Quantitative Analysis of Ginger Maturity and Pulsed Electric Field Thresholds: Effects on Microstructure and Juice’s Nutritional Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Treatment Methods
2.2.1. Sample Preparation
2.2.2. PEF Treatment
2.3. Physicochemical Analysis
2.3.1. Juice Yield
2.3.2. Electrical Conductivity (EC), Temperature, pH, °Brix
2.3.3. Cloud Value, Cloud Stability, and Non-Enzymatic Browning (NEB)
2.4. Phytochemical Analysis
2.4.1. Determination of Total Phenolic Content (TPC)
2.4.2. Determination of Total Flavonoid Content (TFC)
2.4.3. Determination of Total Flavanols
2.4.4. Condensed Tannin Contents
2.5. Antioxidant Analysis
2.5.1. Determination of 1,1-Diphenyl-2-picrylhydrazyl (DPPH•) Radical Scavenging Activity
2.5.2. Determination of 2,2′-Azinobis (3-Ethyl-benzothiozoline-6-sulfonic Acid) Radical Cation (ABTS•+) Decolorization
2.5.3. Metal Chelating Potential
2.6. In Vitro Studies of Ginger
2.6.1. Microstructure Analysis
2.6.2. Optical Properties
2.6.3. Fourier Transform Infrared (FT-IR) Spectroscopy
2.6.4. Determination of Gingerols and Shogaols
2.6.5. Determination of Volatile Compounds by HS-SPME GC-MS
2.6.6. Statistical Analysis
3. Results and Discussion
3.1. Juice Yield, Temperature, pH, and °Brix %, Electrical Conductivity (EC) of Ginger
3.2. Cloud Value and Cloud Stability of Ginger
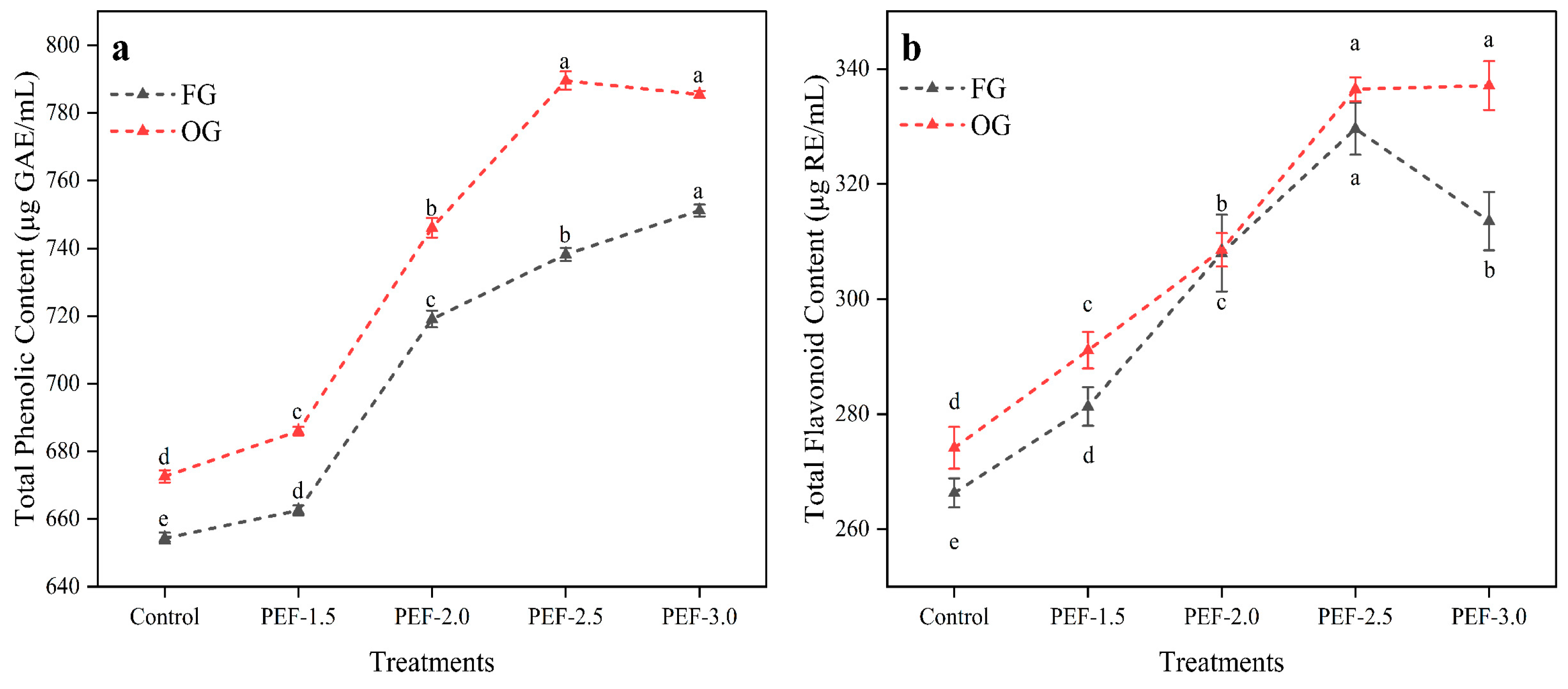
3.3. Total Phenolic and Flavonoid Content of Ginger Juice
3.4. Total Flavanols and Condensed Tannin Contents of Ginger Juice
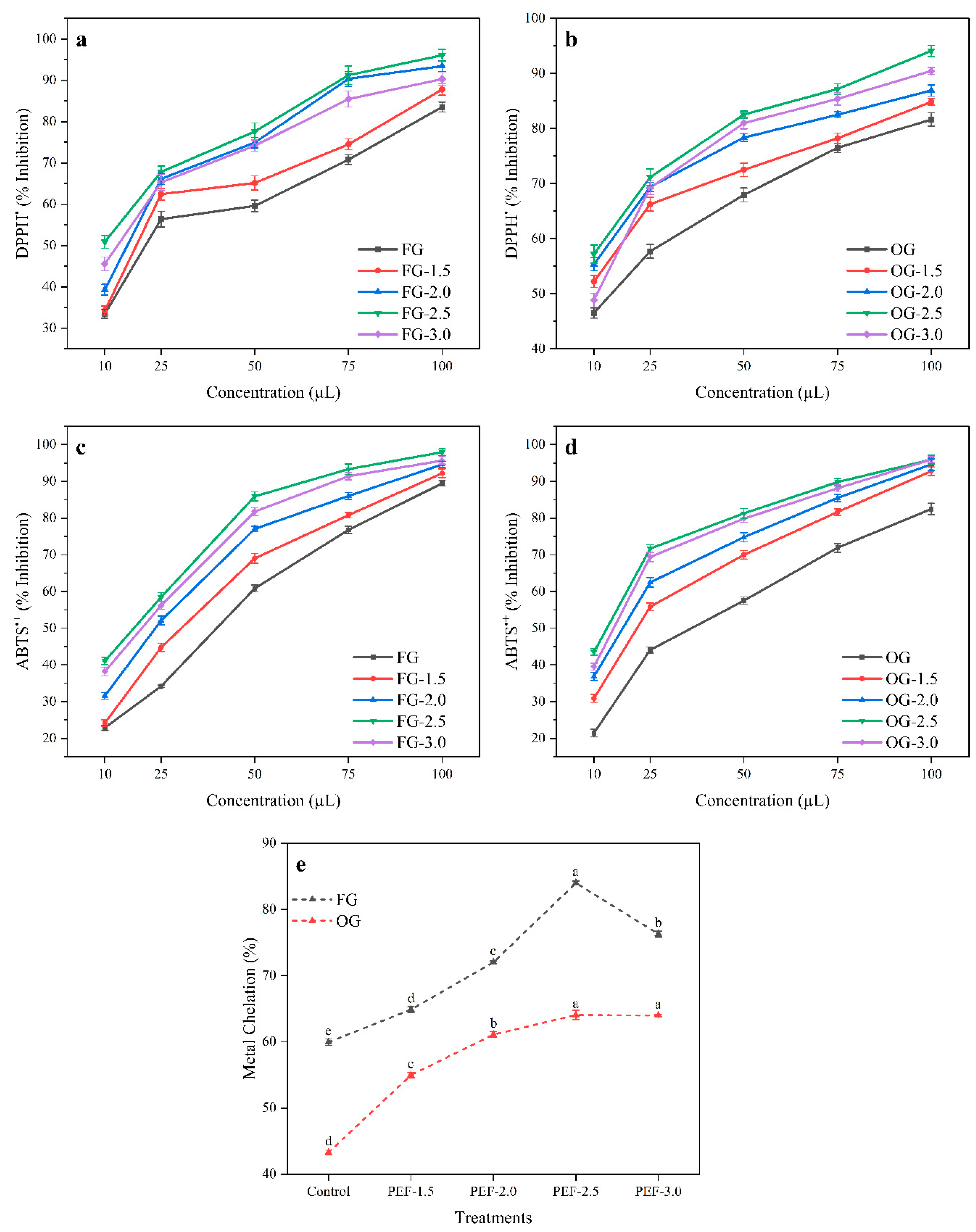
3.5. DPPH• and ABTS•+ Inhibition
3.6. Metal Chelating Potential of Ginger Juice
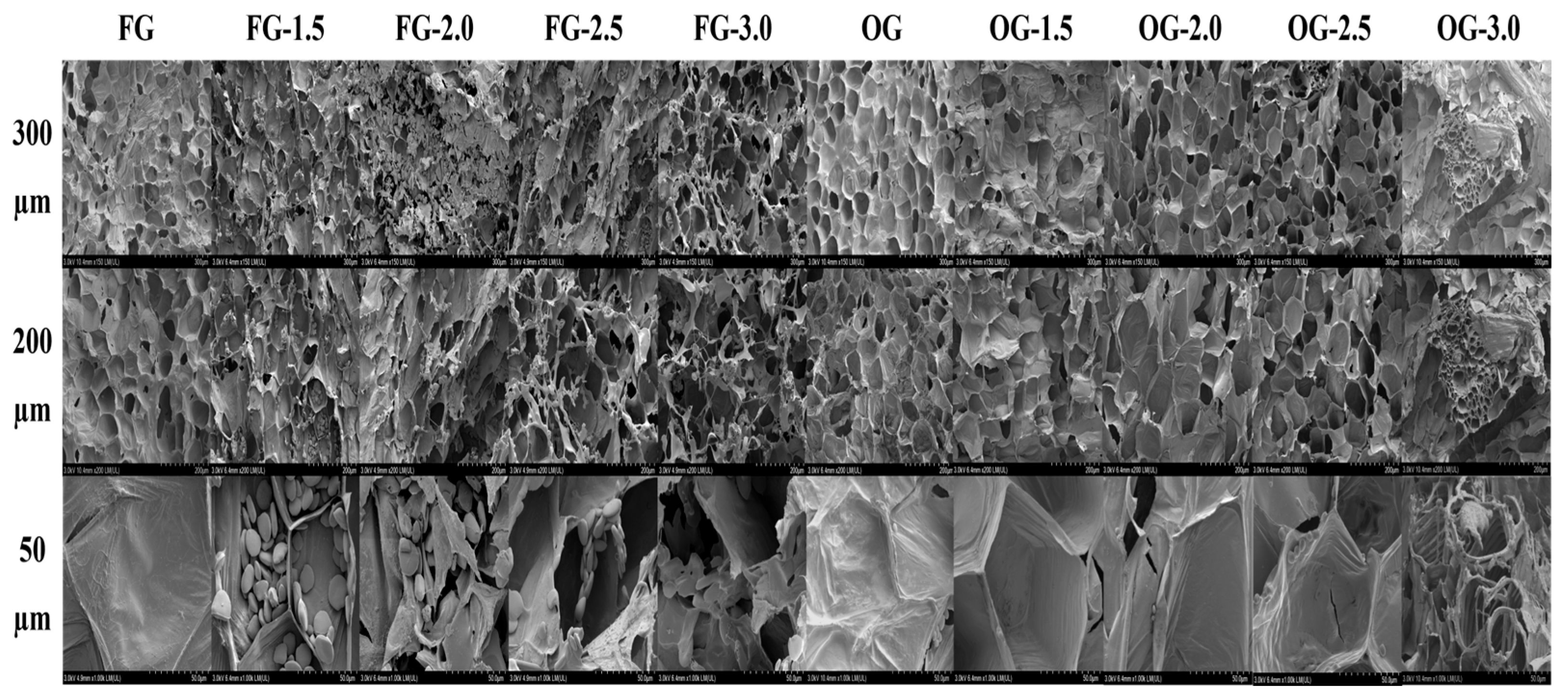
3.7. SEM and Cell Disintegration Degree of Ginger
3.8. Color Parameters of Ginger Juice
3.9. FT-IR Profile of Ginger Juice
3.10. Volatile Aroma Composition of Ginger Juice
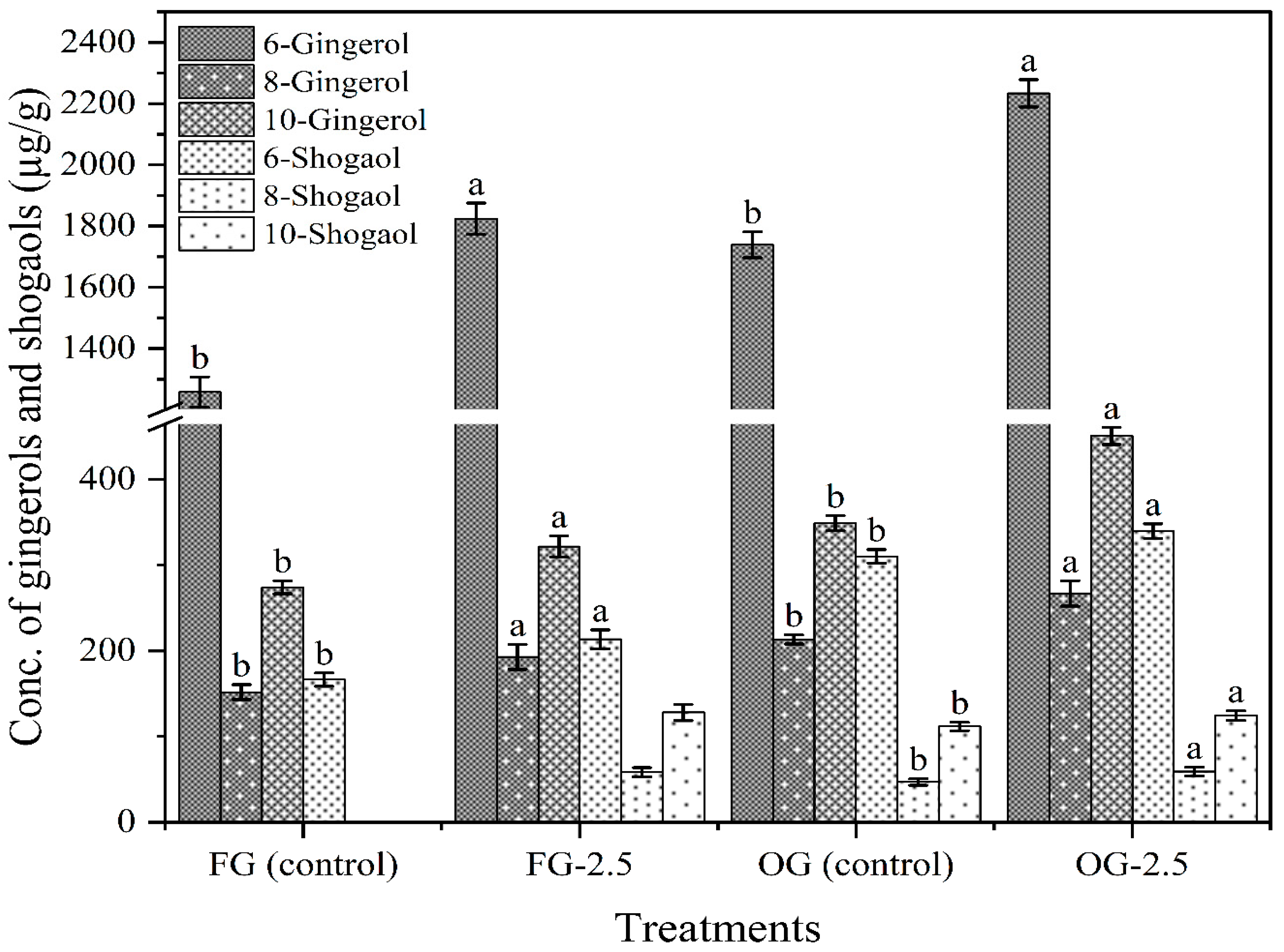
3.11. Gingerols and Shogaols Composition of Ginger Juice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballester, P.; Cerdá, B.; Arcusa, R.; García-Muñoz, A.M.; Marhuenda, J.; Zafrilla, P. Antioxidant activity in extracts from Zingiberaceae family: Cardamom, turmeric, and ginger. Molecules 2023, 28, 4024. [Google Scholar] [CrossRef] [PubMed]
- Laelago Ersedo, T.; Teka, T.A.; Fikreyesus Forsido, S.; Dessalegn, E.; Adebo, J.A.; Tamiru, M.; Astatkie, T. Food flavor enhancement, preservation, and bio-functionality of ginger (Zingiber officinale): A review. Int. J. Food Prop. 2023, 26, 928–951. [Google Scholar] [CrossRef]
- Choi, J.S. Processed Gingers: Current and Prospective Use in Food, Cosmetic, and Pharmaceutical Industry. Recent Pat. Food Nutr. Agric. 2019, 10, 20–26. [Google Scholar] [CrossRef]
- Arslan, H.N.; Çelik, S.Ş. Nonpharmacological nursing interventions in postoperative nausea and vomiting: A systematic review. J. PeriAnesthesia Nurs. 2024, 39, 142–154. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, M.K.; Sharma, K.C.; Tirath; Kumar, L.; Anal, J.M.H.; Upadhyay, S.K.; Bhattacharyya, S.; Kumar, D. Revisiting the therapeutic potential of gingerols against different pharmacological activities. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 633–647. [Google Scholar] [CrossRef]
- O’Connor, E.; Mündel, T.; Barnes, M.J. Nutritional compounds to improve post-exercise recovery. Nutrients 2022, 14, 5069. [Google Scholar] [CrossRef]
- Singh, P.; Mishra, G.; Pottoo, F.H.; Singh, B.; Zeleke, M.M. Zingiber officinale: Its ethanobotanical uses, phytochemistry, and pharmacology. In Edible Plants in Health and Diseases: Volume II: Phytochemical and Pharmacological Properties; Springer: Singapore, 2022; pp. 1–42. [Google Scholar]
- Szymczak, J.; Grygiel-Górniak, B.; Cielecka-Piontek, J. Zingiber officinale roscoe: The antiarthritic potential of a popular spice—Preclinical and clinical evidence. Nutrients 2024, 16, 741. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Grigsby, J.; Mbemi, A.; Nelson, D.; Mildort, B.; Latinwo, L.; Tchounwou, P.B. The management of diabetes mellitus using medicinal plants and vitamins. Int. J. Mol. Sci. 2023, 24, 9085. [Google Scholar] [CrossRef]
- Han, Z.; Xiao, W.; Geng, Y.; Cai, D.; Zeng, X.; Zhao, W.; Gao, W.; Ma, J. Variations in the Impact of Gingerols’ Conversion to Shogaols on the Properties of Corn Starch with Different Amylose Contents. Foods 2024, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Bayoï, J.R.; Tobias, T. Comparative Investigation of Quality Features and Microbiological Safety of the Spicy “Ndjindja” (Zingiber officinale) Drink Made from Various Production Sites in Far North of Cameroon. J. Food Qual. 2025, 2025, 9631365. [Google Scholar] [CrossRef]
