Zinc Sulfate Stress Enhances Flavonoid Content and Antioxidant Capacity from Finger Millet Sprouts for High-Quality Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Finger Millet Sprouts
2.2. Physiological Metabolism
2.3. Flavonoid Contents
2.4. Antioxidant Capacity
2.5. Antioxidant Enzyme Activity
2.6. Flavone Synthase Activity
2.7. Expression Levels of Antioxidants or Flavonoid Synthesis Genes
2.8. Statistical Analysis
3. Results
3.1. Physiological Metabolism
| Categories | Sprout’s Length (cm) | Fresh Weight (mg) | Dry Weight (mg) | Soluble Sugar Content (mg/g) | Chloroplast Pigment Content (μg/g) | |
|---|---|---|---|---|---|---|
| CK | 2 d | 0.59 ± 0.09 c* | 69.75 ± 1.71 c | 21.75 ± 1.04 a | 9.80 ± 0.26 a | 83.21 ± 5.51 c |
| 4 d | 1.29 ± 0.13 b* | 120.53 ± 12.77 b* | 16.75 ± 1.10 b | 3.59 ± 0.05 b | 200.53 ± 2.54 a | |
| 6 d | 1.37 ± 0.09 a* | 140.53 ± 8.50 a* | 13.50 ± 1.22 c | 0.99 ± 0.08 c | 177.43 ± 5.45 b | |
| ZnSO4 | 2 d | 0.43 ± 0.09 b | 66.50 ± 5.25 b | 24.50 ± 0.58 a* | 9.18 ± 0.13 a | 78.26 ± 7.45 c |
| 4 d | 0.80 ± 0.13 ab | 97.52 ± 7.14 ab | 20.25 ± 2.10 b* | 3.96 ± 0.15 b | 219.57 ± 8.98 a* | |
| 6 d | 1.01 ± 0.11 a | 103.01 ± 4.83 a | 16.75 ± 1.04 c* | 2.12 ± 0.04 c* | 195.19 ± 3.92 b* | |
3.2. The Contents of MDA, H2O2, and
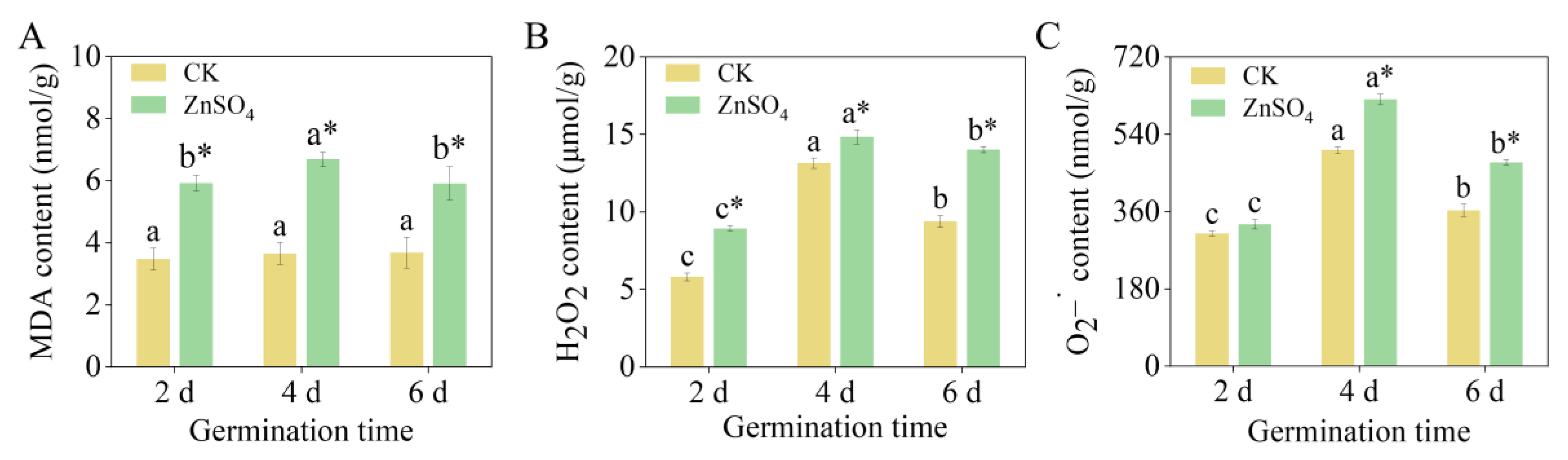
3.3. Flavonoid Content
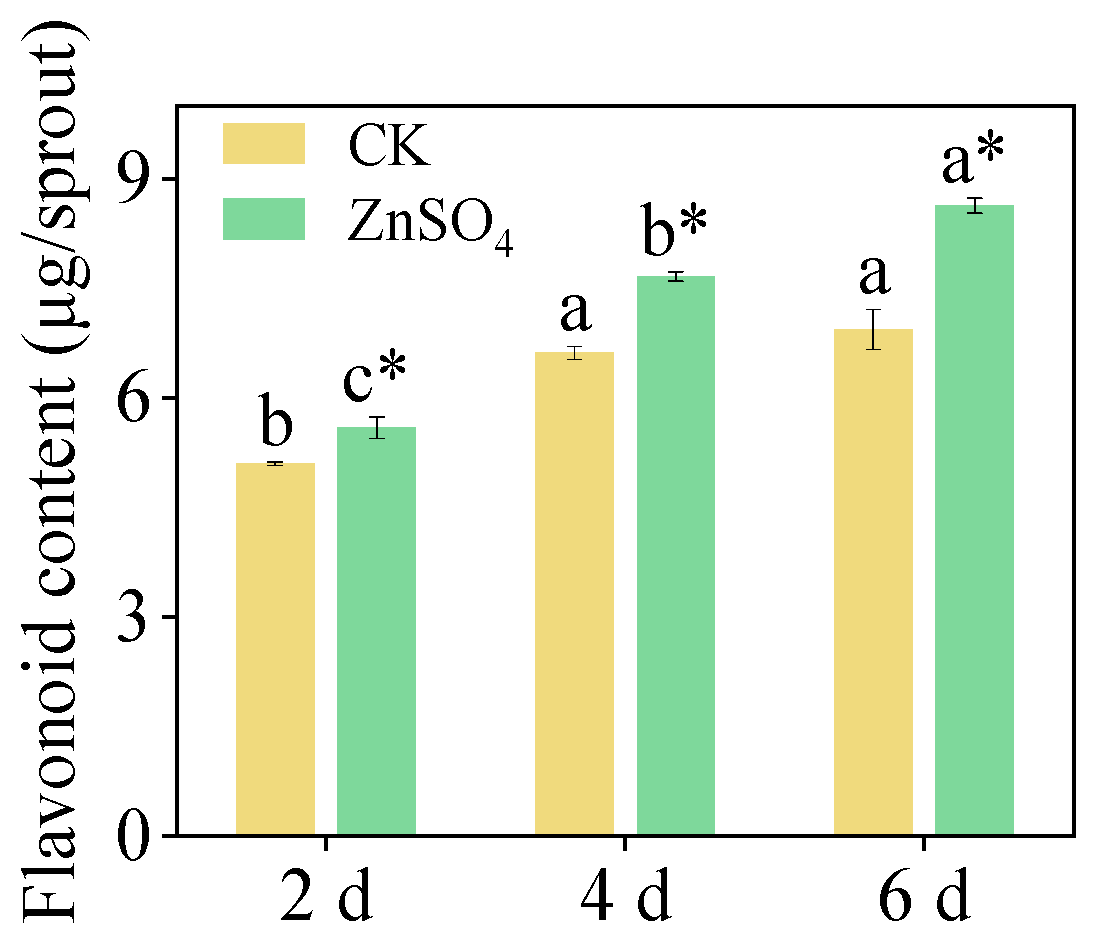
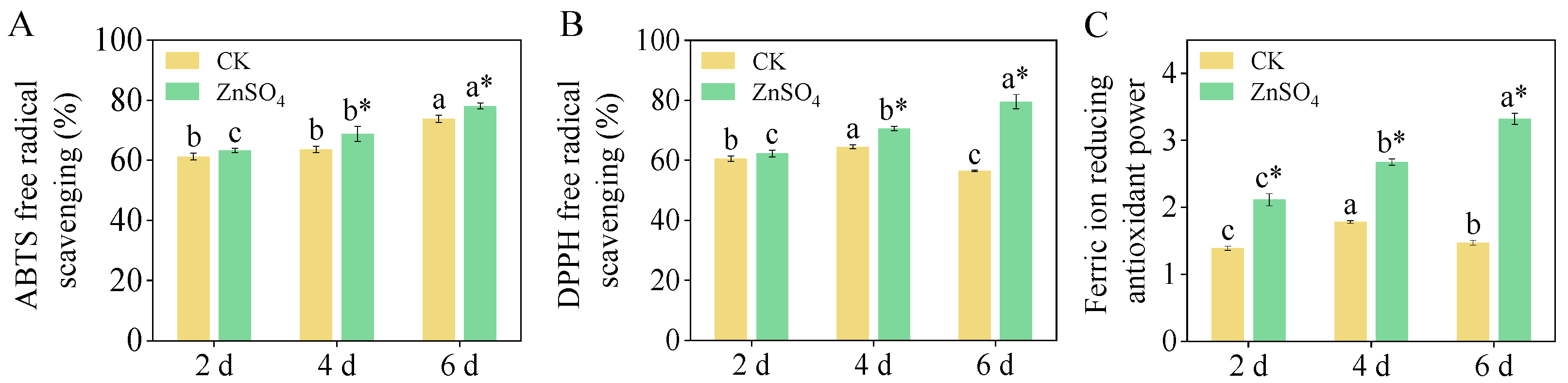
3.4. Antioxidant Capacity
3.5. Antioxidant Enzyme Activities
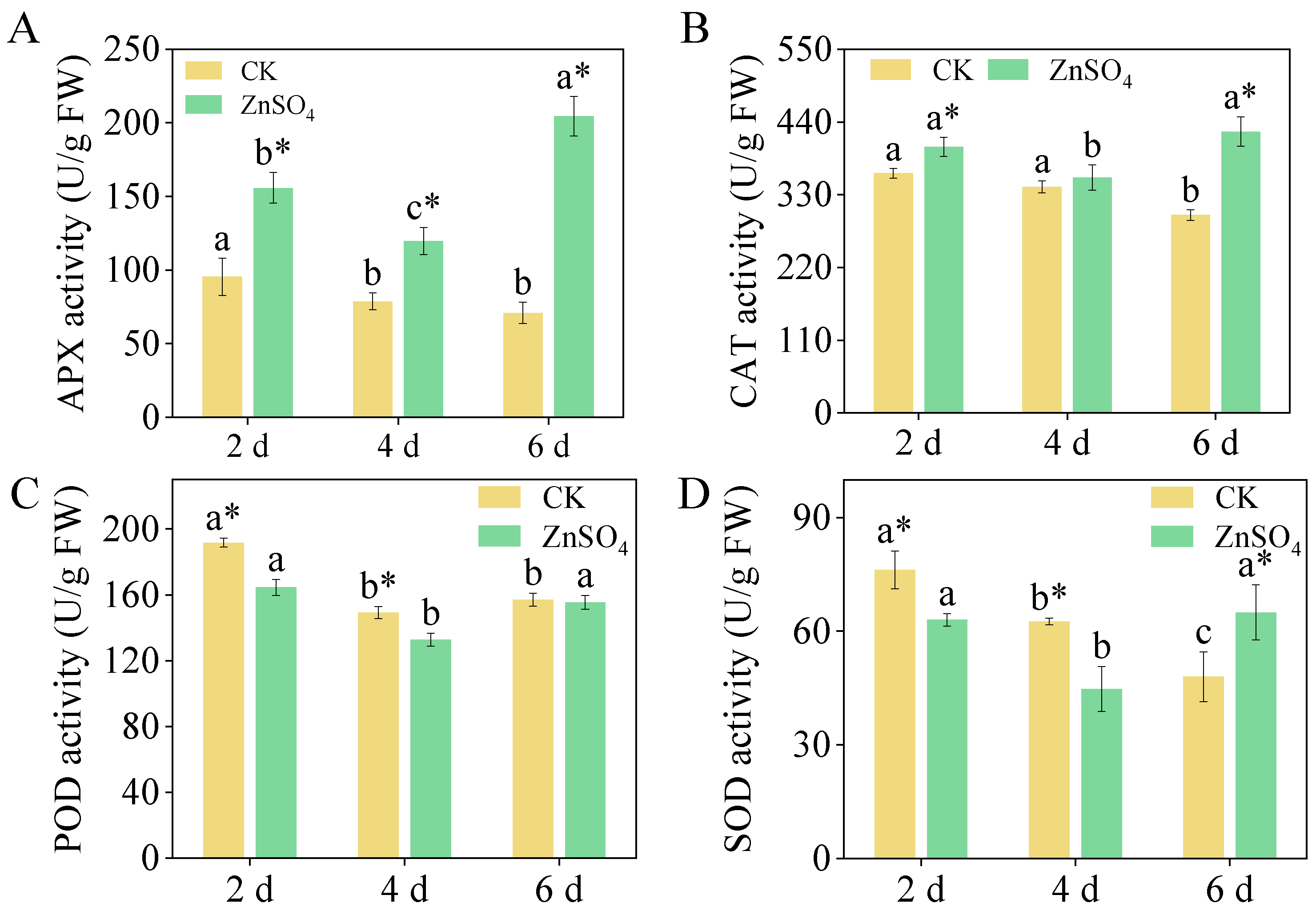
3.6. Activities of PAL, C4H, and 4CL
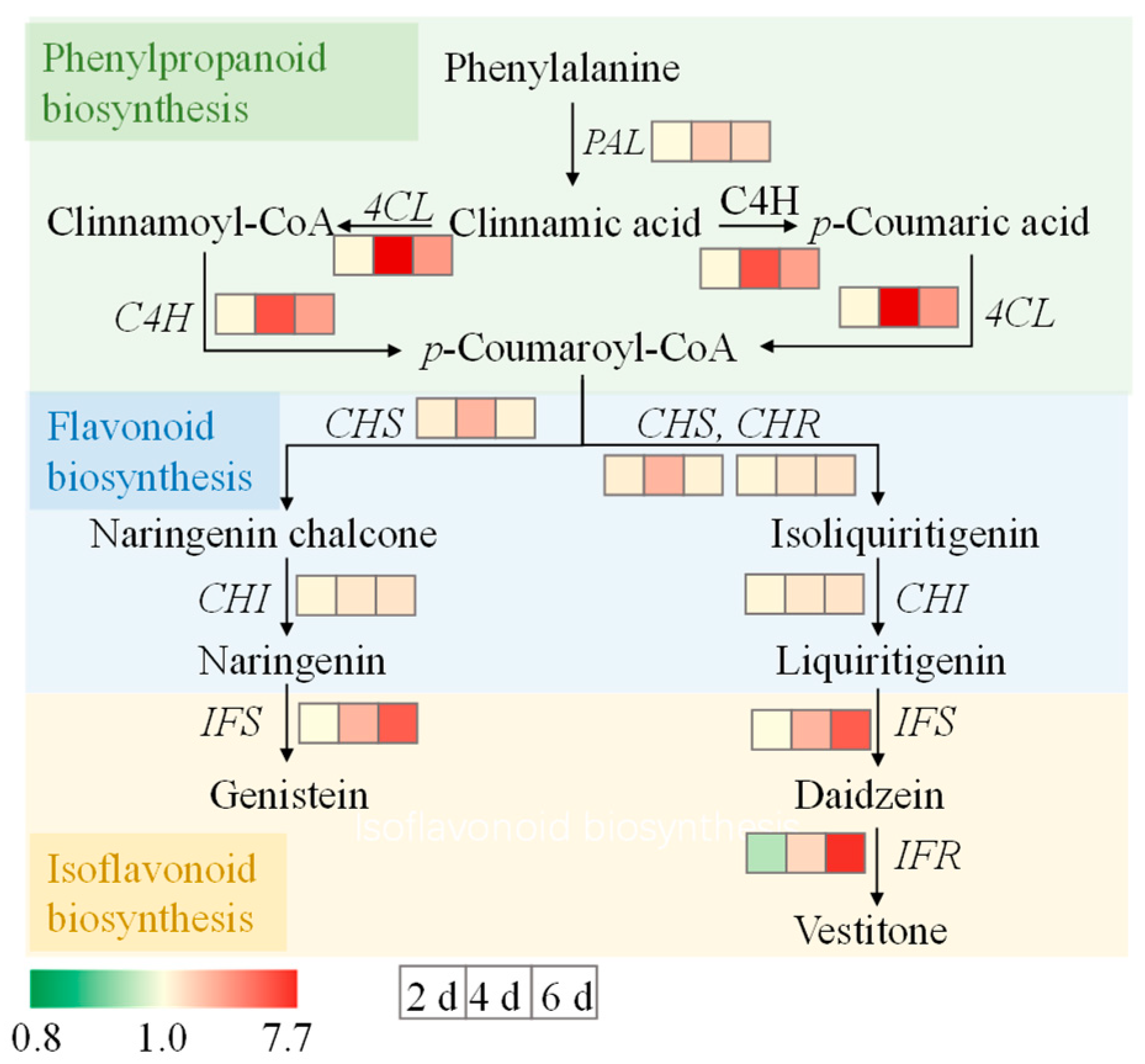
3.7. Gene Expression Levels
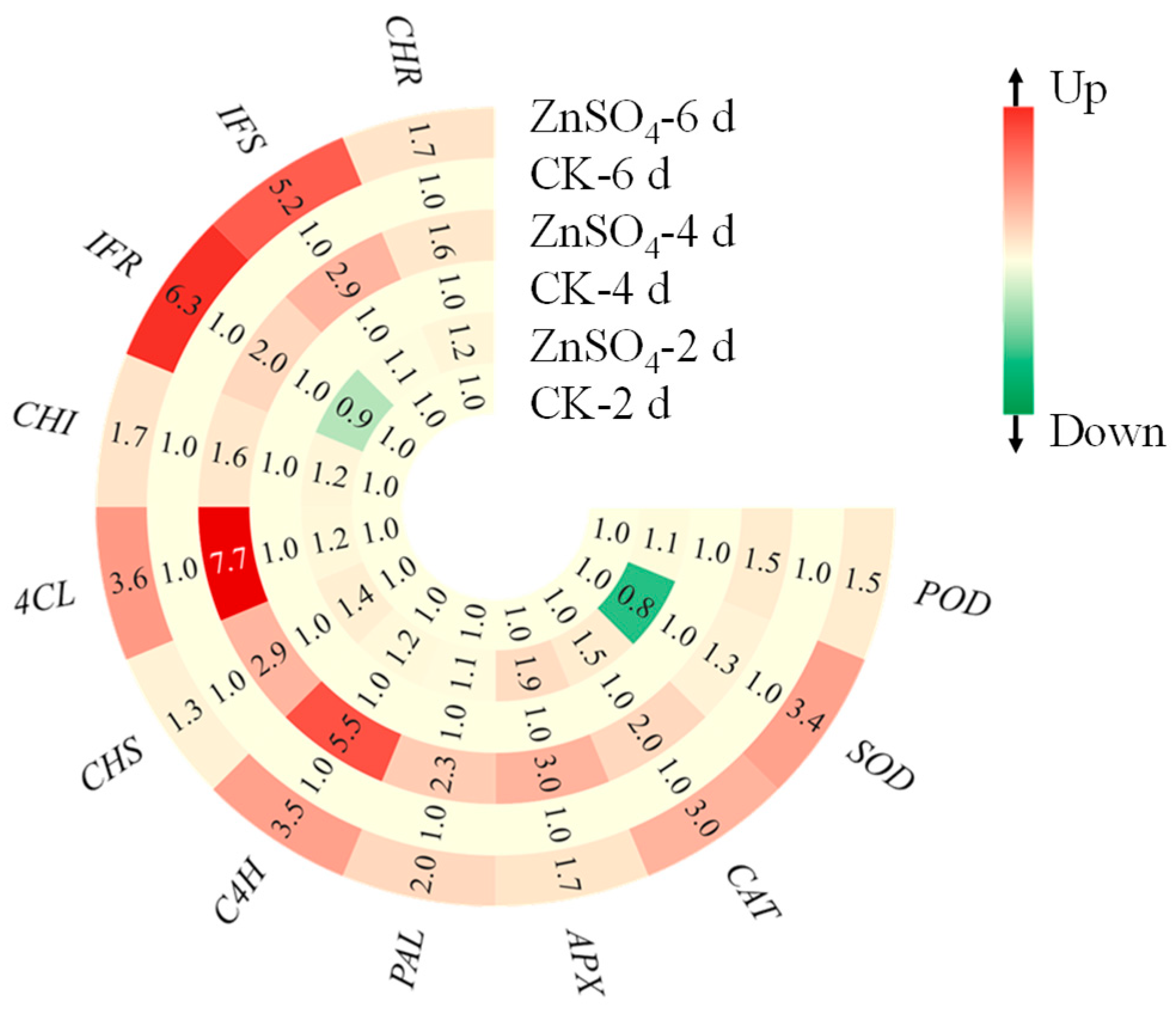
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rurinda, J.; Mapfumo, P.; van Wijk, M.T.; Mtambanengwe, F.; Rufino, M.C.; Chikowo, R.; Giller, K.E. Comparative assessment of maize, finger millet and sorghum for household food security in the face of increasing climatic risk. Eur. J. Agron. 2014, 55, 29–41. [Google Scholar] [CrossRef]
- Kaur, S.; Kumari, A.; Seem, K.; Kaur, G.; Kumar, D.; Verma, S.; Singh, N.; Kumar, A.; Kumar, M.; Jaiswal, S.; et al. Finger millet (Eleusine coracana L.): From staple to superfood-a comprehensive review on nutritional, bioactive, industrial, and climate resilience potential. Planta 2024, 260, 82. [Google Scholar] [CrossRef] [PubMed]
- Anuratha, A.; Krishnan, V.; Chitra, M.; Shibi, S.; Tamilzharasi, M.; Kamalasundari, S.; Sangeetha, M.; Arulselvi, S.; Ahiladevi, P.; Ravi, G. Comparative evaluation of nutritional quality attributes of millets in comparison with wheat and rice. Appl. Ecol. Environ. Res. 2024, 22, 4541–4561. [Google Scholar] [CrossRef]
- Yasir, M.; Bhasin, A.; Maibam, B.D.; Sharma, M. Review on finger millet (Eleusine coracana L.): Nutritional composition, modification, its effect on physicochemical, structural, functional properties, and its applications. J. Food Compos. Anal. 2024, 135, 106623. [Google Scholar] [CrossRef]
- Majumder, S.; Ghosh, A.; Saha, S.; Acharyya, S.; Chakraborty, S.; Subba, P.; Nandi, S.; Sarkar, S.; Bhattacharya, M. In vitro bioactivities and gastrointestinal simulation validate ethnomedicinal efficacy of five fermented kodo-based Himalayan traditional drinks and bioaccessibility of bioactive components. Food Prod. Process. Nutr. 2024, 6, 4. [Google Scholar] [CrossRef]
- Kuljarusnont, S.; Iwakami, S.; Iwashina, T.; Tungmunnithum, D. Flavonoids and other phenolic compounds for physiological roles, plant species delimitation, and medical benefits: A promising view. Molecules 2024, 29, 5351. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Dummi Mahadevan, G.; Anand Iyer, V.; Mukherjee, T.K.; Haribhai Patel, V.; Kumar, R.; Siddiqui, N.; Nayak, M.; Maurya, P.K.; Kumar, P. Dietary flavonoids in health and diseases: A concise review of their role in homeostasis and therapeutics. Food Chem. 2025, 487, 144674. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J. The relationship between dietary flavonols intake and metabolic syndrome in polish adults. Nutrients 2023, 15, 854. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.G.; Wang, G.C. Advances in research on flavonoids in tumor immunotherapy (Review). Mol. Med. Rep. 2025, 31, 150. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yu, Y. Therapeutic potential of flavonoids in gastrointestinal cancer: Focus on signaling pathways and improvement strategies (Review). Mol. Med. Rep. 2025, 31, 109. [Google Scholar] [CrossRef] [PubMed]
- Nakarani, U.M.; Singh, D.; Suthar, K.P.; Karmakar, N.; Faldu, P.; Patil, H.E. Nutritional and phytochemical profiling of nutracereal finger millet (Eleusine coracana L.) genotypes. Food Chem. 2021, 341, 128271. [Google Scholar] [CrossRef] [PubMed]
- Chinma, C.E.; Adedeji, O.E.; Jolayemi, O.S.; Ezeocha, V.C.; Ilowefah, M.A.; Rosell, C.M.; Adebo, J.A.; Wilkin, J.D.; Adebo, O.A. Impact of germination on the techno-functional properties, nutritional composition, and health-promoting compounds of brown rice and its products: A review. J. Food Sci. 2024, 89, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Saxena, A.; Kumar, Y.; Maity, T.; Tarafdar, A. Understanding the antinutritional factors and bioactive compounds of kodo millet (Paspalum scrobiculatum) and little millet (Panicum sumatrense). J. Food Qual. 2022, 2022, 1578448. [Google Scholar] [CrossRef]
- Dubey, A.; Tripathy, P.P. Evaluation and multivariate analysis of antinutrients, bioactive and techno-functional attributes of finger millet and kodo millet as influenced by various germination regimes. J. Food Meas. Charact. 2024, 18, 6035–6049. [Google Scholar] [CrossRef]
- Liu, H.Y.; Liu, Y.; Li, M.Y.; Ge, Y.Y.; Geng, F.; He, X.Q.; Xia, Y.; Guo, B.L.; Gan, R.Y. Antioxidant capacity, phytochemical profiles, and phenolic metabolomics of selected edible seeds and their sprouts. Front. Nutr. 2022, 9, 1067597. [Google Scholar] [CrossRef] [PubMed]
- Kuruburu, M.G.; Bovilla, V.R.; Naaz, R.; Leihang, Z.; Madhunapantula, S.V. Variations in the anticancer activity of free and bound phenolics of finger millet (Eleusine coracana L.) Seeds. Phytomedicine Plus 2022, 2, 100276. [Google Scholar] [CrossRef]
- Shingare, S.P.; Thorat, B.N. Effect of drying temperature and pretreatment on protein content and color changes during fluidized bed drying of finger millets (Ragi, Eleusine coracana) Sprouts. Dry. Technol. 2013, 31, 507–518. [Google Scholar] [CrossRef]
- Tian, X.; Hu, M.X.; Yang, J.; Yin, Y.Q.; Fang, W.M. Ultraviolet-B radiation stimulates flavonoid biosynthesis and antioxidant systems in buckwheat sprouts. Foods 2024, 13, 3650. [Google Scholar] [CrossRef] [PubMed]
- García-Mosqueda, C.; Cerón-García, A.; León-Galván, M.F.; Ozuna, C.; López-Malo, A.; Sosa-Morales, M.E. Changes in phenolics and flavonoids in amaranth and soybean sprouts after UV-C treatment. J. Food Sci. 2023, 88, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.R.; Kwon, S.J.; Kim, J.H.; Duan, S.C.; Eom, S.H. Light-induced antioxidant phenolic changes among the sprouts of lentil cultivar. Antioxidants 2024, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G.; Dong, M.Y.; Feng, H. Hybrid laser/ultrasound technology enhances morphometric, phytochemical, and techno-functional properties of seeds, sprouts, and microgreens of broccoli, radish, and kale. Food Bioprocess Technol. 2025, 18, 1–14. [Google Scholar] [CrossRef]
- Yin, Y.; Tian, X.; He, X.; Yang, J.; Yang, Z.; Fang, W. Exogenous melatonin stimulated isoflavone biosynthesis in NaCl-stressed germinating soybean (Glycine max L.). Plant Physiol Biochem. 2022, 185, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Tantharapornrerk, N.; Photchanachai, S.; Boonyaritthongchai, P.; Techavuthiporn, C. ZnSO4 spraying affects the growth and phytochemicals of Chinese kale microgreens. J. Plant Nutr. 2022, 46, 1960–1974. [Google Scholar] [CrossRef]
- Song, C.Z.; Liu, M.Y.; Meng, J.F.; Shi, P.B.; Zhang, Z.W.; Xi, Z.M. Influence of foliage-sprayed zinc sulfate on grape quality and wine aroma characteristics of Merlot. Eur. Food Res. Technol. 2016, 242, 609–623. [Google Scholar] [CrossRef]
- Tian, X.; Liu, C.; Yang, Z.F.; Zhu, J.Y.; Fang, W.M.; Yin, Y.Q. Crosstalk between ethylene and melatonin activates isoflavone biosynthesis and antioxidant systems to produce high-quality soybean sprouts. Plant Sci. 2024, 347, 112197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Qiu, T.; Duan, C.; Chen, L.; Zhao, S.; Zhang, X.; Fang, L. Microplastics addition reduced the toxicity and uptake of cadmium to Brassica chinensis L. Sci. Total Environ. 2022, 852, 158353. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Mazrou, Y.S.A.; Hafez, Y.M. Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity and fruit yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Du, G.R.; Li, M.J.; Ma, F.W.; Liang, D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. In vitro Antioxidant Activities and Polyphenol Contents of Seven Commercially Available Fruits. Pharmacogn. Res. 2016, 8, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tian, X.; Yang, J.; Yang, Z.; Tao, J.; Fang, W. Melatonin mediates isoflavone accumulation in germinated soybeans (Glycine max L.) under ultraviolet-B stress. Plant Physiol. Biochem. 2022, 175, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. NaCl stress on physio-biochemical metabolism and antioxidant capacity in germinated hulless barley (Hordeum vulgare L.). J. Sci. Food Agric. 2019, 99, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Ojha, P.; Maharjan, S.; Manandhar, U.; Maharjan, S. Optimization of the germination time of proso and foxtail millets to enhance the bioactive properties, antioxidant activity, and enzymatic power and reduce antinutritional factor. Curr. Res. Food Sci. 2025, 10, 100987. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y. Beta-amylase in germinating millet seeds. Phytochemistry 2003, 64, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Bhavadharani, P.V.; Gurumoorthi, P. Impact of germination on nutritional components, antinutritional, and functional properties of proso and barnyard millets. Food Chem. Adv. 2025, 6, 100896. [Google Scholar] [CrossRef]
- Zhao, C.N.; Wang, Z.D.; Liao, Z.K.; Liu, X.J.; Li, Y.J.; Zhou, C.W.; Sun, C.; Wang, Y.; Cao, J.P.; Sun, C.D. Integrated metabolomic-transcriptomic analyses of flavonoid accumulation in citrus fruit under exogenous melatonin treatment. Int. J. Mol. Sci. 2024, 25, 6632. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, R.T.; Ma, Y.X.; Li, M.; Bai, M.J.; Wei, G.; Wang, J.W.; Feng, L.G. Metabolite and transcriptome profiling analysis provides new insights into the distinctive effects of exogenous melatonin on flavonoids biosynthesis in Rosa rugosa. Int. J. Mol. Sci. 2024, 25, 9248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.H.; Du, R.; Han, Y.L.; Yang, Z.H.; Qiu, X.; Li, Y.Q.; Zhang, J.G.; Cheng, Z.W. Effects of methyl jasmonate and salicylhydroxamic acid on the biosynthesis of flavonoids in Glycyrrhiza glabra L. hairy roots. Plant Stress. 2025, 15, 100774. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, X.; Wang, R.J.; Zhang, Y.Y.; Hao, F.; Niu, X.G.; Zhang, H.; Lin, G.L. Effects of exogenous calcium on flavonoid biosynthesis and accumulation in peanut roots under salt stress through multi-omics. Front. Nutr. 2024, 11, 1434170. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.B.; Han, K.N.; Ma, W.L.; Zhang, J.; Xie, J.M. Exogenous melatonin application enhances pepper (Capsicum annuum L.) fruit quality via activation of the phenylpropanoid metabolism. Foods 2025, 14, 1247. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wu, W.-Z.; Ma, Y.-G. Phenylpropanoid metabolism pathway in plants. Zhongguo Shengwu Huaxue Yu Fenzi Shengwu Xuebao 2022, 38, 1467–1476. [Google Scholar] [CrossRef]
- Tao, X.Y.; Wu, Q.; Li, J.Y.; Huang, S.Q.; Cai, L.Y.; Mao, L.C.; Luo, Z.S.; Li, L.; Ying, T.J. Exogenous methyl jasmonate regulates phenolic compounds biosynthesis during postharvest tomato ripening. Postharvest Biol. Technol. 2022, 184, 111760. [Google Scholar] [CrossRef]
- Qian, C.; Sun, Y.; Zhang, B.; Shao, Y.; Liu, J.; Kan, J.; Zhang, M.; Xiao, L.; Jin, C.; Qi, X. Effects of melatonin on inhibiting quality deterioration of postharvest water bamboo shoots. Food Chem. Mol. Sci. 2024, 8, 100208. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Jiang, C.; Long, M.Q.; Hu, X.X.; Xu, S.H.; Huo, H.T.; Shi, R.X.; Xu, Q.; Xie, S.Q.; Li, Z.H.; et al. Overexpression of the Glycyrrhiza uralensis phenylalanine ammonia-lyase gene GuPAL1 promotes flavonoid accumulation in Arabidopsis thaliana. Int. J. Mol. Sci. 2025, 26, 4073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Wen, X.Y.; Zhou, G.S.; Cui, P.J.; Li, F.; Han, Y.L.; Wang, Y. Physiological differences between Mo17 and B73 maize inbred lines under salt stress. N. Z. J. Bot. 2025, 1–12. [Google Scholar] [CrossRef]
- Altinci, N.T.; Cangi, R. Periodic determination of physical and physiological responses to high temperature stress in cv. narince grapes. S. Afr. J. Enol. Vitic. 2024, 45, 5906. [Google Scholar] [CrossRef]
- Shirvani, H.; Mehrabi, A.A.; Farshadfar, M.; Safari, H.; Arminian, A.; Fatehi, F.; Pouraboughadareh, A.; Poczai, P. Investigation of the morphological, physiological, biochemical, and catabolic characteristics and gene expression under drought stress in tolerant and sensitive genotypes of wild barley Hordeum vulgare subsp. spontaneum (K. Koch) Asch. & Graebn. BMC Plant Biol. 2024, 24, 214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.R.; Zou, X.; Wang, Y.; Yang, Z.; Qiu, X.P.; Wang, S.J.; Yang, Y.X.; Yang, D.J.; Kim, H.S.; Jia, X.Y.; et al. Overexpression of dehydroascorbate reductase gene IbDHAR1 improves the tolerance to abiotic stress in sweet potato. Transgenic Res. 2024, 33, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Huang, D.J.; Man, X.L.; Li, A.L.; Fang, H.; Lu, S.T.; Yang, D.; Liao, W.B. SlDCD and SlLCD increased the salt tolerance in tomato seedlings by enhancing antioxidant and photosynthesis capacity. Plant Cell Rep. 2025, 44, 117. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.X.; Li, D.Q.; Mai, H.F.; Lin, X.Y.; Lu, X.; Chen, K.; Wang, R.T.; Riaz, M.; Tian, J.; Liang, C.Y. GmSTOP1-3 regulates flavonoid synthesis to reduce ROS accumulation and enhance aluminum tolerance in soybean. J. Hazard. Mater. 2024, 480, 136074. [Google Scholar] [CrossRef] [PubMed]
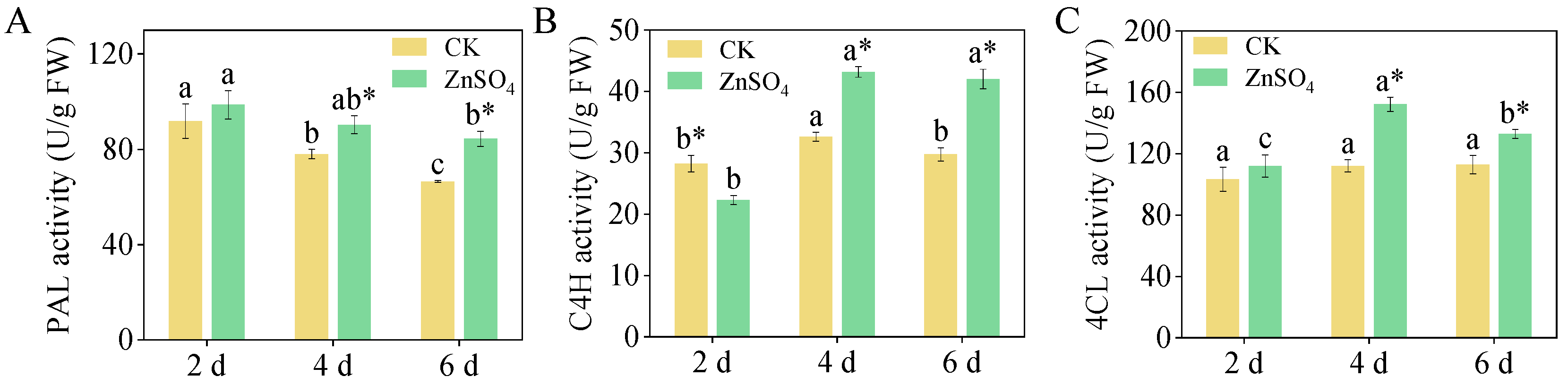
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Zhang, J.; Ye, Z.; Fang, W.; Ding, X.; Yin, Y. Zinc Sulfate Stress Enhances Flavonoid Content and Antioxidant Capacity from Finger Millet Sprouts for High-Quality Production. Foods 2025, 14, 2563. https://doi.org/10.3390/foods14152563
Tian X, Zhang J, Ye Z, Fang W, Ding X, Yin Y. Zinc Sulfate Stress Enhances Flavonoid Content and Antioxidant Capacity from Finger Millet Sprouts for High-Quality Production. Foods. 2025; 14(15):2563. https://doi.org/10.3390/foods14152563
Chicago/Turabian StyleTian, Xin, Jing Zhang, Zhangqin Ye, Weiming Fang, Xiangli Ding, and Yongqi Yin. 2025. "Zinc Sulfate Stress Enhances Flavonoid Content and Antioxidant Capacity from Finger Millet Sprouts for High-Quality Production" Foods 14, no. 15: 2563. https://doi.org/10.3390/foods14152563
APA StyleTian, X., Zhang, J., Ye, Z., Fang, W., Ding, X., & Yin, Y. (2025). Zinc Sulfate Stress Enhances Flavonoid Content and Antioxidant Capacity from Finger Millet Sprouts for High-Quality Production. Foods, 14(15), 2563. https://doi.org/10.3390/foods14152563





