Egg Quality and Nutritional Profile of Three Sicilian Autochthonous Chicken Breeds: Siciliana, Cornuta di Caltanissetta, and Valplatani
Abstract
1. Introduction
2. Materials and Methods
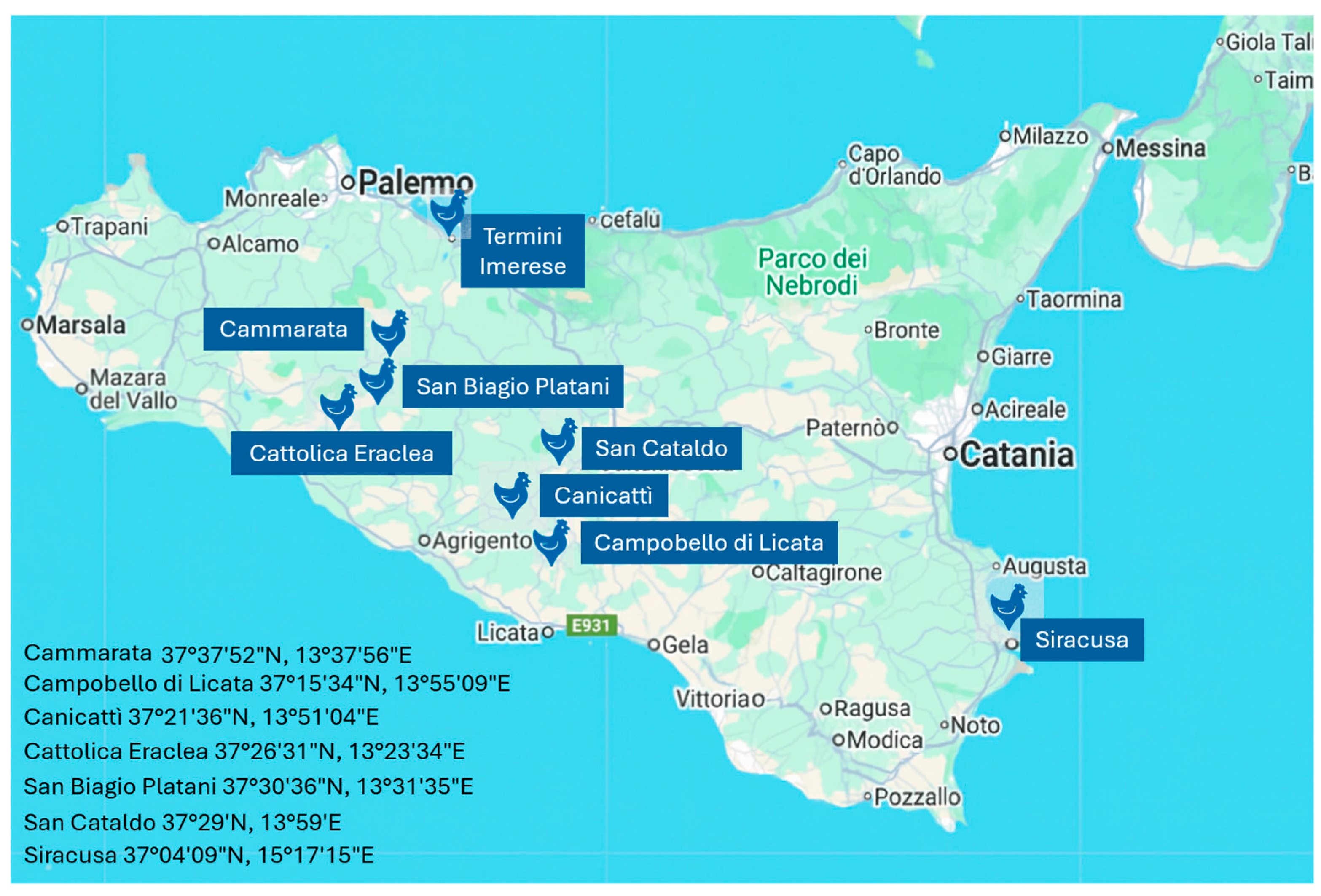
2.1. Sample Collection and Origin
2.2. Chemical Analysis
2.3. Nutritional Indices
2.4. Statistical Analysis
2.5. Language Editing Support
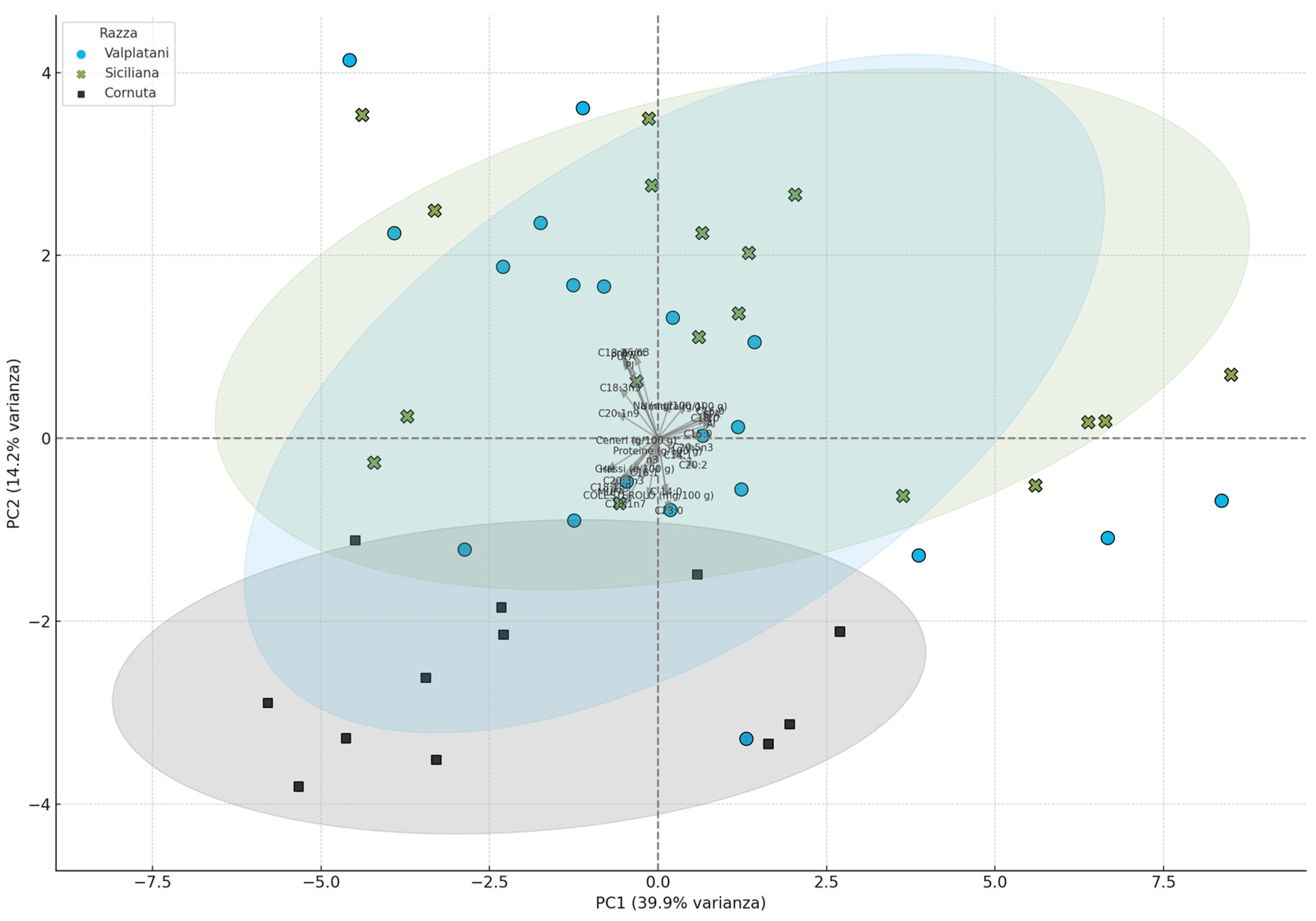
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rischkowsky, B.; Pilling, D. (Eds.) The State of the World’s Animal Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007; Available online: https://www.fao.org/3/a1250e/a1250e.pdf (accessed on 10 May 2025).