- Jovita, O.N.; Patrick, U.S.; Ebisindor, A.V.; Jacob, B.S.; Ngozi, O.; Amanda, O.U.A. Heavy Metals Health-Risk Assessment of Zingiber officinale in Akwa Ibom and Enugu following High Consumption During the COVID-19 Era. East Afr. Sch. J. Med. Sci. 2025, 8, 32–44. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Valencia, G.A.; Monteiro, A.R. Impact of drying and extractions processes on the recovery of gingerols and shogaols, the main bioactive compounds of ginger. Food Res. Int. 2022, 154, 111043. [Google Scholar] [CrossRef]
- Zagórska, J.; Czernicka-Boś, L.; Kukula-Koch, W.; Iłowiecka, K.; Koch, W. Impact of thermal processing on the selected biological activities of ginger rhizome—A review. Molecules 2023, 28, 412. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Zuo, C.; Zhang, J.; Liang, W.; Liu, Y.; Yu, W.; Yu, H.; Peng, C. Different origins and processing methods affect the intrinsic quality of ginger: A novel approach to evaluating ginger quality. Front. Chem. 2023, 11, 1296712. [Google Scholar] [CrossRef]
- Li, L.; Yang, R.; Zhao, W. The Effect of Pulsed Electric Fields (PEF) Combined with Temperature and Natural Preservatives on the Quality and Microbiological Shelf-Life of Cantaloupe Juice. Foods 2021, 10, 2606. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.L.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.; Augustin, M.A. Food processing needs, advantages and misconceptions. Trends Food Sci. Technol. 2021, 108, 103–110. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Gullon, P.; Hesari, J.; Gullón, B.; Alirezalu, K.; Lorenzo, J. Quality aspects and safety of pulsed electric field (PEF) processing on dairy products: A comprehensive review. Food Rev. Int. 2022, 38, 96–117. [Google Scholar] [CrossRef]
- Terefe, N.S.; Buckow, R.; Versteeg, C. Quality-related enzymes in plant-based products: Effects of novel food processing technologies part 2: Pulsed electric field processing. Crit. Rev. Food Sci. Nutr. 2015, 55, 1–15. [Google Scholar] [CrossRef]
- Pollini, L.; Cossignani, L.; Juan, C.; Manes, J. Extraction of Phenolic Compounds from Fresh Apple Pomace by Different Non-Conventional Techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef]
- Niu, D.; Ren, E.-F.; Li, J.; Zeng, X.-A.; Li, S.-L. Effects of pulsed electric field-assisted treatment on the extraction, antioxidant activity and structure of naringin. Sep. Purif. Technol. 2021, 265, 118480. [Google Scholar] [CrossRef]
- Maza, M.A.; Martínez, J.M.; Delso, C.; Camargo, A.; Raso, J.; Álvarez, I. PEF-dependency on polyphenol extraction during maceration/fermentation of Grenache grapes. Innov. Food Sci. Emerg. Technol. 2020, 60, 102303. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; Ouyang, H.-F.; Li, Q.-M.; Kong, Y.-Q.; Su, X.-J.; Wang, F. Effects of PEF pretreatment, combined with different drying methods, on the physicochemical properties, bioactive components, antioxidant potential as well as structural and thermal properties of dried yams. Dry. Technol. 2024, 42, 1077–1088. [Google Scholar] [CrossRef]
- Visockis, M.; Bobinaitė, R.; Ruzgys, P.; Barakauskas, J.; Markevičius, V.; Viškelis, P.; Šatkauskas, S. Assessment of plant tissue disintegration degree and its related implications in the pulsed electric field (PEF)–assisted aqueous extraction of betalains from the fresh red beetroot. Innov. Food Sci. Emerg. Technol. 2021, 73, 102761. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed electric field (PEF): Avant-garde extraction escalation technology in food industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Wang, L.; Boussetta, N.; Lebovka, N.; Vorobiev, E. Cell disintegration of apple peels induced by pulsed electric field and efficiency of bio-compound extraction. Food Bioprod. Process. 2020, 122, 13–21. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Han, Z.; Sun, D.W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Faisal Manzoor, M.; Ahmed, Z.; Ahmad, N.; Karrar, E.; Rehman, A.; Muhammad Aadil, R.; Al-Farga, A.; Waheed Iqbal, M.; Rahaman, A.; Zeng, X.A. Probing the combined impact of pulsed electric field and ultra-sonication on the quality of spinach juice. J. Food Process. Preserv. 2021, 45, e15475. [Google Scholar] [CrossRef]
- Ahmed, Z.; Manzoor, M.F.; Begum, N.; Khan, A.; Shah, I.; Farooq, U.; Siddique, R.; Zeng, X.-A.; Rahaman, A.; Siddeeg, A. Thermo-Ultrasound-Based Sterilization Approach for the Quality Improvement of Wheat Plantlets Juice. Processes 2019, 7, 518. [Google Scholar] [CrossRef]
