The Road to Re-Use of Spice By-Products: Exploring Their Bioactive Compounds and Significance in Active Packaging
Abstract
1. Introduction
2. Classification, Extraction, and Stability of Bioactive Compounds in Spice By-Products
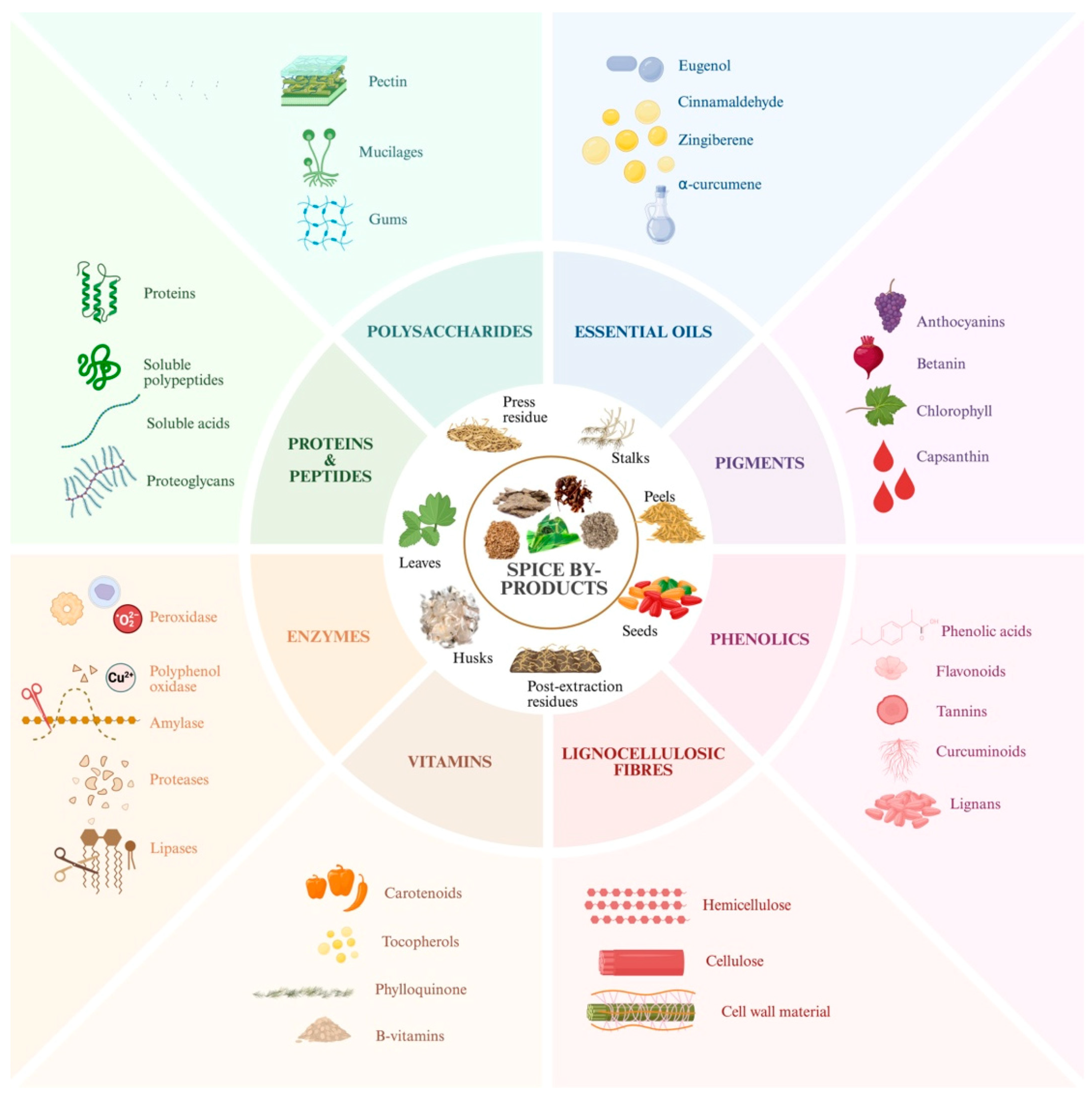
2.1. Spice By-Products and Their Bioactive Compounds
2.1.1. Phenolic Compounds
2.1.2. Polysaccharides
2.1.3. Proteins, Peptides, and Enzymes
2.1.4. Essential Oils
2.1.5. Pigments
2.1.6. Dietary Fiber
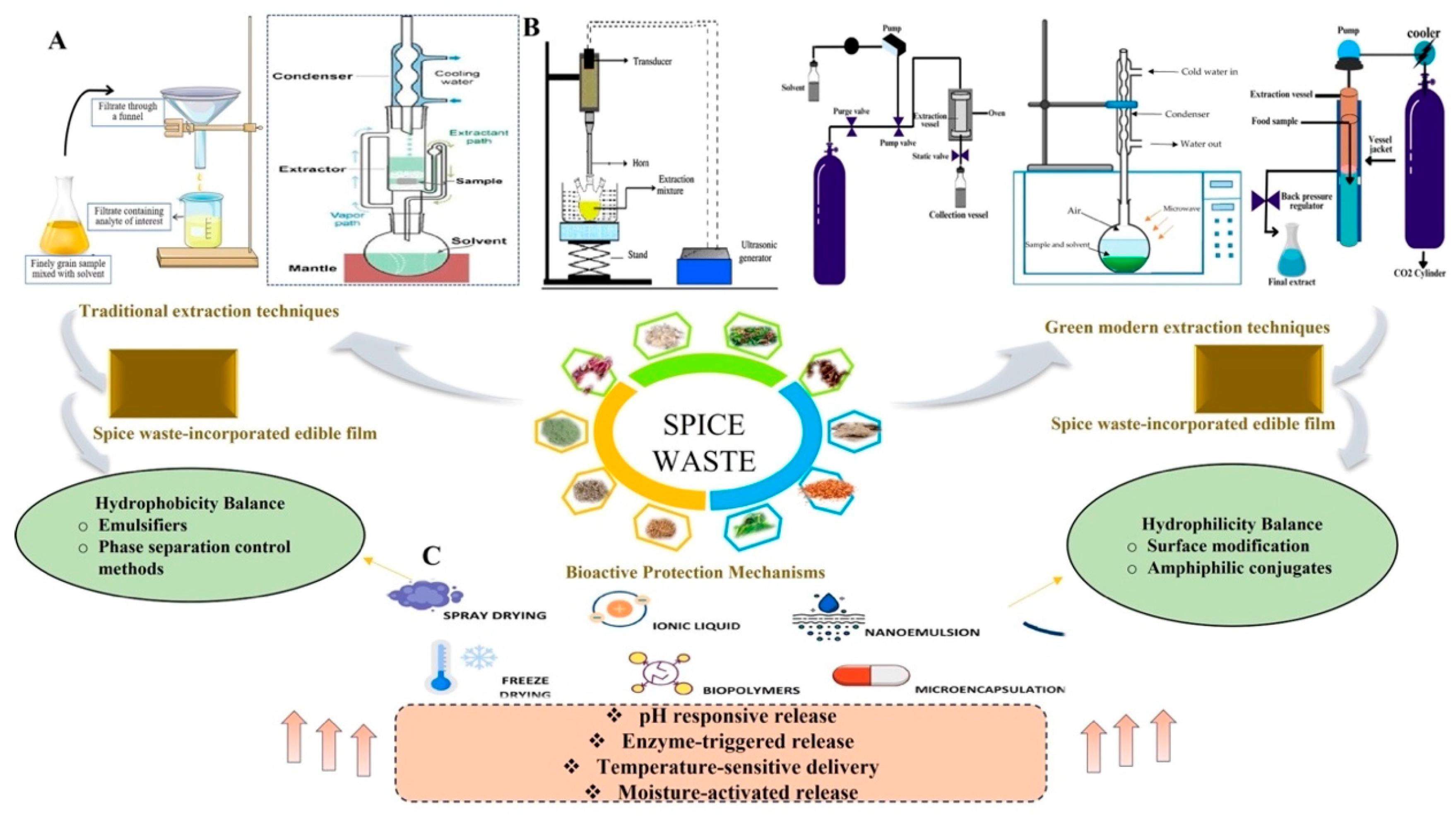
2.2. Extraction of Spice By-Products’ Bioactive Compounds for Packaging Applications
2.3. Stability of Spice By-Products’ Bioactive Compounds for Enhanced Packaging Functionality
3. Spice By-Products in Food Packaging Systems
3.1. Biodegradable Films: Regulatory Framework and Environmental Impact
3.1.1. Spice By-Products as Sources of Lignocellulosic Fibers for Biodegradable Packaging
3.1.2. Processing Methods for Spice By-Product Fibers
3.1.3. Mechanical Properties and Processing Techniques for Biodegradable Packaging
3.2. Edible Coatings
3.3. Pickering and Nanoemulsions
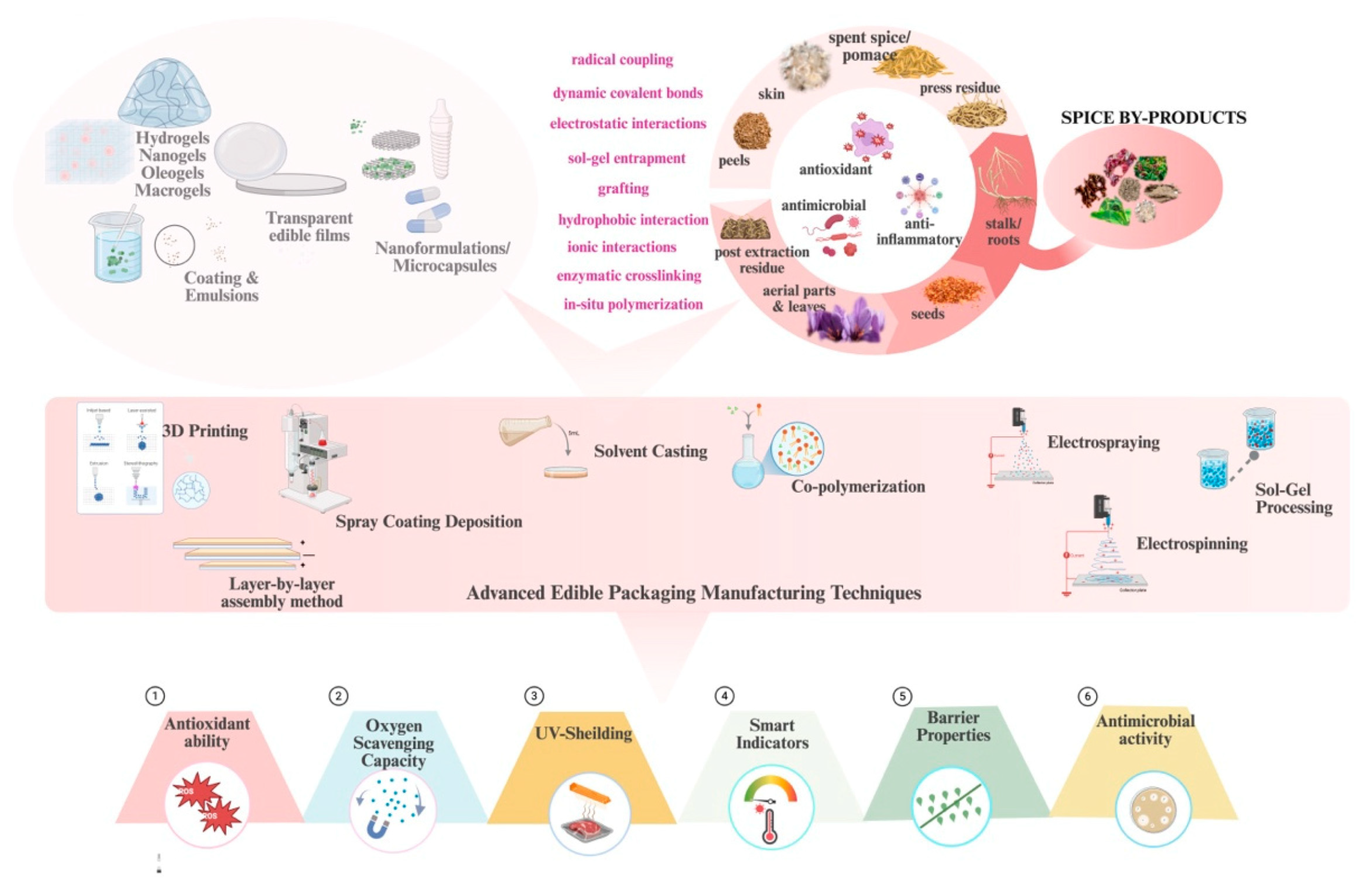
4. Value-Added Benefits of Spice By-Products in Active Packaging
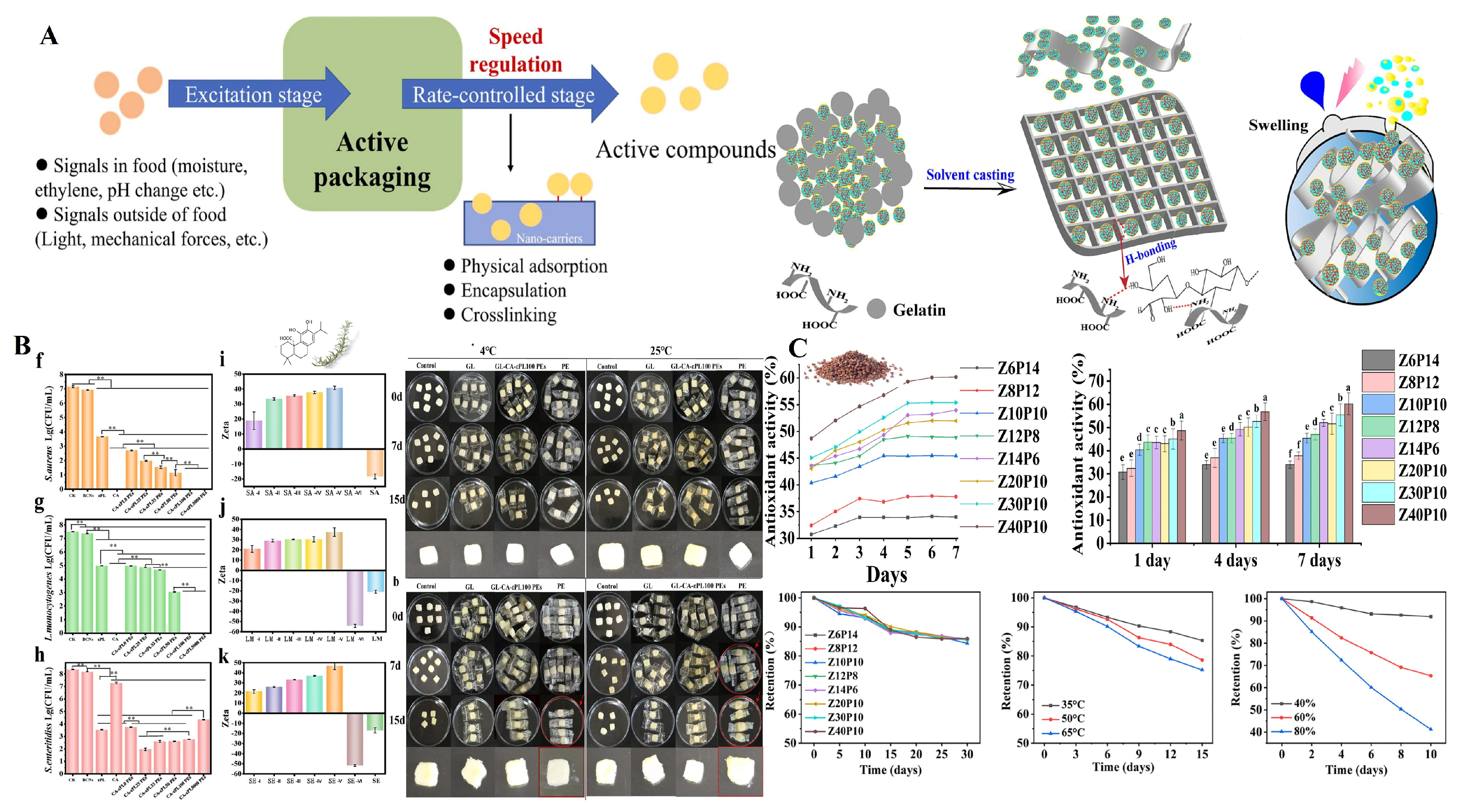
4.1. Antioxidant Packaging Systems
4.1.1. Mechanism of Action
4.1.2. Sources and Efficacy
4.1.3. Major Spice By-Products in Antioxidant Packaging
4.2. Antimicrobial Packaging Systems
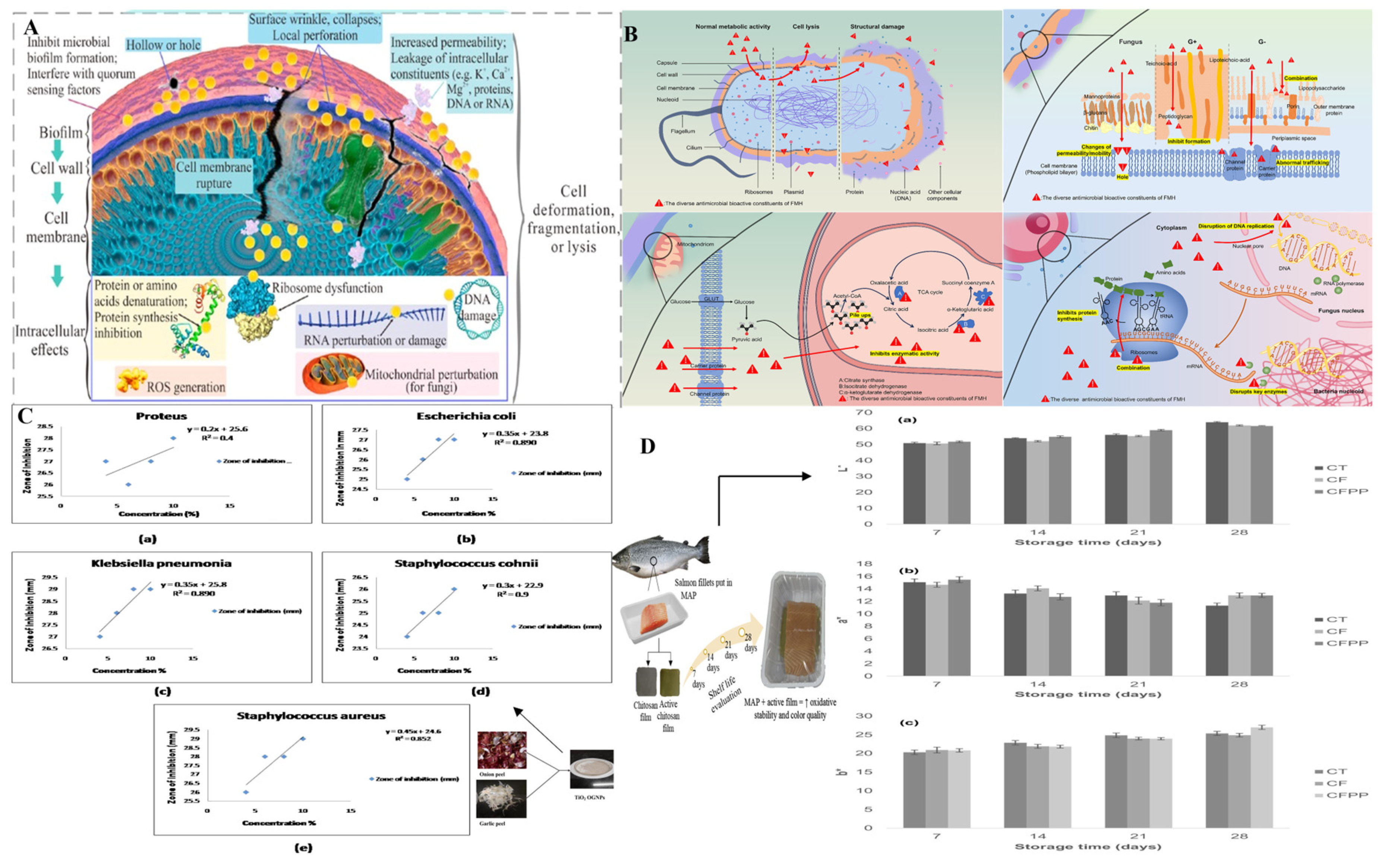
4.3. Shelf-Life Extension Packaging Systems
4.4. Barrier Properties
4.5. Controlled Release Systems
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EFCs | edible films and coatings |
| MIC | minimum inhibitory concentration |
| PCs | phenolic compounds |
| GPPs | ginger peel polysaccharides |
| BPs | bioactive peptides |
| POD | peroxidases |
| PPO | polyphenol oxidases |
| CNCs | cellulose nanocrystals |
| SWE | subcritical water extraction |
| SFE | supercritical fluid extraction |
| VOCs | volatile organic compounds |
| UAE | ultrasound-assisted extraction |
| MAE | Microwave-assisted extraction |
| PLE | pressurized liquid extraction |
| EAE | Enzyme-assisted extraction |
| HC | hydrodynamic cavitation |
| NaOH | sodium hydroxide |
| SPI | soy protein isolate |
| SDF | soluble dietary fiber |
| LDPE | low density polyethylene |
| PP | polypropylene |
| W/O/W | water-in-oil-in-water |
| TPC | total polyphenol content |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| TVC | total viable count |
| ORAC | oxygen radical absorbance capacity |
| RSTE | red onions shallot tunic extracts |
| GL/CC | gelatin/carboxymethylcellulose |
| CA | carvacrol |
| TVB-N | total volatile base nitrogen |
| PLA | poly lactic acid |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| PFE | pulsed electric field extraction |
| HPP | high pressure processing |
| NaDESs | Natural deep eutectic solvent |
| DES | deep eutectic solvent |
| EtOH | ethanol |
| MeOH | methanol |
| L/S | liquid to solid ratio |
| WVP | water vapor permeability |
| YM | Young’s modulus |
| TS | tensile strength |
| CFU | colony forming unit |
| GNC | ginger waste nanocellulose |
| WCA | water contact angle |
| OTR | oxygen transmission rate |
References
- Atta, O.M.; Manan, S.; Ahmed, A.A.Q.; Awad, M.F.; Ul-Islam, M.; Subhan, F.; Ullah, M.W.; Yang, G. Development and characterization of yeast-incorporated antimicrobial cellulose biofilms for edible food packaging application. Polymers 2021, 13, 2310. [Google Scholar] [CrossRef]