- Ben Larbi, M.; M’Hamdi, N.; Rekik, B. The Role of Genetic Diversity Conservation in Indigenous Poultry Production. J. New Sci. 2018, 52, 3508–3511. Available online: https://www.jnsciences.org/agri-biotech/78-volume-52/499-the-role-of-genetic-diversity-conservation-in-indigenous-poultry-production.html (accessed on 10 May 2025).
- Castillo, A.; Gariglio, M.; Franzoni, A.; Soglia, D.; Sartore, S.; Buccioni, A.; Mannelli, F.; Cassandro, M.; Cendron, F.; Castellini, C.; et al. Overview of Native Chicken Breeds in Italy: Conservation Status and Rearing Systems in Use. Animals 2021, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Cendron, F.; Perini, F.; Mastrangelo, S.; Tolone, M.; Criscione, A.; Bordonaro, S.; Iaffaldano, N.; Castellini, C.; Marzoni, M.; Buccioni, A.; et al. Genome-Wide SNP Analysis Reveals the Population Structure and the Conservation Status of 23 Italian Chicken Breeds. Animals 2020, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, A.R.; Chiofalo, B.; Lo Presti, V.; Chiofalo, V.; Liotta, L. Egg Quality Traits in Siciliana and Livorno Breeds under Organic Management. Animals 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C.; Cendron, F.; Penasa, M.; Cassandro, M. Egg Quality of Italian Local Chicken Breeds: I. Yield Performance and Physical Characteristics. Animals 2023, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Tolone, M.; Sardina, M.T.; Criscione, A.; Lasagna, E.; Senczuk, G.; Rizzuto, I.; Riggio, S.; Moscarelli, A.; Macaluso, V.; Di Gerlando, R.; et al. High-Density Single Nucleotide Polymorphism Markers Reveal the Population Structure of Two Local Chicken Genetic Resources. Poult. Sci. 2023, 102, 102692. [Google Scholar] [CrossRef] [PubMed]
- Soglia, D.; Sartore, S.; Lasagna, E.; Castellini, C.; Cendron, F.; Perini, F.; Cassandro, M.; Marzoni, M.; Iaffaldano, N.; Buccioni, A.; et al. Genetic Diversity of 17 Autochthonous Italian Chicken Breeds and Their Extinction Risk Status. Front. Genet. 2021, 12, 715656. [Google Scholar] [CrossRef] [PubMed]
- Perini, F.; Cendron, F.; Lasagna, E.; Cassandro, M.; Penasa, M. Genomic Insights into Shank and Eggshell Color in Italian Local Chickens. Poult. Sci. 2024, 103, 103677. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità (ISS). Analytical Methods for Food Analysis; ISTISAN Reports 1996/34; Istituto Superiore di Sanità: Rome, Italy, 1996. [Google Scholar]
- ISO 1871:2009; Food and Feed Products—General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2009.
- AOAC International. Official Method 994.10. Cholesterol in Foods. In Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- UNI EN ISO 17294-2:2016; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Ente Nazionale Italiano di Unificazione: Milan, Italy, 2016.
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters. International Organization for Standardization: Geneva, Switzerland, 2017.
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of Genotype, Feeding System and Slaughter Weight on the Quality of Light Lambs: II. Fatty Acid Composition of Meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Arakawa, K.; Sagai, M. Species Differences in Lipid Peroxide Levels in Lung Tissue and Investigation of Their Determining Factors. Lipids 1986, 21, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution. New York, USA. 2025. Available online: https://www.xlstat.com (accessed on 10 May 2025).
- Laudadio, V.; Ceci, E.; Lastella, N.M.B.; Tufarelli, V. Dietary High-Polyphenols Extra-Virgin Olive Oil Is Effective in Reducing Cholesterol Content in Eggs. Lipids Health Dis. 2015, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Persson, K. Factors Affecting Shell Quality in Laying Hens. Bachelor’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2009. Available online: https://stud.epsilon.slu.se/228/ (accessed on 8 July 2025).