- Mustafa, I.; Chin, N.L. Antioxidant Properties of Dried Ginger (Zingiber officinale Roscoe) var. Bentong. Foods 2023, 12, 178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Tanweer, S.; Mehmood, T.; Zainab, S.; Ahmad, Z.; Shehzad, A. Comparison and HPLC quantification of antioxidant profiling of ginger rhizome, leaves and flower extracts. Clin. Phytosci. 2020, 6, 12. [Google Scholar] [CrossRef]
- Bayoï, J.R. Unveiling the traditional preparation, biochemical, phytochemical components, antioxidant and microbial properties of “jenja”, a ready-to-serve ginger-based beverage vended and consumed in northern Cameroon. Appl. Food Res. 2024, 4, 100495. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Siddhuraju, P.; Manian, S. Antioxidant and free radical scavenging capacity of the underutilized legume, Vigna vexillata (L.) A. Rich. J. Food Compos. Anal. 2011, 24, 160–165. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Wang, R.; Wen, Q.H.; Li, J.; Lin, J.W.; Zeng, X.A. The interaction between bovine serum albumin and [6]-,[8]- and [10]-gingerol: An effective strategy to improve the solubility and stability of gingerol. Food Chem. 2022, 372, 131280. [Google Scholar] [CrossRef] [PubMed]
- Varakumar, S.; Umesh, K.V.; Singhal, R.S. Enhanced extraction of oleoresin from ginger (Zingiber officinale) rhizome powder using enzyme-assisted three phase partitioning. Food Chem. 2017, 216, 27–36. [Google Scholar] [CrossRef]
- Yu, D.X.; Guo, S.; Wang, J.M.; Yan, H.; Zhang, Z.Y.; Yang, J.; Duan, J.A. Comparison of Different Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-GC-MS Coupled with Fast GC E-Nose. Foods 2022, 11, 1611. [Google Scholar] [CrossRef]
- Chen, D.; Pan, S.; Chen, J.; Pang, X.; Guo, X.; Gao, L.; Liao, X.; Wu, J. Comparing the Effects of High Hydrostatic Pressure and Ultrahigh Temperature on Quality and Shelf Life of Cloudy Ginger Juice. Food Bioprocess Technol. 2016, 9, 1779–1793. [Google Scholar] [CrossRef]
- Jaeger, H.; Meneses, N.; Knorr, D. Impact of PEF treatment inhomogeneity such as electric field distribution, flow characteristics and temperature effects on the inactivation of E. coli and milk alkaline phosphatase. Innov. Food Sci. Emerg. Technol. 2009, 10, 470–480. [Google Scholar] [CrossRef]
- Astráin-Redín, L.; Moya, J.; Alejandre, M.; Beitia, E.; Raso, J.; Calvo, B.; Cebrian, G.; Álvarez, I. Improving the microbial inactivation uniformity of pulsed electric field ohmic heating treatments of solid products. LWT 2022, 154, 112709. [Google Scholar] [CrossRef]
- Yan, Z.; Yin, L.; Hao, C.; Liu, K.; Qiu, J. Synergistic effect of pulsed electric fields and temperature on the inactivation of microorganisms. AMB Express 2021, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Zderic, A.; Zondervan, E. Polyphenol extraction from fresh tea leaves by pulsed electric field: A study of mechanisms. Chem. Eng. Res. Des. 2016, 109, 586–592. [Google Scholar] [CrossRef]
- Meneses, N.; Jaeger, H.; Knorr, D. pH-changes during pulsed electric field treatments—Numerical simulation and in situ impact on polyphenoloxidase inactivation. Innov. Food Sci. Emerg. Technol. 2011, 12, 499–504. [Google Scholar] [CrossRef]
- Kayalvizhi, V.; Pushpa, A.J.S.; Sangeetha, G.; Antony, U. Effect of pulsed electric field (PEF) treatment on sugarcane juice. J. Food Sci. Technol. 2016, 53, 1371–1379. [Google Scholar] [CrossRef]
- Nowacka, M.; Tappi, S.; Wiktor, A.; Rybak, K.; Miszczykowska, A.; Czyzewski, J.; Drozdzal, K.; Witrowa-Rajchert, D.; Tylewicz, U. The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot. Foods 2019, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Padilla-Zakour, O.I. Evaluation of pulsed electric field and high-pressure processing on the overall quality of refrigerated Concord grape juice. Lwt-Food Sci. Technol. 2024, 198, 116002. [Google Scholar] [CrossRef]
- Jaeger, H.; Schulz, M.; Lu, P.; Knorr, D. Adjustment of milling, mash electroporation and pressing for the development of a PEF assisted juice production in industrial scale. Innov. Food Sci. Emerg. Technol. 2012, 14, 46–60. [Google Scholar] [CrossRef]
- Schilling, S.; Alber, T.; Toepfl, S.; Neidhart, S.; Knorr, D.; Schieber, A.; Carle, R. Effects of pulsed electric field treatment of apple mash on juice yield and quality attributes of apple juices. Innov. Food Sci. Emerg. Technol. 2007, 8, 127–134. [Google Scholar] [CrossRef]
- Lamanauskas, N.; Pataro, G.; Bobinas, C.; Satkauskas, S.; Viskelis, P.; Bobinaite, R.; Ferrari, G. Impact of pulsed electric field treatment on juice yield and recovery of bioactive compounds from raspberries and their by-products. Zemdirb.-Agric. 2016, 103, 83–90. [Google Scholar] [CrossRef]
