Caseinate–Carboxymethyl Chitosan Composite Edible Coating with Soybean Oil for Extending the Shelf Life of Blueberry Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coating Solutions
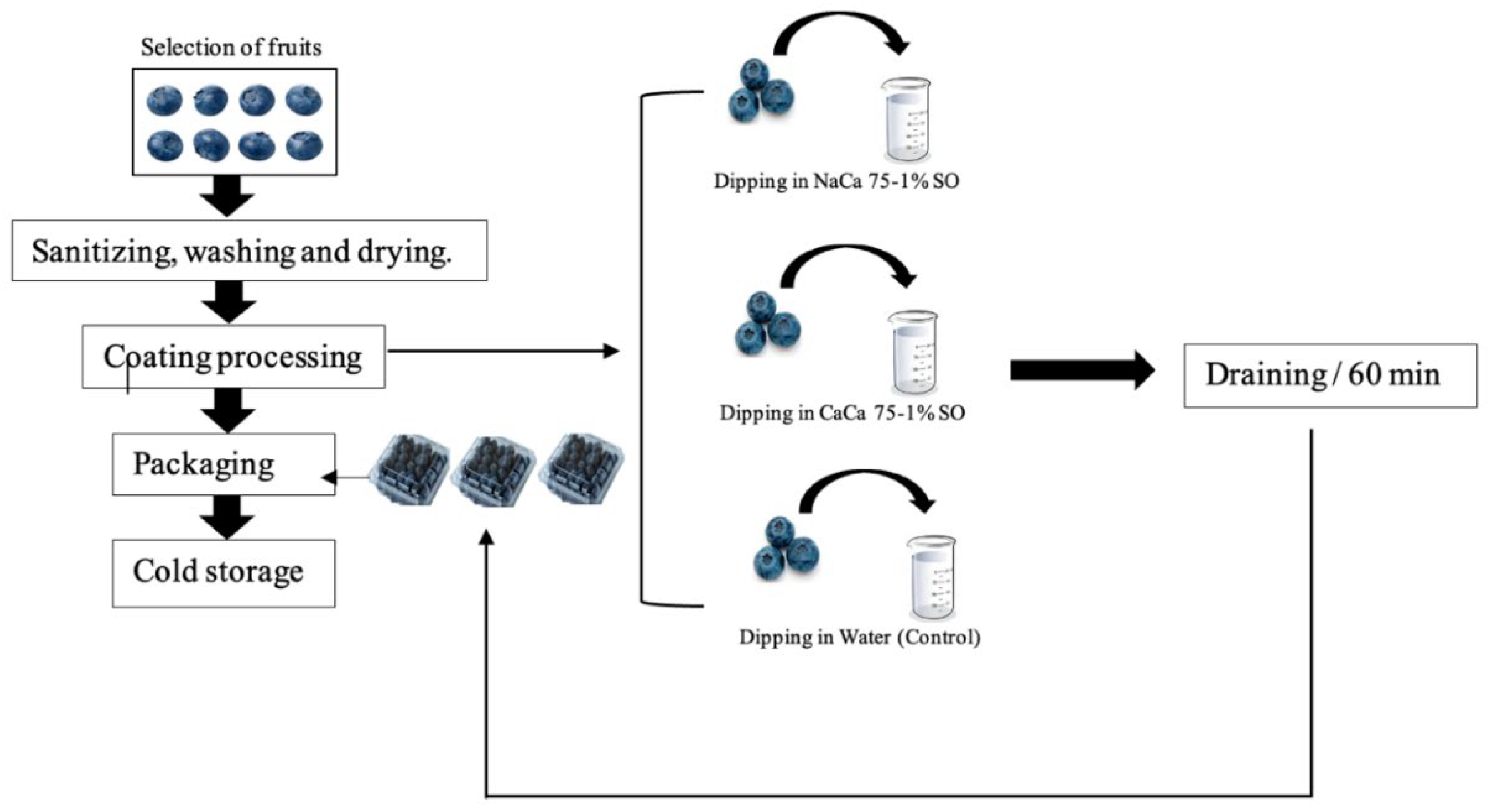
2.3. Sample Preparation
2.4. Shelf Life Studies
2.5. pH, Brix, Titratable Acidity, and Weight Loss
2.6. Textural Analysis