- Hussain, S.; Akhter, R.; Maktedar, S.S. Advancements in sustainable food packaging: From eco-friendly materials to innovative technologies. Sustain. Food Technol. 2024, 2, 1297–1364. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepúlveda, D.R.; Acosta-Muñiz, C.H. Contributing factors to the migration of antimicrobials in active packaging films. Food Res. Int. 2025, 200, 115514. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, M.; Alirezalu, K.; Hadi, F.; Marcinkowska-Lesiak, M.; Ismail-Fitry, M.R.; Abd El-Aty, A.M.; Oz, E.; Oz, F. Trends in food science & technology probiotic-incorporated active packaging solutions for meat and meat products: A review of benefits and recent applications. Trends Food Sci. Technol. 2025, 156, 104848. [Google Scholar] [CrossRef]
- Sethi, S.; Gupta, S. Antimicrobial Spices: Use in Antimicrobial Packaging. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Chapter 35; Academic Press: San Diego, CA, USA, 2016; pp. 433–444. [Google Scholar]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Khalil, H.P.S.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, A.K.; Singh, A. Bioconversion of food industry waste to value added products: Current technological trends and prospects. Food Biosci. 2023, 55, 102935. [Google Scholar] [CrossRef]
- Procopio, F.R.; Ferraz, M.C.; Paulino, B.N.; do Amaral Sobral, P.J.; Hubinger, M.D. Spice oleoresins as value-added ingredient for food industry: Recent advances and perspectives. Trends Food Sci. Technol. 2022, 122, 123–139. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Bin Mokaizh, A.A.; Baarimah, A.O.; Al-Zghoul, T. From agro-waste to bioactive wealth: Analyzing nutraceutical extraction and applications. Case Stud. Chem. Environ. Eng. 2025, 11, 101066. [Google Scholar] [CrossRef]
- Huang, M.; Yu, J.; Guo, M.; Zhang, J.; Ren, L. Recent advances in the preservation effects of spice essential oils on fruits and vegetables. Food Chem. 2025, 464, 141827. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.; Liu, H.; Sun, B. Medicine and food homology substances: A review of bioactive ingredients, pharmacological effects and applications. Food Chem. 2025, 463, 141111. [Google Scholar] [CrossRef] [PubMed]
- Eric Chan, W.C.; Lim, Y.Y.; Wong, S.K. Antioxidant properties of ginger leaves: An overview. Free Radic. Antioxid. 2011, 1, 6–16. [Google Scholar] [CrossRef]
- Zhang, S.L.; Deng, P.; Xu, Y.C.; LÜ, S.W.; Wang, J.J. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J. Integr. Agric. 2016, 15, 2175–2181. [Google Scholar] [CrossRef]
- Sowbhagya, H.B. Value-added processing of by-products from spice industry. Food Qual. Saf. 2019, 3, 73–80. [Google Scholar] [CrossRef]
- Karnwal, A.; Rauf, A.; Jassim, A.Y.; Selvaraj, M.; Al-Tawaha, A.R.M.S.; Kashyap, P.; Kumar, D.; Malik, T. Advanced starch-based films for food packaging: Innovations in sustainability and functional properties. Food Chem. 2025, 29, 102662. [Google Scholar] [CrossRef]
- Embuscado, M.E. Bioactives From Spices and Herbs. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 497–514. [Google Scholar]
- Gao, Z.; Zhang, J.; Zhang, Z.; Lu, H. Morphological identification of selected spices by starches, calciphytoliths, and phytoliths. Rev. Palaeobot. Palynol. 2025, 334, 105269. [Google Scholar] [CrossRef]
