Effect of Rearing, Physiological, and Processing Conditions on the Volatile Profile of Atlantic Salmon (Salmo salar) Using SIFT-MS
Abstract
1. Introduction
2. Materials and Methods
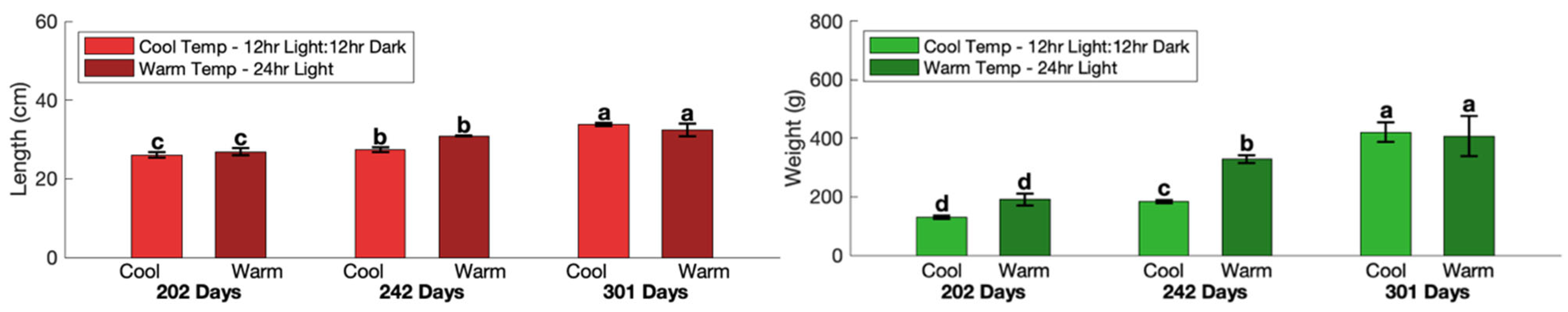
2.1. Experimental Design and Rearing Conditions
2.2. Harvest and Sample Preparation
2.3. SIFT-MS Headspace Analysis of Volatile Compounds
2.4. Statistical Analysis
3. Results and Discussion
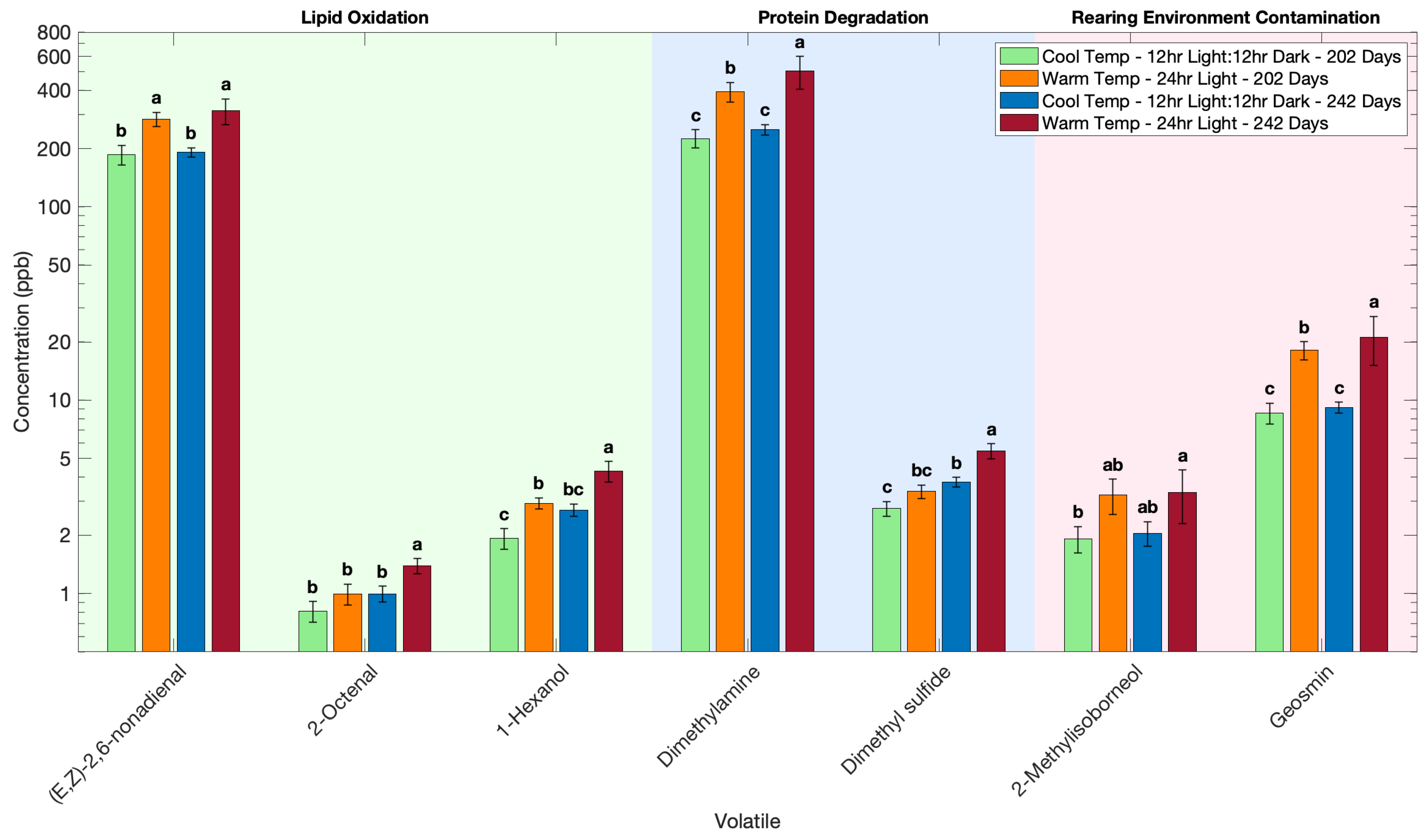
3.1. Effect of Rearing Temperature and Light on the Volatile Profile of Atlantic Salmon
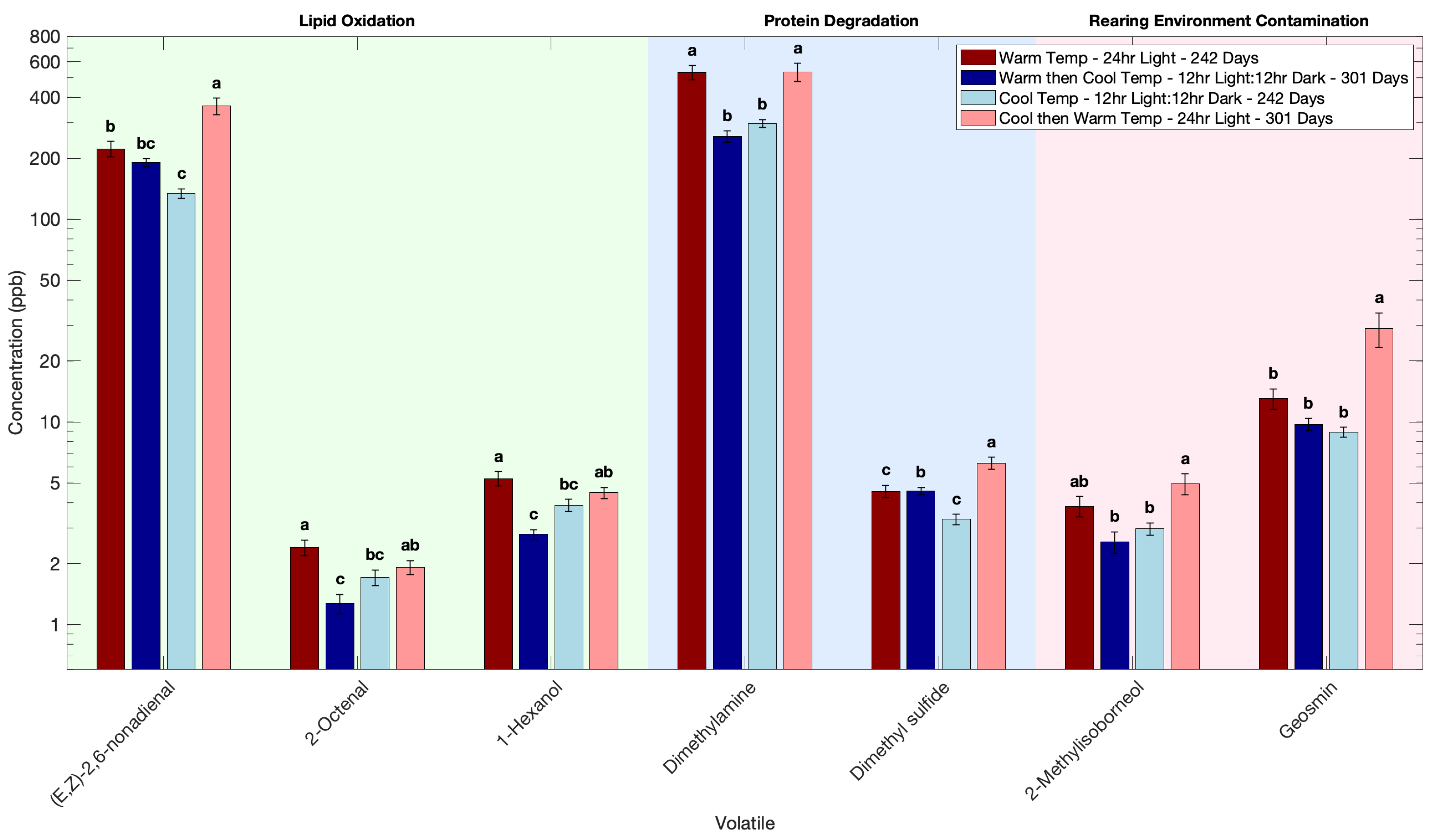
3.2. Effect of Flipping Rearing Conditions on the Volatile Profile of Atlantic Salmon
3.3. Effect of Harvest Time on the Volatile Profile of Atlantic Salmon
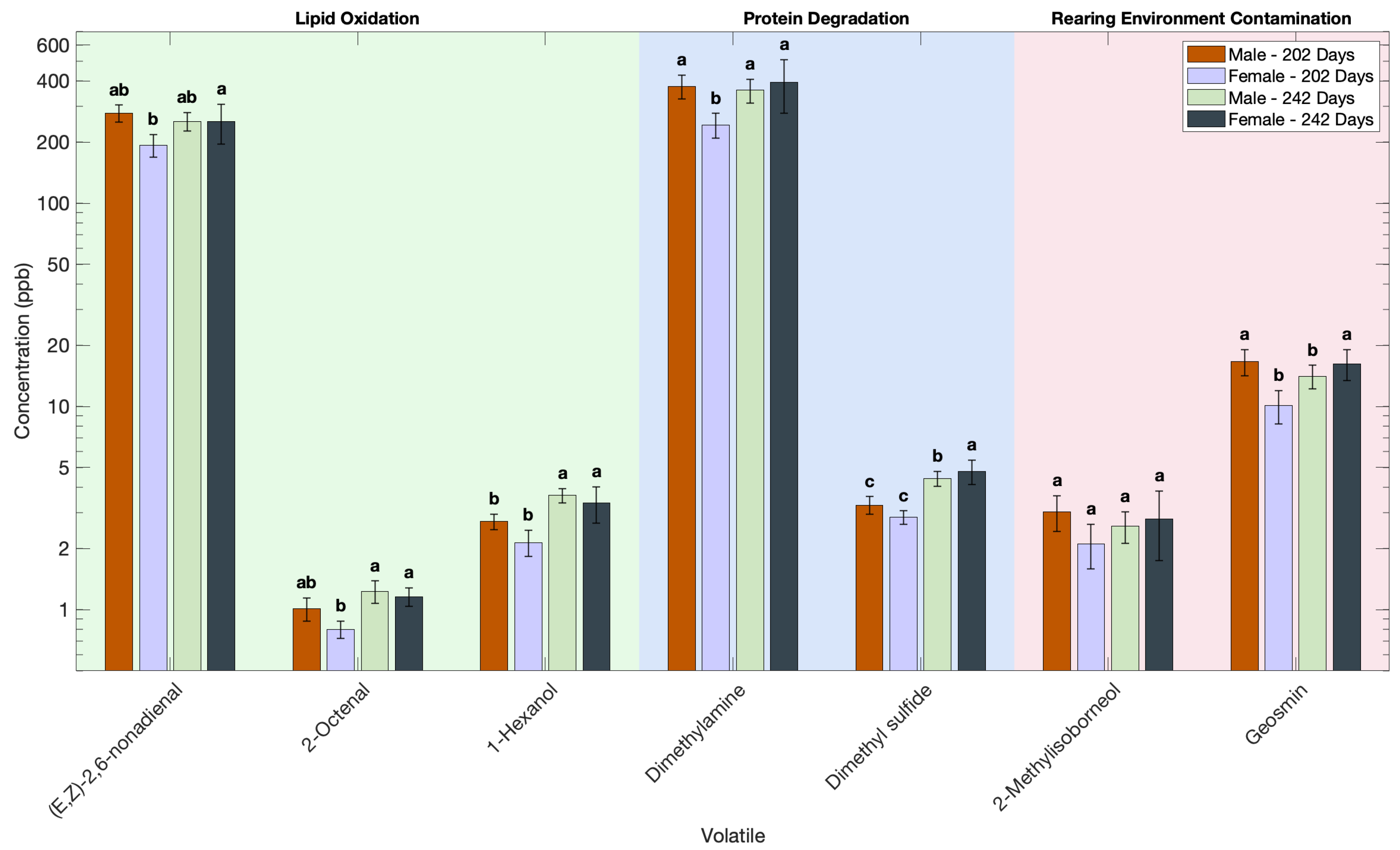
3.4. Effect of Sex on the Volatile Profile of Atlantic Salmon
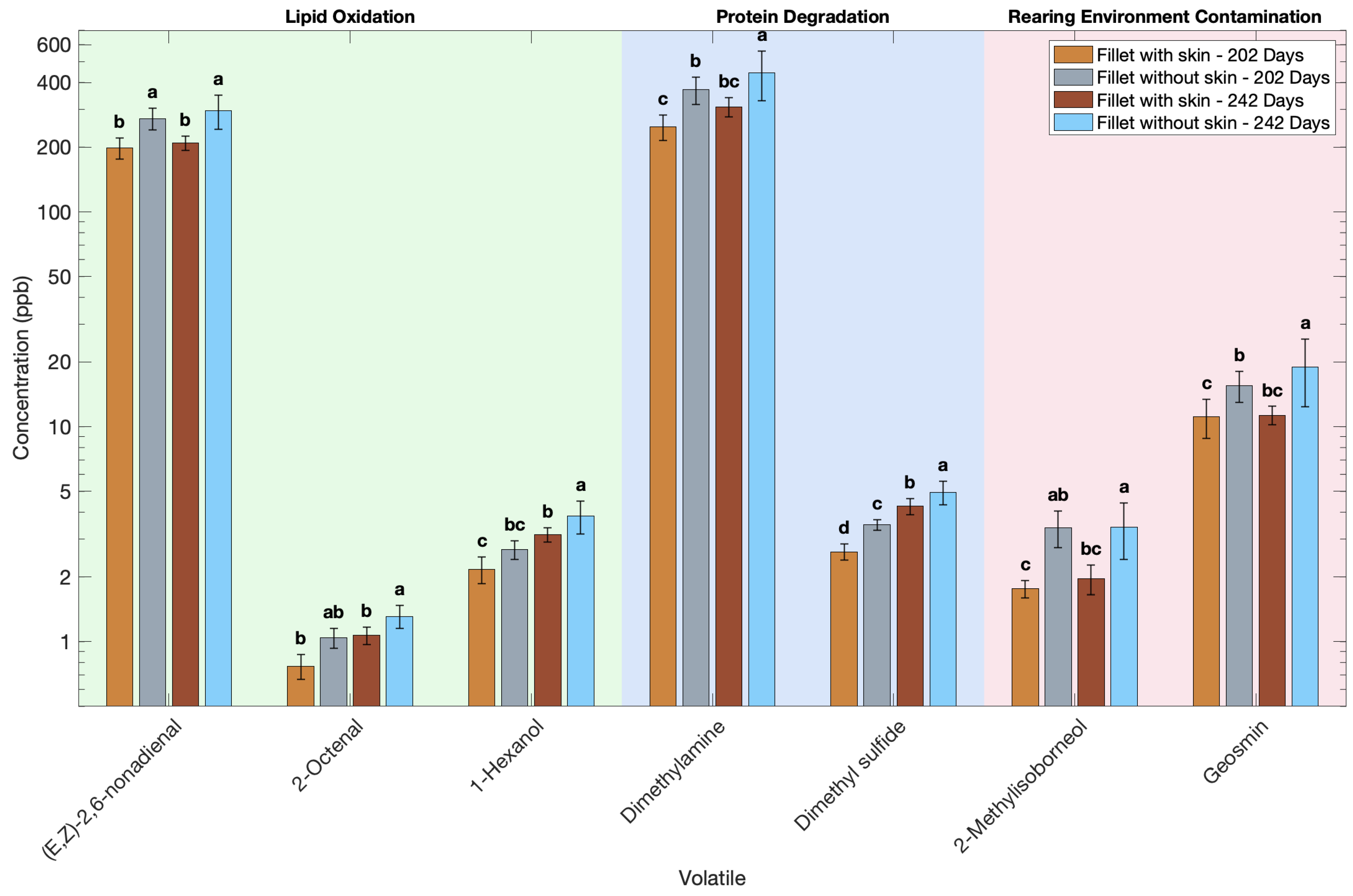
3.5. Effect of Skin Presence on the Volatile Profile of Atlantic Salmon
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Volatile | Cool Temp 12 h L:12 h D (204 Days) | Cool Temp 12 h L:12 h D (242 Days) | Warm Temp 24 h L (204 Days) | Warm Temp 24 h L (242 Days) |
|---|---|---|---|---|
| (E, Z)-2,6-nonadienal | 186 b | 191 b | 284 a | 314 a |
| (E)-2-pentenal | 93.2 b | 95.1 b | 129 a | 141 a |
| 1-hexanol | 1.93 c | 2.70 bc | 2.93 b | 4.29 a |
| 1-octanol | 1.79 c | 2.16 bc | 2.72 ab | 2.57 a |
| 1-octen-3-ol | 343 c | 339 c | 654 b | 833 a |
| 1-octen-3-one | 0.79 a | 0.53 a | 0.78 a | 0.86 a |
| 1-pentanol | 6.48 b | 8.70 ab | 8.89 ab | 10.8 a |
| 1-penten-3-ol | 8.75 b | 10.2 ab | 10.73 ab | 12.0 a |
| 2-butenal | 1.32 c | 1.78 ab | 1.65 bc | 2.02 a |
| 2-decanone | 1.24 a | 0.98 ab | 1.04 b | 0.97 ab |
| 2-decenal | 1.78 b | 1.97 b | 3.02 a | 2.69 a |
| 2-heptanone | 5.27 ab | 4.63 b | 5.49 ab | 5.96 a |
| 2-heptenal | 8.94 c | 8.89 c | 12.0 b | 13.7 a |
| 2-hexenal | 8.33 bc | 7.45 c | 11.9 ab | 13.9 a |
| 2-methyl naphthalene | 7.40 b | 6.76 b | 11.2 a | 10.4 a |
| 2-nonanone | 0.98 a | 0.87 a | 1.24 a | 1.03 a |
| 2-nonenal | 38.4 c | 36.2 c | 71.2 b | 91.0 a |
| 2-octenal | 0.81 b | 1.00 b | 0.99 b | 1.39 a |
| 2-octene | 5.35 b | 5.59 b | 7.63 b | 9.60 a |
| 2-pentanone | 1.00 b | 1.03 b | 1.39 a | 1.26 ab |
| 2-penten-1-ol | 6.45 b | 7.53 ab | 7.91 ab | 8.82 a |
| 2-pentene | 9.59 b | 12.9 ab | 13.2 ab | 16.0 a |
| 2-undecanone | 0.79 c | 0.47 b | 0.76 b | 0.99 a |
| 2,4-decadienal | 0.60 a | 0.49 a | 0.58 a | 0.70 a |
| 2,4-heptadienal | 25.1 ab | 20.2 b | 29.1 a | 21.7 b |
| 3-hexen-1-ol | 1974 b | 1932 b | 2627 a | 2786 a |
| 3-hexenal | 3.53 a | 3.33 a | 3.68 a | 4.28 a |
| decanal | 1.80 a | 1.63 a | 1.61 a | 1.74 a |
| heptanal | 4.36 ab | 3.75 b | 5.03 a | 3.99 ab |
| heptane | 198 b | 244 b | 267 b | 390 a |
| hexanal | 5.84 a | 4.78 b | 6.43 a | 5.82 ab |
| hexanoic acid | 0.88 b | 0.90 b | 1.14 ab | 1.17 a |
| nonanal | 11.3 c | 11.0 c | 18.8 b | 19.5 a |
| oct-2-en-1-ol | 296 c | 293 c | 564 b | 718 a |
| octanal | 2.22 a | 2.34 a | 2.41 a | 2.51 a |
| octane | 37.5 c | 36.1 c | 47.4 b | 52.7 a |
| pentanal | 4.61 b | 6.65 a | 4.81 b | 6.11 a |
| pentane | 2324 b | 3081 ab | 3087 ab | 3702 a |
| propanal | 16.9 ab | 13.6 b | 19.6 a | 14.6 b |
| trans-2-undecenal | 5.79 c | 5.72 c | 8.58 b | 10.4 a |
| 2-isopropyl-3-methoxypyrazine | 5.47 c | 4.91 c | 8.96 b | 9.19 a |
| 2-methylisoborneol | 1.92 b | 2.05 ab | 3.23 ab | 3.32 a |
| 2,3-butanediol | 40.4 b | 61.2 a | 53.3 ab | 66.1 a |