2.7. Color Measurements
2.8. Respiration Rate (RR)
2.9. Microbial Growth
2.10. Data Analyses
3. Results and Discussion
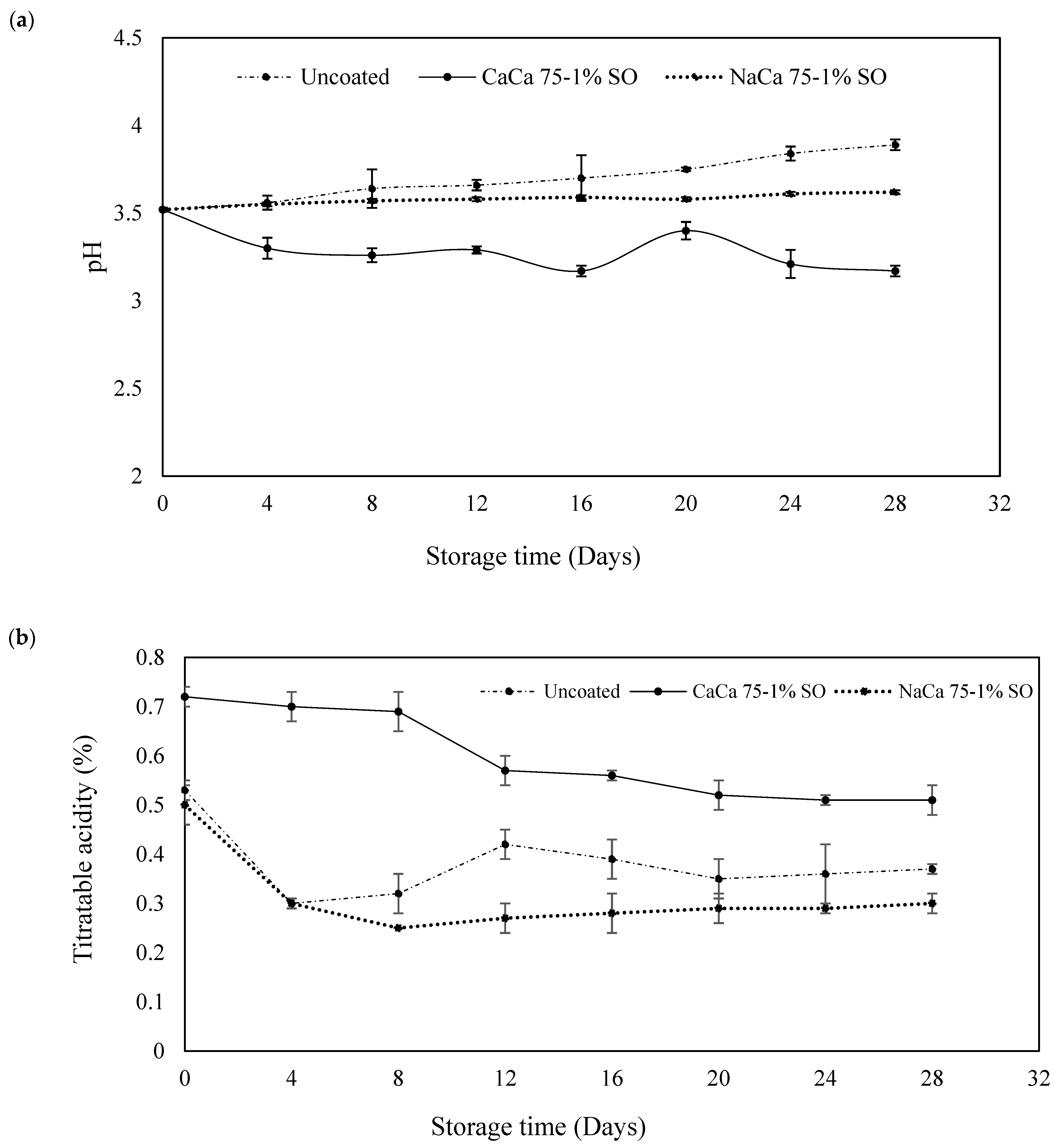
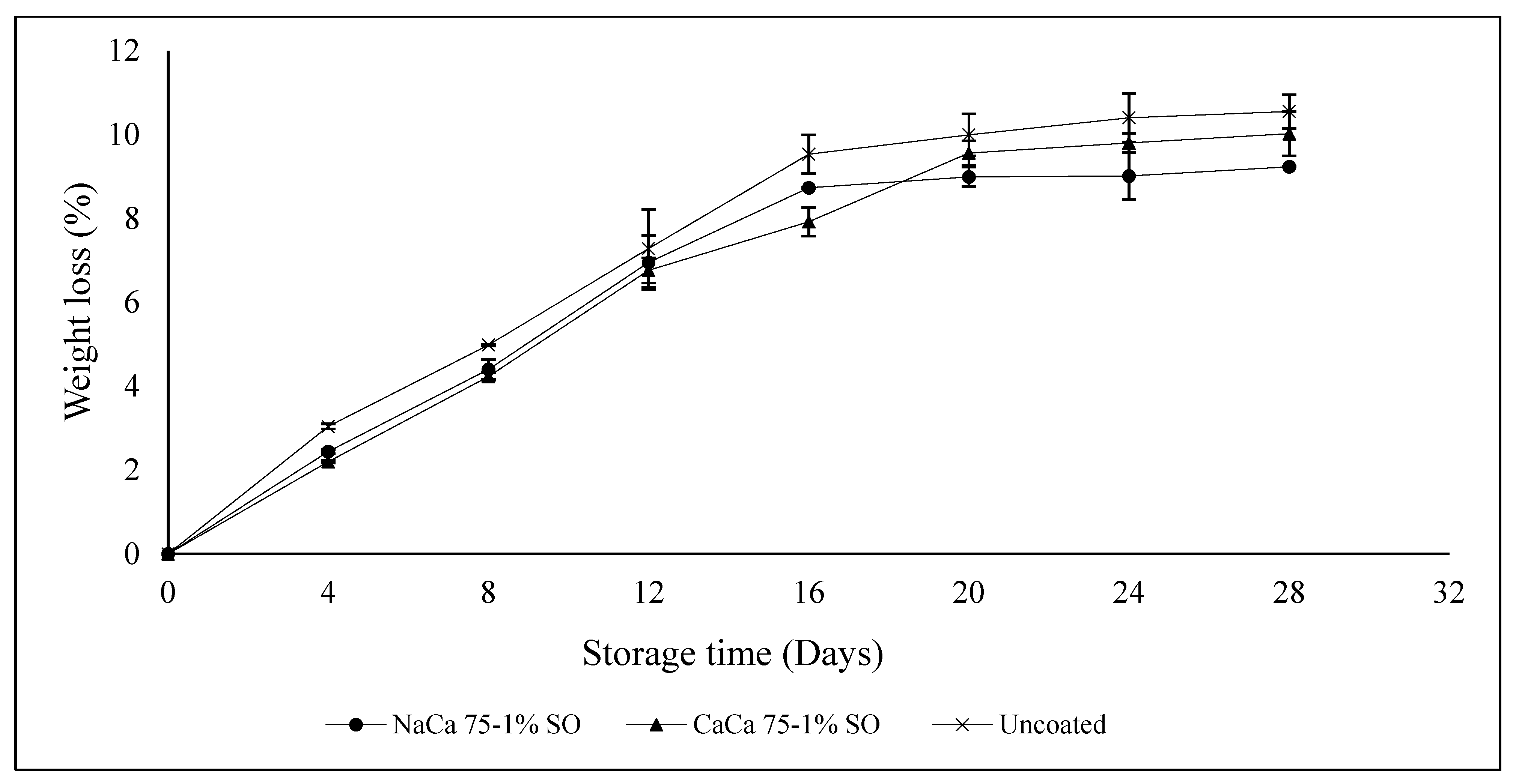
3.1. pH, Soluble Solids Content, Titratable Acidity, and Weight Loss
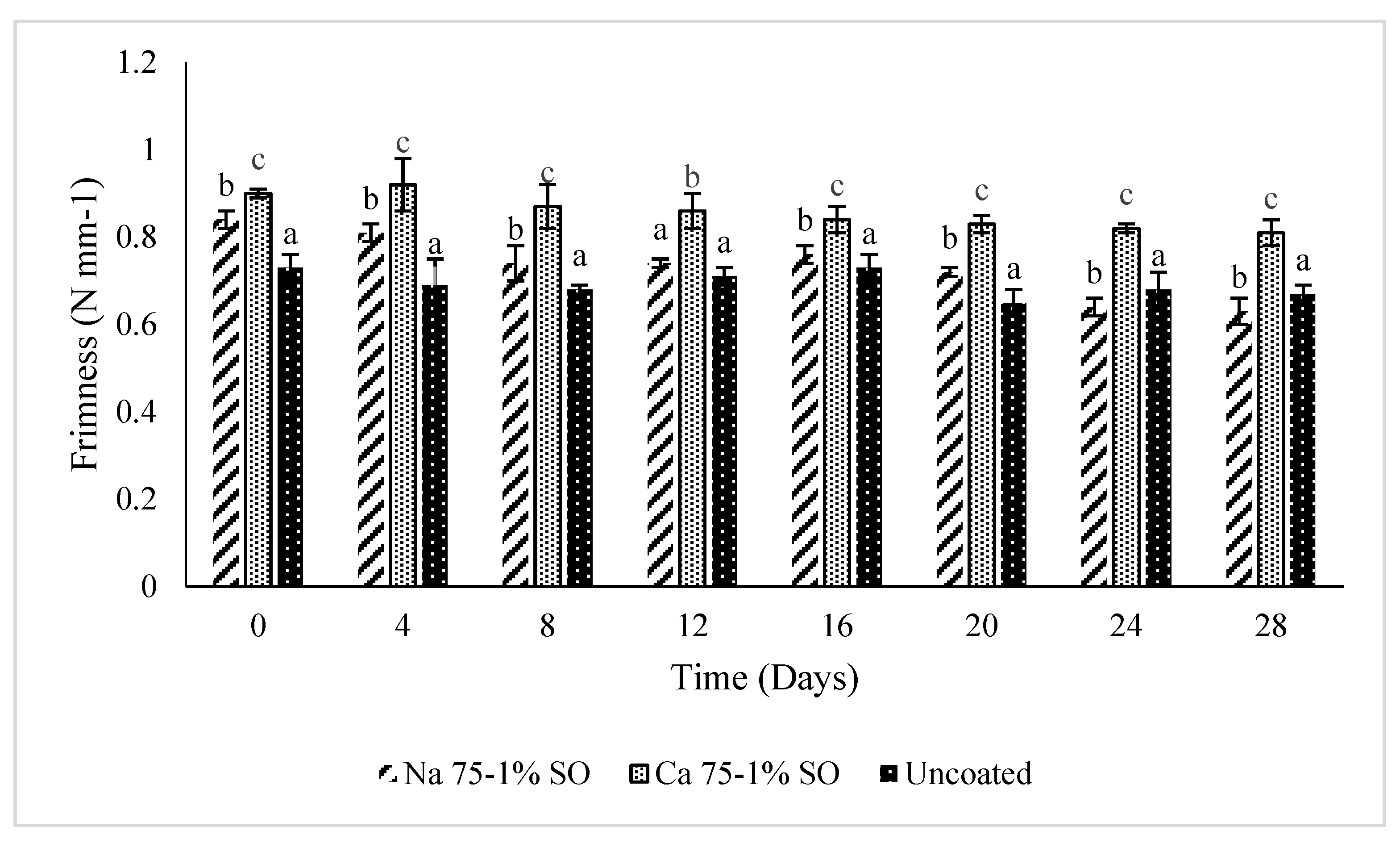
3.2. Textural Analysis
3.3. Color