- Jayashree, E.; Shakkira, P.K.; Anees, K. Turmeric press residue—A high value by-product of tumeric juice powder. Indian. J. Hortic. 2023, 118–125. [Google Scholar] [CrossRef]
- Visakh, N.U.; Pathrose, B.; Chellappan, M.; Ranjith, M.T.; Sindhu, P.V.; Mathew, D. Extraction and chemical characterisation of agro-waste from turmeric leaves as a source of bioactive essential oils with insecticidal and antioxidant activities. Waste Manag. 2023, 169, 1–10. [Google Scholar] [CrossRef]
- Cortes-Ferre, H.E.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A. Enzyme-assisted extraction of anti-inflammatory compounds from habanero chili pepper (Capsicum chinense) seeds. Front. Nutr. 2022, 9, 942805. [Google Scholar] [CrossRef]
- Khadfy, Z.; Atifi, H.; Mamouni, R.; Jadouali, S.M.; Chartier, A.; Nehmé, R.; Karra, Y.; Tahiri, A. Nutraceutical and cosmetic applications of bioactive compounds of Saffron (Crocus Sativus L.) stigmas and its by-products. S. Afr. J. Bot. 2023, 163, 250–261. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Udhayakumar, S.; Mani, S.; Dhanapal, M.; Vijaykumar, S.D. Surface modification of Fe2O3 and MgO nanoparticles with agrowastes for the treatment of chlorosis in Glycine max. Nano Converg. 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, R.; Afzaal, M.; Saeed, F.; Samar, N.; Shahbaz, A.; Ateeq, H.; Farooq, M.U.; Akram, N.; Asghar, A.; Rasheed, A.; et al. Bio valorization and industrial applications of ginger waste: A review. Int. J. Food Prop. 2023, 26, 2772–2780. [Google Scholar] [CrossRef]
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S.E. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind. Crops Prod. 2016, 87, 287–296. [Google Scholar] [CrossRef]
- Yang, D.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of Australian herb and spices (garlic, ginger, and onion) and potential antioxidant activity. J. Food Process. Preserv. 2020, 44, e14497. [Google Scholar] [CrossRef]
- Khemariya, P.; Agrawal, A.; Kumar, K.; Tiwari, A.K. Quantitative Analysis of Eugenol in Different Parts of Clove. Int. J. Biotechnol. 2022, 11, 12–23. [Google Scholar] [CrossRef]
- Bao, Y.; Yang, L.; Fu, Q.; Fu, Y.; Tian, Q.; Wang, C.; Huang, Q. The current situation of Zanthoxylum bungeanum industry and the research and application prospect. A review. Fitoterapia 2023, 164, 105380. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, F.; Li, S.; Peng, Z. Effects of pepper (Zanthoxylum bungeanum Maxim.) leaf extract on the antioxidant enzyme activities of salted silver carp (Hypophthalmichthys molitrix) during processing. J. Funct. Foods 2015, 18, 1179–1190. [Google Scholar] [CrossRef]
- Merah, O.; Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Cerny, M.; Grivot, S.; Evon, P.; Hijazi, A. Biochemical Composition of Cumin Seeds, and Biorefining Study. Biomolecules 2020, 10, 1054. [Google Scholar] [CrossRef]
- Maleš, I.; Dragović-Uzelac, V.; Jerković, I.; Zorić, Z.; Pedisić, S.; Repajić, M.; Garofulić, I.E.; Dobrinčić, A. Non-Volatile and Volatile Bioactives of Salvia officinalis L., Thymus serpyllum L. and Laurus nobilis L. Extracts with Potential Use in the Development of Functional Beverages. Antioxidants 2022, 11, 1140. [Google Scholar] [CrossRef]