| alpha-terpinene | 1.62 a | 1.65 a | 1.51 a | 1.78 a |
| beta-caryophyllene | 0.54 a | 0.86 a | 0.97 a | 0.75 a |
| ethyl acetate | 4.89 ab | 4.42 b | 4.66 ab | 5.05 a |
| geosmin | 8.58 c | 9.14 c | 18.1 b | 21.2 a |
| isobutyl alcohol | 8294 b | 8093 b | 11,404 a | 11,873 a |
| 3-methyl-1-butanol | 16.3 b | 18.5 b | 22.5 ab | 23.0 a |
| acetic acid | 7.03 b | 9.67 ab | 9.30 ab | 11.2 a |
| acetoin | 4.40 ab | 3.98 b | 4.19 ab | 4.55 a |
| acetone | 9.37 c | 12.5 b | 13.6 b | 16.8 a |
| carbon disulfide | 1004 c | 932 c | 1562 b | 1727 a |
| dimethyl disulfide | 4820 b | 4638 b | 7488 a | 8032 a |
| dimethyl sulfide | 2.75 c | 3.76 b | 3.37 bc | 5.45 a |
| formaldehyde | 1197 b | 1148 b | 1429 a | 1423 a |
| hydrogen sulfide | 0.80 a | 0.72 a | 0.55 a | 0.76 a |
| indole | 0.71 a | 0.81 a | 0.96 a | 1.08 a |
| methyl mercaptan | 9265 b | 8915 b | 12,292 a | 12,523 a |
| trimethylamine | 21.4 a | 21.7 a | 25.7 a | 26.8 a |
| ammonia | 483 b | 560 b | 566 b | 823 a |
| dimethylamine | 225 c | 250 c | 394 b | 503 a |
| 2-acetyl furan | 1.32 a | 1.24 a | 1.45 a | 1.74 a |
| 2-ethyl-2,5, -dimethylpyrazine | 1.87 b | 1.86 b | 3.11 ab | 3.26 a |
| 2-ethylfuran | 17.2 b | 17.1 b | 28.1 a | 30.9 a |
| trimethylpyrazine | 13.9 c | 12.6 c | 23.7 b | 27.9 a |
| Volatile | Warm Temp 24 h L (242 Days) | Warm then Cool Temp (301 Days) | Cool Temp 12 h L:12 h D (242 Days) | Cool then Warm Temp (301 Days) |
|---|---|---|---|---|
| (E,Z)-2,6-nonadienal | 223 b | 362 a | 134 c | 190 bc |
| (E)-2-pentenal | 126 ab | 147 a | 78.2 c | 89.3 bc |
| 1-hexanol | 5.26 a | 4.47 ab | 3.89 bc | 2.79 c |
| 1-octanol | 5.17 a | 5.34 a | 5.00 ab | 3.90 b |
| 1-octen-3-ol | 439 b | 1018 a | 211 b | 339 b |
| 1-octen-3-one | 1.62 a | 1.47 a | 1.46 a | 1.35 a |
| 1-pentanol | 17.3 a | 17.9 a | 13.5 b | 10.9 b |
| 1-penten-3-ol | 27.5 a | 23.0 a | 23.8 a | 15.2 b |
| 2-acetyl furan | 2.99 a | 2.06 b | 2.02 bc | 1.48 c |
| 2-butenal | 3.64 a | 2.07 bc | 2.99 ab | 1.59 c |
| 2-decanone | 2.78 a | 1.76 a | 2.09 a | 1.60 a |
| 2-decenal | 4.79 a | 3.42 ab | 4.04 a | 2.20 b |
| 2-ethyl-2,5,-dimethylpyrazine | 3.22 b | 5.24 a | 2.23 b | 2.41 b |
| 2-ethylfuran | 21.8 b | 37.5 a | 13.5 b | 18.0 b |
| 2-heptanone | 4.61 b | 6.81 a | 4.12 b | 5.24 b |
| 2-heptenal | 14.5 b | 19.2 a | 11.1 b | 9.55 b |
| 2-hexenal | 15.0 a | 17.5 a | 11.1 b | 9.25 b |
| 2-isopropyl-3-methoxypyrazine | 14.7 a | 9.66 ab | 10.6 ab | 5.38 b |
| 2-methyl naphthalene | 15.7 b | 38.3 a | 9.72 b | 22.2 b |
| 2-methylisoborneol | 3.84 ab | 4.95 a | 2.97 b | 2.56 b |
| 2-nonanone | 2.53 a | 1.78 a | 2.10 a | 1.63 a |
| 2-nonenal | 42.8 b | 119 a | 23.3 b | 39.8 b |
| 2-octenal | 2.40 a | 1.92 ab | 1.71 bc | 1.27 c |
| 2-octene | 8.53 b | 12.5 a | 5.85 b | 5.54 b |
| 2-pentanone | 2.01 a | 1.92 a | 1.77 b | 1.17 c |
| 2-penten-1-ol | 20.3 a | 17.0 a | 17.6 a | 11.2 b |
| 2-pentene | 25.6 a | 26.5 a | 19.9 b | 16.2 b |
| 2-undecanone | 1.76 a | 1.63 a | 1.72 a | 1.40 a |
| 2,3-butanediol | 102 a | 89.3 ab | 93.8 bc | 64.9 c |
| 2,4-decadienal | 1.22 a | 0.96 ab | 1.01 ab | 0.76 b |
| 2,4-heptadienal | 63.8 a | 20.4 b | 38.6 ab | 19.5 b |
| 3-hexen-1-ol | 2651 a | 2952 a | 1782 b | 1799 b |
| 3-hexenal | 6.80 a | 6.08 a | 5.94 a | 4.61 a |
| 3-methyl-1-butanol | 32.1 a | 29.3 ab | 19.5 b | 18.7 b |
| acetic acid | 21.0 a | 13.9 b | 17.0 b | 10.0 c |
| acetoin | 5.64 a | 5.88 a | 4.54 b | 4.42 b |
| acetone | 20.8 a | 17.9 ab | 16.8 b | 11.9 c |
