Antimicrobial Properties of Thermally Processed Oyster Shell Powder for Use as Calcium Supplement
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermal Processed Oyster Shell Production
2.2. Analytical Methods
2.2.1. Particle Size Analysis
2.2.2. Solubility Measurement
2.2.3. pH Measurement of Calcium Samples
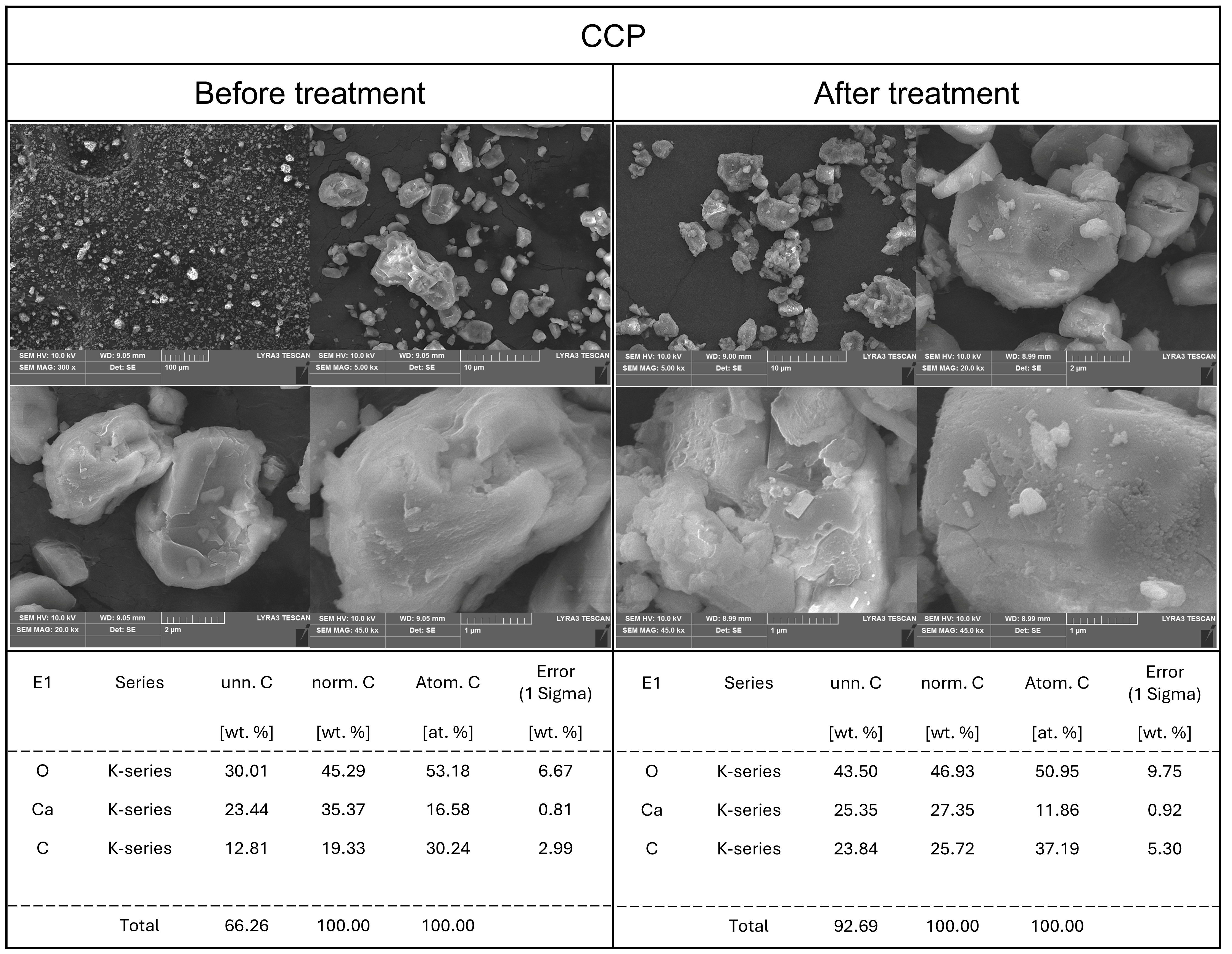
2.2.4. SEM-EDS Analysis
2.2.5. X-Ray Diffraction (XRD) Analysis
2.3. Evaluation of Antibacterial Activity
2.4. Effects of Calcium Sample Application on Rice Cake
2.4.1. Sample Preparation
2.4.2. Antifungal Activity Test
2.4.3. Color and pH Changes
Color Measurement of Rice Cake Samples
pH Measurement of Rice Cake Samples
2.4.4. Antimicrobial Activity Test
2.4.5. Texture Analysis of Rice Cake
3. Results and Discussion
3.1. Analytical Results
3.1.1. Powder Characteristics
3.1.2. Solubility Analysis
3.1.3. pH Changes in Aqueous Solutions
3.1.4. Characteristics After Dissolution
3.1.5. XRD Analysis
3.2. Antibacterial Activity
3.3. Effects of Sample Application on Rice Cake
3.3.1. Color Changes and Antifungal Properties
3.3.2. Antibacterial Properties in Rice Cake Matrix
3.3.3. Hardness and Chewiness Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellei, P.; Torres, I.; Solstad, R.; Flores-Colen, I. Potential Use of Oyster Shell Waste in the Composition of Construction Composites: A Review. Buildings 2023, 13, 1546. [Google Scholar] [CrossRef]
- de Alvarenga, R.A.F.; Galindro, B.M.; de Fátima Helpa, C.; Soares, S.R. The recycling of oyster shells: An environmental analysis using Life Cycle Assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Hart, A. Mini-review of waste shell-derived materials’ applications. Waste Manag. Res. 2020, 38, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The potential use of oyster shell waste in new value-added by-product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- Ke, H.; Ma, R.; Liu, X.; Xie, Y.; Chen, J. Highly effective peptide-calcium chelate prepared from aquatic products processing wastes: Stickwater and oyster shells. Lwt 2022, 168, 113947. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, H.J.; Jin, S.K.; Lee, H.J.; Choi, Y.I. Effect of Oyster Shell Calcium Powder on the Quality of Restructured Pork Ham. Korean J. Food Sci. Anim. Resour. 2014, 34, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, Y.M.; Noh, D.O.; Cho, S.Y.; Suh, H.J. The effect of oyster shell powder on the extension of the shelf life of tofu. Food Chem. 2007, 103, 155–160. [Google Scholar] [CrossRef]