- Ertugay, M.F.; Baslar, M.; Ortakci, F. Effect of pulsed electric field treatment on polyphenol oxidase, total phenolic compounds, and microbial growth of apple juice. Turk. J. Agric. For. 2013, 37, 772–780. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.A.; Farooq, M.A.; Kumari, A.; Murtaza, M.A.; Ahmad, N.; Manzoor, M.F.; Hassan, S.; Ahmad, Z.; Bo-Ru, C. Effect of pulsed electric fields processing on physiochemical properties and bioactive compounds of apricot juice. J. Food Process Eng. 2020, 43, e13449. [Google Scholar] [CrossRef]
- Wibowo, S.; Essel, E.A.; De Man, S.; Bernaert, N.; Van Droogenbroeck, B.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Comparing the impact of high pressure, pulsed electric field and thermal pasteurization on quality attributes of cloudy apple juice using targeted and untargeted analyses. Innov. Food Sci. Emerg. Technol. 2019, 54, 64–77. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Mastwijk, H.C.; Knol, J.J.; Quataert, M.C.J.; Vervoort, L.; Van der Plancken, I.; Hendrickx, M.E.; Matser, A.M. Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice. Part I: Impact on overall quality attributes. Innov. Food Sci. Emerg. Technol. 2011, 12, 235–243. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Boioli, P.; Barbosa-Cánovas, G.V. Assessment of the effects of ultrasonics and pulsed electric fields on nutritional and rheological properties of raspberry and blueberry purees. Food Bioprocess Technol. 2016, 9, 520–531. [Google Scholar] [CrossRef]
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving polyphenol extraction from lemon residues by pulsed electric fields. Waste Biomass Valorization 2017, 10, 889–897. [Google Scholar] [CrossRef]
- Radnia, M.R.; Mahdian, E.; Sani, A.M.; Hesarinejad, M.A. Comparison of microwave and pulsed electric field methods on extracting antioxidant compounds from Arvaneh plant (Hymenocrater platystegius Rech. F). Sci. Rep. 2024, 14, 25903. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.; Kollie, L.; Dong, J.E.; Liang, Z.S. Molecular networks of secondary metabolism accumulation in plants: Current understanding and future challenges. Ind. Crops Prod. 2023, 201, 116901. [Google Scholar] [CrossRef]
- Ahmed, Z.; Manzoor, M.F.; Hussain, A.; Hanif, M.; Zia-ud-Din; Zeng, X.A. Study the impact of ultra-sonication and pulsed electric field on the quality of wheat plantlet juice through FTIR and SERS. Ultrason. Sonochem. 2021, 76, 105648. [Google Scholar] [CrossRef]
- Iqbal, N.; Poór, P. Plant protection by tannins depends on defence-related phytohormones. J. Plant Growth Regul. 2025, 44, 22–39. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Brunetti, C.; dos Santos Nascimento, L.B.; Gori, A.; Piccolo, E.L.; Tattini, M. Antioxidants by nature: An ancient feature at the heart of flavonoids’ multifunctionality. New Phytol. 2024, 245, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Zhang, Z.; Dang, Z.M.; Xu, J.; Ren, X.L. New insights on phenolic compound metabolism in pomegranate fruit during storage. Sci. Hortic. 2021, 285, 110138. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Z.; Liu, Y.; Yang, Z.; Wang, L.; Guo, Y.; Xie, C.; Yu, Z. Storage temperature affects the accumulation of phenolics in fresh-cut melon by accelerating the sucrose decomposition. Horticulturae 2024, 10, 488. [Google Scholar] [CrossRef]
- Delso, C.; Berzosa, A.; Sanz, J.; Alvarez, I.; Raso, J. Two-Step PEF Processing for Enhancing the Polyphenol Concentration and Decontaminating a Red Grape Juice. Foods 2022, 11, 621. [Google Scholar] [CrossRef]
- Grzelka, K.; Matkowski, A.; Chodaczek, G.; Jaspinska, J.; Pawlikowska-Bartosz, A.; Slupski, W.; Lechniak, D.; Szumacher-Strabel, M.; Olorunlowu, S.; Szulc, K.; et al. Pulsed Electric Field (PEF) Treatment Results in Growth Promotion, Main Flavonoids Extraction, and Phytochemical Profile Modulation of Scutellaria baicalensis Georgi Roots. Int. J. Mol. Sci. 2024, 26, 100. [Google Scholar] [CrossRef]
- Yang, S.; Li, S.; Li, G.; Li, C.; Li, W.; Bi, Y.; Wei, J. Pulsed electric field treatment improves the oil yield, quality, and antioxidant activity of virgin olive oil. Food Chem. X 2024, 22, 101372. [Google Scholar] [CrossRef]
- Maza, M.A.; Pereira, C.; Martínez, J.M.; Camargo, A.; Alvarez, I.; Raso, J. PEF treatments of high specific energy permit the reduction of maceration time during vinification of and grapes. Innov. Food Sci. Emerg. Technol. 2020, 63, 102375. [Google Scholar] [CrossRef]