- Dong, Y.; Zhang, K.; Han, M.; Miao, Z.; Liu, C.; Li, J. Low Level of Dietary Organic Trace Minerals Improved Egg Quality and Modulated the Status of Eggshell Gland and Intestinal Microflora of Laying Hens during the Late Production Stage. Front. Vet. Sci. 2022, 9, 920418. [Google Scholar] [CrossRef] [PubMed]
- Sah, N.; Kuehu, D.L.; Lee, C.N.; Jha, R.; Mishra, B. RNA sequencing-based analysis of laying hen uterus reveals novel genes and biological pathways involved in eggshell biomineralization. Sci. Rep. 2018, 8, 16853. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 Fatty Acids in Health and Disease and in Growth and Development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.M. Fatty acid and cholesterol profiles and hypocholesterolemic, atherogenic, and thrombogenic indices of table eggs in the retail market. Lipids Health Dis. 2015, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Laudadio, V. Influence of genotype on the lipid and protein content of yolk and albumen in local and commercial laying hens. Arch. Anim. Breed. 2017, 60, 183–188. [Google Scholar] [CrossRef]
- Baião, N.C.; Lara, L.J.C. Oil and Fat in Broiler Nutrition. Rev. Bras. Ciênc. Avíc. 2005, 7, 129–141. [Google Scholar] [CrossRef]
- Surai, P.F. Natural Antioxidants in Avian Nutrition and Reproduction; Nottingham University Press: Nottingham, UK, 2002. [Google Scholar]
- Pashtetsky, V.; Ostapchuk, P.; Il’yazov, R.; Zubochenko, D.; Kuevda, T. Use of Antioxidants in Poultry Farming (Review). IOP Conf. Ser. Earth Environ. Sci. 2019, 341, 012042. [Google Scholar] [CrossRef]
- Zita, L.; Tůmová, E.; Štolc, L. Effects of genotype, age and their interaction on egg quality in brown-egg laying hens. Acta Vet. Brno 2009, 78, 85–91. [Google Scholar] [CrossRef]
- Küçükyılmaz, K.; Bozkurt, M.; Herken, E.N.; Çınar, M.; Çatlı, A.U.; Bintaş, E.; Çöven, F. Effects of rearing systems on performance, egg characteristics and immune response in two layer hen genotype. Asian-Australas. J. Anim. Sci. 2012, 25, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Tůmová, E.; Gous, R.M. Interaction of hen production type, age, and temperature on laying pattern and egg quality. Poult. Sci. 2012, 91, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Fonsatti, E.; Bortoletti, M.; Birolo, M.; Bordignon, F.; Xiccato, G.; Trocino, A.; Bertotto, D.; Vascellari, M.; Radaelli, G.; Ballarin, C. Histochemical and immunohistochemical evaluation of the effects of a low-input diet on different chicken breeds. Animals 2025, 15, 696. [Google Scholar] [CrossRef] [PubMed]
- Mugnai, C.; Sossidou, E.N.; Dal Bosco, A.; Ruggeri, S.; Mattioli, S.; Castellini, C. The Effects of Husbandry System on the Grass Intake and Egg Nutritive Characteristics of Laying Hens. J. Sci. Food Agric. 2014, 94, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Sergin, S.; Jambunathan, V.; Garg, E.; Rowntree, J.E.; Fenton, J.I. Fatty Acid and Antioxidant Profile of Eggs from Pasture-Raised Hens Fed a Corn- and Soy-Free Diet and Supplemented with Grass-Fed Beef Suet and Liver. Foods 2022, 11, 3404. [Google Scholar] [CrossRef] [PubMed]
- González-Muñoz, M.J.; Bastida, S.; Jiménez, O.; Lorenzo, C.; Vergara, G.; Sánchez-Muniz, F.J. The Effect of Dietary Fat on the Fatty Acid Composition and Cholesterol Content of the Eggs from Hy-Line and Warren Hens. Grasas Aceites 2009, 60, 350–359. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Al-Sagan, A.A.; Alqurashi, A.D.; Korish, M.A.; Abdulsalam, N.M.; Olal, M.J.; Bovera, F. Dietary Supplementation with Different ω-6 to ω-3 Fatty Acid Ratios Affects the Sustainability of Performance, Egg Quality, Fatty Acid Profile, Immunity and Egg Health Indices of Laying Hens. Agriculture 2022, 12, 1712. [Google Scholar] [CrossRef]
- Dedousi, A.; Kritsa, M.-Z.; Đukić Stojčić, M.; Sfetsas, T.; Sentas, A.; Sossidou, E. Production Performance, Egg Quality Characteristics, Fatty Acid Profile and Health Lipid Indices of Produced Eggs, Blood Biochemical Parameters and Welfare Indicators of Laying Hens Fed Dried Olive Pulp. Sustainability 2022, 14, 3157. [Google Scholar] [CrossRef]
- Dedousi, A.; Kotzamanidis, C.; Dimitropoulou, G.; Sfetsas, T.; Malousi, A.; Giantzi, V.; Sossidou, E. The Beneficial Dietary Effect of Dried Olive Pulp on Some Nutritional Characteristics of Eggs Produced by Mid- and Late-Laying Hens. Foods 2024, 13, 4152. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, R.; Rondón-Barragán, I.S.; Oviedo-Rondón, E.O. Egg Quality, Yolk Fatty Acid Profiles from Laying Hens Housed in Conventional Cage and Cage-Free Production Systems in the Andean Tropics. Animals 2024, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- González Ariza, A.; Arando Arbulu, A.; Navas González, F.J.; Nogales Baena, S.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E. The Study of Growth and Performance in Local Chicken Breeds and Varieties: A Review of Methods and Scientific Transference. Animals 2021, 11, 2492. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; Resolution A/RES/70/1 adopted by the UN General Assembly; United Nations: New York, NY, USA, 2015; Available online: https://sdgs.un.org/2030agenda (accessed on 10 May 2025).
- FAO. The Global Plan of Action for Animal Genetic Resources and the Interlaken Declaration; Commission on Genetic Resources for Food and Agriculture, Food and Agriculture Organization of the United Nations: Rome, Italy, 2007; Available online: https://www.fao.org/3/a1404e/a1404e.pdf (accessed on 10 May 2025).
- European Commission. Farm to Fork Strategy: For a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2020; Available online: https://food.ec.europa.eu/system/files/2020-05/f2f_action-plan_2020_strategy-info_en.pdf (accessed on 10 May 2025).
| Parameter | Siciliana (Mean ± SD) | Cornuta (Mean ± SD) | Valplatani (Mean ± SD) | p -Value |
|---|---|---|---|---|
| Samples | 61 | 59 | 60 | - |
| Energy (kJ/100 g) | 545–131 | 616–148 | 596–143 | ns |
| Moisture (%) | 75.43 a ± 1.85 | 72.86 b ± 2.32 | 73.66 ab ± 1.77 | <0.05 |
| Fat (%) | 9.08 a ± 1.55 | 10.95 b ± 1.26 | 10.33 ab ± 0.68 | <0.05 |
| Protein (%) | 12.29 ± 0.87 | 12.40 ± 0.96 | 12.57 ± 0.63 | ns |
| Ash (%) | 1.29 ± 0.29 | 1.40 ± 0.31 | 1.46 ± 0.23 | ns |
| Cholesterol (mg/100 g yolk) | 1464 ac ± 52 | 1789 b ± 48 | 1477 ac ± 50 | <0.05 |
| Salt (%) | 0.33 ± 0.02 | 0.33 ± 0.01 | 0.35 ± 0.02 | ns |
| Element | Siciliana (Mean ± SD) | Cornuta (Mean ± SD) | Valplatani (Mean ± SD) | p-Value |
|---|---|---|---|---|
| Samples | 61 | 59 | 60 | - |
| Sodium | 133.54 a ± 9.72 | 133.20 a ± 5.69 | 139.92 b ± 6.51 | <0.05 |
| Potassium | 142.60 ± 5.34 | 141.33 ± 10.52 | 143.98 ± 8.92 | ns |
| Phosphorus | 216.10 ± 34.42 | 219.36 ± 12.87 | 224.24 ± 17.68 | ns |
| Calcium | 58.03 ± 11.79 | 58.35 ± 6.84 | 59.44 ± 5.41 | ns |
| Magnesium | 12.55 ± 1.78 | 12.58 ± 1.00 | 12.34 ± 0.87 | ns |
| Iron | 2.55 ± 1.14 | 2.63 ± 0.58 | 2.19 ± 0.33 | ns |
| Zinc | 1.23 ± 0.24 | 1.27 ± 0.22 | 1.26 ± 0.21 | ns |
| Copper | 0.16 ± 0.08 | 0.08 ± 0.01 | 0.10 ± 0.06 | ns |
| Selenium | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | ns |
| Manganese | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | ns |
| Fatty Acid(%) | Siciliana (Mean ± SD) | Cornuta (Mean ± SD) | Valplatani (Mean ± SD) | p-Value |
|---|---|---|---|---|
| Samples | 61 | 59 | 60 | - |
| C14:0 | 1.10 a ± 0.09 | 0.95 b ± 0.08 | 1.02 a ± 0.09 | <0.05 |
| C16:0 | 37.19 a ± 1.8 | 28.31 b ± 1.5 | 34.79 a ± 1.7 | <0.05 |
| C18:0 | 10.52 ± 0.8 | 9.29 ± 0.7 | 9.88 ± 0.8 | ns |
| C18:1 n-9 | 39.92 a ± 2.1 | 50.74 b ± 1.9 | 43.36 a ± 2.0 | <0.05 |
| C18:2 n-6 | 8.40 a ± 0.7 | 6.58 b ± 0.6 | 7.95 a ± 0.7 | <0.05 |
| C18:3 n-3 | 0.63 a ± 0.05 | 0.90 b ± 0.06 | 0.68 a ± 0.05 | <0.05 |
| C20:4 n-6 | 1.45 ± 0.12 | 1.21 ± 0.10 | 1.34 ± 0.11 | ns |
| C20:5 n-3 | 0.21 a ± 0.02 | 0.41 b ± 0.03 | 0.31 a ± 0.02 | <0.05 |
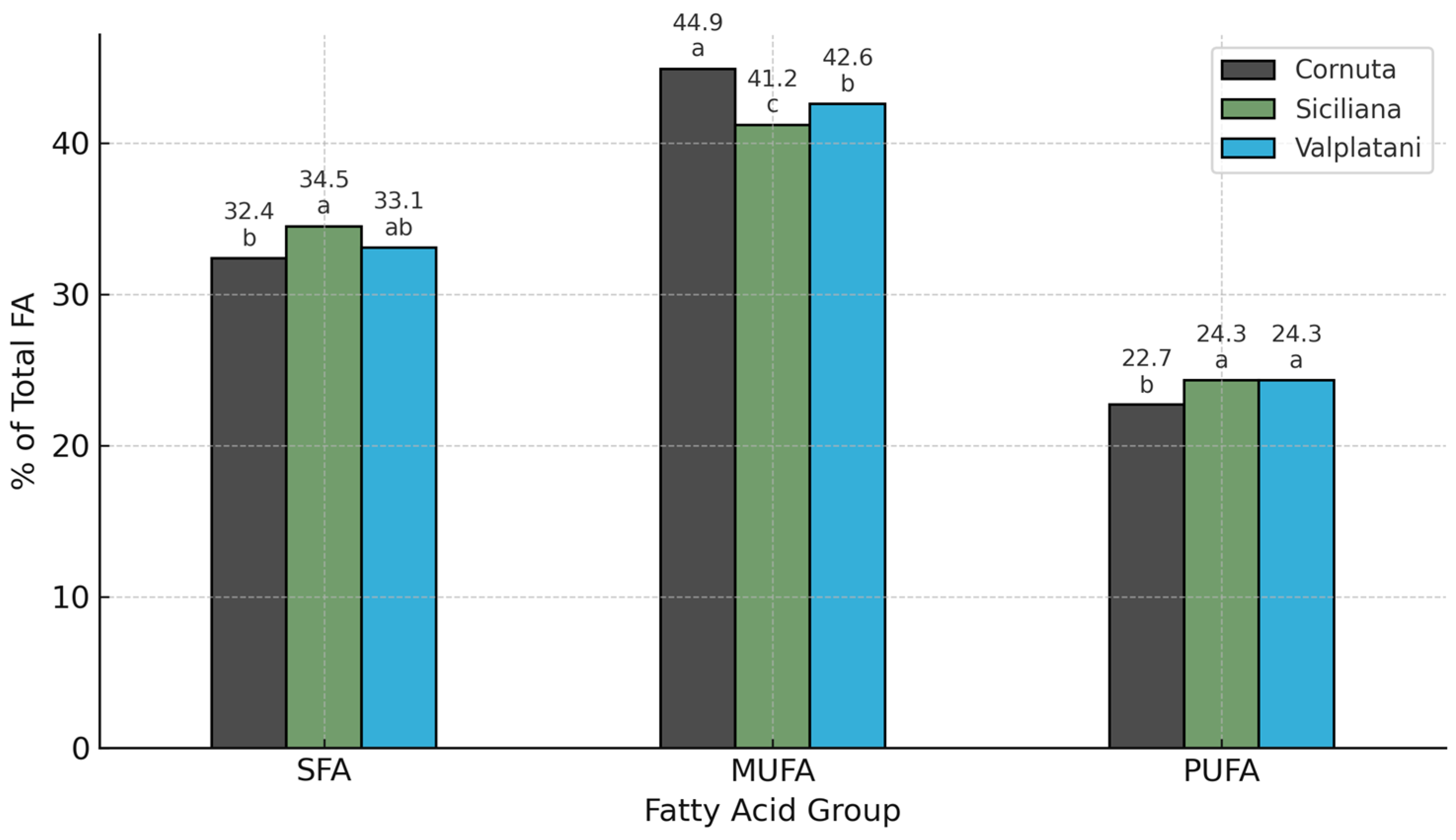
| SFA | 48.81 a ± 8.24 | 38.55 b ± 6.93 | 45.92 a ± 7.15 | <0.05 |
| MUFA | 48.16 a ± 5.91 | 56.22 b ± 5.49 | 48.16 a ± 5.91 | <0.05 |
| PUFA | 6.40 ± 2.62 | 5.03 ± 2.27 | 5.84 ± 3.10 | ns |
| Omega 3 | 6.18 ± 2.71 | 4.45 ± 2.18 | 5.32 ± 3.16 | ns |
| Omega 6 | 0.49 ± 0.24 | 0.61 ± 0.22 | 0.54 ± 0.28 | ns |
| Index | Siciliana | Cornuta | Valplatani | p-Value |
|---|---|---|---|---|
| n-6/n-3 | 13.21 a | 7.35 b | 12.34 a | <0.05 |
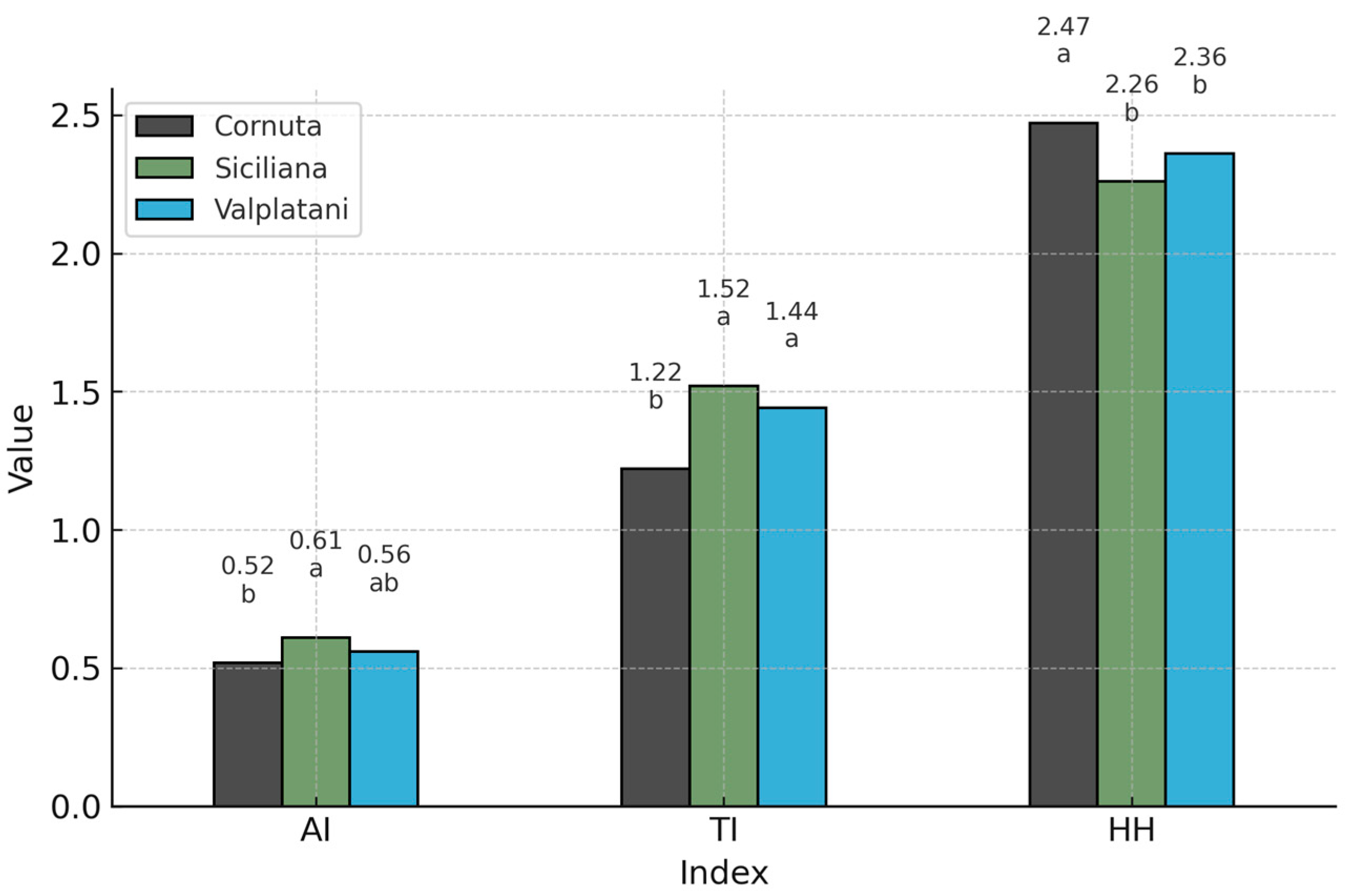
| AI | 0.82 a | 0.52 b | 0.72 a | <0.05 |
| TI | 1.91 a | 1.22 b | 1.66 a | <0.05 |
| HH | 1.30 a | 2.02 b | 1.44 a | <0.05 |
| PI | 25.1 | 24.8 | 25.0 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Presti, V.; Accetta, F.; Furfaro, M.E.; Virga, A.N.; Di Rosa, A.R. Egg Quality and Nutritional Profile of Three Sicilian Autochthonous Chicken Breeds: Siciliana, Cornuta di Caltanissetta, and Valplatani. Foods 2025, 14, 2571. https://doi.org/10.3390/foods14152571
Lo Presti V, Accetta F, Furfaro ME, Virga AN, Di Rosa AR. Egg Quality and Nutritional Profile of Three Sicilian Autochthonous Chicken Breeds: Siciliana, Cornuta di Caltanissetta, and Valplatani. Foods. 2025; 14(15):2571. https://doi.org/10.3390/foods14152571
Chicago/Turabian StyleLo Presti, Vittorio, Francesca Accetta, Maria Elena Furfaro, Antonino Nazareno Virga, and Ambra Rita Di Rosa. 2025. "Egg Quality and Nutritional Profile of Three Sicilian Autochthonous Chicken Breeds: Siciliana, Cornuta di Caltanissetta, and Valplatani" Foods 14, no. 15: 2571. https://doi.org/10.3390/foods14152571
APA StyleLo Presti, V., Accetta, F., Furfaro, M. E., Virga, A. N., & Di Rosa, A. R. (2025). Egg Quality and Nutritional Profile of Three Sicilian Autochthonous Chicken Breeds: Siciliana, Cornuta di Caltanissetta, and Valplatani. Foods, 14(15), 2571. https://doi.org/10.3390/foods14152571