- Nutrizio, M.; Gajdoš Kljusurić, J.; Badanjak Sabolović, M.; Bursać Kovačević, D.; Šupljika, F.; Putnik, P.; Semenčić Čakić, M.; Dubrović, I.; Vrsaljko, D.; Maltar-Strmečki, N.; et al. Valorization of sage extracts (Salvia officinalis L.) obtained by high voltage electrical discharges: Process control and antioxidant properties. Innov. Food Sci. Emerg. Technol. 2020, 60, 102284. [Google Scholar] [CrossRef]
- Pavlić, B.; Bera, O.; Teslić, N.; Vidović, S.; Parpinello, G.; Zeković, Z. Chemical profile and antioxidant activity of sage herbal dust extracts obtained by supercritical fluid extraction. Ind. Crops Prod. 2018, 120, 305–312. [Google Scholar] [CrossRef]
- Inthalaeng, N.; Gao, Y.; Remón, J.; Dugmore, T.I.J.; Ozel, M.Z.; Sulaeman, A.; Matharu, A.S. Ginger waste as a potential feedstock for a zero-waste ginger biorefinery: A review. RSC Sustain. 2023, 1, 213–223. [Google Scholar] [CrossRef]
- Chen, G.T.; Yuan, B.; Wang, H.X.; Qi, G.H.; Cheng, S.J. Characterization and antioxidant activity of polysaccharides obtained from ginger pomace using two different extraction processes. Int. J. Biol. Macromol. 2019, 139, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, B.C.; Silveira, T.M.G.; Tapia-Blácido, D.R. Starch isolation from turmeric dye extraction residue and its application in active film production. Int. J. Biol. Macromol. 2022, 202, 508–519. [Google Scholar] [CrossRef]
- Patil, S.S.; Rathod, V.K. Simultaneous extraction and partial purification of proteins from spent turmeric powder using ultrasound intensified three phase partitioning and its potential as antidiabetic agent. Chem. Eng. Process—Process Intensif. 2022, 172, 108788. [Google Scholar] [CrossRef]
- Kim, S.; Ko, S.C.; Kim, Y.S.; Ha, S.K.; Park, H.Y.; Park, Y.; Lee, S.H. Determination of Curcuma longa L. (Turmeric) Leaf Extraction Conditions Using Response Surface Methodology to Optimize Extraction Yield and Antioxidant Content. J. Food Qual. 2019, 2019, 7575206. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Mora, Z.V.-d.l.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef]
- Cheikh Rouhou, M.; Abdelmoumen, S.; Atrous, H.; Lung, A.; Vaca-Medina, G.; Raynaud, C.; Ghorbel, D. Pilot scale production of dietary fibers from Tunisian tomato and red pepper by-products. Sustain. Chem. Pharm. 2024, 39, 101521. [Google Scholar] [CrossRef]
- Hussain, A.; Arif, M.R.; Ahmed, A.; Fiaz, I.; Zulfiqar, N.; Ali, M.Q.; Firdous, N.; Fatima, H.; Shehzad, A.; Elkhedir, A.E. Evaluation of Leaves, Flowers, and Seeds of Coriander (Coriandrum sativum L.) through Microwave Drying and Ultrasonic-Assisted Extraction, for Biologically Active Components. J. Food Process. Preserv. 2024, 2024, 2378604. [Google Scholar] [CrossRef]
- Ahmadian-Kouchaksaraie, Z.; Niazmand, R. Supercritical carbon dioxide extraction of antioxidants from Crocus sativus petals of saffron industry residues: Optimization using response surface methodology. J. Supercrit. Fluids 2017, 121, 19–31. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, Y.; Lei, F.; Li, P.; Jiang, J. Efficient co-production of xylo-oligosaccharides and fermentable sugars from sugarcane bagasse by glutamic acid pretreatment. Bioresour. Technol. 2023, 387, 129704. [Google Scholar] [CrossRef]
- Kallel, F.; Ellouz Chaabouni, S. Perspective of garlic processing wastes as low-cost substrates for production of high-added value products: A review. Environ. Prog. Sustain. Energy 2017, 36, 1765–1777. [Google Scholar] [CrossRef]