| alpha-terpinene | 2.96 a | 2.55 ab | 2.14 bc | 1.56 c |
| ammonia | 547 b | 861 a | 469 b | 564 b |
| beta-caryophyllene | 2.44 a | 1.43 ab | 2.39 a | 1.28 b |
| carbon disulfide | 1149 b | 2365 a | 718 b | 1120 b |
| decanal | 2.91 a | 2.90 ab | 2.60 ab | 2.00 b |
| dimethyl disulfide | 5607 b | 10,081 a | 3415 b | 4948 b |
| dimethyl sulfide | 4.55 bc | 6.27 a | 3.31 c | 4.56 b |
| dimethylamine | 531 a | 534 a | 297 b | 257 b |
| ethyl acetate | 6.27 a | 6.53 a | 5.05 b | 4.92 b |
| formaldehyde | 1507 b | 1659 a | 1291 c | 1241 c |
| geosmin | 13.1 b | 28.9 a | 8.90 b | 9.75 b |
| heptanal | 5.90 b | 10.0 a | 4.78 b | 6.61 b |
| heptane | 278 b | 341 a | 214 b | 208 b |
| hexanal | 13.2 b | 8.63 a | 8.04 b | 6.86 b |
| hexanoic acid | 1.79 ab | 1.78 a | 1.41 ab | 1.19 b |
| hydrogen sulfide | 2.43 a | 2.03 a | 2.20 a | 1.28 a |
| indole | 1.52 b | 1.84 b | 1.84 b | 0.94 a |
| isobutyl alcohol | 10,371 b | 14,787 a | 7508 b | 8890 b |
| methyl mercaptan | 11,842 b | 15,759 a | 8919 c | 10,019 bc |
| nonanal | 15.0 b | 45.6 a | 10.6 b | 23.7 b |
| oct-2-en-1-ol | 379 b | 878 a | 182 b | 293 b |
| octanal | 5.30 a | 6.31 a | 4.08 b | 4.87 b |
| octane | 54.1 b | 71.2 a | 43.7 b | 38.5 b |
| pentanal | 9.10 a | 7.25 bc | 11.0 ab | 6.36 c |
| pentane | 4681 ab | 5251 a | 4645 bc | 3672 c |
| propanal | 42.9 a | 13.7 b | 25.9 ab | 13.1 b |
| trans-2-undecenal | 9.53 b | 14.7 a | 7.74 b | 7.90 b |
| trimethylamine | 36.5 a | 30.5 ab | 27.0 b | 22.5 b |
| trimethylpyrazine | 16.3 b | 37.7 a | 9.26 b | 15.7 b |
| Volatile | Female (202 Days) | Female (242 Days) | Male (202 Days) | Male (242 Days) |
|---|---|---|---|---|
| (E,Z)-2,6-nonadienal | 193 b | 252 a | 277 ab | 253 ab |
| (E)-2-pentenal | 96.2 b | 118 a | 126 ab | 119 ab |
| 1-hexanol | 2.14 b | 3.34 a | 2.71 b | 3.65 a |
| 1-octanol | 1.92 b | 2.65 a | 2.59 ab | 2.08 b |
| 1-octen-3-ol | 369 b | 620 a | 629 ab | 552 b |
| 1-octen-3-one | 0.78 a | 0.73 a | 0.78 a | 0.67 a |
| 1-pentanol | 7.20 b | 9.95 a | 8.18 b | 9.56 ab |
| 1-penten-3-ol | 9.08 a | 11.2 a | 10.4 a | 11.0 a |
| 2-butenal | 1.35 c | 1.93 a | 1.62 bc | 1.87 ab |
| 2-decanone | 0.81 b | 0.86 b | 1.47 a | 1.08 ab |
| 2-decenal | 1.83 b | 2.31 ab | 2.97 a | 2.35 ab |
| 2-heptanone | 5.10 a | 5.43 a | 5.66 a | 5.16 a |
| 2-heptenal | 8.92 c | 12.0 b | 12.0 a | 10.7 bc |
| 2-hexenal | 8.92 a | 11.0 a | 11.3 a | 10.4 a |
| 2-methyl naphthalene | 7.62 a | 8.64 a | 11.0 a | 8.52 a |
| 2-nonanone | 0.90 b | 1.22 a | 1.33 a | 0.68 b |
| 2-nonenal | 41.1 b | 67.7 a | 68.5 a | 59.5 b |
| 2-octenal | 0.80 b | 1.16 a | 1.01 ab | 1.23 a |
| 2-octene | 5.05 b | 7.70 a | 7.93 a | 7.50 ab |
| 2-pentanone | 1.01 a | 1.13 a | 1.37 a | 1.16 a |
| 2-penten-1-ol | 6.69 a | 8.24 a | 7.66 a | 8.10 a |
| 2-pentene | 10.5 b | 14.7 a | 12.1 b | 14.1 ab |
| 2-undecanone | 0.79 ab | 0.88 a | 0.75 ab | 0.57 b |
| 2,4-decadienal | 0.53 a | 0.59 a | 0.65 a | 0.60 a |
| 2,4-heptadienal | 25.3 ab | 20.7 b | 29.0 a | 21.2 b |
| 3-hexen-1-ol | 2031 b | 2342 a | 2570 ab | 2376 ab |
| 3-hexenal | 3.50 a | 3.96 a | 3.71 a | 3.65 a |
| decanal | 1.43 c | 1.90 ab | 1.98 a | 1.48 bc |
| heptanal | 4.35 ab | 3.91 ab | 5.05 a | 3.84 b |
| heptane | 186 c | 344 a | 279 b | 290 b |
| hexanal | 5.53 ab | 5.35 ab | 6.74 a | 5.26 b |
| hexanoic acid | 0.96 a | 1.12 a | 1.05 a | 0.95 a |
| nonanal | 12.5 b | 16.4 ab | 17.6 a | 14.1 b |
| oct-2-en-1-ol | 318 b | 535 ab | 542 a | 476 b |
| octanal | 2.06 a | 2.49 a | 2.57 a | 2.35 a |
| octane | 37.3 c | 46.2 ab | 47.6 a | 42.6 bc |
| pentanal | 4.62 b | 6.82 a | 4.80 b | 5.94 a |
| pentane | 2487 b | 3497 a | 2924 b | 3286 ab |
| propanal | 17.0 ab | 13.9 b | 19.5 a | 14.3 b |
| trans-2-undecenal | 6.10 b | 8.58 a | 8.27 b | 7.52 b |
| 2-isopropyl-3-methoxypyrazine | 5.21 b | 7.69 a | 9.22 a | 6.40 b |
| 2-methylisoborneol | 2.11 a | 2.79 a | 3.03 a | 2.58 a |
| 2,3-butanediol | 42.9 b | 67.0 a | 50.8 b | 60.4 ab |
| alpha-terpinene | 1.70 a | 1.68 a | 1.43 a | 1.75 a |
| beta-caryophyllene | 0.39 a | 0.74 a | 1.13 a | 0.88 a |
| ethyl acetate | 4.69 a | 4.82 a | 4.85 a | 4.65 a |
| geosmin | 10.1 b | 16.2 a | 16.6 a | 14.1 b |
| isobutyl alcohol | 8593 b | 9927 ab | 11,106 a | 10,039 ab |
| 3-methyl-1-butanol | 16.0 b | 21.0 a | 22.8 a | 20.5 ab |
| acetic acid | 7.53 a | 11.6 a | 8.79 a | 9.25 a |
| acetoin | 4.22 a | 4.34 a | 4.37 a | 4.18 a |
| acetone | 9.79 c | 14.9 a | 13.2 b | 14.4 ab |
| carbon disulfide | 1043 b | 1314 a | 1523 a | 1345 ab |
| dimethyl disulfide | 5070 b | 6330 ab | 7238 a | 6341 ab |
| dimethyl sulfide | 2.85 c | 4.79 a | 3.27 c | 4.42 b |
| formaldehyde | 1215 a | 1267 a | 1411 a | 1304 a |
| hydrogen sulfide | 0.65 a | 0.76 a | 0.71 a | 0.72 a |
| indole | 0.94 a | 0.84 a | 0.73 a | 1.06 a |
| methyl mercaptan | 9534 a | 10,613 a | 12,023 a | 10,825 a |
| trimethylamine | 20.9 a | 25.3 a | 26.2 a | 23.1 a |
| ammonia | 487 c | 711 a | 562 bc | 672 b |
| dimethylamine | 243 b | 393 a | 376 bc | 360 b |
| 2-acetyl furan | 1.19 a | 1.39 a | 1.58 a | 1.60 a |
| 2-ethyl-2,5, dimethylpyrazine | 1.84 b | 3.06 a | 3.14 ab | 2.06 b |
| 2-ethylfuran | 18.7 b | 23.9 a | 26.6 ab | 23.9 ab |
| trimethylpyrazine | 14.8 b | 20.7 ab | 22.9 a | 19.8 b |
| Volatile | Fillet Without Skin (202 Days) | Fillet Without Skin (242 Days) | Fillet with Skin (202 Days) | Fillet with Skin (242 Days) |
|---|---|---|---|---|
| (E,Z)-2,6-nonadienal | 199 b | 209 b | 272 a | 295 a |
| (E)-2-pentenal | 99.4 c | 103 bc | 123 ab | 133 a |
| 1-hexanol | 2.17 c | 3.15 b | 2.68 bc | 3.84 a |
| 1-octanol | 1.86 b | 1.88 b | 2.66 a | 2.86 a |
| 1-octen-3-ol | 373 c | 390 c | 624 b | 782 a |
| 1-octen-3-one | 0.82 a | 0.62 a | 0.75 a | 0.77 a |
| 1-pentanol | 6.68 b | 8.70 b | 8.70 b | 10.81 a |
| 1-penten-3-ol | 7.63 b | 11.0 a | 11.9 a | 11.1 a |
| 2-butenal | 1.24 c | 1.76 ab | 1.72 b | 2.04 a |
| 2-decanone | 1.26 a | 0.93 a | 1.02 a | 1.01 a |
| 2-decenal | 2.35 a | 2.21 a | 2.45 a | 2.45 a |
| 2-heptanone | 5.08 ab | 4.63 b | 5.68 ab | 5.96 a |
| 2-heptenal | 8.44 c | 8.65 c | 12.5 b | 13.9 a |
| 2-hexenal | 8.27 c | 8.74 bc | 12.0 ab | 12.7 b |
| 2-methyl naphthalene | 5.95 b | 5.48 b | 12.7 a | 11.7 a |
| 2-nonanone | 0.99 ab | 0.83 b | 1.23 ab | 1.08 a |
| 2-nonenal | 40.2 c | 40.5 c | 69.4 b | 86.8 a |
| 2-octenal | 0.77 b | 1.07 b | 1.04 ab | 1.31 a |
| 2-octene | 4.92 c | 6.39 bc | 8.06 ab | 8.81 a |
| 2-pentanone | 1.14 a | 1.26 a | 1.25 a | 1.03 a |
| 2-penten-1-ol | 5.62 b | 8.13 a | 8.73 a | 8.21 a |
| 2-pentene | 9.87 b | 12.9 b | 12.9 ab | 15.99 a |
| 2-undecanone | 0.54 c | 0.66 bc | 1.00 a | 0.79 ab |
| 2,4-decadienal | 0.62 ab | 0.50 b | 0.56 ab | 0.69 a |
| 2,4-heptadienal | 25.4 ab | 21.5 b | 28.8 a | 20.1 b |
| 3-hexen-1-ol | 2087 b | 2084 b | 2514 a | 2634 a |
| 3-hexenal | 3.55 a | 3.30 a | 3.66 a | 4.31 a |
| decanal | 1.40 b | 1.32 b | 2.01 a | 2.05 a |
| heptanal | 4.29 b | 3.05 c | 5.11 a | 4.69 ab |
| heptane | 216 b | 249 b | 249 b | 385 a |
| hexanal | 5.50 b | 5.13 b | 6.77 a | 5.47 b |
| hexanoic acid | 1.05 ab | 0.86 b | 0.96 b | 1.21 a |
| nonanal | 10.2 b | 10.2 b | 19.9 a | 20.3 a |
| oct-2-en-1-ol | 322 c | 337 c | 538 b | 674 a |
| octanal | 1.96 b | 2.22 ab | 2.67 a | 2.63 a |
| octane | 35.0 c | 33.6 c | 49.9 b | 55.2 a |
| pentanal | 3.80 c | 7.93 a | 5.62 b | 4.83 b |
| pentane | 2235 b | 3379 a | 3177 a | 3405 a |
| propanal | 17.1 ab | 14.4 b | 19.4 a | 13.7 b |
| trans-2-undecenal | 5.98 c | 6.63 bc | 8.39 b | 9.48 a |
| 2-isopropyl-3-methoxypyrazine | 6.18 b | 5.92 b | 8.25 a | 8.18 a |
| 2-methylisoborneol | 1.76 c | 1.96 bc | 3.39 ab | 3.41 a |
| 2,3-butanediol | 39.5 b | 69.1 a | 54.2 ab | 58.3 a |
| alpha-terpinene | 1.46 a | 1.76 a | 1.66 a | 1.67 a |
| beta-caryophyllene | 0.60 a | 0.70 a | 0.92 a | 0.91 a |
| ethyl acetate | 4.40 a | 4.70 a | 5.14 a | 4.77 a |
| geosmin | 11.1 c | 11.3 bc | 15.5 b | 19.0 a |
| isobutyl alcohol | 8486 b | 8467 b | 11,213 a | 11,499 a |
| 3-methyl-1-butanol | 17.7 a | 20.2 a | 21.2 a | 21.3 a |
| acetic acid | 6.84 a | 11.0 a | 9.49 a | 9.89 a |
| acetoin | 3.96 a | 4.23 a | 4.63 a | 4.30 a |
| acetone | 10.1 c | 13.8 b | 12.9 b | 15.6 a |
| carbon disulfide | 1016 b | 1016 b | 1550 a | 1642 a |
| dimethyl disulfide | 5011 b | 4982 b | 7297 a | 7688 a |
| dimethyl sulfide | 2.62 d | 4.26 b | 3.50 c | 4.95 a |
| formaldehyde | 1201 b | 1171 b | 1425 a | 1400 a |
| hydrogen sulfide | 0.68 a | 0.60 a | 0.68 a | 0.88 a |
| indole | 0.58 a | 0.94 a | 1.09 a | 0.95 a |
| methyl mercaptan | 9404 b | 9264 b | 12,153 a | 12,174 a |
| trimethylamine | 22.8 a | 23.7 a | 24.3 a | 24.7 a |
| ammonia | 443 c | 527 bc | 605 b | 856 a |
| dimethylamine | 249 c | 309 bc | 371 b | 444 a |
| 2-acetyl furan | 1.31 a | 1.40 a | 1.46 a | 1.58 a |
| 2-ethyl-2,5, -dimethylpyrazine | 2.00 b | 2.11 b | 2.98 ab | 3.01 a |
| 2-ethylfuran | 18.1 b | 19.1 b | 27.3 a | 28.7 a |
| trimethylpyrazine | 14.3 b | 14.5 b | 23.3 a | 25.9 a |
References
- Jørgensen, L.V.; Huss, H.H.; Dalgaard, P.J. Significance of Volatile Compounds Produced by Spoilage Bacteria in Vacuum-Packed Cold-Smoked Salmon (Salmo salar) Analyzed by GC-MS and Multivariate Regression. Agric. Food Chem. 2001, 49, 2376–2381. [Google Scholar] [CrossRef] [PubMed]
- Jónsdóttir, R.; Ólafsdóttir, G.; Chanie, E.; Haugen, J.-E. Volatile compounds suitable for rapid detection as quality indicators of cold smoked salmon (Salmo salar). Food Chem. 2008, 109, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Frank, D.; Arcot, J. Creating alternative seafood flavour from non-animal ingredients: A review of key flavour molecules relevant to seafood. Food Chem. X 2024, 22, 101400. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.B.; Silva, M.V.; Lannes, S.C.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. (Campinas) 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Fontes, P.R.; Gomide, L.A.M.; Romas, E.M.; Stringheta, P.C. Color evaluation of carbon monoxide treated porcine blood. Meat Sci. 2005, 68, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Conz, A.; Davoli, E.; Franchi, C.; Diomede, L. Seafood loss prevention and waste reduction. Food Qual. Saf. 2021, 8, fyae017. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Undercurrent News. Norway Led US Atlantic Salmon Import Rebound in Q1. Available online: https://www.undercurrentnews.com/2025/05/26/norway-led-us-atlantic-salmon-import-rebound-in-q1/ (accessed on 8 July 2025).
- Yu, Y.J.; Yang, S.P.; Lin, T.; Qian, Y.F.; Xie, J.; Hu, C. Effect of Cold Chain Logistic Interruptions on Lipid Oxidation and Volatile Organic Compounds of Salmon (Salmo salar) and Their Correlations With Water Dynamics. Front. Nutr. 2020, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Bendiksen, E.; Jobling, M. Effects of temperature and feed composition on essential fatty acid (n-3 and n-6) retention in Atlantic salmon (Salmo salar L.) parr. Fish Physiol. Biochem. 2003, 29, 133–140. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Björnsson, B.T.; Stefansson, S.O. Long-term effects of photoperiod, temperature and their interaction on growth, gill Na+, K+-ATPase activity, seawater tolerance and plasma growth-hormone levels in Atlantic salmon Salmo salar. J. Fish Biol. 2013, 83, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Pino-Martínez, E.; Balseiro, P.; Pedrosa, C.; Haugen, T.S.; Fleming, M.S.; Handeland, S.O. The effect of photoperiod manipulation on Atlantic salmon growth, smoltification and sexual maturation: A case study of a commercial RAS. Aquac. Res. 2021, 52, 2593–2608. [Google Scholar] [CrossRef]
- Clercin, N.; Druschel, G. Influence of Environmental Factors on the Production of MIB and Geosmin Metabolites by Bacteria in a Eutrophic Reservoir. Water Resour. Res. 2019, 55, 5413–5430. [Google Scholar] [CrossRef]
- Suurnäkki, S.; Gómez-Saez, G.V.; Rantala-Ylinen, A.; Jokela, J.; Fewer, D.P.; Sivonen, K. Identification of geosmin and 2-methylisoborneol in cyanobacteria and molecular detection methods for the producers of these compounds. Water Res. 2015, 68, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Anagnostopoulos, D.A.; Karamani, E.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Growth and Volatile Organic Compound Production of Pseudomonas Fish Spoiler Strains on Fish Juice Agar Model Substrate at Different Temperatures. Microorganisms 2023, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.E. Microbial Food Spoilage—Losses and Control Strategies; Food Research Institute, University of Wisconsin-Madison: Madison, WI, USA, 2007; Available online: https://fri.wisc.edu/files/Briefs_File/2017-07-18_0857_FRI_Brief_Microbial_Food_Spoilage_7_07.pdf (accessed on 2 May 2025).
- Zhao, S.; Yu, J.; Xi, L.; Kong, X.; Pei, J.; Jiang, P.; Gao, R.; Jin, W. Sex-Specific Lipid Profiles and Flavor Volatiles in Giant Salamander (Andrias davidianus) Tails Revealed by Lipidomics and GC-IMS. Foods 2024, 13, 3048. [Google Scholar] [CrossRef] [PubMed]
- Chaliha, M.; Cusack, A.; Currie, M.; Sultanbawa, Y.; Smyth, H. Effect of Packaging Materials and Storage on Major Volatile Compounds in Three Australian Native Herbs. J. Agric. Food Chem. 2013, 61, 5738–5745. [Google Scholar] [CrossRef] [PubMed]
- Sendón García, R.; Sanches Silva, A.; Cooper, I.; Franz, R.; Paseiro Losada, P. Revision of analytical strategies to evaluate different migrants from food packaging materials. Trends Food Sci. Technol. 2006, 17, 354–366. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging Role of Phenolic Compounds as Natural Food Additives in Fish and Fish Products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Sae-leaw, T.; Benjakul, S. Fatty acid composition, lipid oxidation, and fishy odour development in seabass (Lates calcarifer) skin during iced storage. Eur. J. Lipid Sci. Technol. 2014, 116, 885–894. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C.; Vielma, J. Controlling of geosmin and 2-methylisoborneol induced off-flavours in recirculating aquaculture system farmed fish—A review. Aquac. Res. 2019, 50, 9–28. [Google Scholar] [CrossRef]
- Pino Martinez, E.; Balseiro, P.; Fleming, M.S.; Stefansson, S.O.; Norberg, B.; Imsland, A.K.D.; Handeland, S.O. Interaction of Temperature and Photoperiod on Male Postsmolt Maturation of Atlantic Salmon (Salmo salar L.). Aquaculture 2023, 568, 739325. [Google Scholar] [CrossRef]
- Venkateshwarlu, G.; Let, M.B.; Meyer, A.S.; Jacobsen, C. Chemical and Olfactometric Characterization of Volatile Flavor Compounds in a Fish Oil Enriched Milk Emulsion. J. Agric. Food Chem. 2004, 52, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Piveteau, F.; Guen, S.; Gandemer, G.; Baud, J.; Prost, C.; Demaimay, M. Aroma of Fresh Oysters Crassostrea gigas: Composition and Aroma Notes. J. Agric. Food Chem. 2000, 48, 4851–4857. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Smith, D. Use of Volatiles as Indicators of Lipid Oxidation in Muscle Foods. Compr. Rev. Food Sci. Food Saf. 2006, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.M.; Hurley, M.A. A functional model for maximum growth of Atlantic Salmon parr, Salmo salar, from two populations in northwest England. Funct. Ecol. 1997, 11, 592–603. [Google Scholar] [CrossRef]
- Bernthal, F.R.; Seaman, B.W.; Rush, E.; Armstrong, J.D.; McLennan, D.; Nislow, K.H.; Metcalfe, N.B. High summer temperatures are associated with poorer performance of underyearling Atlantic salmon (Salmo salar) in upland streams. J. Fish Biol. 2023, 102, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Forseth, T.; Hurley, M.; Jensen, A.; Elliott, J. Functional models for growth and food consumption of Atlantic salmon parr, Salmo salar, from a Norwegian river. Freshw. Biol. 2001, 46, 173–186. [Google Scholar] [CrossRef]
- Feidantsis, K.; Pörtner, H.; Antonopoulou, E.; Michaelidis, B. Synergistic effects of acute warming and low pH on cellular stress responses of the gilthead seabream Sparus aurata. J. Comp. Physiol. B 2014, 185, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Mittakos, I.; Nathanailides, C.I.; Kokokiris, L.E.; Barbouti, A.; Bitchava, K.; Gouva, E.; Kolygas, M.N.; Terzidis, M.A.; Kontominas, M.G. Antioxidant Capacity, Lipid Oxidation, and Quality Traits of Slow- and Fast-Growing Meagre (Argyrosomus regius) Fillets During Cold Storage. Antioxidants 2025, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Osako, K.; Kuwahara, K.; Nozaki, Y. Seasonal variations in gel-forming ability of rabbit fish. Fish. Sci. 2003, 69, 1281–1289. [Google Scholar] [CrossRef]
- Yin, P.; Björnsson, B.T.; Fjelldal, P.G.; Saito, T.; Remø, S.C.; Edvardsen, R.B.; Hansen, T.; Sharma, S.; Olsen, R.E.; Hamre, K. Impact of Antioxidant Feed and Growth Manipulation on the Redox Regulation of Atlantic Salmon Smolts. Antioxidants 2022, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Nemova, N.N.; Nefedova, Z.A.; Pekkoeva, S.N.; Voronin, V.P.; Shulgina, N.S.; Churova, M.V.; Murzina, S.A. The Effect of the Photoperiod on the Fatty Acid Profile and Weight in Hatchery-Reared Underyearlings and Yearlings of Atlantic Salmon Salmo salar L. Biomolecules 2020, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Saito, T.; Fjelldal, P.G.; Björnsson, B.T.; Remø, S.C.; Hansen, T.J.; Sharma, S.; Olsen, R.E.; Hamre, K. Seasonal Changes in Photoperiod: Effects on Growth and Redox Signaling Patterns in Atlantic Salmon Postsmolts. Antioxidants 2023, 12, 1546. [Google Scholar] [CrossRef] [PubMed]
- Heise, K.; Puntarulo, S.; Nikinmaa, M.; Abele, D.; Pörtner, H. Oxidative stress during stressful heat exposure and recovery in the North Sea eelpout Zoarces viviparus L. J. Exp. Biol. 2006, 209, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.; Vikeså, V.; Lie, K.; Hevrøy, E. Transcriptional responses to temperature and low oxygen stress in Atlantic salmon studied with next-generation sequencing technology. BMC Genom. 2013, 14, 817. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.D.; Blanch, E.W.; Torley, P.J. Chemical Indicators of Atlantic Salmon Quality. Food Rev. Int. 2023, 40, 1426–1456. [Google Scholar] [CrossRef]
- Kaur, M.; Atif, F.; Ali, M.; Rehman, H.; Raisuddin, S.J. Heat stress-induced alterations of antioxidants in the freshwater fish Channa punctata Bloch. Fish Biol. 2005, 67, 1653–1665. [Google Scholar] [CrossRef]
- Dalsvåg, H.; Cropotova, J.; Jambrak, A.; Janči, T.; Španěl, P.; Dryahina, K.; Rustad, T. Mass Spectrometric Quantification of Volatile Compounds Released by Fresh Atlantic Salmon Stored at 4 °C under Modified Atmosphere Packaging and Vacuum Packaging for up to 16 Days. ACS Food Sci. Technol. 2021, 2, 400–414. [Google Scholar] [CrossRef]
- Ólafsdóttir, G.; Jónsdóttir, R.; Lauzon, H.L.; Luten, J.B.; Kristbergsson, K. Characterization of Volatile Compounds in Chilled Cod (Gadus morhua) Fillets by Gas Chromatography and Detection of Quality Indicators by an Electronic Nose. J. Agric. Food Chem. 2005, 53, 10140–10147. [Google Scholar] [CrossRef] [PubMed]
- Lindholm-Lehto, P.C.; Koskela, J.; Kaseva, J.; Vielma, J. Accumulation of Geosmin and 2-methylisoborneol in European Whitefish Coregonus lavaretus and Rainbow Trout Oncorhynchus mykiss in RAS. Fishes 2020, 5, 13. [Google Scholar] [CrossRef]
- Gómez-Boronat, M.; Sáiz, N.; Delgado, M.; Pedro, N.; Isorna, E. Time-Lag in Feeding Schedule Acts as a Stressor That Alters Circadian Oscillators in Goldfish. Front. Physiol. 2018, 9, 1749. [Google Scholar] [CrossRef] [PubMed]
- Imsland, A.K.D.; Roth, B.; Døskeland, I.; Fjelldal, P.G.; Stefansson, S.O.; Handeland, S.; Mikalsen, B. Flesh quality of Atlantic salmon smolts reared at different temperatures and photoperiods. Aquac. Res. 2019, 50, 1795–1801. [Google Scholar] [CrossRef]
- Sáiz, N.; Gómez-Boronat, M.; Pedro, N.; Delgado, M.; Isorna, E. The Lack of Light-Dark and Feeding-Fasting Cycles Alters Temporal Events in the Goldfish (Carassius auratus) Stress Axis. Animals 2021, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.; Yamaka, S.; Pornsopin, P.; Jaturasitha, S.; Faggio, C. Proximate and Nutritional Content of Rainbow Trout (Oncorhynchus mykiss) Flesh Cultured in a Tropical Highland Area. Braz. Arch. Biol. Technol. 2020, 63, e20180234. [Google Scholar] [CrossRef]
- Tang, H.; Chen, L.; Xiao, C.; Wu, T.J. Fatty acid profiles of muscle from large yellow croaker (Pseudosciaena crocea R.) of different age. Zhejiang Univ. Sci. B 2009, 10, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Stansby, M.E. Composition of certain species of fresh-water fish. I. Introduction: The determination of the variation of composition of fish. J. Food Sci. 1954, 19, 231–234. [Google Scholar] [CrossRef]
- Jacquot, R. CHAPTER 6–Organic Constituents of Fish and Other Aquatic Animal Foods. In Fish as Food; Academic Press: Cambridge, MA, USA, 1961; pp. 145–209. [Google Scholar] [CrossRef]
- Matulić, D.; Blažina, M.; Pritišanac, E.; Čolak, S.; Bavčević, L.; Barić, R.; Križanac, S.; Vitlov, B.; Šuran, J.; Perović, I.S.; et al. Growth, Fatty Acid Profile and Malondialdehyde Concentration of Meagre Argyrosomus regius Fed Diets with Different Lipid Content. Appl. Sci. 2024, 14, 4842. [Google Scholar] [CrossRef]
- Kunyaboon, S.; Thumanu, K.; Park, J.W.; Khongla, C.; Yongsawatdigul, J. Evaluation of Lipid Oxidation, Volatile Compounds and Vibrational Spectroscopy of Silver Carp (Hypophthalmichthys molitrix) during Ice Storage as Related to the Quality of Its Washed Mince. Foods 2021, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Meng, Y.; Zhang, W.; Mai, K. Comparative study on the organoleptic quality of wild and farmed large yellow croaker Larimichthys crocea. J. Oceanol. Limnol. 2019, 38, 260–274. [Google Scholar] [CrossRef]
- Sarika, A.R.; Lipton, A.P.; Aishwarya, M.S. Biopreservative Efficacy of Bacteriocin GP1 of Lactobacillus rhamnosus GP1 on Stored Fish Filets. Front. Nutr. 2019, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Hasanah, F.; Pratoko, D.K.; Kristiningrum, N. Colorimetric Paper-Based Dual Indicator Label for Real-Time Monitoring of Fish Freshness. Food Technol. Biotechnol. 2022, 60, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Bower, C.; Malemute, C.; Bechtel, P. ENDOGENOUS PROTEASE ACTIVITY IN BY-PRODUCTS OF PINK SALMON (ONCORHYNCHUS GORBUSCHA). J. Food Biochem. 2011, 35, 628–637. [Google Scholar] [CrossRef]
- Duflos, G.; Coin, V.M.; Cornu, M.; Antinelli, J.F.; Malle, P. Determination of volatile compounds to characterize fish spoilage using headspace/mass spectrometry and solid-phase microextraction/gas chromatography/mass spectrometry. J. Sci. Food Agric. 2005, 86, 600–611. [Google Scholar] [CrossRef]
- Schrader, K.; Summerfelt, S.T. Distribution of Off-Flavor Compounds and Isolation of Geosmin-Producing Bacteria in a Series of Water Recirculating Systems for Rainbow Trout Culture. N. Am. J. Aquac. 2010, 72, 1–9. [Google Scholar] [CrossRef]
- Tucker, C.S. Off-Flavor Problems in Aquaculture. Rev. Fish. Sci. 2000, 8, 45–88. [Google Scholar] [CrossRef]
- Percival, S.; Drabsch, P.; Glencross, B. Determining factors affecting muddy-flavour taint in farmed barramundi, Lates calcarifer. Aquaculture 2008, 284, 136–143. [Google Scholar] [CrossRef]
- Howgate, P. Tainting of farmed fish by geosmin and 2-methyl-iso-borneol: A review of sensory aspects and of uptake/depuration. Aquaculture 2004, 234, 155–181. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2008, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- House, A.H.; Debes, P.V.; Kurko, J.; Erkinaro, J.; Käkelä, R.; Primmer, C.R. Sex-specific lipid profiles in the muscle of Atlantic salmon juveniles. Biorxiv 2020, 100810. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhao, S.; Li, J.; Cheng, K.; Xi, L.; Pei, J.; Gao, R.; Jiang, P. Unraveling sex-specific lipids and flavor volatiles in giant salamander (Andrias davidianus) livers via lipidomics and GC-IMS. Food Chem. X 2024, 23, 101786. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.B.; Houlihan, D.F.; Talbot, C.; Palmer, R.M. Protein metabolism during sexual maturation in female Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 1993, 12, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lu, S.; Tao, Y.; Hua, J.; Zhuge, Y.; Chen, W.; Qiang, J. Characteristic Muscle Quality Parameters of Male Largemouth Bass (Micropterus salmoides) Distinguished from Female and Physiological Variations Revealed by Transcriptome Profiling. Biology 2024, 13, 1029. [Google Scholar] [CrossRef] [PubMed]
- Dhurmeea, Z.; Pethybridge, H.; Appadoo, C.; Bodin, N. Lipid and fatty acid dynamics in mature female albacore tuna (Thunnus alalunga) in the western Indian Ocean. PLoS ONE 2018, 13, e0194558. [Google Scholar] [CrossRef] [PubMed]
- Karbsri, W.; Hamzeh, A.; Yongsawatdigul, J. Changes in volatile compounds and lipid oxidation in various tissues of Nile tilapia (Oreochromis niloticus) during ice storage. J. Food Sci. 2024, 89, 2261–2276. [Google Scholar] [CrossRef] [PubMed]
- Ackman, R.; Heras, H.; Zhou, S. SALMON LIPID STORAGE SITES AND THEIR ROLE IN CONTAMINATION WITH WATER-SOLUBLE PETROLEUM MATERIALS. J. Food Lipids 1996, 3, 161–170. [Google Scholar] [CrossRef]
- Aursand, M.; Bleivik, B.; Rainuzzo, J.; Jørgensen, L.; Mohr, V. Lipid distribution and composition of commercially farmed atlantic salmon (salmosalar). J. Sci. Food Agric. 1994, 64, 239–248. [Google Scholar] [CrossRef]
- Głowacz-Różyńska, A.; Tynek, M.; Malinowska-Pańczyk, E.; Martysiak-Żurowska, D.; Pawłowicz, R.; Kołodziejska, I. Comparison of oil yield and quality obtained by different extraction procedures from salmon (Salmo salar) processing byproducts. Eur. J. Lipid Sci. Technol. 2016, 118, 1759–1767. [Google Scholar] [CrossRef]
- Wójciak, K.; Dolatowski, Z.; Kołożyn-Krajewska, D.; Trząskowska, M. Comparison of oil yield and quality obtained by different extraction procedures from salmon (Salmo salar) processing byproducts. J. Food Qual. 2012, 35, 353–365. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, S.; Wu, H.; Jatt, A.; Pan, Y.; Zeng, M. Quorum Sensing Involved in the Spoilage Process of the Skin and Flesh of Vacuum-Packaged Farmed Turbot (Scophthalmus maximus) Stored at 4 °C. J. Food Sci. 2016, 81, M2776–M2784. [Google Scholar] [CrossRef] [PubMed]
- Jyothylakshmi, K.; Nandakumar, S.; Kumar, M.G.S. Bacterial pollution indicators associated in the tissues of an estuarine fish mugil cephalusfrom Ashtamudi lake, aRAMSAR site(Kerala, India). Sustain. Agri. Food Environ. Res. 2020, 9, 4. [Google Scholar] [CrossRef]
- Webster, T.; Consuegra, S.; Hitchings, M.; Leániz, C. Inter-population variation in the Atlantic salmon microbiome reflects environmental and genetic diversity. Biorxiv 2018, 84, 16. [Google Scholar] [CrossRef]
- Ellison, A.; Wilcockson, D.; Cable, J. Circadian dynamics of the teleost skin immune-microbiome interface. Biorxiv 2021, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, J.; Xie, J. Progress on odor deterioration of aquatic products: Characteristic volatile compounds, analysis methods, and formation mechanisms. Food Biosci. 2023, 53, 102666. [Google Scholar] [CrossRef]
- Ganguly, S.; Mahanty, A.; Mitra, T.; Raman, R.K.; Mohanty, B.P. Volatile compounds in hilsa (Tenualosa ilisha, Hamilton) as detected by static headspace gas chromatography and mass spectrometry. J. Food Process. Preserv. 2017, 41, e13212. [Google Scholar] [CrossRef]
- Natale, C.D.; Ólafsdóttir, G. Electronic Nose and Electronic Tongue. Fish. Prod. 2009, 105–126. [Google Scholar] [CrossRef]
- Di Lucia, F.; Lacivita, V.; Nobile, M.A.D.; Conte, A. Improving the Storability of Cod Fish-Burgers According to the Zero-Waste Approach. Foods 2021, 10, 1972. [Google Scholar] [CrossRef]
- Oveland, E.; Bøkevoll, A.; Araujo, P.; Hemre, G. Frozen storage procedures for salmon and plaice samples: Nutrient composition and implications for preservation. J. Food Sci. 2024, 89, 4660–4670. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.V.; Phan, L.M.T. Influences of Bleeding Conditions on the Quality and Lipid Degradation of Cobia (Rachycentron canadum) Fillets During Frozen Storage. Turk. J. Fish. Aquat. Sci. 2018, 18, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, K.; Majumdar, R.K.; Choudhury, J.; Priyadarshini, B.M.; Dhar, B.; Roy, D.; Saha, A.; Maurya, P. Protein Degradation and Instrumental Textural Changes in Fresh Nile Tilapia (Oreochromis niloticus) during Frozen Storage. J. Food Process. Preserv. 2015, 39, 2206–2214. [Google Scholar] [CrossRef]

| Volatile | Reagent | k (10−9 cm3/s) | Mass (m/z) | Product |
|---|---|---|---|---|
| (E,Z)-2,6-nonadienal | NO+ | 2.5 | 137 | C9H13O+ |
| (E)-2-pentenal | NO+ | 4 | 83 | C5H7O+ |
| 1-hexanol | NO+ | 2.4 | 101 | C6H13O+ |
| 1-octanol | NO+ | 2.3 | 129 | C8H17O+ |
| 1-octen-3-ol | H3O+ | 2.5 | 111 | C8H15+ |
| 1-octen-3-one | NO+ | 2.5 | 156 | C8H14.NO+ |
| 1-pentanol | H3O+ | 2.8 | 71 | C5H11+ |
| 1-penten-3-ol | H3O+ | 2.6 | 69 | C5H9+ |
| 2-butenal | NO+ | 4.1 | 69 | C4H5O+ |
| 2-decanone | NO+ | 2.5 | 186 | C10H20O.NO+ |
| 2-decenal | NO+ | 2.1 | 153 | C10H17O+ |
| 2-heptanone | NO+ | 3.4 | 144 | C7H14O.NO+ |
| 2-heptenal | H3O+ | 4.7 | 113 | C7H13O+ |
| 131 | C7H13O+.H2O | |||
| 2-hexenal | H3O+ | 4.6 | 99 | C6H11O+ |
| 117 | C6H11O+.H2O | |||
| 2-methyl naphthalene | H3O+ | 2.5 | 143 | C11H11+ |
| 2-nonanone | NO+ | 2.7 | 172 | C9H18O.NO+ |
| 2-nonenal | H3O+ | 4.8 | 141 | C9H17O+ |
| 159 | C9H17O+.H2O | |||
| 2-octenal | NO+ | 4.1 | 125 | C8H13O+ |
| 2-octene | NO+ | 2.1 | 112 | C8H16+ |
| 2-pentanone | NO+ | 3.1 | 116 | NO+.C5H10O |
| 2-penten-1-ol | H3O+ | 3 | 69 | C5H9+ |
| 2-pentene | H3O+ | 1.9 | 71 | C5H11+ |
| 2-undecanone | NO+ | 3.4 | 200 | C11H22O.NO+ |
| 2,4-decadienal | NO+ | 4.2 | 151 | C10H15O+ |
| 2,4-heptadienal | NO+ | 2.1 | 57 | C3H5O+ |
| 3-hexen-1-ol | H3O+ | 3.2 | 83 | C6H11+ |
| 3-hexenal | H3O+ | 4.2 | 81 | C6H9+ |
| decanal | NO+ | 3.3 | 155 | C10H19O+ |
| heptanal | NO+ | 3.3 | 113 | C7H13O+ |
| heptane | H3O+ | 2.6 | 119 | H3O+.C7H16 |
| hexanal | NO+ | 2.5 | 99 | C6H11O+ |
| hexanoic acid | NO+ | 2.5 | 146 | C6H12O2.NO+ |
| nonanal | NO+ | 2.7 | 141 | C9H17O+ |
| oct-2-en-1-ol | H3O+ | 2.8 | 111 | C8H14.H+ |
| octanal | NO+ | 3 | 127 | C8H15O+ |
| octane | H3O+ | 9 | 113 | C8H17+ |
| pentanal | NO+ | 3 | 85 | C5H9O+ |
| pentane | O2+ | 1.6 | 43 | C3H7+ |
| propanal | NO+ | 2.5 | 57 | C3H5O+ |
| trans-2-undecenal | H3O+ | 3 | 169 | C11H20O.H+ |
| 2-isopropyl-3-methoxypyrazine | H3O+ | 3 | 153 | C8H12N2O.H+ |
| 2-methylisoborneol | H3O+ | 2.9 | 151 | C11H19+ |
| 2,3-butanediol | NO+ | 2.3 | 89 | C4H9O2+ |
| 107 | C4H9O2+.H2O | |||
| alpha-terpinene | NO+ | 2 | 136 | C10H16+ |
| beta-caryophyllene | NO+ | 2.7 | 204 | C15H24+ |
| ethyl acetate | NO+ | 2.7 | 118 | NO+.CH3COOC2H5 |
| geosmin | NO+ | 2.5 | 112 | C8H16+ |
| isobutyl alcohol | NO+ | 2.4 | 73 | C4H9O+ |
| 3-methyl-1-butanol | H3O+ | 2.8 | 71 | C5H11+ |
| acetic acid | NO+ | 9 | 90 | NO+.CH3COOH |
| acetoin | NO+ | 3 | 118 | C4H8O2.NO+ |
| acetone | NO+ | 1.2 | 88 | NO+.C3H6O |
| carbon disulfide | O2+ | 4 | 76 | CS2+ |
| dimethyl disulfide | NO+ | 2.4 | 94 | (CH3)2S2+ |
| dimethyl sulfide | NO+ | 2.2 | 62 | (CH3)2S+ |
| formaldehyde | H3O+ | 3.4 | 31 | CH3O+ |
| 49 | H2CO.H+.H2O | |||
| hydrogen sulfide | H3O+ | 1.6 | 35 | H3S+ |
| indole | H3O+ | 3.3 | 118 | C8H8N+ |
| methyl mercaptan | H3O+ | 1.8 | 49 | CH4S.H+ |
| trimethylamine | NO+ | 1.6 | 59 | (CH3)3N+ |
| ammonia | H3O+ | 2.6 | 18 | NH4+ |
| 36 | NH4+.H2O | |||
| dimethylamine | H3O+ | 2.1 | 46 | (CH3)2NH.H+ |
| 2-acetyl furan | NO+ | 2 | 110 | C6H6O2+ |
| 2-ethyl-2,5, dimethylpyrazine | O2+ | 2.5 | 136 | C8H12N2+ |
| 2-ethylfuran | NO+ | 2.9 | 96 | C6H8O+ |
| trimethylpyrazine | NO+ | 2.5 | 122 | C7H10N2+ |
| Harvest Time | Total Body Weight (g) | Length (cm) | GSI (%) | |
|---|---|---|---|---|
| Male | Female | |||
| 202 Days | 161 b ± 16.2 | 26.4 b ± 0.54 | 0.00 ± 0 | 0.25 ± 0.02 |
| 242 Days | 256 a ± 32.9 | 29.1 a ± 0.84 | 0.18 ± 0.08 | 0.27 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, M.; Dabrowski, K.; Fisher, K.J.; Hossain, M.Z.; Barringer, S. Effect of Rearing, Physiological, and Processing Conditions on the Volatile Profile of Atlantic Salmon (Salmo salar) Using SIFT-MS. Foods 2025, 14, 2540. https://doi.org/10.3390/foods14142540
Kaur M, Dabrowski K, Fisher KJ, Hossain MZ, Barringer S. Effect of Rearing, Physiological, and Processing Conditions on the Volatile Profile of Atlantic Salmon (Salmo salar) Using SIFT-MS. Foods. 2025; 14(14):2540. https://doi.org/10.3390/foods14142540
Chicago/Turabian StyleKaur, Manpreet, Konrad Dabrowski, Kevin J. Fisher, Md Zakir Hossain, and Sheryl Barringer. 2025. "Effect of Rearing, Physiological, and Processing Conditions on the Volatile Profile of Atlantic Salmon (Salmo salar) Using SIFT-MS" Foods 14, no. 14: 2540. https://doi.org/10.3390/foods14142540
APA StyleKaur, M., Dabrowski, K., Fisher, K. J., Hossain, M. Z., & Barringer, S. (2025). Effect of Rearing, Physiological, and Processing Conditions on the Volatile Profile of Atlantic Salmon (Salmo salar) Using SIFT-MS. Foods, 14(14), 2540. https://doi.org/10.3390/foods14142540







