis and chlorophyll degradation. Fruit Res. 2023, 3, 3. [Google Scholar] [CrossRef]
- Han, Y.C.; Kuang, J.F.; Chen, J.Y.; Liu, X.C.; Xiao, Y.Y.; Fu, C.C.; Wang, J.N.; Wu, K.Q.; Lu, W.J. Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol. 2016, 171, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Inaba, A.; Liu, X.; Yokotani, N.; Yamane, M.; Lu, W.J.; Nakano, R.; Kubo, Y. Differential feedback regulation of ethylene biosynthesis in pulp and peel tissues of banana fruit. J. Exp. Bot. 2007, 58, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Hu, Y.Y.; Shen, J.Y.; Meng, X.C.; Suo, J.W.; Zhang, Z.Y.; Song, L.L.; Wu, J.S. Acceleration of aril cracking by ethylene in Torreya grandis during nut maturation. Front. Plant Sci. 2021, 12, 761139. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.N.; Li, B.J.; Grierson, D.; Chen, K.S. Insights into cell wall changes during fruit softening from transgenic and naturally occurring mutants. Plant Physiol. 2023, 192, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lai, X.; Wang, L.; Li, X.; Chen, W.; Zhu, X.; Song, Z. Ethylene Response Factor MaERF012 Modulates Fruit Ripening by Regulating Chlorophyll Degradation and Softening in Banana. Foods 2022, 11, 3882. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Wang, K.; Wu, C.X.; Zhao, Y.; Yin, X.R.; Zhang, B.; Don, G.; Chen, K.S.; Xu, C.J. Effect of ethylene on cell wall and lipid metabolism during alleviation of postharvest chilling injury in peach. Cells 2019, 8, 1612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Hu, L.Y.; Li, Y.T.S.; Chen, X.F.; Zhang, Z.J.; Liu, B.B.; Li, P.M.; Gong, X.Q.; Ma, F.W. MdUGT88F1-Mediated Phloridzin Biosynthesis Regulates Apple Development and Valsa Canker Resistance. Plant Physiol. 2019, 180, 2290–2305. [Google Scholar] [CrossRef] [PubMed]
- Overmyer, K.; Brosché, M.; Kangasjärvi, J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003, 8, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Hu, K.D.; Wang, S.S.; Hu, L.Y.; Chen, X.Y.; Li, Y.H.; Yang, Y.; Yang, F.; Zhang, H. Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS ONE 2017, 2, e0180113. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars—Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Recent advances of polyphenol oxidases in plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef] [PubMed]
| Compounds | ‘Fengza No. 1’ (Day 2) | ‘Brazil’ (Day 8) | ||||
|---|---|---|---|---|---|---|
| Control | Myo-Inositol | FC | Control | Myo-Inositol | FC | |
| Maltose | 265.22 ± 6.34 | 196.91 ± 59.61 | 0.74 | 44.32 ± 8.69 | 23.1 ± 0.56 | 0.52 * |
| Trehalose | 22.92 ± 0.12 | 13.56 ± 0.17 | 0.59 *** | 8.73 ± 0.35 | 8.57 ± 0.26 | 0.98 |
| Sucrose | 58.56 ± 0.76 | 41.69 ± 2.47 | 0.71 *** | 37.44 ± 1.37 | 35.77 ± 0.07 | 0.96 |
| Mannose (a) | 36.90 ± 1.55 | 32.88 ± 3.14 | 0.89 | 29.48 ± 1.88 | 24.32 ± 0.50 | 0.83 * |
| Glucuronic acid | 12.39 ± 0.04 | 9.56 ± 0.40 | 0.77 *** | 5.01 ± 0.55 | 5.33 ± 0.12 | 1.07 |
| Galacturonic acid | 3.64 ± 0.01 | 2.95 ± 0.11 | 0.81 *** | 2.54 ± 0.07 | 2.49 ± 0.03 | 0.98 |
| Xylose | 8.20 ± 0.08 | 3.65 ± 0.38 | 0.44 *** | 5.23 ± 0.17 | 3.75 ± 0.08 | 0.71 *** |
| Sorbitol | 95.59 ± 1.87 | 75.09 ± 6.82 | 0.79 ** | 22.16 ± 0.56 | 14.81 ± 0.02 | 0.67 *** |
| Ribono-1,4-lactone | 1.35 ± 0.13 | 0.57 ± 0.28 | 0.42 ** | 0.99 ± 0.03 | 0.63 ± 0.17 | 0.64 * |
| Glucose (a) | 20.54 ± 0.49 | 11.62 ± 0.86 | 0.57 *** | 8.27 ± 0.75 | 5.37 ± 0.09 | 0.65 ** |
| Galactose | 433.55 ± 11.26 | 196.22 ± 34.41 | 0.45 *** | 82.74 ± 0.84 | 59.63 ± 0.63 | 0.72 ** |
| Fucose | 47.96 ± 0.84 | 37.52 ± 1.10 | 0.78 *** | 105.86 ± 7.06 | 91.66 ± 2.59 | 0.87 * |
| Fructose (a) | 15.25 ± 0.42 | 8.50 ± 0.59 | 0.56 *** | 6.40 ± 0.38 | 4.17 ± 0.08 | 0.65 ** |
| Arabinose | 9.04 ± 0.28 | 8.12 ± 0.16 | 0.90 ** | 9.78 ± 0.63 | 8.61 ± 0.30 | 0.88 * |
| Arabinitol | 3.30 ± 0.75 | 1.52 ± 0.25 | 0.46 * | 1.67 ± 0.34 | 0.50 ± 0.26 | 0.30 ** |
| 2-Acetamido-2-deoxy-D-glucopyranose | 8.65 ± 0.08 | 8.36 ± 0.08 | 0.97 | 6.58 ± 0.16 | 6.00 ± 0.48 | 0.91 |
| Rhamnose | 4.66 ± 0.01 | 5.11 ± 0.15 | 1.10 | 7.68 ± 1.45 | 7.64 ± 0.18 | 1.00 |
| Levoglucosan | 23.52 ± 0.29 | 25.49 ± 1.46 | 1.08 | 16.43 ± 0.13 | 15.87 ± 1.92 | 0.97 |
| Myo-inositol | 28.70 ± 0.27 | 38.81 ± 2.17 | 1.35 ** | 49.18 ± 7.58 | 70.81 ± 0.99 | 1.44 ** |
| Raffinose | 55.88 ± 0.40 | 69.76 ± 5.84 | 1.25 * | 19.73 ± 0.82 | 23.05 ± 1.62 | 1.17 * |
| Total sugars (a) | 95.41 ± 1.65 | 62.54 ± 4.01 | 0.66 *** | 52.53 ± 2.50 | 45.68 ± 0.15 | 0.87 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Li, Y.; Zhou, K.; Shi, K.; Niu, Y.; Qu, F.; Zhang, S.; He, W.; Wu, Y. Postharvest Application of Myo-Inositol Extends the Shelf-Life of Banana Fruit by Delaying Ethylene Biosynthesis and Improving Antioxidant Activity. Foods 2025, 14, 2638. https://doi.org/10.3390/foods14152638
Hu L, Li Y, Zhou K, Shi K, Niu Y, Qu F, Zhang S, He W, Wu Y. Postharvest Application of Myo-Inositol Extends the Shelf-Life of Banana Fruit by Delaying Ethylene Biosynthesis and Improving Antioxidant Activity. Foods. 2025; 14(15):2638. https://doi.org/10.3390/foods14152638
Chicago/Turabian StyleHu, Lingyu, Yi Li, Kun Zhou, Kaili Shi, Yi Niu, Feng Qu, Shenglin Zhang, Weidi He, and Yuanli Wu. 2025. "Postharvest Application of Myo-Inositol Extends the Shelf-Life of Banana Fruit by Delaying Ethylene Biosynthesis and Improving Antioxidant Activity" Foods 14, no. 15: 2638. https://doi.org/10.3390/foods14152638
APA StyleHu, L., Li, Y., Zhou, K., Shi, K., Niu, Y., Qu, F., Zhang, S., He, W., & Wu, Y. (2025). Postharvest Application of Myo-Inositol Extends the Shelf-Life of Banana Fruit by Delaying Ethylene Biosynthesis and Improving Antioxidant Activity. Foods, 14(15), 2638. https://doi.org/10.3390/foods14152638






