Footprint of Domestic Processing on Safety and Functional Properties of Italian Black Garlic
Abstract
1. Introduction
2. Materials and Methods
2.1. Garlic Samples and Maturation Process
2.2. Microbial Sampling
2.3. Microbial Strains and Growth Conditions
2.4. Evaluation of Minimum Inhibitory Concentration (MIC) Versus Food Borne Pathogens and Intestinal Beneficial Bacteria
2.5. Evaluation of Prebiotic Score of Garlic Samples
2.6. Quantification of Bacterial DNA by qPCR
2.7. pH, Aw, and Moisture Content
2.8. Brown Intensity and Mechanical Properties
2.9. Volatilome Analyses by Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry (SPME-GC-MS)
2.10. Data Processing and Statistical Analysis
3. Results and Discussion
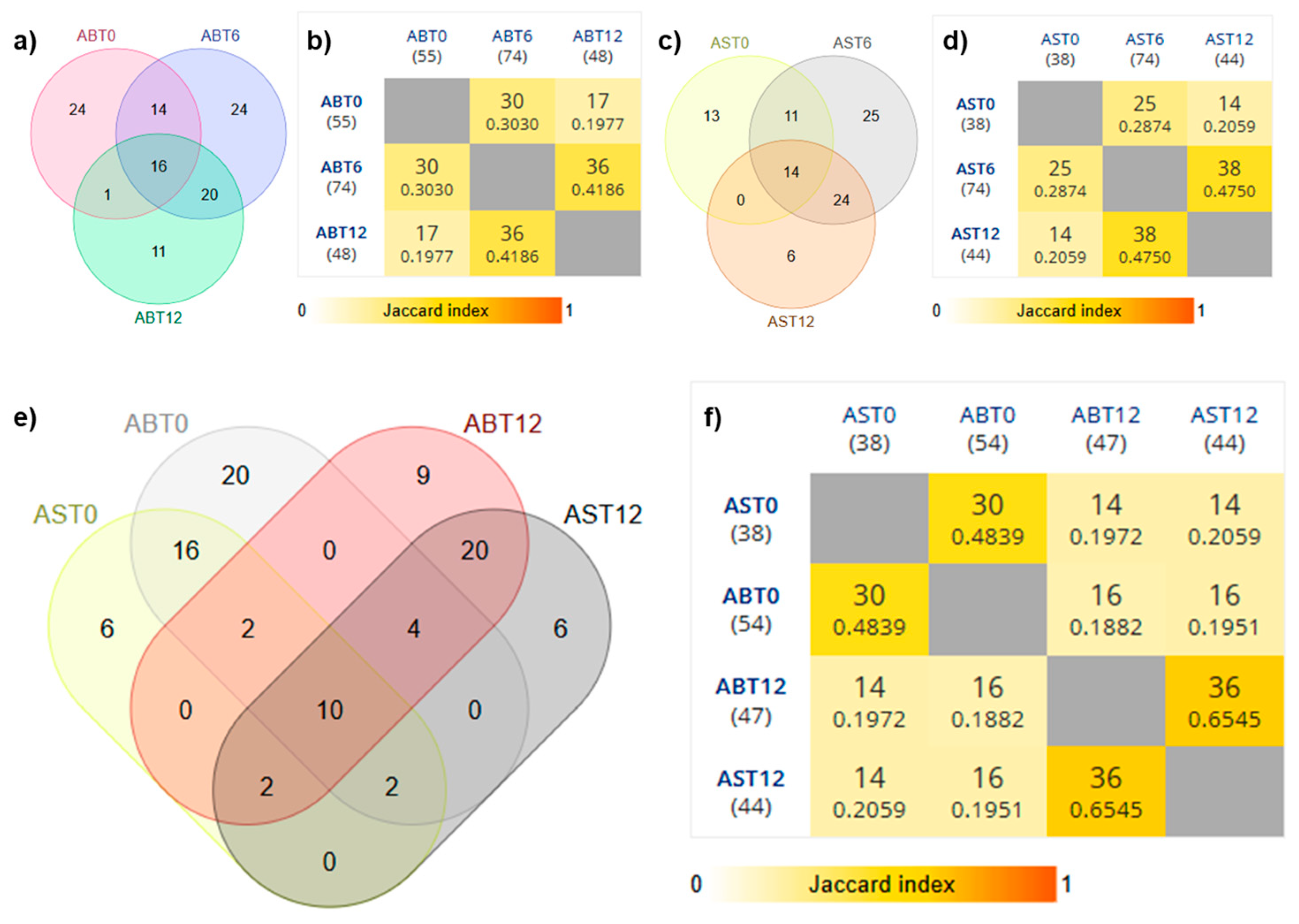
3.1. Evolution of the Growth of Endophytic Mesophiles and Yeasts
3.2. pH, Aw, and Moisture
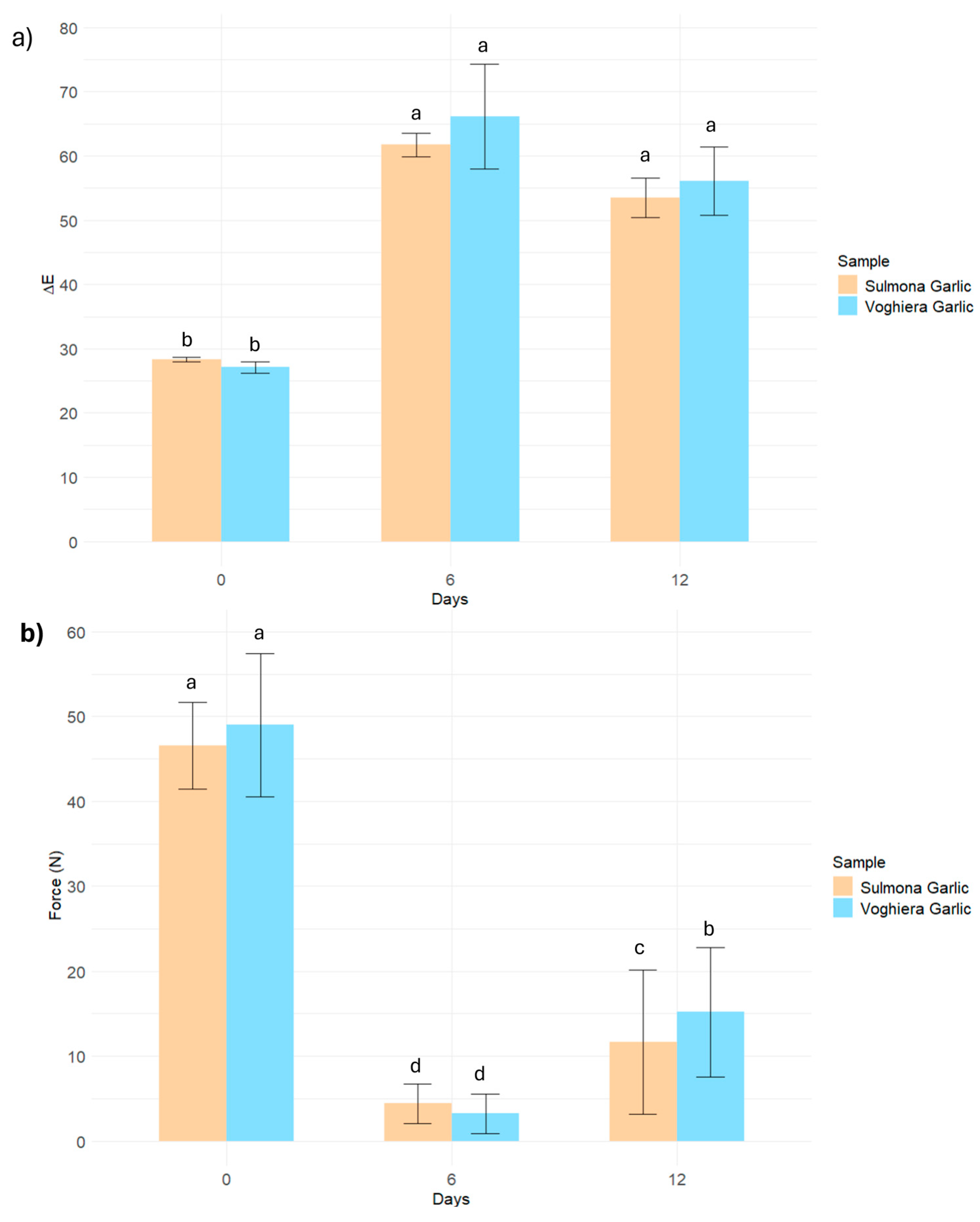
3.3. Brown Intensity and Mechanical Properties
3.4. Antimicrobial Properties
3.5. Prebiotic Activity
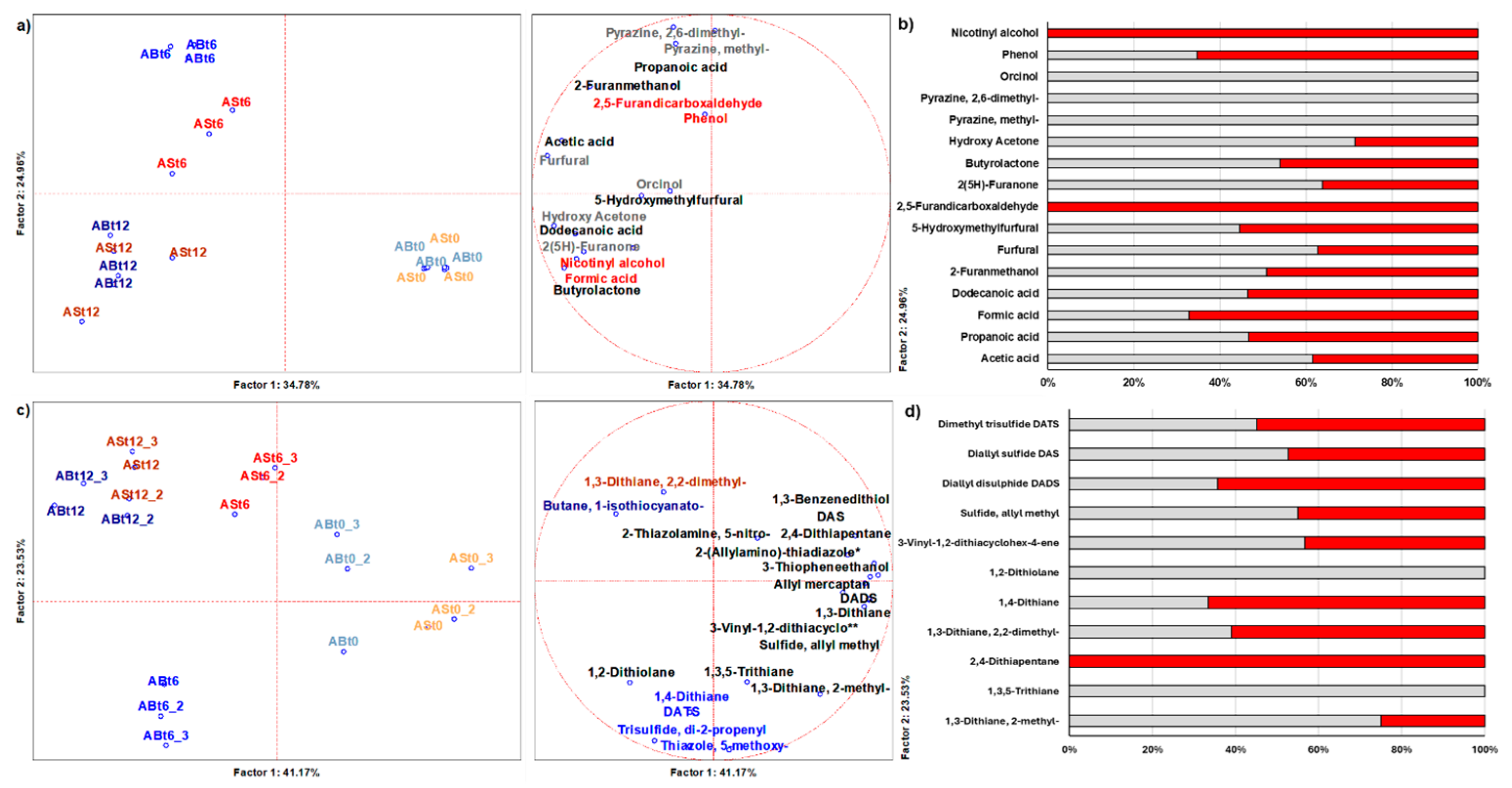
3.6. Volatile Organic Compounds Related to the Transformation Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Anti-Cancer and Anti-Inflammatory Properties of Black Garlic. Int. J. Mol. Sci. 2024, 25, 1801. [Google Scholar] [CrossRef] [PubMed]
- Utama, G.L.; Rahmi, Z.; Sari, M.P.; Hanidah, I. Psychochemical changes and functional properties of organosulfur and polysaccharide compounds of black garlic (Allium sativum L.). Curr. Res. Food Sci. 2024, 8, 100717. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-G.; Jin, N.; Deng, Y.-F.; Shen, X.-F.; Liu, C.-Y.; Ding, B.-Y.; Guo, L.-H.; Liu, Y.-X.; Huang, Z.-R.; Li, L.; et al. Protective action of selenium-enriched black garlic extract in rats with lipopolysaccharide/D-galactosamine-induced acute liver failure. J. Funct. Foods 2024, 115, 106123. [Google Scholar] [CrossRef]