- Azmat, F.; Imran, A.; Islam, F.; Afzaal, M.; Zahoor, T.; Akram, R.; Aggarwal, S.; Rehman, M.; Naaz, S.; Ashraf, S.; et al. Valorization of the phytochemical profile, nutritional composition, and therapeutic potentials of garlic peel: A concurrent review. Int. J. Food Prop. 2023, 26, 2642–2655. [Google Scholar] [CrossRef]
- Alexandri, M.; Christaki, S.; Gkatzionis, K.; Mourtzinos, I.; Tsouko, E. Residual biomass from major aromatic and medicinal flora of the Mediterranean: Challenges towards sustainable integration into food systems within the circular bioeconomy. Trends Food Sci. Technol. 2023, 139, 104123. [Google Scholar] [CrossRef]
- Areti, H.A.; Muleta, M.D.; Abo, L.D.; Hamda, A.S.; Adugna, A.A.; Edae, I.T.; Daba, B.J.; Gudeta, R.L. Innovative uses of agricultural by-products in the food and beverage sector: A review. Food Chem. Adv. 2024, 5, 100838. [Google Scholar] [CrossRef]
- Hitlamani, V.; Inamdar, A.A. Valorization and the potential use of garlic (Allium sativum L.) skin in food industries. Food Humanit. 2024, 3, 100437. [Google Scholar] [CrossRef]
- Cetó, X.; Sarma, M.; Valle, M.d. Analysis of spices & herbs and its phenolic content by means of an electronic tongue. LWT 2024, 191, 115578. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.-J. Microencapsulated saffron floral waste extracts as functional ingredients for antioxidant fortification of yogurt: Stability during the storage. LWT 2023, 184, 114976. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S. A review on health benefits of phenolics derived from dietary spices. Curr. Res. Food Sci. 2022, 5, 1508–1523. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Wang, Y.; Chen, J.; Huang, Y.; Yan, Y.; Li, L.; Li, Z.; Ren, Y.; Xiao, Y. Total phenolics, capsaicinoids, antioxidant activity, and α-glucosidase inhibitory activity of three varieties of pepper seeds. Int. J. Food Prop. 2020, 23, 1016–1035. [Google Scholar] [CrossRef]
- Edo, G.I.; Nwachukwu, S.C.; Ali, A.B.M.; Yousif, E.; Jikah, A.N.; Zainulabdeen, K.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; et al. A review on the composition, extraction and applications of phenolic compounds. Ecol. Front. 2024, 45, 7–23. [Google Scholar] [CrossRef]
- Tylewicz, U.; Nowacka, M.; Martín-García, B.; Wiktor, A.; Gómez Caravaca, A.M. Target sources of polyphenols in different food products and their processing by-products. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–175. [Google Scholar]
- Nambiraj, M.; Suresh Kumar, K. Impact of essential oils from agro waste as antioxidants on biodiesel long-term storage stability for CI engines: Fresh and aged evaluations. Process Saf. Environ. Prot. 2024, 186, 1214–1228. [Google Scholar] [CrossRef]
- Chhouk, K.; Uemori, C.; Wahyudiono; Kanda, H.; Goto, M. Extraction of phenolic compounds and antioxidant activity from garlic husk using carbon dioxide expanded ethanol. Chem. Eng. Process. Process Intensif. 2017, 117, 113–119. [Google Scholar] [CrossRef]
- Chen, X.; Wei, Z.; Zhu, L.; Yuan, X.; Wei, D.; Peng, W.; Wu, C. Efficient Approach for the Extraction and Identification of Red Pigment from Zanthoxylum bungeanum Maxim and Its Antioxidant Activity. Molecules 2018, 23, 1109. [Google Scholar] [CrossRef]
- Hoseinzadeh Moghaddam, S.; Vatankhah, A.; Armide, N.; Keshavarzi, Z. Revisiting the protective effects of ginger phenolic compounds on the kidneys: A narrative review. Food Humanit. 2024, 3, 100442. [Google Scholar] [CrossRef]
- Nam, D.G.; Kim, M.; Choi, A.J.; Choe, J.S. Health Benefits of Antioxidant Bioactive Compounds in Ginger (Zingiber officinale) Leaves by Network Pharmacology Analysis Combined with Experimental Validation. Antioxidants 2024, 13, 652. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kang, M.-C.; Lee, H.H.L.; Cho, C.H.; Choi, I.; Park, Y.; Lee, S.-H. Antioxidant Effects of Turmeric Leaf Extract against Hydrogen Peroxide-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish. Antioxidants 2021, 10, 112. [Google Scholar] [CrossRef]
- Belyagoubi, L.; Loukidi, B.; Belyagoubi-Benhammou, N.; Gismondi, A.; Di Marco, G.; D’Agostino, A.; Canini, A.; Benmahieddine, A.; Rouigueb, K.; Ben Menni, D.; et al. Valorization of Algerian Saffron: Stigmas and Flowers as Source of Bioactive Compounds. Waste Biomass Valorization 2021, 12, 6671–6683. [Google Scholar] [CrossRef]
- Masala, V.; Jokić, S.; Aladić, K.; Molnar, M.; Tuberoso, C.I.G. Exploring Phenolic Compounds Extraction from Saffron (C. sativus) Floral By-Products Using Ultrasound-Assisted Extraction, Deep Eutectic Solvent Extraction, and Subcritical Water Extraction. Molecules 2024, 29, 2600. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Makris, D.P. Saffron Processing Wastes as a Bioresource of High-Value Added Compounds: Development of a Green Extraction Process for Polyphenol Recovery Using a Natural Deep Eutectic Solvent. Antioxidants 2019, 12, 586. [Google Scholar]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. LC-MS Identification and Quantification of Phenolic Compounds in Solid Residues from the Essential Oil Industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Jasicka-Misiak, I.; Poliwoda, A.; Petecka, M.; Buslovych, O.; Shlyapnikov, V.A.; Wieczorek, P.P. Antioxidant Phenolic Compounds in Salvia officinalis L. and Salvia sclarea L. Ecol. Chem. Eng. 2018, 25, 133–142. [Google Scholar] [CrossRef]
- Ianni, F.; Scandar, S.; Mangiapelo, L.; Blasi, F.; Marcotullio, M.C.; Cossignani, L. NADES-Assisted Extraction of Polyphenols from Coriander Seeds: A Systematic Optimization Study. Antioxidants 2023, 12, 2048. [Google Scholar] [CrossRef]
- Sriti, J.; Bettaieb, I.; Bachrouch, O.; Talou, T.; Marzouk, B. Chemical composition and antioxidant activity of the coriander cake obtained by extrusion. Arab. J. Chem. 2019, 12, 1765–1773. [Google Scholar] [CrossRef]
- Yang, L.C.; Li, R.; Tan, J.; Jiang, Z.T. Polyphenolics composition of the leaves of Zanthoxylum bungeanum maxim. grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 1772–1778. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, X.; Battino, M.; Wei, X.; Shi, J.; Zhao, L.; Liu, S.; Xiao, J.; Shi, B.; Zou, X. A comparative overview on chili pepper (capsicum genus) and sichuan pepper (zanthoxylum genus): From pungent spices to pharma-foods. Trends Food Sci. Technol. 2021, 117, 148–162. [Google Scholar] [CrossRef]
- Zhai, X.X.; Meng, X.H.; Wang, C.B.; Zhao, Y.M.; Yang, J.L. Anti-hypoxic active constituents from the twigs of Zanthoxylum armatum DC. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2022, 104, 104480. [Google Scholar] [CrossRef]
- Ivane, N.M.A.; Wang, W.; Ma, Q.; Wang, J.; Liu, Y.; Sun, J. Antioxidative effects of incorporated ginger peel extracts on beef patties subjected to refrigerated storage. Food Humanit. 2024, 2, 100251. [Google Scholar] [CrossRef]
- Mobin, L.; Haq, M.A.; Ali, R.; Naz, S.; Saeed, S.G. Antibacterial and antioxidant potential of the phenolic extract and its fractions isolated from Allium ascalonicum (onion) peel. Nat. Prod. Res. 2022, 36, 3163–3167. [Google Scholar] [CrossRef]
- Alimi, D.; Hajri, A.; Jallouli, S.; Sebai, H. Toxicity, repellency, and anti-cholinesterase activities of bioactive molecules from clove buds Syzygium aromaticum L. as an ecological alternative in the search for control Hyalomma scupense (Acari: Ixodidae). Heliyon 2023, 9, e18899. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Kaplan, M.; Milošević, S.; Božović, D.; Sknepnek, A.; Miletić, D.; Lazarević Mrkonjić, I.; Rakić, D.; Zeković, Z.; Pavlić, B. Green Extraction Approach for Isolation of Bioactive Compounds in Wild Thyme (Thymus serpyllum L.) Herbal Dust—Chemical Profile, Antioxidant and Antimicrobial Activity and Comparison with Conventional Techniques. Plants 2024, 13, 897. [Google Scholar] [CrossRef]
- Murphy, E.J.F.; Fehrenbach, G.W.; Abidin, I.Z.; Buckley, C.; Montgomery, T.; Pogue, R.; Murray, P.; Major, I.; Rezoagli, E. Polysaccharides—Naturally Occurring Immune Modulators. Polymers 2023, 15, 2373. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Chen, Z.; Yuan, Q.; Ma, M.; Gao, C.; Zhou, Y.; Zhou, H.; Wu, X.; Wu, D.; Farag, M.A.; et al. Ginger polysaccharides relieve ulcerative colitis via maintaining intestinal barrier integrity and gut microbiota modulation. Int. J. Biol. Macromol. 2022, 219, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Li, W.; Xiao, H.; Ji, W.; Li, L.; Zhu, W.; Lan, T.; Zheng, Z. Developing ginger polysaccharide-Cr (III) complexes using ginger peel to provide enhanced in vivo anti-inflammatory activity: Fabrication, structural characterization and activity evaluation. Food Chem. Adv. 2024, 4, 100572. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, M.; Radha; Lorenzo, J.M.; Sharma, D.; Puri, S.; Pundir, A.; Dhumal, S.; Bhuyan, D.J.; Jayanthy, G.; et al. Onion and garlic polysaccharides: A review on extraction, characterization, bioactivity, and modifications. Int. J. Biol. Macromol. 2022, 219, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-H.; Liu, F.; Li, L.-Q.; Jin, M.-Y.; Yu, X.; Liu, X.; Li, Y.; Li, L.; Yan, J.-K. Recent advances in dietary polysaccharides from Allium species: Preparation, characterization, and bioactivity. Int. J. Biol. Macromol. 2024, 277, 134130. [Google Scholar] [CrossRef]
- Sagar, N.A.; Kumar, Y.; Singh, R.; Nickhil, C.; Kumar, D.; Sharma, P.; Om Pandey, H.; Bhoj, S.; Tarafdar, A. Onion waste based-biorefinery for sustainable generation of value-added products. Bioresour. Technol. 2022, 362, 127870. [Google Scholar] [CrossRef]
- Kallel, F.; Driss, D.; Bouaziz, F.; Belghith, L.; Zouari-Ellouzi, S.; Fatma Chaari, F.C.; Haddar, A.; Chaabouni, S.E.; Ghorbel, R. Polysaccharide from garlic straw: Extraction, structural data, biological properties and application to beef meat preservation. RSC Adv. 2015, 5, 6728–6741. [Google Scholar] [CrossRef]
- Aksoylu Özbek, Z.; Kawata, K.; Zhou, H.; Chung, C.; Park, J.H.; McClements, D.J. Isolation and characterization of nettle (Urtica dioica L.) seed proteins: Conversion of underutilized by-products of the edible oil industry into food emulsifiers. Food Chem. 2024, 456, 139878. [Google Scholar] [CrossRef]
- Qin, J.; Liu, L.; Miao, C.; Lan, B.; Liao, T.; Tian, X.; Wu, Z. Impact of Co-60 γ-ray irradiation on the cross-linking and stability of fish collagen: Structural changes and digestibility. Food Hydrocoll. 2024, 157, 110445. [Google Scholar] [CrossRef]
- Assiéné Agamou, J.A.; Djeukeu Asongni, W.; Assiéné Oyong, D.S.; Mbida Mbida, I.Y.; Nyangono Biyegue, C.F. Nutritional and bioactive potentials of a powder and a decoction made from Ceylon cinnamon bark, Laurus nobilis leaves, and Curcuma longa Linn rhizome. Appl. Food Res. 2024, 4, 100436. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Dhiman, A.; Kumar, S.; Suhag, R. Garlic (Allium sativum L.): A review on bio-functionality, allicin’s potency and drying methodologies. S. Afr. J. Bot. 2024, 171, 129–146. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Zadeh, A.N.; Tayefe, M.; Zolqadri, R.; Ramezani, A.; Mazloomi, N.; Sarabandi, K. Biological conversion of red garlic (Allium sativum L) protein to bioactive peptides: ACE- DPP-IV inhibitory, and Techno-functional features. Appl. Food Res. 2024, 4, 100551. [Google Scholar] [CrossRef]
- Chidike Ezeorba, T.P.; Ezugwu, A.L.; Chukwuma, I.F.; Anaduaka, E.G.; Udenigwe, C.C. Health-promoting properties of bioactive proteins and peptides of garlic (Allium sativum). Food Chem. 2024, 435, 137632. [Google Scholar] [CrossRef]
- Deng, Z.; Du, X.; Liu, S.; Xiong, Y.; Wang, Y.; Rao, L.; Liu, M.; Zhao, L.; Liao, X. Modification of pepper seed protein isolate to improve its functional characteristic by high hydrostatic pressure. Food Chem. 2025, 464, 141594. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Liu, X.; Liu, Y.; Hou, A.; Wang, Y.; Li, L.; Peng, X.; Xiao, Y. Discrimination and characterization of volatile organic compounds and nutritional values of three varieties of chopped pepper seeds. Food Chem. 2024, 21, 101150. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yang, H.-J.; Lee, K.-Y.; Song, K.B. Physical properties and application of a red pepper seed meal protein composite film containing oregano oil. Food Hydrocoll. 2016, 55, 136–143. [Google Scholar] [CrossRef]
- Golubkina, N.; Caruso, G. Onion. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–87. [Google Scholar]
- Mazou Ma, D.A.J.; Tchobo, F.P.; Villeneuve, P.B.; Soumanou, M.M. Catalytic properties of lipase from Ficus trichopoda and Euphorbia unispina latex: Study of their typoselectivity. J. Appl. Biosci. 2017, 110, 10790–10801. [Google Scholar] [CrossRef][Green Version]
- Makris, D.P. Recovery and applications of enzymes from food wastes. In Food Waste Recovery; Elsevier: Amsterdam, The Netherlands, 2015; pp. 361–379. [Google Scholar]
- Prathapan, A.; Lukhman, M.; Arumughan, C.; Sundaresan, A.; Raghu, K.G. Effect of heat treatment on curcuminoid, colour value and total polyphenols of fresh turmeric rhizome. Int. J. Food Sci. Technol. 2009, 44, 1438–1444. [Google Scholar] [CrossRef]
- Yasin, M.; Li, L.; Donovan-Mak, M.; Chen, Z.H.; Panchal, S.K. Capsicum Waste as a Sustainable Source of Capsaicinoids for Metabolic Diseases. Foods 2023, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, Y.S.; Mahnashi, M.H.; Alyami, B.A.; Alqarni, A.O.; Huneif, M.A.; Nahari, M.H.; Ali, A.; Javed, Q.; Ilyas, H.; Rafiq, M. Preparation of Spice Extracts: Evaluation of Their Phytochemical, Antioxidant, Antityrosinase, and Anti-α-Glucosidase Properties Exploring Their Mechanism of Enzyme Inhibition with Antibrowning and Antidiabetic Studies In Vivo. BioMed Res. Int. 2022, 2022, 9983124. [Google Scholar] [CrossRef]
- Topčagić, A.; Ćavar Zeljković, S.; Kezić, M.; Sofić, E. