Occurrence of Potentially Toxic Metals Detected in Milk and Dairy Products in Türkiye: An Assessment in Terms of Human Exposure and Health Risks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ICP-MS Analysis
2.2.1. Reagents and Standards
2.2.2. Equipments
2.2.3. Sample Preparation and Analysis
2.2.4. Quality Control
2.3. Health Risk Assessment
2.4. PTMs Contamination Level
2.5. Statistical Analysis
3. Results and Discussion
3.1. PTMs Levels in Milk and Dairy Products
3.2. Relationship Between PTMs
3.3. Dietary Exposure
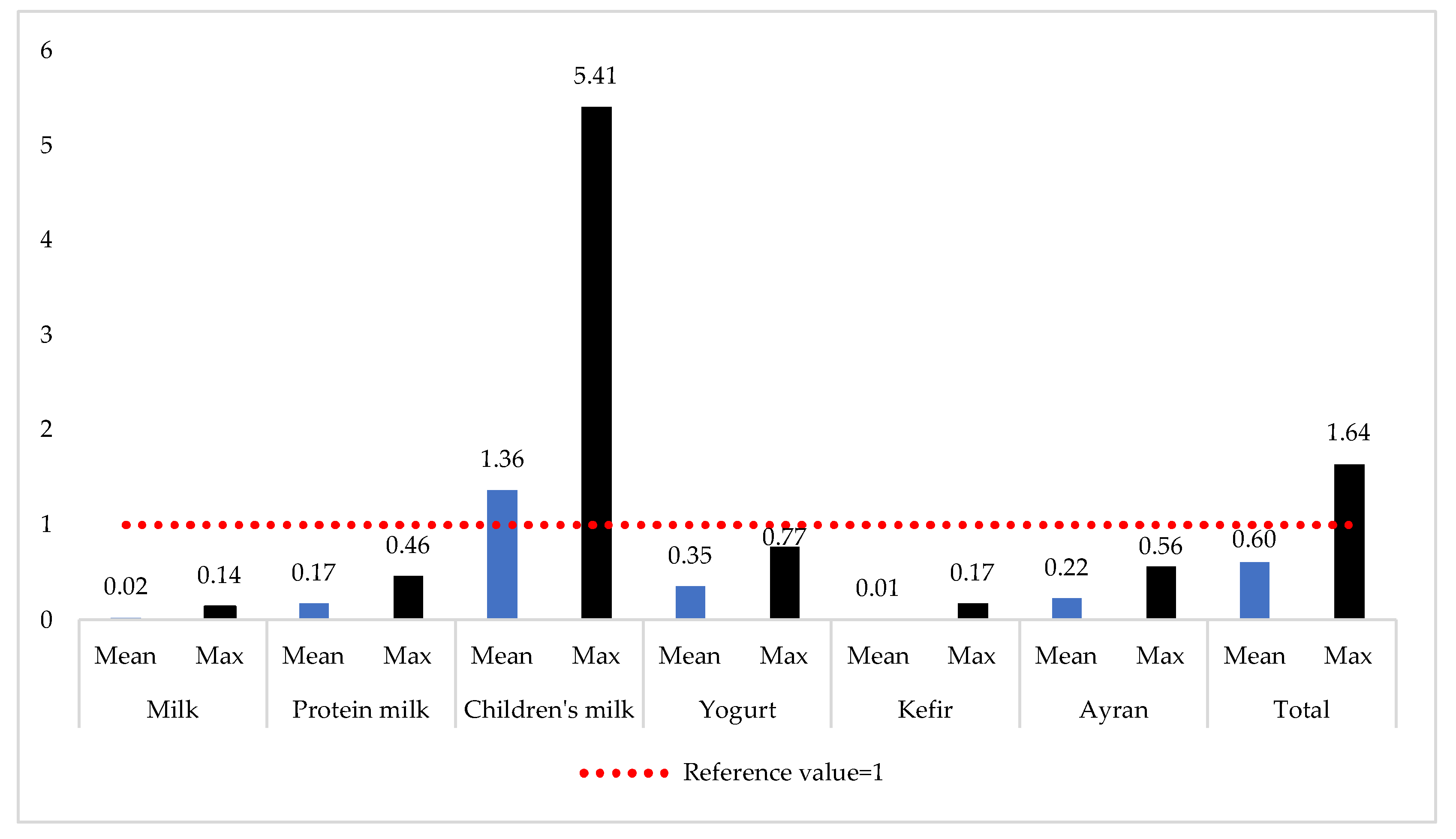
3.4. Non-Carcinogenic Risk Assessment
3.5. PTMs Contamination Level
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cimmino, F.; Catapano, A.; Petrella, L.; Villano, I.; Tudisco, R.; Cavaliere, G. Role of milk micronutrients in human health. Front. Biosci.-Landmark 2023, 28, 41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, S.; Shrivas, K.; Kant, T. Progress in analytical methods for monitoring of heavy metals and metalloid in milk and global health risk assessment. J. Food Compos. Anal. 2024, 135, 106568. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Zawada, A.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Milk and dairy products: Good or bad for human bone? practical dietary recommendations for the prevention and management of osteoporosis. Nutrients 2021, 13, 1329. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Dairy products and bone health. Aging Clin. Exp. Res. 2022, 34, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M. Introductory Chapter: Cheese as a Natural Functional Food. In Recent Trends on Cheese as Functional Food with Great Nutritive and Health Benefits; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Kazimierska, K.; Kalinowska-Lis, U. Milk proteins—Their biological activities and use in cosmetics and dermatology. Molecules 2021, 26, 3253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dong, X.; Huang, Z.; Li, X.; Zhao, Y.; Wang, Y.; Zhu, H.; Fang, A.; Giovannucci, E.L. Cheese consumption and multiple health outcomes: An umbrella review and updated meta-analysis of prospective studies. Adv. Nutr. 2023, 14, 1170–1186. [Google Scholar] [CrossRef] [PubMed]
- Basaran, B.; Turk, H. The levels, single and multiple health risk assessment of 23 metals in enteral nutrition formulas. Food Chem. Toxicol. 2024, 192, 114914. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Dasharathy, S.; Arjunan, S.; Maliyur Basavaraju, A.; Murugasen, V.; Ramachandran, S.; Keshav, R.; Murugan, R. Mutagenic, carcinogenic, and teratogenic effect of heavy metals. Evid.-Based Complement. Altern. Med. 2022, 2022, 8011953. [Google Scholar] [CrossRef] [PubMed]
- Sanajou, S.; Erkekoğlu, P.; Şahin, G.; Baydar, T. Role of aluminum exposure on Alzheimer’s disease and related glycogen synthase kinase pathway. Drug Chem. Toxicol. 2023, 46, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Mattison, D.R.; Momoli, F.; Alyanak, C.; Aschner, M.; Baker, M.; Cashman, N.; Dydak, U.; Farhat, N.; Guilarte, R.T.; Karyakina, N.; et al. Diagnosis of manganism and manganese neurotoxicity: A workshop report. Med. Int. 2024, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, V.I.; Hendricks, M.; Jones, K.S. Lead toxicity in children: An unremitting public health problem. Pediatr. Neurol. 2020, 113, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wu, N.; Du, X.; Li, H.; Mei, X.; Song, Y. Toxic nephropathy secondary to chronic mercury poisoning: Clinical characteristics and outcomes. Kidney Int. Rep. 2022, 7, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Mehrjerdi, F.Z.; Raeini, A.S.; Zebhi, F.S.; Hafizi, Z.; Mirjalili, R.; Aghda, F.A. Berberine hydrochloride improves cognitive function and hippocampal antioxidant status in subchronic and chronic lead poisoning. Chin. J. Integr. Med. 2025, 31, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinath, S.C.B.; et al. The toxicity of mercury and its chemical compounds: Molecular mechanisms and environmental and human health implications: A comprehensive review. ACS Omega 2024, 9, 5100–5126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, P.; Zhao, F.J. Dietary cadmium exposure, risks to human health and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 939–963. [Google Scholar] [CrossRef]
- Bhat, A.A.; Moglad, E.; Bansal, P.; Kaur, H.; Deorari, M.; Thapa, R.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; Kukreti, N.; et al. Pollutants to pathogens: The role of heavy metals in modulating TGF-β signaling and lung cancer risk. Pathol.-Res. Pract. 2024, 256, 155260. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.; Chaudhary, V.; Sharma, M.; Kumari, S.; Murti, K.; Meenakshi, S.; Pal, B. Serum levels of heavy metals in patients with prostate cancer: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2025, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hossini, H.; Shafie, B.; Niri, A.D.; Nazari, M.; Esfahlan, A.J.; Ahmadpour, M.; Nazmara, Z.; Ahmadimanesh, M.; Makhdoumi, P.; Mirzaei, N.; et al. A comprehensive review on human health effects of chromium: Insights on induced toxicity. Environ. Sci. Pollut. Res. 2022, 29, 70686–70705. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Lee, S.M.; Jang, Y.; Lee, J.; Lee, C.M.; Cho, E.M.; Seo, Y.R. Adverse human health effects of chromium by exposure route: A comprehensive review based on toxicogenomic approach. Int. J. Mol. Sci. 2023, 24, 3410. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T. Copper biology in health and disease: Copper in the tumor microenvironment and tumor metastasis. J. Clin. Biochem. Nutr. 2022, 71, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Mondal, M.H.; Bhattarai, A.; Saha, B. A comprehensive review on the sources, essentiality and toxicological profile of nickel. RSC Adv. 2022, 12, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Linna, A.; Uitti, J.; Oksa, P.; Toivio, P.; Virtanen, V.; Lindholm, H.; Halkosaari, M.; Sauni, R. Effects of occupational cobalt exposure on the heart in the production of cobalt and cobalt compounds: A 6-year follow-up. Int. Arch. Occup. Environ. Health 2020, 93, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Midander, K.; Werner, P.; Isaksson, M.; Wisgrill, L.; Lidén, C.; Fyhrquist, N.; Julander, A. Cobalt nanoparticles cause allergic contact dermatitis in humans. Br. J. Dermatol. 2023, 188, 278–287. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Recommendation of 4 April 2014 on the Reduction of the Presence of Cadmium in Foodstuffs (Text with EEA Relevance) (2014/193/EU); European Commission: Brussels, Belgium, 2014.
- European Commission. Commission Recommendation (EU) 2015/1381 of 10 August 2015 on the Monitoring of Arsenic in Food; European Commission: Brussels, Belgium, 2015.
- WHO. Joint FAO/WHO Expert Committee on Food Additives. In Proceedings of the Summary and Conclusions—72nd Meeting, Rome, Italy, 16–25 February 2010. [Google Scholar]
- Naseem, R.; Abbasi, A.M.; Ajab, H.; Khan, L.; Yaqub, A. Quantification of toxic metals contamination and health risk assessment in processed and raw dairy products in Abbottabad city. J. Hazard. Mater. Adv. 2025, 17, 100537. [Google Scholar] [CrossRef]
- Yan, M.; Niu, C.; Li, X.; Wang, F.; Jiang, S.; Li, K.; Yao, Z. Heavy metal levels in milk and dairy products and health risk assessment: A systematic review of studies in China. Sci. Total Environ. 2022, 851, 158161. [Google Scholar] [CrossRef] [PubMed]
- Ziarati, P.; Shirkhan, F.; Mostafidi, M.; Zahedi, M.T. An overview of the heavy metal contamination in milk and dairy products. Acta Sci. Pharm. Sci. 2018, 2, 1–14. [Google Scholar]
- Basaran, B.; Sadighara, P. Selenium content in milk and dairy products: Estimation of the daily intake and assessment of the potential risk to public health. Food Chem. Toxicol. 2025, 201, 115486. [Google Scholar] [CrossRef] [PubMed]
- Türkiye Nutrition and Health Survey. 2019. Available online: https://krtknadmn.karatekin.edu.tr/files/sbf/TBSA_RAPOR_KITAP_20.08.pdf (accessed on 11 June 2025).
- Neyzi, O.; Günöz, H.; Furman, A.; Bundak, R.; Gökçay, G.; ve Darendeliler, F. Türk çocuklarında vücut ağırlığı, boy uzunluğu, baş çevresi ve vücut kitle indeksi referans değerleri. Çocuk Sağlığı Hastalık. Derg. 2008, 51, 1–14. [Google Scholar]
- United States Environmental Protection Agency. Risk Assessment. Regional Screening Levels (RSLs)-Generic Tables. Tables as of: May 2024. Summary Table. 2024. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 11 June 2025).
- Su, C.; Zheng, N.; Gao, Y.; Huang, S.; Yang, X.; Wang, Z.; Yang, H.; Wang, J. Content and dietary exposure assessment of toxic elements in infant formulas from the Chinese market. Foods 2020, 9, 1839. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Guidance Manual for Assessing Human Health Risks from Chemically Contaminated, Fish and Shellfish [EPA-503/8-89-002]; U.S. Environmental Protection Agency: Washington, DC, USA, 1989.
- Salazar-Rojas, T.; Cejudo-Ruiz, F.R.; Gutiérrez-Soto, M.V.; Calvo-Brenes, G. Assessing heavy metal pollution load index (PLI) in biomonitors and road dust from vehicular emission by magnetic properties modeling. Environ. Sci. Pollut. Res. 2023, 30, 91248–91261. [Google Scholar] [CrossRef] [PubMed]
- Sigamani, S.; Dhrisha, J.A.; YT, D.M.; Subiksha, S.; Balaji, U.; Kolandhasamy, P.; Syed, A.; Elgorban, A.M. Bioaccumulation and health risk of metal contamination from different tiers of food chain in Ennore estuary, Southeast coast of India. Mar. Pollut. Bull. 2024, 200, 116154. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, S.; Kashmar, R.; Tzenios, N.; Hoteit, M.; Tannous, T.; Matta, J. Heavy Metal Contamination in Yogurt from Lebanon: Evaluating Lead (Pb) and Cadmium (Cd) Concentrations Across Multiple Regions. Toxics 2025, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.; Elarnaoutti, M.S. Heavy Metals Transfer from Milk into Milk Products. Turk. J. Agric.-Food Sci. Technol. 2025, 13, 900–906. [Google Scholar] [CrossRef]
- Pipoyan, D.; Hovhannisyan, A.; Beglaryan, M.; Mantovani, A. Risk assessment of potentially toxic trace elements via consumption of dairy products sold in the city of Yerevan, Armenia. Food Chem. Toxicol. 2022, 163, 112922. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, M.A.R.; de Carvalho, T.C.; da Silva, I.F. Concentration of heavy metals in UHT dairy milk available in the markets of São Luís, Brazil, and potential health risk to children. Food Chem. 2021, 346, 128961. [Google Scholar] [CrossRef] [PubMed]
- Năstăsescu, V.; Mititelu, M.; Goumenou, M.; Docea, A.O.; Renieri, E.; Udeanu, D.I.; Oprea, E.; Arsene, A.L.; Dinu-Pîrvu, C.E.; Ghica, M. Heavy metal and pesticide levels in dairy products: Evaluation of human health risk. Food Chem. Toxicol. 2020, 146, 111844. [Google Scholar] [CrossRef] [PubMed]
- Sujka, M.; Pankiewicz, U.; Kowalski, R.; Mazurek, A.; Ślepecka, K.; Góral, M. Determination of the content of Pb, Cd, Cu, Zn in dairy products from various regions of Poland. Open Chem. 2019, 17, 694–702. [Google Scholar] [CrossRef]
- Chekri, R.; Le Calvez, E.; Zinck, J.; Leblanc, J.-C.; Sirot, V.; Hulin, M.; Noël, L.; Guérin, T. Trace element contents in foods from the first French total diet study on infants and toddlers. J. Food Compos. Anal. 2019, 78, 108–120. [Google Scholar] [CrossRef]
- Capcarova, M.; Harangozo, L.; Toth, T.; Schwarczova, L.; Bobkova, A.; Stawarz, R.; Guidi, A.; Massanyi, P. Detection of selected trace elements in yogurt components. J. Environ. Sci. Health Part B 2017, 52, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y.; Ahmadi, F.; Fakhari, F. Voltammetric determination of Pb, Cd, Zn, Cu and Se in milk and dairy products collected from Iran: An emphasis on permissible limits and risk assessment of exposure to heavy metals. Food Chem. 2016, 192, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Luis, G.; Rubio, C.; Revert, C.; Espinosa, A.; González-Weller, D.; Gutiérrez, A.J.; Hardisson, A. Dietary intake of metals from yogurts analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES). J. Food Compos. Anal. 2015, 39, 48–54. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Fu, Q.L.; Li, L. Health risk assessment of Al and heavy metals in milk products for different age groups in China. Pol. J. Environ. Stud. 2015, 24, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Jeong, I.S.; Hwang, I.M.; Kim, J.S.; Choi, S.H.; Nho, E.Y.; Choi, J.Y.; Park, K.S.; Kim, K.S. Analysis of minor and trace elements in milk and yogurts by inductively coupled plasma-mass spectrometry (ICP-MS). Food Chem. 2014, 147, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Report of the 50th Session of the Codex Committee on Food Additives and Contaminants; Codex Alimentarius Commission: Hague, The Netherlands, 2011.
- European Commission. Regulation (EU) 2023/915 on Maximum Levels for Certain Contaminants in Food. 2023. Available online: https://eur-lex.europa.eu/EN/legal-content/summary/maximum-levels-for-certain-contaminants-in-food.html?fromSummary=30 (accessed on 14 June 2025).
- Pandelova, M.; Lopez, W.L.; Michalke, B.; Schramm, K.W. Ca, Cu, Fe, Mg, Mn, Zn, As, Cd, Pb and Ni levels in infant formulae and baby foods consumed in Germany and risk assessment. Food Chem. 2012, 217, 248–256. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Raghunath, R.; Krishnamoorthy, T.M. Dietary intake of heavy metals in Bombay city, India. Sci. Total Environ. 2019, 208, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, G.; Panjagari, N. Review on metal packaging: Materials, forms, food applications, safety and recyclability. J. Food Sci. Technol. 2020, 57, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2021, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Y.; Ippolito, J.A.; Xing, W.; Zuo, Q.; Wang, F. Fermentation affects heavy metal bioaccessibility in Chinese mantou. Environ. Sci. Pollut. Res. 2023, 30, 59013–59026. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on the essential composition of infant and follow-on formulae. EFSA J. 2013, 11, 3408. [Google Scholar] [CrossRef]
- Kaur, I.; Behl, T.; Aleya, L.; Rahman, M.H.; Kumar, A.; Arora, S.; Akter, R. Role of metallic pollutants in neurodegeneration: Effects of aluminum, lead, mercury, and arsenic in mediating brain impairment events and autism spectrum disorder. Environ. Sci. Pollut. Res. 2021, 28, 8989–9001. [Google Scholar] [CrossRef] [PubMed]
- Coulson, J.M.; Hughes, B.W. Dose-response relationships in aluminium toxicity in humans. Clin. Toxicol. 2022, 60, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Singh, R.K. Neurotoxic effects of aluminium exposure as a potential risk factor for Alzheimer’s disease. Pharmacol. Rep. 2022, 74, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Tanaka, K.I.; Kato-Negishi, M. Neurotoxicity of aluminum and its link to neurodegenerative diseases. Met. Res. 2021, 1, rev-47. [Google Scholar] [CrossRef]
- National Institutes Health. Nutrient Recommendations and Databases. 2019. Available online: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx (accessed on 29 May 2025).
- Krawic, C.; Zhitkovich, A. Chemical mechanisms of DNA damage by carcinogenic chromium (VI). In Advances in Pharmacology; Academic Press: Cambridge, MA, USA, 2023; Volume 96, pp. 25–46. [Google Scholar] [CrossRef]
- Mahiout, S.; Kiilunen, M.; Vermeire, T.; Viegas, S.; Woutersen, M.; Santonen, T. Occupational exposure to Cr (VI) in Finland in 1980–2016 and related lung cancer risk assessment. Regul. Toxicol. Pharmacol. 2022, 136, 105276. [Google Scholar] [CrossRef] [PubMed]
- Khan, C.; Malik, R.N.; Chen, J. Human exposure to chromite mining pollution, the toxicity mechanism and health impact. Heliyon 2024, 10, e40083. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xu, X.; Qin, Q.; Ye, K.; Wu, W.; Huo, X. Heavy metal exposure has adverse effects on the growth and development of preschool children. Environ. Geochem. Health 2019, 41, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.; Rahaman, M.S.; Perez, E.; Khan, K.M. Associations of environmental exposure to arsenic, manganese, lead, and cadmium with Alzheimer’s disease: A review of recent evidence from mechanistic studies. J. Xenobiotics 2025, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Martins, A.C.; Oliveira-Paula, G.H.; Skalny, A.V.; Zaitseva, I.P.; Bowman, A.B.; Kirichuk, A.A.; Santamaria, A.; Tizabi, Y.; Tinkov, A.A. Manganese in autism spectrum disorder and attention deficit hyperactivity disorder: The state of the art. Curr. Res. Toxicol. 2024, 6, 100170. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.T.G.; Silva, A.d.C.; Tinkov, A.A.; Khan, H.; Santamaría, A.; Skalnaya, M.G.; Skalny, A.V.; Tsatsakis, A.; Bowman, A.B.; Aschner, M.; et al. The impact of manganese on neurotransmitter systems. J. Trace Elem. Med. Biol. 2020, 61, 126554. [Google Scholar] [CrossRef] [PubMed]
- Agence Française de S’ecurit’e Sanitaire des Aliments. Opinion of the French Food Safety Agency on a Request for Scientific and Technical Support Regarding the Migration of Cobalt from Porcelain Oven-Dishes Intended to Come in Contact with Food, 11 May 2010, Request No. 2010-SA-0095; Agence Française de S’ecurit’e Sanitaire des Aliments: Maisons-Alfort, France, 2010. Available online: https://www.anses.fr/en/content/opinion-french-food-safety-agency-request-scientific-and-technical-support-regarding (accessed on 10 June 2025).
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-I.; Hong, J.A.; Kim, M.-S.; Lee, S.E.; Jung, S.-H.; Yoon, P.W.; Song, J.S.; Kim, J.-J. Severe cardiomyopathy due to arthroprosthetic cobaltism: Report of two cases with different outcomes. Cardiovasc. Toxicol. 2019, 19, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liao, S.; Lu, X.; Shi, S.; Gong, D.; Cheang, I.; Zhu, X.; Zhang, H.; Li, X. Cobalt exposure in relation to cardiovascular disease in the United States general population. Environ. Sci. Pollut. Res. 2021, 28, 41834–41842. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, F.; Zachariae, C.; Thyssen, J.P.; Johansen, J.D. Causative exposures and temporal development of cobalt allergy in Denmark between 2002 and 2017. Contact Dermat. 2019, 81, 242–248. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Update of the risk assessment of nickel in food and drinking water. EFSA J. 2020, 18, e06268. [Google Scholar] [CrossRef]
- Goverment of Canada. Dietary Reference İntakes Tables: Reference Values for Elements. 2023. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/dietary-reference-intakes/tables/reference-values-elements.html (accessed on 1 June 2025).
- European Food Safety Authority. Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. 2015, 13, 4002. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Peng, Y.; Cai, L. Cardio-metabolic effects of nickel: A narrative review. Cardiovasc. Toxicol. 2025, 25, 944–954. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. 2025. Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 11 June 2025).
- Yang, L.; Chen, X.; Cheng, H.; Zhang, L. Dietary copper intake and risk of stroke in adults: A case-control study based on national health and nutrition examination survey 2013–2018. Nutrients 2022, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of copper on mitochondrial function and metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The role of Fe, Zn, and Cu in pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ban, X.-X.; Wan, H.; Wan, X.-X.; Tan, Y.-T.; Hu, X.-M.; Ban, H.-X.; Chen, X.-Y.; Huang, K.; Zhang, Q.; Xiong, K. Copper metabolism and cuproptosis: Molecular mechanisms and therapeutic perspectives in neurodegenerative diseases. Curr. Med. Sci. 2024, 44, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hermon, T.; Gao, X.; Dixon, D.; Xiao, H. Arsenic and diabetes mellitus: A putative role for the immune system. All Life 2023, 16, 2167869. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Khamutian, S.; Doosti-Irani, A.; Shokoohizadeh, M.J.; Shirmohammadi-Khorram, N.; Sahraeei, F.; Khodabakhshi, M.; Ahangaran, N. The association of arsenic exposure with mortality due to cancer, diabetes, Alzheimer’s and congenital anomalies using Poisson regression. Sci. Rep. 2023, 13, 15456. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on lead in food. Scientific opinion of the panel on contaminants in the food chain. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- Heidari, S.; Mostafaei, S.; Razazian, N.; Rajati, M.; Saeedi, A.; Rajati, F. Correlation between lead exposure and cognitive function in 12-year-old children: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2021, 28, 43064–43073. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on cadmium in food. Scientific opinion of the panel on contaminants in the food chain. EFSA J. 2009, 980, 1–139. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on cadmium exposure in the population and intervention strategies against cadmium toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Mercury as undesirable substance in animal feed-Scientific opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 6, 654. [Google Scholar] [CrossRef]
