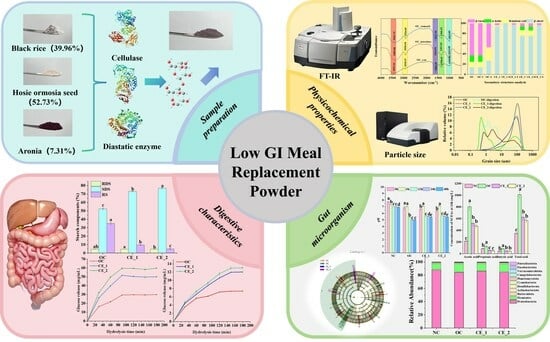
Enzymolysis-Driven Development of a Gut-Targeted Aronia melanocarpa Meal Replacement Powder with Glycemic Control and Microbial Homeostasis Benefits
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Meal Replacement Powder
2.3. Color Difference Analysis
2.4. Determination of Dispersion, Wettability and Hydration Properties
2.5. Determination of Water and Oil Holding Capacity
2.5.1. Determination of Water-Holding Capacity (WHO)
2.5.2. Determination of Oil Holding Capacity (OHC)
2.6. Determination of Antioxidant Capacities
2.7. Determination of Particle Size
2.8. Fourier Transform-Infrared Spectroscopy (FT-IR)
2.9. Absorption Characteristics
2.9.1. In Vitro Sodium Cholate Adsorption
2.9.2. Cholesterol Adsorption Capacity (CAC)
2.10. In Vitro Digestion Characterization
2.11. In Vitro Gastrointestinal Digestive Characterization
2.12. Everted Intestinal Sac Model
2.13. In Vitro Fecal Fermentation Characterization
2.13.1. Sample Collection
2.13.2. Determination of Short-Chain Fatty Acids Content
2.13.3. 16S rDNA Sequencing Analysis
2.14. Statistical Analysis
3. Result and Discussion
3.1. Effect of Enzymatic Hydrolysis Treatment on Color
3.2. Effect of Enzymatic Hydrolysis Treatment on Dispersion, Wettability and Hydration Characteristics
3.3. Effect of Enzymatic Hydrolysis Treatment on Oil and Water Holding Capacity
3.4. Effect of Enzymatic Hydrolysis Treatment on Cholate/Cholesterol Adsorption and Antioxidant Capacity
3.5. Effect of Enzymatic Hydrolysis Treatment on In Vitro Digestion Characteristics
3.5.1. In Vitro Digestion Characteristics
3.5.2. Estimated Glycemic Index
3.5.3. Simulated Gastrointestinal Digestion Characteristics
3.5.4. Fourier Transform Infrared Spectroscopy (FT-IR)
3.5.5. Particle Size Distribution
3.6. Glucose Absorption Properties
3.7. In Vitro Fermentation Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Obesity Federation. World Obesity Atlas. 2023. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023 (accessed on 21 May 2025).
- Fryk, E.; Silva, V.R.R.; Jansson, P.A. Galectin-1 in Obesity and Type 2 Diabetes. Metabolites 2022, 12, 930. [Google Scholar] [CrossRef]
- Nagano, T.; Higashimura, Y.; Nakano, M.; Nishiuchi, T.; Lelo, A.P. High-viscosity dietary fibers modulate gut microbiota and liver metabolism to prevent obesity in high-fat diet-fed mice. Int. J. Biol. Macromol. 2025, 298, 139962. [Google Scholar] [CrossRef] [PubMed]
- Renee, K.; Megan, K.; Swanson, K.S. Effects of oats on gastrointestinal health as assessed by in vitro, animal, and human studies. Nutr. Rev. 2019, 78, 343–363. [Google Scholar]
- Kouadio, J.E.-P.; Kouamé, A.F.M.; Bora, X.D.; Li, L.; Coulibaly, I.; Sun, Y.; Hussain, M. New insights into functional cereal foods as an alternative for dairy products: A review. Food Biosci. 2023, 55, 102840. [Google Scholar]
- Heymsfield, S.B.; Mierlo, C.A.J.V.; Van Der Knaap, H.C.M.; Heo, M.; Frier, H.I. Weight management using a meal replacement strategy: Meta and pooling analysis from six studies. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Čiž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Struyf, N.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Investigating the impact of α-amylase, α-glucosidase and glucoamylase action on yeast-mediated bread dough fermentation and bread sugar levels. J. Cereal Sci. 2017, 75, 35–44. [Google Scholar] [CrossRef]
- Deng, H.; Xue, B.; Wang, M. TMT-based quantitative proteomics analyses reveal the antibacterial mechanisms of anthocyanins from Aronia melanocarpa against Escherichia coli O157:H7. J. Agric. Food Chem. 2022, 70, 8032–8042. [Google Scholar] [CrossRef]
- Dong, J.; Wang, L.; Bai, Y.; Huang, X.; Chen, C.; Liu, Y. Study on the physicochemical properties and immune regulatory mechanism of polysaccharide fraction from Aronia Melanocarpa fruit. Int. J. Biol. Macromol. 2024, 283 Pt 2, 137696. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. Aronia in the Type 2 Diabetes Treatment Regimen. Nutrients. 2023, 15, 4188. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, J.; Tong, Y.; Kong, Y.; Tan, C.; Wang, M.; Wan, M.; Meng, X. Antibacterial characteristics and mechanisms of action of Aronia melanocarpa anthocyanins against Escherichia coli. LWT 2021, 150, 112018. [Google Scholar] [CrossRef]
- Cassidy, Y.M.; McSorley, E.M.; Allsopp, P.J. Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Foods 2018, 46, 423–439. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.: A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.3-2016; National Food Safety Standard—Determination of Moisture in Foods. National Food Safety Standard in China: Beijing, China, 2016.
- GB 5009.5-2025; National Food Safety Standard—Determination of Protein in Foods. National Food Safety Standard in China: Beijing, China, 2025.
- GB 5009.4-2016; National Food Safety Standard—Determination of Ash in Foods. National Food Safety Standard in China: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standard—Determination of Fat in Foods. National Food Safety Standard in China: Beijing, China, 2016.
- Huang, Y.; Wu, P.; Chen, X.D. Mechanistic insights into the influence of flavonoids from dandelion on physicochemical properties and in vitro digestibility of cooked potato starch. Food Hydrocolloids 2022, 130, 107714. [Google Scholar] [CrossRef]
- Sangnark, A.; Noomhorm, A. Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagass. Food Chem. 2003, 80, 221–229. [Google Scholar] [CrossRef]
- Heo, S.; Jeon, S.; Lee, S. Utilization of Lentinus edodes mushroom β-glucan to enhance the functional properties of gluten-free rice noodles. LWT 2014, 55, 627–631. [Google Scholar] [CrossRef]
- Chau, C.F.; Huang, Y.L. Comparison of the chemical composition and physicochemical properties of different fibers prepared from the peel of Citrus sinensis L. cv. Liucheng. J. Agric. Food Chem. 2003, 51, 2615–2618. [Google Scholar] [CrossRef]
- Donoso-Bustamante, V.; Osorio, E.; Arias-Santé, M.F.; De Camargo, A.C.; Rincón-Cervera, M.Á.; Amalraj, J.; Carrasco, B.; Palomo, I.; Araya-Maturana, R. Antioxidant activity of sinapic acid anilides: DPPH, ABTS, FRAP, electrochemical and theoretical analysis. LWT 2025, 222, 117656. [Google Scholar] [CrossRef]
- Sekar, G.; Sivakumar, A.; Mukherjee, A.; Chandrasekaran, N. Existence of hydroxylat MWCNTs demotes the catalysis effect of amylases against starch degradation. Int. J. Biol. Macromol. 2016, 86, 250–261. [Google Scholar] [CrossRef]
- Chen, L.; He, X.; Pu, Y.; Wang, H.; Cao, J.; Jiang, W. Adsorption removal properties of β-cyclodextrin-modified pectin on cholesterol and sodium cholate. Food Chem. 2024, 430, 137059. [Google Scholar] [CrossRef]
- Wu, W.; Hu, J.; Gao, H.; Chen, H.; Fang, X.; Mu, H.; Han, Y.; Liu, R. The potential cholesterol-lowering and prebiotic effects of bamboo shoot dietary fibers and their structural characteristics. Food Chem. 2020, 332, 127372. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33–S50. [Google Scholar] [PubMed]
- Granfeldt, Y.; Björck, I.; Drews, A.; Östman, E. An in vitro procedure based on chewing to predict metabolic response to starch in cereal and legume products. Eur. J. Clin. Nutr. 1992, 46, 649–660. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Razavi, S.M. Modeling of glucose release from native and modified wheat starch gels during in vitro gastrointestinal digestion using artificial intelligence methods. Int. J. Biol. Macromol. 2017, 97, 752–760. [Google Scholar] [CrossRef]
- Li, M.; Yang, S.; Yi, H.; Wang, Z.; Xu, B.; Li, G.; Ma, C.; Yuan, C.; Wang, Z. Preparation and evaluation of licochalcone A-integrated casein-pectin nanodelivery system: Insights into its gastrointestinal digestibility and bioavailability. Food Chem. 2025, 486, 144633. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, F.; Wang, J.; Wang, R.; Strappe, P.; Zheng, B.; Zhou, Z.; Chen, L. Manipulation of the internal structure of starch by propionyl treatment and its diverse influence on digestion and in vitro fermentation characteristics. Carbohydr. Polym. 2021, 270, 118390. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; de Mejía, E.G. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Ou, S.J.L.; Yu, J.; Zhou, W.; Liu, M.H. Effects of anthocyanins on bread microstructure and their combined impact on starch digestibility. Food Chem. 2022, 374, 131744. [Google Scholar] [CrossRef]
- Bangar, S.P.; Ashogbon, A.O.; Singh, A.; Chaudhary, V.; Whiteside, W.S. Enzymatic modification of starch: A green approach for starch applications. Carbohydr. Polym. 2022, 287, 119265. [Google Scholar] [CrossRef]
- Calliope, S.; Wagner, J.; Samman, S. Physicochemical and functional characterization of potato starch (Solanum Tuberosum ssp. Andigenum) from the Quebrada De Humahuaca, Argentina. Starch-Stärke 2020, 72, 1900069. [Google Scholar] [CrossRef]
- Singh, V.; Johnston, D.B. Pasting properties and surface characteristics of starch obtained from an enzymatic corn wet-milling process. Cereal Chem. 2002, 79, 523–527. [Google Scholar] [CrossRef]
- Miłek, J.; Lamkiewicz, J. The starch hydrolysis by α-amylase Bacillus spp.: An estimation of the optimum temperatures, the activation and deactivation energies. J. Therm. Anal. Calorim. 2022, 147, 14459–14466. [Google Scholar] [CrossRef]
- Rahmadani, M.; Fidriyanto, R.; Nahrowi, L.K.; Jayanegara, A. Investigating the impact of modified cassava starch with tannic acid and heat moisture treatment on physicochemical and in vitro starch digestibility. J. Agr. Food Res. 2025, 19, 101686. [Google Scholar] [CrossRef]
- Jing, Y.; Cao, R.-X.; Lei, X.; Wang, Z.-L.; Huang, X.-L.; Di, J.-R.; Mi, Z.-X.; Zhao, X.; Wang, M.; Jiang, M.-M.; et al. Structural characterization of polysaccharide from the peel of Trichosanthes kirilowii Maxim and its anti-hyperlipidemia activity by regulating gut microbiota and inhibiting cholesterol absorption. Bioorg. Chem. 2024, 149, 107487. [Google Scholar] [CrossRef]
- Li, J.; Bollati, C.; D’aDduzio, L.; Fanzaga, M.; Cruz-Chamorro, I.; Arnoldi, A.; Sirtori, C.R.; Lammi, C. Food-derived peptides with hypocholesterolemic activity: Production, transepithelial transport and cellular mechanisms. Trends Food Sci. Tech. 2024, 143, 104279. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, R.; Chen, J.; Chen, L.; Xie, F. Favored CH-π interaction between enzymatically modified high amylose starch and resveratrol improves digestion resistance. Food Hydrocolloids 2024, 154, 110137. [Google Scholar] [CrossRef]
- ISO 26642:2010; Food Products—Determination of Free and Total Glycerol and Mono-, Di- and Triglycerides by Gas Chromatography. ISO: Geneva, Switzerland, 2010.
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analysis of Prospective Cohort Studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Zhang, Y.; Xu, F.; Tan, L.; Wu, G.; Zhu, K.; Zhang, Y. The multi-scale structure and in vitro digestive kinetics of underutilized Chinese seedless breadfruit starch. Int. J. Biol. Macromol. 2024, 281 Pt 2, 136134. [Google Scholar] [CrossRef]
- Leow, J.W.H.; Chan, E.C.Y. Atypical Michaelis-Menten kinetics in cytochrome P450 enzymes: A focus on substrate inhibition. Biochem. Pharmacol. 2019, 169, 113615. [Google Scholar] [CrossRef]
- Stani, C.; Vaccari, L.; Mitri, E.; Birarda, G. FTIR investigation of the secondary structure of type I collagen: New insight into the amide III band. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 229, 118006. [Google Scholar] [CrossRef]
- Yang, J.; Dong, M.; Fang, F.; Li, Y.; Li, C. Effects of varied preparation processes on polyphenol-rice starch complexes, in vitro starch digestion, and polyphenols release. Food Chem. 2024, 450, 139330. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wang, S. Impact of trypsin on interfacial conformational evolution of soy protein isolate/soy hull polysaccharide emulsion. Int. J. Biol. Macromol. 2025, 308 Pt 3, 142507. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Petersen, B.L.; Liu, X.; Li, H.; Kirkensgaard, J.J.K.; Enemark-Rasmussen, K.; Khakimov, B. Characterization of different high amylose starch granules. part Ⅱ: Structure evolution during digestion and distinct digestion mechanisms. Food Hydrocoll. 2024, 149, 109593. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Inoue, R.; Matsumoto, M.; Yajima, T.; Ushida, K.; Iwanaga, T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem. Cell Biol. 2011, 135, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Disca, V.; Capuano, E.; Arlorio, M. Colonic fermentation of enzymatically treated cocoa bean shells (CBSs) and short chain fatty acids (SCFAs) production. LWT 2024, 202, 116311. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Li, M.; Wang, F.; Wang, J.; Wang, A.; Yao, X.; Strappe, P.; Zhou, Z.; Wu, Q.; Guo, T. Starch acylation of different short-chain fatty acids and its corresponding influence on gut microbiome and diabetic indexes. Food Chem. 2022, 389, 133089. [Google Scholar] [CrossRef]
- Rauf, A.; Khalil, A.A.; Rahman, U.U.; Khalid, A.; Naz, S.; Shariati, M.A.; Rebezov, M.; Urtecho, E.Z.; de Albuquerque, R.D.D.G.; Anwar, S.; et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6034–6054. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]





| Sample | L* | a* | b* | c* | h* |
|---|---|---|---|---|---|
| OC | 61.32 ± 0.73 a | 5.31 ± 0.03 a | 6.00 ± 0.14 a | 7.57 ± 0.14 a | 52.13 ± 0.72 a |
| CE_1 | 55.13 ± 1.34 b | 4.66 ± 0.08 b | 3.02 ± 0.13 b | 6.10 ± 0.06 b | 29.53 ± 1.04 b |
| CE_2 | 52.98 ± 0.89 c | 4.75 ± 0.20 b | 2.68 ± 0.17 c | 5.32 ± 0.32 c | 26.63 ± 2.45 b |
| Sample | Dispersibility (s) | Wettability (s) | WAI (g/g) | WSI (%) | SP (g/g) | OHC (g/g) | WHC (g/g) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 °C | 100 °C | 25 °C | 100 °C | 25 °C | 100 °C | |||||
| OC | 68.89 ± 1.21 c | 28.78 ± 1.28 b | 5.39 ± 0.34 b | 8.46 ± 0.61 b | 45.04 ± 1.39 b | 45.78 ± 1.29 b | 2.38 ± 0.18 a | 3.48 ± 0.37 a | 1.90 ± 0.08 b | 4.99 ± 0.44 a |
| CE_1 | 51.18 ± 0.95 b | 18.34 ± 1.03 a | 6.57 ± 0.27 a | 10.00 ± 0.90 a | 64.14 ± 1.63 a | 76.06 ± 3.15 a | 1.43 ± 0.11 b | 1.14 ± 0.11 b | 2.14 ± 0.13 a | 4.66 ± 0.09 a |
| CE_2 | 45.05 ± 0.62 a | 16.38 ± 1.16 a | 7.29 ± 0.32 a | 9.70 ± 0.67 ab | 65.26 ± 2.07 a | 80.04 ± 0.81 a | 1.43 ± 0.07 b | 0.89 ± 0.11 b | 2.21 ± 0.07 a | 4.13 ± 0.08 b |
| Sample | Sodium Taurocholate (μmol/100 mg) | Sodium Glycine Cholate (μmol/100 mg) | pH = 2 Cholesterol (mg/g) | pH = 7 Cholesterol (mg/g) | ABTS+ (%) | DPPH+ (%) | HI | eGI |
|---|---|---|---|---|---|---|---|---|
| OC | 2.37 ± 0.06 c | 3.65 ± 0.09 c | 0.32 ± 0.01 a | 2.34 ± 0.04 a | 92.11 ± 1.91 b | 81.08 ± 0.94 c | 36.75 ± 0.25 c | 39.88 ± 0.22 c |
| CE_1 | 2.60 ± 0.05 b | 5.12 ± 0.08 b | 0.27 ± 0.02 b | 1.60 ± 0.02 b | 96.34 ± 0.75 a | 85.40 ± 1.40 b | 46.09 ± 0.34 b | 47.93 ± 0.29 b |
| CE_2 | 3.89 ± 0.05 a | 5.64 ± 0.12 a | 0.26 ± 0.01 b | 0.75 ± 0.02 c | 96.81 ± 0.43 a | 87.98 ± 0.66 a | 50.11 ± 0.16 a | 51.40 ± 0.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, Z.; Ji, H.; Yang, S.; Guo, R.; Zhang, J.; He, H.; Xu, B.; Li, M. Enzymolysis-Driven Development of a Gut-Targeted Aronia melanocarpa Meal Replacement Powder with Glycemic Control and Microbial Homeostasis Benefits. Foods 2025, 14, 2456. https://doi.org/10.3390/foods14142456
Li Y, Hu Z, Ji H, Yang S, Guo R, Zhang J, He H, Xu B, Li M. Enzymolysis-Driven Development of a Gut-Targeted Aronia melanocarpa Meal Replacement Powder with Glycemic Control and Microbial Homeostasis Benefits. Foods. 2025; 14(14):2456. https://doi.org/10.3390/foods14142456
Chicago/Turabian StyleLi, Yongxing, Zhihui Hu, Haiyu Ji, Shuang Yang, Ruihan Guo, Jinfang Zhang, Hongjun He, Bo Xu, and Mei Li. 2025. "Enzymolysis-Driven Development of a Gut-Targeted Aronia melanocarpa Meal Replacement Powder with Glycemic Control and Microbial Homeostasis Benefits" Foods 14, no. 14: 2456. https://doi.org/10.3390/foods14142456
APA StyleLi, Y., Hu, Z., Ji, H., Yang, S., Guo, R., Zhang, J., He, H., Xu, B., & Li, M. (2025). Enzymolysis-Driven Development of a Gut-Targeted Aronia melanocarpa Meal Replacement Powder with Glycemic Control and Microbial Homeostasis Benefits. Foods, 14(14), 2456. https://doi.org/10.3390/foods14142456