- Choi, Y.M.; Whang, J.H.; Kim, J.M.; Suh, H.J. The effect of oyster shell powder on the extension of the shelf-life of Kimchi. Food Control 2006, 17, 695–699. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kerry, J.P.; Kelly, A.L. Changes in the microbiological and physicochemical quality of high-pressure-treated oysters (Crassostrea gigas) during chilled storage. Food Control 2008, 19, 1139–1147. [Google Scholar] [CrossRef]
- Li, M.; Yao, Z.T.; Chen, T.; Lou, Z.H.; Xia, M. The antibacterial activity and mechanism of mussel shell waste derived material. Powder Technol. 2014, 264, 577–582. [Google Scholar] [CrossRef]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Qin, Y.; Guan, X.; Liu, S.; Yu, H.; Li, P. Comparison of antifungal activities of scallop shell, oyster shell and their pyrolyzed products. Egypt. J. Aquat. Res. 2013, 39, 83–90. [Google Scholar] [CrossRef]
- Ulagesan, S.; Krishnan, S.; Nam, T.-J.; Choi, Y.-H. A review of bioactive compounds in oyster shell and tissues. Front. Bioeng. Biotechnol. 2022, 10, 913839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yan, G. Facile preparation of oyster shells–derived versatile Ca–Fe–O porous materials as Fenton-like reagent for environmental remediation. Funct. Mater. Lett. 2020, 13, 2050034. [Google Scholar] [CrossRef]
- Kim, B.-G.; Khirul, M.A.; Cho, D.; Kwon, S.-H. The effect of calcium peroxide originating from oyster shell powder on control of phosphorus compounds in oceanic sediment. Korean J. Chem. Eng. 2020, 37, 105–110. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, J.; Nuss, A.; Berghoff, B.; Klug, G. Singlet oxygen stress in microorganisms. Adv. Microb. Physiol. 2011, 58, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Yen, L.-T.; Weng, C.-H.; Than, N.A.T.; Tzeng, J.-H.; Jacobson, A.R.; Iamsaard, K.; Dang, V.D.; Lin, Y.-T. Mode of inactivation of Staphylococcus aureus and Escherichia coli by heated oyster-shell powder. Chem. Eng. J. 2022, 432, 134386. [Google Scholar] [CrossRef]
- Claisse, P.A. Civil Engineering Materials; Butterworth-Heinemann: Woburn, MA, USA, 2015. [Google Scholar]
- Lee, Y.-K.; Jung, S.K.; Chang, Y.H.; Kwak, H.-S. Highly bioavailable nanocalcium from oyster shell for preventing osteoporosis in rats. Int. J. Food Sci. Nutr. 2017, 68, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Njoyim-Tamungang, E.; Laminsi, S.; Ghogomu, P.; Njopwouo, D.; Brisset, J.-L. Pollution control of surface waters by coupling gliding discharge treatment with incorporated oyster shell powder. Chem. Eng. J. 2011, 173, 303–308. [Google Scholar] [CrossRef]