- Azar, J.; Yousef, M.H.; El-Fawal, H.A.; Abdelnaser, A. Mercury and Alzheimer’s disease: A look at the links and evidence. Metab. Brain Dis. 2021, 36, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; Lowe, M.; Chan, H.M. Mercury exposure, cardiovascular disease, and mortality: A systematic review and dose-response meta-analysis. Environ. Res. 2021, 193, 110538. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Awaad, S.S.; Shehta, H.A.; Hegab, O.W. Dietary exposure and health risk assessment of selected toxic and essential metals in various flavored dairy products. Biol. Trace Elem. Res. 2025, 1–21. [Google Scholar] [CrossRef] [PubMed]


| Cutoff Point | Formula | Variables | Criteria |
|---|---|---|---|
| Estimated Daily Intake (EDI) (µg/kg/day) | C is the concentration of each PTM in each sample (μg/L–kg), IR is the intake rate of each sample (g/day), bw is the body weight (kg), and 1000 is the conversion factor. Milk and yogurt consumption of the general population (>15 years old) is 35 mL and 113 g, respectively, and body weight is 70 kg [34]. The body weight of children aged 3–6 is 17.5 kg [35]. a The consumption amount of protein milk, children’s milk, kefir, and ayran was accepted as 500, 200, 250 and 200 mL, respectively. | PTM exposure levels should not exceed the Tolerable Daily Intake (TDI) or the Recommended Dietary Allowance (RDA). | |
| Target Hazard Quotient (THQ) (It has no units) | RfD is the oral reference dose (μg/bw/day). RfD for Cr, Mn, Al, Co, Ni, Cu, As, Cd, Hg, and Pb is 3, 140, 143, 0.3, 20, 40, 0.3, 1, 0.1, and 4 μg/kg/day, respectively [36,37]. | While THQ indicates a significant health problem that is not carcinogenic, THQ < 1 means that there is an insignificant risk of health hazard [38]. | |
| Hazard Index (HI) (It has no units) | _____ | While HI ≥ 1 indicates a significant health problem that is not carcinogenic, HI < 1 means that there is an insignificant risk of health hazard [38]. |
| CF | Contamination Level | Color Assigned to the Contamination Level |
|---|---|---|
| CF < 1 | low contamination | |
| 1 ≤ CF < 3 | moderate contamination | |
| 3 ≤ CF < 6 | considerable contamination | |
| CF ≥ 6 | very high contamination | |
| PLI | ||
| PLI < 1 | no pollution | |
| PLI = 1 | baseline contamination level | |
| PLI > 1 | progressive degradation | |
| Countries | Products | Al | Cr | Mn | Co | Ni | Cu | As | Cd | Hg | Pb | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Türkiye | Milk | 281 ± 644 | 5 ± 11 | 16 ± 5 | 2 ± 1 | 12 ± 13 | 19 ± 8 | 8 ± 12 | <LOD | 0.01 ± 0.02 | 0.03 ± 0.08 | This study |
| Protein milk | 424 ± 495 | 15 ± 22 | 122 ± 195 | 7 ± 4 | 40 ± 56 | 99 ± 27 | 6 ± 7 | 0.11 ± 0.27 | <LOD | 0.04 ± 0.09 | ||
| Children’s milk | 895 ± 1145 | 58 ± 58 | 300 ± 328 | 18 ± 26 | 88 ± 78 | 256 ± 17 | 6 ± 3 | 0.10 ± 0.15 | <LOD | <LOD | ||
| Yogurt * | 946 ± 815 | 56 ± 41 | 155 ± 149 | 10 ± 10 | 72 ± 100 | 183 ± 108 | 42 ± 37 | 0.02 ± 0.07 | 0.02 ± 0.03 | 0.01 ± 0.02 | ||
| Kefir | 32 ± 57 | 9 ± 9 | 29 ± 16 | 2 ± 1 | 14 ± 18 | 19 ± 7 | 4 ± 2 | <LOD | 0.02 ± 0.02 | <LOD | ||
| Ayran | 30 ± 40 | 11 ± 19 | 55 ± 97 | 3 ± 4 | 29 ± 43 | 53 ± 81 | 17 ± 23 | 0.01 ± 0.02 | 0.02 ± 0.03 | 0.09 ± 0.15 | ||
| Pakistan | Milk | ___a | 22–473 | ___a | ___a | ___a | ___a | ___a | 7–69 | ___a | <LOD–608 | [30] |
| Yogurt | ___a | 48–441 | ___a | ___a | ___a | ___a | ___a | 15–59 | ___a | 133–465 | ||
| Lebanon | Yogurt | ___a | ___a | ___a | ___a | ___a | ___a | ___a | 6.75 | ___a | 13.7 | [41] |
| Libya | Ayran | ___a | ___a | ___a | ___a | ___a | 129 ± 57.0 | ___a | 7.00 ± 1.00 | ___a | 88.0 ± 41.0 | [42] |
| Armenia | Milk | ___a | ___a | ___a | ___a | ___a | 226 ± 0.00 | ___a | 2.17 ± 0.00 | <LOD | 0.79 ± 0.00 | [43] |
| Brazil | Milk | ___a | ___a | ___a | 1050 ± 840 | 150 ± 310 | 200 ± 151 | ___a | ___a | 6800 ± 3220 | 3550 ± 3310 | [44] |
| Romania | Milk | ___a | ___a | ___a | ___a | ___a | 2400 ± 62 | ___a | 3.90 ± 1.10 | ___a | 120 ± 44 | [45] |
| Poland | Milk | ___a | ___a | ___a | ___a | ___a | 360 ± 110 | ___a | <0.00 ± 0.00 | ___a | 12.0 ± 4.00 | [46] |
| Kefir | ___a | ___a | ___a | ___a | ___a | 1250 ± 220 | ___a | <0.00 ± 0.00 | ___a | 156 ± 22.0 | ||
| France | Milk | 69.3 ± 23.7 | 10.7 ± 1.15 | ___a | 1.23 ± 0.68 | 25.0 ± 0.00 | ___a | 1.00 ± 0.00 | 0.30 ± 0.00 | ___a | ___a | [47] |
| Slovak Republic | Yogurt | ___a | 120 | 130 | ___a | 200 | 10 | ___a | <0.00 | <0.00 | 110 | [48] |
| Iran | Milk | ___a | ___a | ___a | ___a | ___a | 378 ± 159 | ___a | 1.00 ± 0.49 | ___a | 9.59 ± 1.99 | [49] |
| Yogurt | ___a | ___a | ___a | ___a | ___a | 399 ± 125 | ___a | 0.99 ± 0.40 | ___a | 7.54 ± 1.76 | ||
| Spain | Yogurt | 720 ± 570 | 20 ± 10 | 20 ± 4 | 2 ± 1 | 10 ± 3 | 290 ± 80 | ___a | <0.00 | ___a | 3 ± 3 | [50] |
| China | Milk | 173 ± 92.0 | 10.6 ± 3.30 | ___a | 3.80 ± 0.07 | 55.2 ± 81.3 | 439 ± 15.0 | ___a | 1.90 ± 1.10 | ___a | 6.20 ± 2.20 | [51] |
| Republic of Korea | Milk | ___a | 365 ± 0.41 | 134 ± 0.47 | 5.71 ± 0.02 | 153 ± 0.26 | 383 ± 0.50 | 1.90 ± 0.07 | 2.38 ± 0.02 | ___a | 3.35 ± 0.08 | [52] |
| Yogurt | ___a | 204 ± 0.32 | 79.3 ± 0.132 | 3.74 ± 0.01 | 87.6 ± 0.15 | 138 ± 0.26 | 0.26 ± 0.06 | 1.77 ± 0.02 | ___a | 4.21 ± 0.07 |
| Products | Al | Cr | Mn | Co | Ni | Cu | As | Cd | Hg | Pb |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | |
| Milk | 0.14 ± 0.32 (0.00–1.36) | <0.00 ± 0.00 (0.00–0.02) | 0.01 ± 0.00 (0.01–0.01) | <0.00 ± 0.00 (0.00–0.01) | 0.01 ± 0.01 (0.00–0.02) | 0.01 ± 0.00 (0.00–0.02) | <0.00 ± 0.01 (0.00–0.03) | <0.00 ± 0.00 (0.00–0.00) | <0.00 ± 0.00 (0.00–0.00) | <0.00 ± 0.00 (0.00–0.00) |
| Protein milk | 1.20 ± 1.41 (0.40–4.10) | 0.04 ± 0.06 (0.00–0.20) | 0.35 ± 0.56 (0.10–1.50) | 0.02 ± 0.01 (0.00–0.01) | 0.11 ± 0.16 (0.00–0.40) | 0.28 ± 0.44 (0.10–1.20) | 0.02 ± 0.02 (0.00–0.03) | <0.00 ± 0.00 (0.00–0.01) | <0.00 ± 0.00 (0.00–0.00) | <0.00 ± 0.00 (0.00–0.00) |
| Children milk (3–7 years old) | 10.2 ± 13.1 (0.00–37.5) | 0.59 ± 0.69 (0.00–1.95) | 3.37 ± 3.80 (0.04–8.30) | 0.20 ± 0.30 (0.00–1.09) | 0.86 ± 0.88 (0.00–2.06) | 2.89 ± 2.96 (0.02–7.41) | 0.07 ± 0.04 (0.00–0.10) | <0.00 ± 0.00 (0.00–0.01) | <0.00 ± 0.00 (0.00–0.00) | <0.00 ± 0.00 (0.00–0.00) |
| Yogurt | 1.53 ± 1.31 (0.00–4.08) | 0.09 ± 0.07 (0.01–0.12) | 0.25 ± 0.24 (0.05–0.31) | 0.02 ± 0.02 (0.00–0.03) | 0.12 ± 0.16 (0.00–0.13) | 0.30 ± 0.18 (0.09–0.45) | 0.07 ± 0.06 (0.00–0.17) | <0.00 ± 0.00 (0.00–0.01) | <0.00 ± 0.00 (0.00–0.00) | <0.00 ± 0.00 (0.00–0.00) |
| Kefir | 0.11 ± 0.18 (0.00–0.04) | <0.00 ± 0.00 (0.00–0.09) | <0.00 ± 0.00 (0.00–0.97) | <0.00 ± 0.00 (0.00–0.02) | <0.00 ± 0.00 (0.00–0.16) | <0.00 ± 0.00 (0.00–0.66) | <0.00 ± 0.00 (0.00–0.01) | <0.00 ± 0.00 (0.00–0.00) | <0.00 ± 0.00 (0.00–0.00) | <0.00 ± 0.00 (0.00–0.00) |
| Ayran | 0.09 ± 0.11 (0.00–0.34) | 0.03 ± 0.05 (0.00–0.07) | 0.16 ± 0.28 (0.00–0.25) | 0.01 ± 0.01 (0.00–0.01) | 0.08 ± 0.13 (0.00–0.09) | 0.15 ± 0.23 (0.00–0.30) | 0.05 ± 0.07 (0.00–0.15) | <0.00 ± 0.00 (0.00–0.00) | <0.00 ± 0.00 (0.00–0.00) | <0.00 ± 0.00 (0.00–0.00) |
| Total * | 1.87 (0.00–5.82) | 0.12 (0.00–0.30) | 0.42 (0.06–1.54) | 0.03 (0.00–0.07) | 0.20 (0.00–0.40) | 0.46 (0.09–1.43) | 0.12 (0.00–0.36) | <0.00 (0.00–0.00) | <0.00 (0.00–0.00) | <0.00 (0.00–0.00) |
| Products | Al | Cr | Mn | Co | Ni | Cu | As | Cd | Hg | Pb |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | |
| Milk | <0.00 ± 0.00 (0.00–0.01) | <0.00 ± 0.00 (0.00–0.01) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–0.01) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) | 0.01 ± 0.02 (0.00–0.09) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) |
| Protein milk | 0.01 ± 0.01 (0.00–0.03) | 0.01 ± 0.02 (0.00–0.05) | <0.00 ± 0.00 (0.00–0.01) | 0.07 ± 0.04 (0.02–0.13) | <0.00 ± 0.00 (0.00–0.01) | 0.01 ± 0.02 (0.00–0.06) | 0.06 ± 0.06 (0.00–0.15) | <0.00 ± 0.00 (0.00–0.02) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) |
| Children’s milk (3–7 years old) | 0.07 ± 0.09 (0.00–0.26) | 0.20 ± 0.23 (0.00–0.65) | 0.02 ± 0.02 (0.00–0.06) | 0.67 ± 0.99 (0.01–3.63) | 0.02 ± 0.02 (0.00–0.05) | 0.14 ± 0.15 (0.00–0.37) | 0.22 ± 0.12 (0.00–0.34) | 0.01 ± 0.01 (0.00–0.05) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) |
| Yogurt | 0.01 ± 0.01 (0.00–0.03) | 0.03 ± 0.02 (0.00–0.04) | <0.00 ± 0.00 (0.00–<0.01) | 0.06 ± 0.05 (0.00–0.10) | <0.00 ± 0.00 (0.00–<0.01) | 0.01 ± 0.01 (0.00–0.02) | 0.23 ± 0.20 (0.00–0.58) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) |
| Kefir | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–0.03) | <0.00 ± 0.00 (0.00–0.01) | <0.00 ± 0.00 (0.00–0.06) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–0.03) | <0.00 ± 0.00 (0.00–0.04) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) |
| Ayran | <0.00 ± 0.00 (0.00–<0.01) | 0.01 ± 0.02 (0.00–(0.02) | <0.00 ± 0.00 (0.00–<0.01) | 0.03 ± 0.04 (0.00–0.03) | <0.00 ± 0.00 (0.00–<0.01) | 0.01 ± 0.01 (0.00–0.01) | 0.16 ± 0.22 (0.00–0.50) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) | <0.00 ± 0.00 (0.00–<0.01) |
| Total * | 0.01 (0.00–0.05) | 0.04 (0.00–0.10) | <0.00 (0.00–0.01) | 0.09 (0.00–0.20) | 0.01 (0.00–0.01) | 0.02 (0.00–0.06) | 0.40 (0.00–1.21) | <0.00 (0.00–<0.01) | <0.00 (0.00–<0.01) | <0.00 (0.00–<0.01) |
| Products | PTMs | Color Assigned to the Contamination Level |
|---|---|---|
| Milk | Mn, Cd, Hg | moderate contamination |
| Co, Cu, Pb | considerable contamination | |
| Al, Cr, Ni, As | very high contamination | |
| Protein milk | Hg | low contamination |
| As | considerable contamination | |
| Al, Cr, Mn, Co, Ni, Cu, Cd, Pb | very high contamination | |
| Children’s milk | Hg, Pb | low contamination |
| As | considerable contamination | |
| Al, Cr, Mn, Co, Ni, Cu, Cd | very high contamination | |
| Yogurt | Hg, Pb | considerable contamination |
| Al, Cr, Mn, Co, Ni, Cu, As, Cd | very high contamination | |
| Kefir | Cd, Pb | low contamination |
| Mn, Co, Cu, As, Hg | considerable contamination | |
| Al, Cr, Ni | very high contamination | |
| Ayran | Hg | moderate contamination |
| Pb | considerable contamination | |
| Al, Cr, Mn, Co, Ni, Cu, As, Cd | very high contamination | |
| PLI | ||
| Milk, protein milk, children’s milk, yogurt, kefir, and ayran | progressive degradation | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basaran, B. Occurrence of Potentially Toxic Metals Detected in Milk and Dairy Products in Türkiye: An Assessment in Terms of Human Exposure and Health Risks. Foods 2025, 14, 2561. https://doi.org/10.3390/foods14152561
Basaran B. Occurrence of Potentially Toxic Metals Detected in Milk and Dairy Products in Türkiye: An Assessment in Terms of Human Exposure and Health Risks. Foods. 2025; 14(15):2561. https://doi.org/10.3390/foods14152561
Chicago/Turabian StyleBasaran, Burhan. 2025. "Occurrence of Potentially Toxic Metals Detected in Milk and Dairy Products in Türkiye: An Assessment in Terms of Human Exposure and Health Risks" Foods 14, no. 15: 2561. https://doi.org/10.3390/foods14152561
APA StyleBasaran, B. (2025). Occurrence of Potentially Toxic Metals Detected in Milk and Dairy Products in Türkiye: An Assessment in Terms of Human Exposure and Health Risks. Foods, 14(15), 2561. https://doi.org/10.3390/foods14152561