- Bebek Markovinovic, A.; Stulic, V.; Putnik, P.; Birkic, A.; Jambrovic, M.; Sasko, D.; Ljubicic, J.; Pavlic, B.; Herceg, Z.; Bursac Kovacevic, D. Pulsed Electric Field (PEF) and High-Power Ultrasound (HPU) in the Hurdle Concept for the Preservation of Antioxidant Bioactive Compounds of Strawberry Juice-A Chemometric Evaluation-Part I. Foods 2023, 12, 3172. [Google Scholar] [CrossRef] [PubMed]
- Rahmah, N.; Ahsan, A. Analysis of pulsed electric field (PEF) specific input energy and its effect to the tannin content of Areca (Areca catechu L.) seed powder extract. IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 012031. [Google Scholar] [CrossRef]
- Huang, W.; Feng, Z.; Aila, R.; Hou, Y.; Carne, A.; Bekhit, A.E.A. Effect of pulsed electric fields (PEF) on physico-chemical properties, beta-carotene and antioxidant activity of air-dried apricots. Food Chem. 2019, 291, 253–262. [Google Scholar] [CrossRef]
- Souli, I.; Chaira, N.; Jemni, M.; Tlahig, S.; Ferchichi, A.; Lanoisellé, J.L. Optimization and Intensification of Bioactive Components and Antioxidant Activity of Extracts from Date Fruit (Phoenix dactylifera L.) Using Pulsed Electric Fields (PEF) Technology and Thermal Processing. Processes 2023, 11, 884. [Google Scholar] [CrossRef]
- Basak, S.; Shaik, L.; Chakraborty, S. Effect of ultraviolet and pulsed light treatments on ascorbic acid content in fruit juices-A review of the degradation mechanism. Food Chem. Adv. 2023, 2, 100333. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Han, Z.; Zeng, X.A.; Wang, M.S. The preparation of Fe-glycine complexes by a novel method (pulsed electric fields). Food Chem. 2017, 219, 468–476. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Guo, S.; Xue, X.; Yu, S. Iron-Chelating Properties of Melanoidins and Inducing Effect of Pulsed Electric Field. J. Food Process Eng. 2013, 36, 786–792. [Google Scholar] [CrossRef]
- Huang, W.N.; Nie, Y.T.; Zhu, N.; Yang, Y.F.; Zhu, C.Q.; Ji, M.B.; Wu, D.; Chen, K.S. Hybrid Label-Free Molecular Microscopies for Simultaneous Visualization of Changes in Cell Wall Polysaccharides of Peach at Single- and Multiple-Cell Levels during Postharvest Storage. Cells 2020, 9, 761. [Google Scholar] [CrossRef]
- Li, R.M.; Rosado-Souza, L.; Sampathkumar, A.; Fernie, A.R. The relationship between cell wall and postharvest physiological deterioration of fresh produce. Plant Physiol. Biochem. 2024, 210, 108568. [Google Scholar] [CrossRef] [PubMed]
- Lohani, U.C.; Muthukumarappan, K. Application of the pulsed electric field to release bound phenolics in sorghum flour and apple pomace. Innov. Food Sci. Emerg. Technol. 2016, 35, 29–35. [Google Scholar] [CrossRef]
- Faridnia, F.; Burritt, D.J.; Bremer, P.J.; Oey, I. Innovative approach to determine the effect of pulsed electric fields on the microstructure of whole potato tubers: Use of cell viability, microscopic images and ionic leakage measurements. Food Res. Int. 2015, 77, 556–564. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Castagnini, J.M.; Berrada, H.; Barba, F.J. Pulsed electric field (PEF) recovery of biomolecules from Chlorella: Extract efficiency, nutrient relative value, and algae morphology analysis. Food Chem. 2023, 404, 134615. [Google Scholar] [CrossRef]
- Bi, X.; Liu, F.; Rao, L.; Li, J.; Liu, B.; Liao, X.; Wu, J. Effects of electric field strength and pulse rise time on physicochemical and sensory properties of apple juice by pulsed electric field. Innov. Food Sci. Emerg. Technol. 2013, 17, 85–92. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Guo, S.; Ma, S.; Yu, S.-J. Kinetic modeling of Maillard reaction system subjected to pulsed electric field. Innov. Food Sci. Emerg. Technol. 2013, 20, 121–125. [Google Scholar] [CrossRef]
- Rodier, L.C.; Hartel, R.W. Characterizing Maillard reaction kinetics and rheological changes in white chocolate over extended heating. J. Food Sci. 2021, 86, 2553–2568. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.; Buniowska, M.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M. Influence of pulsed electric field processing on the quality of fruit juice beverages sweetened with Stevia rebaudiana. Food Bioprod. Process. 2017, 101, 214–222. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Roland, W.S.U.; van Kekem, K.; Matser, A.M.; van Boekel, M. Effect of Pasteurization by Moderate Intensity Pulsed Electric Fields (PEF) Treatment Compared to Thermal Treatment on Quality Attributes of Fresh Orange Juice. Foods 2022, 11, 3360. [Google Scholar] [CrossRef]
- Yeom, H.W.; Streaker, C.B.; Zhang, Q.H.; Min, D.B. Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization. J. Agric. Food Chem. 2000, 48, 4597–4605. [Google Scholar] [CrossRef]
- Debbarma, M.; Srivastava, B. Impact of Pulsed Electric Field Pretreatment on Extraction Efficiency and Quality Attributes of Sohiong Fruit Juice. J. Food Process Eng. 2025, 48, e70038. [Google Scholar] [CrossRef]
- Ahmed, T.; Rana, M.R.; Zzaman, W.; Ara, R.; Aziz, M.G. Optimization of substrate composition for pectinase production from Satkara (Citrus macroptera) peel using Aspergillus niger-ATCC 1640 in solid-state fermentation. Heliyon 2021, 7, e08133. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Zeng, X.A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J. Food Sci. Technol. 2019, 56, 2355–2364. [Google Scholar] [CrossRef]
- Lee, H.; Choi, S.; Kim, E.; Kim, Y.-N.; Lee, J.; Lee, D.-U. Effects of pulsed electric field and thermal treatments on microbial reduction, volatile composition, and sensory properties of orange juice, and their characterization by a principal component analysis. Appl. Sci. 2020, 11, 186. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das, B.; Haldar, P.K.; Kar, A.; Chaudhary, S.K.; Singh, K.O.; Bhardwaj, P.K.; Sharma, N.; Mukherjee, P.K. 6-Gingerol contents of several ginger varieties of Northeast India and correlation of their antioxidant activity in respect to phenolics and flavonoids contents. Phytochem. Anal. 2023, 34, 259–268. [Google Scholar] [CrossRef] [PubMed]



| Sample | Temp. (°C) | pH | EC (μS/cm) | °Brix (%) | Yield (%) | Cloud Value | Cloud Stability |
|---|---|---|---|---|---|---|---|
| FG | 24.17 ± 0.61 e | 7.23 ± 0.02 a | 223.37 ± 3.96 e | 2.33 ± 0.11 c | 69.94 ± 1.21 d | 2.46 ± 0.008 d | 71.98 ± 0.32 d |
| FG-1.5 | 26.35 ± 0.54 d | 7.09 ± 0.05 b | 238.00 ± 2.45 d | 2.83 ± 0.09 b | 76.38 ± 1.07 c | 2.53 ± 0.006 c | 88.79 ± 0.49 b |
| FG-2.0 | 29.19 ± 0.28 c | 7.02 ± 0.03 bc | 297.33 ± 4.11 c | 3.07 ± 0.09 ab | 79.77 ± 1.22 b | 2.56 ± 0.011 b | 91.84 ± 0.76 a |
| FG-2.5 | 37.83 ± 0.59 b | 6.99 ± 0.02 c | 348.84 ± 3.44 b | 3.08 ± 0.05 ab | 84.16 ± 1.13 a | 2.59 ± 0.009 a | 92.38 ± 0.57 a |
| FG-3.0 | 43.39 ± 0.31 a | 6.98 ± 0.04 c | 390.33 ± 2.49 a | 3.20 ± 0.08 a | 80.57 ± 0.90 b | 2.57 ± 0.008 ab | 86.68 ± 0.79 c |
| Results are shown as mean values ± SD in triplicate. Values in the same columns showing the letters differ significantly (p < 0.05). | |||||||
| OG | 22.97 ± 0.29 e | 6.51 ± 0.02 a | 254.0 ± 2.64 e | 3.25 ± 0.07 c | 81.93 ± 0.24 d | 2.73 ± 0.008 d | 82.83 ± 0.34 d |
| OG-1.5 | 25.96 ± 0.29 d | 6.45 ± 0.01 b | 258.3 ± 1.70 d | 4.30 ± 0.16 b | 85.21 ± 1.31 c | 2.85 ± 0.005 b | 89.40 ± 0.31 c |
| OG-2.0 | 30.89 ± 0.47 c | 6.41 ± 0.02 c | 332.0 ± 3.27 c | 4.47 ± 0.12 ab | 87.58 ± 0.73 b | 2.85 ± 0.002 b | 91.25 ± 0.19 b |
| OG-2.5 | 36.43 ± 0.24 b | 6.34 ± 0.02 d | 389.7 ± 2.37 a | 4.56 ± 0.06 ab | 90.85 ± 0.68 a | 2.86 ± 0.003 a | 91.96 ± 0.65 a |
| OG-3.0 | 41.99 ± 0.25 a | 6.30 ± 0.02 d | 372.7 ± 2.49 b | 4.60 ± 0.08 a | 89.19 ± 0.39 ab | 2.86 ± 0.001 ab | 90.48 ± 0.34 b |
| Values are means ± standard deviations. Values within each column with different letters are significantly different (p < 0.05). | |||||||
| Sample | L* | a* | b* | ΔE | Hue (hº) | C* | WI | NEB |
|---|---|---|---|---|---|---|---|---|
| FG | 17.73 ± 0.74 c | 0.20 ± 0.08 a | 11.93 ± 0.29 d | - | 89.04 ± 0.39 d | 11.94 ± 0.29 d | 16.87 ± 0.74 d | 0.243 ± 0.003 e |
| FG-1.5 | 27.27 ± 0.24 b | −0.33 ± 0.12 b | 14.67 ± 0.05 b | 10.01 ± 0.87 b | 91.30 ± 0.48 c | 14.67 ± 0.05 b | 25.80 ± 0.23 bc | 0.257 ± 0.003 d |
| FG-2.0 | 28.47 ± 0.47 a | −0.57 ± 0.05 c | 16.13 ± 0.37 a | 11.63 ± 0.32 a | 92.01 ± 0.17 c | 16.14 ± 0.37 a | 26.67 ± 0.40 ab | 0.301 ± 0.004 c |
| FG-2.5 | 28.80 ± 0.29 a | −1.00 ± 0.05 d | 15.47 ± 0.29 a | 11.69 ± 0.37 a | 93.70 ± 0.35 b | 15.50 ± 0.28 a | 27.13 ± 0.25 a | 0.331 ± 0.003 a |
| FG-3.0 | 26.73 ± 0.34 b | −1.23 ± 0.09 e | 13.47 ± 0.41 c | 9.31 ± 0.65 b | 95.25 ± 0.54 a | 13.52 ± 0.40 c | 25.49 ± 0.26 c | 0.315 ± 0.005 b |
| Values are means ± standard deviations. Values within each column with different letters are significantly different (p < 0.05). | ||||||||
| OG | 25.10 ± 0.54 c | −0.90 ± 0.08 a | 6.00 ± 0.29 e | - | 98.51 ± 0.37 b | 6.07 ± 0.30 e | 24.85 ± 0.52 d | 0.281 ± 0.003 e |
| OG-1.5 | 28.6 ± 0.22 b | −1.70 ± 0.08 b | 9.20 ± 0.14 d | 4.92 ± 0.51 c | 100.46 ± 0.37 ab | 9.36 ± 0.15 d | 27.99 ± 0.20 bc | 0.296 ± 0.001 d |
| OG-2.0 | 28.63 ± 0.5 b | −2.20 ± 0.14 c | 10.90 ± 0.08 c | 6.29 ± 0.63 c | 101.41 ± 0.72 a | 11.12 ± 0.08 c | 27.77 ± 0.49 c | 0.315 ± 0.003 c |
| OG-2.5 | 30.77 ± 0.33 ab | −2.73 ± 0.12 e | 15.20 ± 0.29 a | 10.97 ± 0.67 a | 100.21 ± 0.65 ab | 15.44 ± 0.27 a | 29.06 ± 0.27 b | 0.347 ± 0.005 a |
| OG-3.0 | 31.77 ± 0.26 a | −2.50 ± 0.08 d | 12.03 ± 0.42 b | 9.24 ± 0.95 b | 101.76 ± 0.76 a | 12.29 ± 0.39 b | 30.67 ± 0.26 a | 0.335 ± 0.002 b |
| Results are shown as mean values ± SD in triplicate. Values in the same columns showing the letters differ significantly (p < 0.05). | ||||||||
| No. | Peak | FG | FG-1.5 | FG-2.0 | FG-2.5 | FG-3.0 | OG | OG-1.5 | OG-2.0 | OG-2.5 | OG-3.0 | Compounds |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | ||||||||||||
| 1 | 777 | 186.7 | 87.6 | 108.0 | 113.7 | 81.7 | 174.4 | 232.4 | 134.5 | 183.9 | 131.2 | C–H bending out-of-plane vibrations |
| 2 | 1028 | 2336.0 | 2820.8 | 3274.0 | 3951.7 | 2912.9 | 5195.4 | 5995.3 | 6438.8 | 6587.7 | 6130.8 | Cellulose and phenols |
| 3 | 1075 | 1006.2 | 1205.4 | 1609.6 | 1711.8 | 1317.6 | - | - | - | - | - | Cellulose and phenols |
| 4 | 1401 | 1423.2 | 1289.5 | 1790.0 | 1812.2 | 1361.8 | 2753.3 | 2698.5 | 2951.0 | 2549.1 | 2409.7 | CH2 or CH3 bending vibrations, indicating aliphatic chains |
| 5 | 1634 | 1805.9 | 1320.9 | 2622.8 | 1926.9 | 1982.1 | 2839.9 | 2493.5 | 2045.8 | 2342.0 | 2099.9 | C = C stretching in aromatic rings or amide (Terpenes like zingiberene, camphene, β-elemene, limonene) |
| 6 | 1737 | 197.7 | 201.2 | 88.8 | 64.5 | 37.5 | 57.6 | 78.7 | 74.9 | 88.8 | 68.8 | C = O stretching vibrations (aldehydes and ketones) |
| 7 | 2300–1900 | 125.9 | 31.7 | 36.6 | 54.1 | 32.2 | 298.9 | 303.2 | 360.2 | 304.2 | 392.6 | Aromatic combination and overtone bands |
| 8 | 2926 | 642.2 | 661.8 | 671.1 | 859.6 | 763.8 | 1023.2 | 1226.7 | 1094.9 | 1233.2 | 1311.2 | C–H stretching vibrations (carboxylic acids) |
| 9 | 3600–3000 | 7177.9 | 4238.6 | 3900.2 | 6479.6 | 4246.7 | 9810.2 | 10,877.6 | 12,221.3 | 11,622.0 | 11,627.9 | O–H stretching vibrations (water) |
| Sr. No. | RT | Compound | Type | Match Factor | Formula | CAS | Concentration of Compound (µg/mL) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| FG | FG-2.5 | OG | OG-2.5 | |||||||
| 1 | 9.19 | (1R)-2,6,6-Trimethylbicyclo [3.1.1]hept-2-ene | Monoterpene | 98.0 | C10H16 | 7785-70-8 | 2.03 | 3.85 | 5.92 | 13.29 |
| 2 | 10.58 | Camphene | Monoterpene | 98.2 | C10H16 | 79-92-5 | 6.49 | 13.07 | 17.76 | 37.67 |
| 3 | 13.53 | Cyclopentasiloxane, decamethyl- | Organosilicon compound | 97.9 | C10H30O5Si5 | 541-02-6 | 1.43 | 3.13 | 5.59 | 7.29 |
| 4 | 14.11 | Bicyclo [3.1.1]heptane, 6,6-dimethyl-2-methylene-, (1S)- | Monoterpene | 97.1 | C10H16 | 18172-67-3 | 1.05 | 0.68 | 2.96 | 2.66 |
| 5 | 14.20 | α-Phellandrene | Monoterpene | 94.5 | C10H16 | 99-83-2 | 0.34 | 0.55 | 8.52 | 17.14 |
| 6 | 15.57 | D-Limonene | Monoterpene | 98.5 | C10H16 | 5989-27-5 | 1.41 | 2.66 | 3.42 | 7.70 |
| 7 | 15.94 | Eucalyptol | Ether | 97.6 | C10H18O | 470-82-6 | 11.21 | 22.62 | 47.11 | 42.24 |
| 8 | 15.99 | β-Phellandrene | Monoterpene | 93.7 | C10H16 | 555-10-2 | 7.09 | |||
| 9 | 19.16 | 1,3-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- | Monoterpene | 95.3 | C10H16 | 99-86-5 | 0.63 | |||
| 10 | 20.49 | 2-Heptanol | Alcohol | 93.8 | C7H16O | 543-49-7 | 0.46 | 1.03 | 1.51 | |
| 11 | 20.96 | Cyclohexasiloxane, dodecamethyl- | Organosilicon compound | 94.9 | C12H36O6Si6 | 540-97-6 | 0.59 | 1.49 | 2.95 | 4.19 |
| 12 | 21.56 | 5-Hepten-2-one, 6-methyl- | Ketone | 96.4 | C8H14O | 110-93-0 | 0.64 | 1.38 | 4.81 | |
| 13 | 27.66 | 1,2,4-Metheno-1H-indene, octahydro-1,7a-dimethyl-5-(1-methylethyl)-, [1S-(1α,2α,3aβ,4α,5α,7aβ,8S*)]- | Sesquiterpene | 97.2 | C15H24 | 22469-52-9 | 0.42 | 0.54 | 1.65 | 5.48 |
| 14 | 28.07 | Copaene | Sesquiterpene | 96.9 | C15H24 | 3856-25-5 | 0.70 | 0.78 | 3.06 | 10.48 |
| 15 | 29.30 | (+)-2-Bornanone | Ketone | 96.3 | C10H16O | 464-49-3 | 0.63 | |||
| 16 | 30.23 | (+)-3-Carene | Monoterpene | 96.0 | C10H16 | 498-15-7 | 1.22 | 2.31 | 3.90 | 3.39 |
| 17 | 31.78 | Bicyclo [2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S-endo)- | Ester | 97.3 | C12H20O2 | 5655-61-8 | 0.72 | |||
| 18 | 32.11 | Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)-, [1S-(1α,2β,4β)]- | Sesquiterpene | 96.7 | C15H24 | 515-13-9 | 0.97 | 1.20 | 3.05 | 8.65 |
| 19 | 32.39 | 2-Undecanone | Ketone | 95.7 | C11H22O | 112-12-9 | 0.49 | 0.75 | ||
| 20 | 32.61 | 4-Terpinenyl acetate | Ester | 94.0 | C12H20O2 | 4821-04-9 | 0.44 | 0.89 | 1.70 | |
| 21 | 34.36 | Aromandendrene | Sesquiterpene | 93.8 | C15H24 | 489-39-4 | 0.51 | |||
| 22 | 34.36 | Alloaromadendrene | Sesquiterpene | 96.7 | C15H24 | 25246-27-9 | 2.06 | 6.96 | ||
| 23 | 34.99 | 1H-Cycloprop[e]azulene, decahydro-1,1,7-trimethyl-4-methylene- | Sesquiterpene | 85.0 | C15H24 | 72747-25-2 | 0.35 | |||
| 24 | 34.99 | Caryophyllene | Sesquiterpene | 94.0 | C15H24 | 87-44-5 | 1.79 | 5.96 | ||
| 25 | 35.51 | Naphthalene, decahydro-4a-methyl-1-methylene-7-(1-methylethylidene)-, (4aR-trans)- | Sesquiterpene | 95.6 | C15H24 | 515-17-3 | 3.04 | |||
| 26 | 35.81 | Carveol | Alcohol | 95.8 | C10H16O | 99-48-9 | 19.29 | 23.39 | 50.90 | 46.90 |
| 27 | 36.02 | α-Cuprenene | Sesquiterpene | 89.2 | C15H24 | 29621-78-1 | 1.15 | |||
| 28 | 36.04 | .gamma.-Muurolene | Sesquiterpene | 92.1 | C15H24 | 30021-74-0 | 8.66 | |||
| 29 | 36.28 | α-Terpineol | Alcohol | 95.2 | C10H18O | 98-55-5 | 2.63 | 5.93 | 9.68 | 7.45 |
| 30 | 36.53 | endo-Borneol | Alcohol | 96.0 | C10H18O | 507-70-0 | 3.75 | 6.40 | 5.03 | |
| 31 | 36.92 | (+)-epi-Bicyclosesquiphellandrene | Sesquiterpene | 92.8 | C15H24 | 54274-73-6 | 2.63 | 25.52 | ||
| 32 | 37.28 | (3R,3aS,8aS)-3,6,8,8-Tetramethyl-2,3,4,7,8,8a-hexahydro-1H-3a,7-methanoazulene | Sesquiterpene | 93.8 | C15H24 | 22567-43-7 | 31.05 | 3.60 | ||
| 33 | 37.38 | 10s,11s-Himachala-3(12),4-diene | Sesquiterpene | 85.2 | C15H24 | 60909-28-6 | 227.72 | |||
| 34 | 37.60 | β-Bisabolene | Sesquiterpene | 96.4 | C15H24 | 495-61-4 | 5.95 | 5.38 | 27.95 | 71.47 |
| 35 | 37.74 | β-Panasinsene | Sesquiterpene | 95.3 | C15H24 | 1000159-39-0 | 1.75 | 1.79 | 5.52 | |
| 36 | 37.74 | 1H-Cyclopropa[a]naphthalene, decahydro-1,1,3a-trimethyl-7-methylene-, [1aS-(1aα,3aα,7aβ,7bα)]- | Sesquiterpene | 94.0 | C15H24 | 20071-49-2 | 16.20 | |||
| 37 | 37.96 | Neral | Aldehyde | 91.8 | C10H16O | 106-26-3 | 32.60 | 81.83 | 115.18 | |
| 38 | 38.43 | α-Farnesene | Sesquiterpene | 94.3 | C15H24 | 502-61-4 | 7.51 | 7.85 | 43.73 | 101.08 |
| 39 | 38.86 | Bicyclo [3.1.0]hex-2-ene, 4-methyl-1-(1-methylethyl)- | Monoterpene | 85.2 | C10H16 | 28634-89-1 | 0.96 | |||
| 40 | 39.17 | Citronellol | Alcohol | 93.6 | C10H20O | 106-22-9 | 0.94 | 1.64 | 2.48 | |
| 41 | 39.17 | Carotol | Alcohol | 85.9 | C15H26O | 465-28-1 | 0.62 | 3.11 | ||
| 42 | 39.37 | (2S,4aR,8aR)-4a,8-Dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,8a-octahydronaphthalene | Sesquiterpene | 93.0 | C15H24 | 123123-37-5 | 2.20 | |||
| 43 | 39.40 | (-)-α-Panasinsen | Sesquiterpene | 94.4 | C15H24 | 56633-28-4 | 5.57 | |||
| 44 | 39.57 | 1H-3a,7-Methanoazulene, octahydro-3,8,8-trimethyl-6-methylene-, [3R-(3α,3aβ,7β,8aα)]- | Sesquiterpene | 94.9 | C15H24 | 546-28-1 | 11.89 | 10.15 | 4.77 | |
| 45 | 39.61 | Cyclohexene, 3-(1,5-dimethyl-4-hexenyl)-6-methylene-, [S-(R*,S*)]- | Sesquiterpene | 96.6 | C15H24 | 20307-83-9 | 136.93 | |||
| 46 | 39.82 | Benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl- | Sesquiterpene | 98.1 | C15H22 | 644-30-4 | 6.45 | 7.37 | 41.96 | 120.63 |
| 47 | 42.72 | 1,5-Cyclodecadiene, 1,5-dimethyl-8-(1-methylethylidene)-, (E,E)- | Sesquiterpene | 95.4 | C15H24 | 15423-57-1 | 0.61 | 0.69 | 5.31 | |
| 48 | 42.73 | Azulene, 1,2,3,3a,4,5,6,7-octahydro-1,4-dimethyl-7-(1-methylethenyl)-, [1R-(1α,3aβ,4α,7β)]- | Monoterpene | 94.1 | C15H24 | 22567-17-5 | 2.36 | |||
| 49 | 43.46 | (+)-3-Carene | Sesquiterpene | 95.3 | C10H16 | 498-15-7 | 1.26 | 5.70 | 3.59 | 5.37 |
| 50 | 49.80 | (8R,8aS)-8,8a-Dimethyl-2-(propan-2-ylidene)-1,2,3,7,8,8a-hexahydronaphthalene | Sesquiterpene | 89.0 | C15H22 | 27840-40-0 | 0.39 | |||
| Total volatile compounds concentration | 88.17 | 208.98 | 392.73 | 1091.23 | ||||||
| No. of volatile compounds | 26 | 39 | 28 | 35 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; He, P.; Geng, Y.-H.; Manzoor, M.F.; Zeng, X.-A.; Hassan, S.; Afraz, M.T. Quantitative Analysis of Ginger Maturity and Pulsed Electric Field Thresholds: Effects on Microstructure and Juice’s Nutritional Profile. Foods 2025, 14, 2637. https://doi.org/10.3390/foods14152637
Han Z, He P, Geng Y-H, Manzoor MF, Zeng X-A, Hassan S, Afraz MT. Quantitative Analysis of Ginger Maturity and Pulsed Electric Field Thresholds: Effects on Microstructure and Juice’s Nutritional Profile. Foods. 2025; 14(15):2637. https://doi.org/10.3390/foods14152637
Chicago/Turabian StyleHan, Zhong, Pan He, Yu-Huan Geng, Muhammad Faisal Manzoor, Xin-An Zeng, Suqlain Hassan, and Muhammad Talha Afraz. 2025. "Quantitative Analysis of Ginger Maturity and Pulsed Electric Field Thresholds: Effects on Microstructure and Juice’s Nutritional Profile" Foods 14, no. 15: 2637. https://doi.org/10.3390/foods14152637
APA StyleHan, Z., He, P., Geng, Y.-H., Manzoor, M. F., Zeng, X.-A., Hassan, S., & Afraz, M. T. (2025). Quantitative Analysis of Ginger Maturity and Pulsed Electric Field Thresholds: Effects on Microstructure and Juice’s Nutritional Profile. Foods, 14(15), 2637. https://doi.org/10.3390/foods14152637