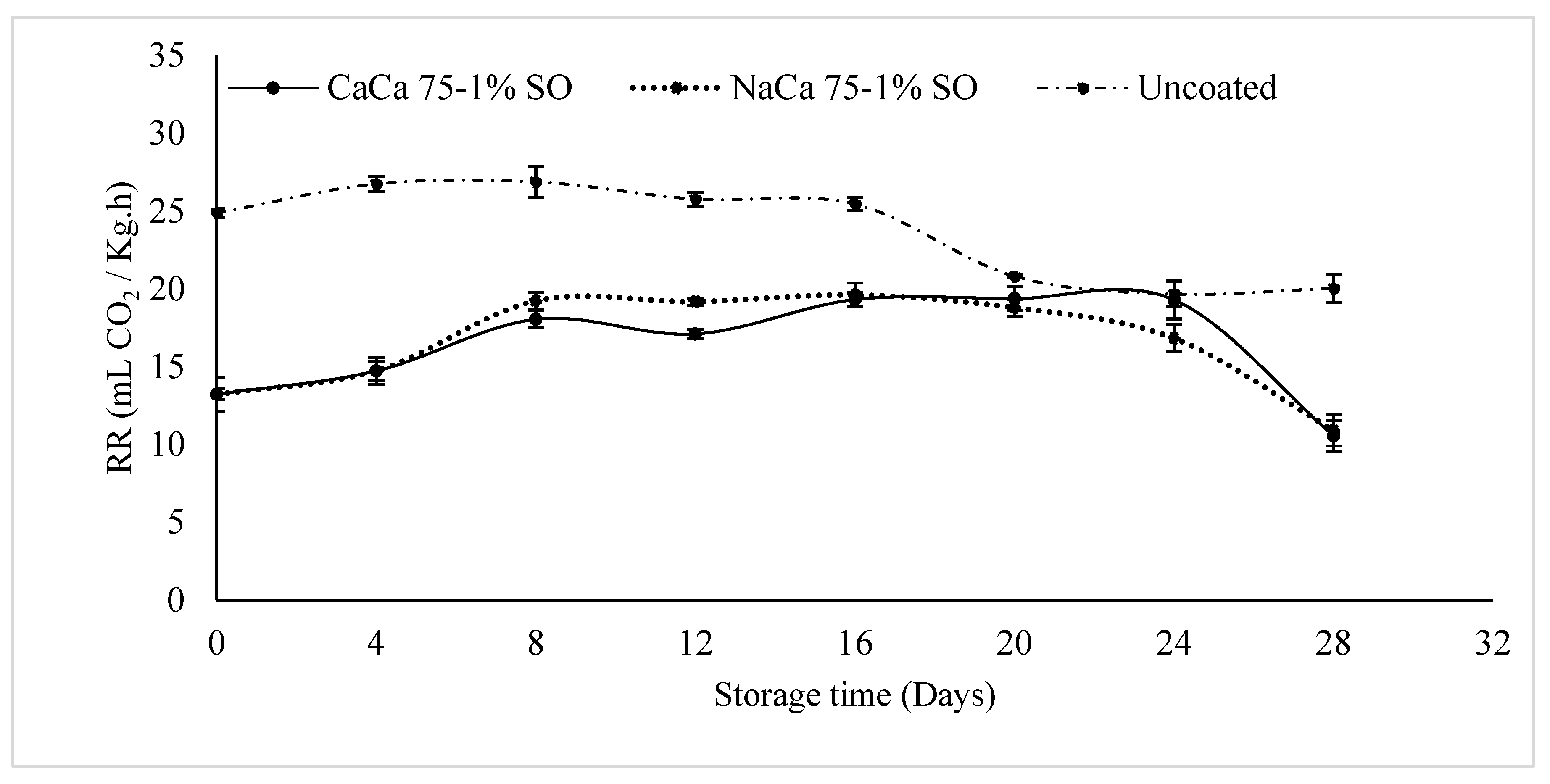
3.4. Respiration Rate
3.5. Microbial Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalska, A.; Lysiak, G. Bioactive compound of blueberries: Post-harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 2015, 8, 18642–18663. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Qiao, L.; Huang, T.; Zhang, Y.; Qu, G.; Kou, X. Melatonin reduces postharvest decay of blueberries by regulating ascorbate-glutathione cycle and membrane lipid metabolism. Postharvest Biol. Technol. 2024, 218, 113185. [Google Scholar] [CrossRef]
- FAO. The State of Food Security and Nutrition in the World; Food and Agriculture Organization: Rome, Italy, 2020. [Google Scholar]
- Harris, J.; Piters, B.S.; McMullin, S.; Bajwa, B.; Jager, I.; Brouwer, I.D. Fruit and vegetable for healthy diets: Priorities for food system research and action. In Science Innovations for Food Systems Transformation; National Center for Biotechnology information: Bethesda, MD, USA, 2023; Available online: https://pubmed.ncbi.nlm.nih.gov/38285842/ (accessed on 22 July 2025).
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; (WHO Technical Report Series, No. 916); World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Gidado, M.J.; Gunny, A.A.N.; Gopinath, S.C.B.; Ali, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of postharvest water loss in fruit: Mechanisms, influencing factors, and effective control strategies—A comprehensive review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- Fan, Y.L.; Yang, J.; Duan, A.B.; Li, X.J. Pectin/sodium alginate/xanthan gum edible composite films as the fresh-cut package. Int. J. Biol. Macromol. 2021, 181, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, R.; Lei, D.; Huang, Y.; Cheng, S.; Zhu, Z.; Wu, Z.; Cravotto, G. Impact of ultrasound, microwaves and high-pressure processing on food components and their interactions. Trends Food Sci. Technol. 2021, 109, 1–15. [Google Scholar] [CrossRef]
- Guo, X.; Guo, Y.; Yu, J.; Gu, T.; Russo, H.B.; Liu, Q.; Du, J.; Bai, J.; Zhang, B.; Kou, L. X-ray irradiation—Nonthermal processing and preservation of fresh winter jujube. Innov. Food Sci. Emerg. Technol. 2022, 81, 103151. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Wang, M.; Li, R.; Dai, J.; Yan, J.; Liu, Y. 3D printing of essential oil/B-cyclodextrin/popping candy modified atmosphere packaging for strawberry preservation. Carbohydr. Polym. 2022, 297, 120037. [Google Scholar] [CrossRef] [PubMed]
- Xie, F. Biopolymer-based multilayer films and coatings for food preservation: An update of the recent development. Curr. Food Sci. Technol. Rep. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Vanaraj, R.; Kumar, S.M.S.; Mayakrishnan, G.; Rathinam, B.; Kim, S.C. A current trend in efficient biopolymer coatings for edible fruits to enhance shelf life. Polymers 2024, 16, 2639. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Avalos, M.C.; Minjares-Fuentes, R.; Femenia, A. Contreras-Esquivel, J.C. Quintero-Ramos, A. Esparza-Rivera, J.R. Meza-Velazquez, J.A. Application of an alginate-chitosan edible film on figs (Ficus carica): Effect on bioactive compounds and antioxidant capacity. Food Bioprocess Technol. 2019, 12, 499–511. [Google Scholar] [CrossRef]
- Tokatli, K.; Demirdoven, A. Effects of chitosan edible film coatings on the physicochemical and microbiocidal qualities of sweet cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656. [Google Scholar] [CrossRef]
- Chen, W.J.; Wang, L.; Yang, F.X.; Chen, C.W. Development of active poly (butylene adipate-co-terephthalate) films incorpated with sodium benzoate and its application in white mushroom (Agaricus bisporus) packaging. J. Food Process. Preserv. 2021, 7, 15666. [Google Scholar]
- Wang, T.; Zhai, X.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Shi, J. Effect of different coating methods on coating quality and mango preservation. Food Packag. Shelf Life 2023, 39, 101133. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, D.; Zhu, C.; Younis, K.; Yousuf, O. Shelf-life extension and quality changes of fresh-cut apple via sago and soy-oil-based edible coatings. Coatings 2024, 12, 1202. [Google Scholar] [CrossRef]
- Falco, I.; Flores-Meraz, P.L.; Randazzo, W.; Sanchez, G.; Lopez-Rubio, A.; Fabra, M.J. Antiviral activity of alginate-oleic acid-based coatings incorporating green tea extract on strawberries and raspberries. Food Hydrocoll. 2019, 87, 611–618. [Google Scholar] [CrossRef]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent development on postharvest application of edible coatings on stone fruit: A review. Sci. Hortic. 2020, 262, 109074. [Google Scholar] [CrossRef]
- Zimet, P.; Mombru, A.W.; Mombru, D.; Castro, A.; Pablo, J.; Pardo, H.; Rufo, C. Physico-chemical and antiliteral properties of nisin incorporated chitosan/carboxymethyl chitosan films. Carbohydr. Polym. 2019, 219, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Cui, Q.L.; Wang, Y.; Shi, F.; Liu, Y.; Liu, J.; Nie, G. Effect of carboxymethyl chitosan- gelatin based edible coatings on the quality and antioxidant properites of sweet cherry during postharvest storage. Sci. Hortic. 2021, 289, 110462. [Google Scholar] [CrossRef]
- Dayarian, S.; Zamani, A.; Moheb, A.; Masoomi, M. Physico- mechanical properties of films of chitosan, carboxymethyl chitosan, and their blend. J. Polym. Environ. 2014, 3, 409–416. [Google Scholar] [CrossRef]
- Bhatia, S.; Shan, Y.A.; Al-Harrasi, A.; Jawad, M.; Koca, E.; Aydemir, L.Y. Novel applications of black pepper essential oil as an antioxidant agent in sodium caseinate and chitosan based active edible films. Int. J. Biol. Macromol. 2024, 254, 128045. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Romero, D.; Castillo, S.; Guillen, F.; Paladine, D.; Zapata, P.J.; Valero, D.; Serrano, M. Rosehip oil coating delays postharvest ripening and maintains quality of European and Japanese plum cultivars. Postharvest Biol. Technol. 2019, 155, 29–36. [Google Scholar] [CrossRef]
- Moghadas, H.C.; Chauhan, R.; Scott Smith, J. Application of plant oils as functional additives in edible films and coatings for food packaging: A review. Foods 2024, 13, 997. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.; Cavella, S.; Masi, P.; Torrieri, E. Effect of solid concentration on structure and properties of chitosan-caseinate blend films. Food Packag. Shelf Life 2017, 13, 76–84. [Google Scholar] [CrossRef]
- Mohamed, A.; Ramaswamy, H.S. Characterization of caseinate- carboxymethyl chitosan-based edible films formulated with and without Transglutaminase enzyme. J. Compos. Sci. 2022, 7, 216. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International. In Official Methods of Analysis of AOAC International, 4th ed.; Association of Official Analytical Chemistry: Washington, DC, USA, 1998; methods 981.12. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Vieira, J.M.; Flores-Lopez, M.L.; Rodrguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan-aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef]
- Marquez, G.R.; Pierro, P.D.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.L.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein edible films. Food Sci. Technol. 2017, 75, 124–130. [Google Scholar]
- Maftoonzad, N. Evaluation of Edible Films and Coatings for Extending the Postharvest Shelf Life of Avocado. Ph.D. Thesis, McGill University Libraries, Montreal, QC, Canada, 2006. Available online: https://escholarship.mcgill.ca/concern/theses/jm214t40r (accessed on 22 July 2025).
- Fan, Y.; Li, C.; Zhu, J.; Sun, L.; Huang, R.; Guo, M.; Wu, Y.; Ge, Y. Organic acids metabolism and GABA shunt involved in maintaining quality of Malus domestica by methyl jasmonate treatment. Food Res. Int. 2022, 160, 111741. [Google Scholar] [CrossRef] [PubMed]
- Abugoch, L.; Tapia, C.; Plasencia, D.; Pastor, A.; Castro-Mandujano, O.; López, L.; Escalona, V.H. Shelf-life of fresh blueberries coated with quinoa protein/chitosan/sunflower oil edible film. J. Sci. Food Agric. 2016, 96, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries. Crop Production Science in Horticulture; Cabi: Cambridge, MA, USA, 2011. [Google Scholar]
- Yamam, O.; Bayoindili, L. Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT-Food Sci. Technol. 2002, 2, 146–150. [Google Scholar] [CrossRef]
- Wang, Y.; Acharya, T.P.; Malladi, A.; Tsai, H.; Nesmith, D.S.; Doyle, J.W.; Nambeesan, S.U. Atypical climacteric and functional ethylene metabolism and signaling during fruit ripening in blueberry (Vaccinium sp.). Front. Plant Sci. 2022, 13, 932642. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Villacorta, L.; Pretell-Vasquez, C.; Hayayumi-Valdivia, M. Optimization of edible coating with essential oils in blueberries. Cienc. E Agrotecnol. 2022, 46, e006022. [Google Scholar] [CrossRef]
- Tahir, H.E.; Zhihua, L.; Mahunu, G.K.; Xiaobo, Z.; Arslan, M.; Xiaowei, H.; Yang, Z.; Mariod, A.A. Effect of gum Arabic edible coating incorporated with African baobab pulp extract on postharvest quality of cold stored blueberries. Food Sci. Biotechnol. 2020, 2, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Han, Z.; Zhao, C.; Jiang, Q.; Tang, Y.; Li, Y.; Cheng, Z. Preparation and characterization of aloe vera polysaccharide-based packaging film and its application in blueberry preservation. Prog. Org. Coat. 2023, 177, 107445. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewics, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT-Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Recent highlights in sustainable bio-based edible films and coatings for fruit and vegetable applications. Foods 2024, 2, 318. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Li, J. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Fangfang, Z.; Xinpeng, B.; Wei, G.; Wang, G.D.; Shi, Z.Z.; Jun, G. Effects of virgin coconut oil on the physicochemical, morphological and antibacterial properties of potato starch-based biodegradable films. Int. J. Food Sci. Technol. 2020, 55, 192–200. [Google Scholar] [CrossRef]



| a* | ||||||||
| Day 0 | Day 4 | Day 8 | Day 12 | Day 16 | Day 20 | Day 24 | Day 28 | |
| Uncoated | 0.47 ± 1.19 a | 0.19 ± 0.18 b | 0.28 ± 0.08 c | 0.40 ± 0.11 d | 0.18 ± 0.17 b | 0.45 ± 0.12 a | 0.18 ± 0.16 b | 0.10 ± 0.29 e |
| 0.20 ± 0.24 a | 0.27 ± 0.11 b | 0.23 ± 0.09 a | 0.28 ± 0.14 b | 0.15 ± 0.21 c | 0.55 ± 0.40 d | 0.25 ± 0.04 e | 0.32 ± 0.16 f | |
| 0.53 ± 0.50 a | 0.73 ± 0.30 b | 0.73 ± 0.37 b | 0.79 ± 0.20 c | 1.24 ± 1.03 d | 1.40 ± 0.34 e | 1.23 ± 0.60 d | 1.10 ± 0.34 f | |
| b* | ||||||||
| Day 0 | Day 4 | Day 8 | Day 12 | Day 16 | Day 20 | Day 24 | Day 28 | |
| Uncoated | −3.08 ± 0.99 a | −2.31 ± 0.64 b | −1.74 ± 1.13 c | −3.17 ± 1.89 d | −3.03 ± 0.61 a | −2.85 ± 0.96 a | −2.63 0.22 e | −0.61 ± 0.81 f |
| −0.45 ± 0.52 a | −0.47 ± 0.28 a | −0.74 ± 0.18 b | −1.12 ± 0.28 c | −1.04 ± 0.14 d | −0.14 ± 0.81 e | −1.05 ± 0.12 d | −0.77 ± 0.22 f | |
| −1.66 ± 0.62 a | −1.98 ± 1.12 b | −1.72 ± 1.36 c | −1.72 ± 1.30 c | −0.51 ± 1.55 d | −1.52 ± 0.98 e | −1.35 ± 0.82 f | −1.33 ± 0.44 f | |
| Yeast and Mold Counts (log 10 cfu g−1) | |||||
| Day 0 | Day 4 | Day 10 | Day 20 | Day 28 | |
| Uncoated | nd | 2.2 0.2 a | 3.1 0.4 a | 3.8 0.2 a | 4.2 0.3 a |
| NaCa 75-1% SO | nd | 0 | 0 | 1.3 0.1 b | 2.3 0.4 b |
| CaCa 75-1% SO | nd | 0 | 0 | 0.9 0.5 c | 1.4 0.2 c |
| Mesophilic aerobic bacteria count (log 10 cfu g−1) | |||||
| Day 0 | Day 4 | Day 10 | Day 20 | Day 28 | |
| Uncoated | nd | 2.9 0.1 a | 3.3 0.2 a | 4.1 0.1 a | 4.2 0.3 a |
| NaCa 75-1% SO | nd | 1.2 0.2 b | 1.5 0.4 b | 2.1 0.2 b | 2.3 0.3 b |
| CaCa 75-1% SO | nd | 0 | 0 | 1.1 0.3 c | 1.4 0.1 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.M.A.; Ramaswamy, H.S. Caseinate–Carboxymethyl Chitosan Composite Edible Coating with Soybean Oil for Extending the Shelf Life of Blueberry Fruit. Foods 2025, 14, 2598. https://doi.org/10.3390/foods14152598
Mohamed AMA, Ramaswamy HS. Caseinate–Carboxymethyl Chitosan Composite Edible Coating with Soybean Oil for Extending the Shelf Life of Blueberry Fruit. Foods. 2025; 14(15):2598. https://doi.org/10.3390/foods14152598
Chicago/Turabian StyleMohamed, Amal M. A., and Hosahalli S. Ramaswamy. 2025. "Caseinate–Carboxymethyl Chitosan Composite Edible Coating with Soybean Oil for Extending the Shelf Life of Blueberry Fruit" Foods 14, no. 15: 2598. https://doi.org/10.3390/foods14152598
APA StyleMohamed, A. M. A., & Ramaswamy, H. S. (2025). Caseinate–Carboxymethyl Chitosan Composite Edible Coating with Soybean Oil for Extending the Shelf Life of Blueberry Fruit. Foods, 14(15), 2598. https://doi.org/10.3390/foods14152598