- Bergström, C.A.; Larsson, P. Computational prediction of drug solubility in water-based systems: Qualitative and quantitative approaches used in the current drug discovery and development setting. Int. J. Pharm. 2018, 540, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Belahcen, L.; Cassan, D.; Canaguier, E.; Robin, M.-H.; Chiffoleau, Y.; Samson, M.-F.; Jard, G. Physicochemical and sensorial characterization of artisanal pasta from the occitanie region in france. Foods 2022, 11, 3208. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Al-Farsi, S.A. Instrumental texture profile analysis (TPA) of date flesh as a function of moisture content. J. Food Eng. 2005, 66, 505–511. [Google Scholar] [CrossRef]
- Lee, I.-Y.; Park, Y.-S.; Shin, W.-S. The particle size of rice flour greatly affects the structural, textural and masticatory properties of steamed rice cake (Baekseolgi). Food Sci. Biotechnol. 2021, 30, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, F.; Sui, Y.; She, Z.; Zhai, W.; Wang, C.; Deng, Y. Effect of particle size on solubility, dissolution rate, and oral bioavailability: Evaluation using coenzyme Q10 as naked nanocrystals. Int. J. Nanomed. 2012, 7, 5733–5744. [Google Scholar] [CrossRef] [PubMed]
- Malešič, J.; Kadivec, M.; Kunaver, M.; Skalar, T.; Cigić, I.K. Nano calcium carbonate versus nano calcium hydroxide in alcohols as a deacidification medium for lignocellulosic paper. Herit. Sci. 2019, 7, 50. [Google Scholar] [CrossRef]
- Olds, W.E.; Tsang, D.C.; Weber, P.A. Lignite-Derived Humic Substances for Treatment of Acid Mine Drainage. Environ. Eng. Sci. 2013, 30, 638–645. [Google Scholar] [CrossRef]
- Kayes, J. Pharmaceutical suspensions: Relation between zeta potential, sedimentation volume and suspension stability. J. Pharm. Pharmacol. 1977, 29, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ershov, V.A.; Ershov, B.G. Effect of Silver Nanoparticle Size on Antibacterial Activity. Toxics 2024, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-B.; Wolthers, M.; Wolf-Gladrow, D.A.; Nehrke, G. Effect of pH and phosphate on calcium carbonate polymorphs precipitated at near-freezing temperature. Cryst. Growth Des. 2015, 15, 1596–1601. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.R.; Kharissova, O.V.; Vázquez, A.; Pena, Y.; Gomez, I. Solubilization, dispersion and stabilization of magnetic nanoparticles in water and non-aqueous solvents: Recent trends. RSC Adv. 2014, 4, 45354–45381. [Google Scholar] [CrossRef]
- Kugler, R.; Bouloussa, O.; Rondelez, F. Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology 2005, 151, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Ihli, J.; Wong, W.C.; Noel, E.H.; Kim, Y.-Y.; Kulak, A.N.; Christenson, H.K.; Duer, M.J.; Meldrum, F.C. Dehydration and crystallization of amorphous calcium carbonate in solution and in air. Nat. Commun. 2014, 5, 3169. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Shiga, H.; Kojima, H. Kinetic analysis of the bactericidal action of heated scallop-shell powder. Int. J. Food Microbiol. 2001, 71, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Koutroulis, A.; Kuehne, S.A.; Cooper, P.R.; Camilleri, J. The role of calcium ion release on biocompatibility and antimicrobial properties of hydraulic cements. Sci. Rep. 2019, 9, 19019. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.H.; Khalaf, A.A. The antimicrobial activity of nanochitosan and nano-CaCO3 against some bacteria. Med. J. Babylon 2023, 20, 540–546. [Google Scholar] [CrossRef]
- Baek, E.; Kim, H.; Choi, H.; Yoon, S.; Kim, J. Antifungal activity of Leuconostoc citreum and Weissella confusa in rice cakes. J. Microbiol. 2012, 50, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhu, K.; Qian, H.; Zhou, H. Effect of water activity and temperature on growth of Penicillium citreoviride and Penicillium citrinum on MiGao (rice cake). Can. J. Microbiol. 2007, 53, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Reepholkul, K.; Charoenrein, S. Effect of sodium carbonate on appearance and textural properties of glutinous rice cake. Int. J. Food Sci. Technol. 2013, 48, 2185–2192. [Google Scholar] [CrossRef]
- Cosan, G.; Ozverel, C.S.; Yigit Hanoglu, D.; Baser, K.H.C.; Tunca, Y.M. Evaluation of antibacterial and antifungal effects of calcium hydroxide mixed with two different essential oils. Molecules 2022, 27, 2635. [Google Scholar] [CrossRef] [PubMed]












| Count Rate (kcps) | Z-Average (d.nm) | PDI | Intercept | Size (d.nm) | St Dev (d.nm) | |
|---|---|---|---|---|---|---|
| TPOS | 141.91 | 3635.5 ± 635 a | 0.557 ± 0.147 a | 1.049 | 946.45 ± 384 ab | 111.77 ± 61.6 ab |
| TPOSc | 302.41 | 1408.1 ± 513 b | 0.972 ± 0.0673 b | 0.740 | 1175.3 ± 479 b | 180.50 ± 80.1 b |
| FCC | 219.08 | 1344.3 ± 233 b | 0.964 ± 0.0509 b | 1.075 | 476.26 ± 75.4 a | 48.910 ± 15.2 a |
| CCP | 327.36 | 1961.5 ± 646 ab | 0.246 ± 0.197 c | 0.720 | 1981.3 ± 618 c | 335.38 ± 119 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.; Lee, S.; Lim, S. Antimicrobial Properties of Thermally Processed Oyster Shell Powder for Use as Calcium Supplement. Foods 2025, 14, 2579. https://doi.org/10.3390/foods14152579
Ahn S, Lee S, Lim S. Antimicrobial Properties of Thermally Processed Oyster Shell Powder for Use as Calcium Supplement. Foods. 2025; 14(15):2579. https://doi.org/10.3390/foods14152579
Chicago/Turabian StyleAhn, Sungmo, Soohwan Lee, and Seokwon Lim. 2025. "Antimicrobial Properties of Thermally Processed Oyster Shell Powder for Use as Calcium Supplement" Foods 14, no. 15: 2579. https://doi.org/10.3390/foods14152579
APA StyleAhn, S., Lee, S., & Lim, S. (2025). Antimicrobial Properties of Thermally Processed Oyster Shell Powder for Use as Calcium Supplement. Foods, 14(15), 2579. https://doi.org/10.3390/foods14152579