- Farhat, Z.; Scheving, T.; Aga, D.S.; Hershberger, P.A.; Freudenheim, J.L.; Hageman Blair, R.; Mammen, M.J.; Mu, L. Antioxidant and Antiproliferative Activities of Several Garlic Forms. Nutrients 2023, 15, 4099. [Google Scholar] [CrossRef] [PubMed]
- Matsuse, K.; Hirata, S.; Abdelrahman, M.; Nakajima, T.; Iuchi, Y.; Kambayashi, S.; Okuda, M.; Kazumura, K.; Manochai, B.; Shigyo, M. Comparative Studies of Bioactivities and Chemical Components in Fresh and Black Garlics. Molecules 2024, 29, 2258. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Ge, S.; Fu, X.; Zhu, J.; Wang, M.; Wang, Y.; Huang, X.; Qin, X.; Tu, Y.; et al. Characterization of a short-term processing technology of black garlic with low 5-HMF content. Food Control. 2024, 165, 110650. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Kačániová, M.; Ormian, M.; Topczewska, J.; Sokołowicz, Z.; Hanus, P. Quality Assessment of Minced Poultry Products Including Black Fermented Garlic. Foods 2024, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, S.; Matei, L.A.; Lafay, C.B.B.; Mitterer-Daltoé, M.L.; de Lima, V.A.; Oldoni, T.L.C. Regrowth age, region, and harvest season on chemical and color parameters of Moringa oleifera from Brazil. S. Afr. J. Bot. 2023, 154, 26–31. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 16 April 2025).
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of microorganisms Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae Part 2: Colony-Count Technique. ISO: Geneva, Switzerland, 2017.
- ISO 17410:2019; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. ISO: Geneva, Switzerland, 2019.
- Rød, S.K.; Hansen, F.; Leipold, F.; Knøchel, S. Cold atmospheric pressure plasma treatment of ready-to-eat meat: Inactivation of Listeria innocua and changes in product quality. Food Microbiol. 2012, 30, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Addazii, D.; Casciano, F.; Danesi, F.; Rodriguez-Estrada, M.T.; Mercatante, D.; Ben Ayache, S.; Lotfi, A.; Argiriou, A.; Ayfantopoulou, G.; et al. Carob Syrup: Prebiotic Potential of a Neglected Functional Beverage of Mediterranean Countries. Foods 2024, 13, 4172. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, C.; Chrubasik-Hausmann, S.; Hellwig, E.; Vach, K.; Al-Ahmad, A. Activity of preparations from Spilanthes oleracea, propolis, Nigella sativa, and black garlic on different microorganisms involved in oral diseases and on total human salivary bacteria: A pilot study. Phytother. Res. 2018, 32, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Gunel, Z.; Torun, M.; Sahin-Nadeem, H. Sugar, d-pinitol, volatile composition, and antioxidant activity of carob powder roasted by microwave, hot air, and combined microwave/hot air. J. Food Process. Preserv. 2020, 44, e14371. [Google Scholar] [CrossRef]
- Pakakaew, P.; Phimolsiripol, Y.; Taesuwan, S.; Kumphune, S.; Klangpetch, W.; Utama-ang, N. The shortest innovative process for enhancing the S-allylcysteine content and antioxidant activity of black and golden garlic. Sci. Rep. 2022, 12, 11493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; de Araújo Calado, V.M.; Jarvis, B. Observations on the use of statistical methods in Food Science and Technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Babini, E.; Gianotti, A. Prebiotic potential and bioactive volatiles of hemp byproduct fermented by lactobacilli. LWT 2021, 151, 112201. [Google Scholar] [CrossRef]
- Nissen, L.; Rollini, M.; Picozzi, C.; Musatti, A.; Foschino, R.; Gianotti, A. Yeast-free doughs by Zymomonas mobilis: Evaluation of technological and fermentation performances by using a metabolomic approach. Microorganisms 2020, 8, 792. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Zheng, Z.; Zhang, B.; Lu, X.; Qiao, X. Characterization of the growth properties of garlic endophytes and their roles in the formation of black garlic. LWT 2021, 147, 111537. [Google Scholar] [CrossRef]
- Liang, T.; Wei, F.; Lu, Y.; Kodani, Y.; Nakada, M.; Miyakawa, T.; Tanokura, M. Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J. Agric. Food Chem. 2015, 63, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Najman, K.; Sadowska, A.; Hallmann, E. Influence of Thermal Processing on the Bioactive, Antioxidant, and Physicochemical Properties of Conventional and Organic Agriculture Black Garlic (Allium sativum L.). Appl. Sci. 2020, 10, 8638. [Google Scholar] [CrossRef]
- Ríos-Ríos, K.L.; Montilla, A.; Olano, A.; Villamiel, M. Physicochemical changes and sensorial properties during black garlic elaboration: A review. Trends Food Sci. Technol. 2019, 88, 459–467. [Google Scholar] [CrossRef]
- Aoudeh, E.; Oz, E.; Kelebek, H.; Brennan, C.; Ahmad, N.; Elobeid, T.; Oz, F. Black garlic production: The influence of ageing temperature and duration on some physicochemical and antioxidant properties, and sugar content. Int. J. Food Sci. Technol. 2023, 58, 3580–3590. [Google Scholar] [CrossRef]
- Fang, L. Effects of Humidity on the Quality of Black Garlic; Shandong Agricultural University: Taian, China, 2017. [Google Scholar]
- Kang, O.J. Physicochemical Characteristics of Black Garlic after Different Thermal Processing Steps. Prev. Nutr. Food Sci. 2016, 21, 348. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Maeda, H.; Yamaya, Y.; Tonosaki, Y.; Pinela, J.; Barros, L.; Dias, M.I. Maillard Reaction Intermediates and Related Phytochemicals in Black Garlic Determined by EPR and HPLC Analyses. Molecules 2020, 25, 4578. [Google Scholar] [CrossRef] [PubMed]
- Arachchi, S.J.T.; Kim, Y.J.; Kim, D.W.; Oh, S.C.; Lee, Y.B. Optimization of Maillard Reaction in Model System of Glucosamine and Cysteine Using Response Surface Methodology. Prev. Nutr. Food Sci. 2017, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M.; Nabavi, S.F.; Jafari, N.J.; Izadi, M.; Ajami, M.; Nabavi, S.M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Chhim, P.; Netzel, M.E.; Sultanbawa, Y. Phytochemical Characteristics and Antimicrobial Activity of Australian Grown Garlic (Allium sativum L.) Cultivars. Foods 2019, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.; Chin, V.K.; Wong, E.H.; Madhavan, P.; Tay, S.T.; Yong, P.V.C.; Chong, P.P. Review: Antimicrobial properties of allicin used alone or in combination with other medications. Folia Microbiol. 2020, 65, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.M.; Liu, X.; Hu, Y.H.; Feng, H.L.; Jia, Y.L.; Guo, Y.J.; Zhou, H.T.; Chen, Q.X. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int. J. Biol. Macromol. 2013, 57, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Jilani, S.B.; Olson, D.G. Mechanism of furfural toxicity and metabolic strategies to engineer tolerance in microbial strains. Microb. Cell Factories 2023, 22, 221. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Atsamnia, D.; Hamadache, M.; Hanini, S.; Benkortbi, O.; Oukrif, D. Prediction of the antibacterial activity of garlic extract on E. coli, S. aureus and B. subtilis by determining the diameter of the inhibition zones using artificial neural networks. LWT Food Sci. Technol. 2017, 82, 287–295. [Google Scholar] [CrossRef]
- Sharma, N.; Behl, T.; Singh, S.; Bansal, A.; Singh, S.K.; Zahoor, I. Expatiating the Therapeutic Profile of Garlic (Allium sativum): A Bench to Bedside Approach. Biointerface Res. Appl. Chem. 2021, 11, 14225–14239. [Google Scholar] [CrossRef]
- Song, X.; Xue, L.; Geng, X.; Wu, J.; Wu, T.; Zhang, M. Structural characteristics and immunomodulatory effects of melanoidins from black garlic. Foods 2023, 12, 2004. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Kim, J.; Kim, S.; Lee, K.J.; Shin, H. Garlic-induced enhancement of Bifidobacterium: Enterotype-specific modulation of gut microbiota and probiotic populations. Microorganisms 2024, 12, 1971. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, F.; Giambanelli, E.; D’Antuono, L.F. Comparison of Two Extraction Techniques (SDE vs. SPME) for the Determination of Garlic and Elephant Garlic Volatile Compounds. Food Anal. Methods 2022, 15, 1867–1879. [Google Scholar] [CrossRef]
- Şaşmaz, H.K.; Sevindik, O.; Güçlü, G.; Selli, S.; Kelebek, H. Effect of encapsulation on aroma and aroma-active components of fresh and black garlic. Int. J. Agric. Environ. Food Sci. 2025, 9, 627–637. [Google Scholar] [CrossRef]
- Molina-Calle, M.; Priego-Capote, F.; Luque de Castro, M.D. Headspace−GC–MS volatile profile of black garlic vs fresh garlic: Evolution along fermentation and behavior under heating. LWT 2017, 80, 98–105. [Google Scholar] [CrossRef]
- Fernandez, S.P.; González, R.E. Nutritional quality, bioactive compounds, and antioxidant activity of nine clones of fresh garlic and its black garlic derivative: A comparative study. Biol. Life Sci. Forum 2024, 40, 29. [Google Scholar]
- Maietti, A.; Marchetti, N.; Baraldo, N.; Fontana, R.; Tedeschi, P. Black Garlic Powder as an Ingredient to Enhance the Functional and Sensorial Properties of Bread and Its Shelf Life. Appl. Sci. 2025, 15, 5174. [Google Scholar] [CrossRef]
- Sasmaz, H.K.; Uzlasir, T.; Selli, S.; Kelebek, H. Black Garlic: Production Process, Bioactive Compounds, and Health Effects. Food Chem. Int. 2025, 1, 24–30. [Google Scholar] [CrossRef]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Masco, L.; Ventura, M.; Zink, R.; Huys, G.; Swings, J. Polyphasic taxonomic analysis of Bifidobacterium animalis and Bifidobacterium lactis reveals relatedness at the subspecies level: Reclassification of Bifidobacterium animalis as Bifidobacterium animalis subsp. animalis subsp. nov. and Bifidobacterium lactis as Bifidobacterium animalis subsp. lactis subsp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Bogan, J.A.; Welch, K.; Pickett, S.R.; Wang, H.J.; Zaritsky, A.; Helmstetter, C.E. Gene transcription and chromosome replication in Escherichia coli. J. Bacteriol. 1997, 179, 163–169. [Google Scholar] [CrossRef] [PubMed]
| Analysis | Garlic Samples at Different Times of Maturation | ||||||
|---|---|---|---|---|---|---|---|
| ABt0 | ASt0 | ABt6 | ASt6 | ABt12 | ASt12 | p-Value | |
| pH | 5.89 ± 0.09 a | 5.97 ± 0.05 a | 4.48 ± 0.07 b | 4.64 ± 0.07 b | 3.68 ± 0.05 c | 3.76 ± 0.07 c | 0.000001 |
| Aw | 0.979 ± 0.002 a | 0.978 ± 0.010 a | 0.855 ± 0.072 a,b | 0.872 ± 0.021 b | 0.63 ± 0.013 c | 0.712 ± 0.011 c | 0.000001 |
| Moisture (%) | 62.61 ± 5.10 a | 65.07 ± 1.71 a | 40 ± 14.21 b | 36.19 ± 7.80 b,c | 28.09 ± 9.11 c | 22.25 ± 9.81 c | 0.000001 |
| Yeast * | 5.84 ± 0.33 | 4.30 ± 0.75 | <10.00 ± 0.00 | <10.00 ± 0.00 | <10.00 ± 0.00 | <10.000 ± 0.00 | 0.476000 |
| Mesophiles * | 6.55 ± 0.07 a | 6.44 ± 0.02 a | 6.99 ± 0.15 b | 6.98 ± 0.25 b | <10.00 ± 0.00 b | <10.00 ± 0.00 b | 0.000002 |
| Strains | Minimal Inhibitory Concentration % (vol:vol) | ||||||
|---|---|---|---|---|---|---|---|
| ASt12 | ABt12 | ASt0 | ABt0 | Rifaximin | Chloramphenicol | p-Value | |
| Listeria innocua | >10.00 ± 0.00 a | >10.00 ± 0.00 a | 2.50 ± 0.00 c | 5.00 ± 1.44 b | 0.62 ± 0.00 d | 0.62 ± 0.00 d | 0.000066 |
| Escherichia coli | >10.00 ± 0.00 a | >10.00 ± 0.00 a | 1.25 ± 0.72 b | 1.25 ± 0.00 b | 0.62 ± 0.00 c | 0.62 ± 0.00 c | 0.000233 |
| Bacillus amyloliquefaciens | >10.00 ± 0.00 a | >10.00 ± 0.00 a | 2.50 ± 0.00 c | 5.00 ± 0.00 b | 0.62 ± 0.00 d | 0.62 ± 0.00 d | 0.00451 |
| Aerobic probiotic mix | >10.00 ± 0.00 a | >10.00 ± 0.00 a | 1.25 ± 0.72 b,c | 2.50 ± 0.00 b | <0.62 ± 0.00 c | 0.62 ± 0.00 c | 0.000741 |
| Probiotic bifidobacteria mix | >10.00 ± 0.00 a | >10.00 ± 0.00 a | 5.00 ± 1.44 b | 10.00 ± 0.00 a | 0.62 ± 0.00 c | 0.62 ± 0.00 c | 0.000006 |
| Probiotic lactobacilli mix | >10.00 ± 0.00 a | >10.00 ± 0.00 a | 2.50 ± 0.00 c | 5.00 ± 1.44 b | 0.62 ± 0.00 d | 0.62 ± 0.00 d | 0.000515 |
| Garlic Samples | Mean ± SD (Log Cells/mL) | ||
|---|---|---|---|
| Escherichia coli Mix | Bifidobacteria Mix | Lactobacilli Mix | |
| ASt12 | 10.31 ± 8.9 c | 10.93 ± 9.23 c | 11.36 ± 10.12 c |
| ABt12 | 12.77 ± 11.12 a | 10.34 ± 9.22 d | 11.08 ± 9.53 d |
| ASt0 | 7.95 ± 6.13 d | 10.88 ± 9.12 c | 10.91 ± 9.04 d |
| ABt0 | 7.97 ± 6.24 d | 11.28 ± 9.81 b | 10.70 ± 8.93 e |
| Glucose | 10.94 ± 9.01 b | 11.35 ± 8.82 b | 11.63 ± 10.25 b |
| FOS | 10.27 ± 8.82 c | 12.32 ± 11.01 a | 12.30 ± 11.22 a |
| p value | 0.000344 | 0.004664 | 0.000252 |
| Garlic Samples | Mean ± SD Prebiotic Index | |
|---|---|---|
| Lactogenic Effect | Bifidogenic Effect | |
| ASt12 | 0.08 ± 0.04 d | 0.13 ± 0.03 d |
| ABt12 | 0.33 ± 0.05 c | 0.20 ± 0.04 d |
| ASt0 | 0.67 ± 0.05 a | 0.67 ± 0.05 a |
| ABt0 | 0.74 ± 0.03 a | 0.63 ± 0.02 a |
| FOS | 0.51 ± 0.03 b | 0.53 ± 0.04 b |
| INU | 0.37 ± 0.01 c | 0.39 ± 0.04 c |
| p value | 0.000231 | 0.000066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addazii, D.; Cevoli, C.; Casciano, F.; Ferioli, F.; Gallina Toschi, T.; Gianotti, A.; Nissen, L. Footprint of Domestic Processing on Safety and Functional Properties of Italian Black Garlic. Foods 2025, 14, 2595. https://doi.org/10.3390/foods14152595
Addazii D, Cevoli C, Casciano F, Ferioli F, Gallina Toschi T, Gianotti A, Nissen L. Footprint of Domestic Processing on Safety and Functional Properties of Italian Black Garlic. Foods. 2025; 14(15):2595. https://doi.org/10.3390/foods14152595
Chicago/Turabian StyleAddazii, Davide, Chiara Cevoli, Flavia Casciano, Federico Ferioli, Tullia Gallina Toschi, Andrea Gianotti, and Lorenzo Nissen. 2025. "Footprint of Domestic Processing on Safety and Functional Properties of Italian Black Garlic" Foods 14, no. 15: 2595. https://doi.org/10.3390/foods14152595
APA StyleAddazii, D., Cevoli, C., Casciano, F., Ferioli, F., Gallina Toschi, T., Gianotti, A., & Nissen, L. (2025). Footprint of Domestic Processing on Safety and Functional Properties of Italian Black Garlic. Foods, 14(15), 2595. https://doi.org/10.3390/foods14152595







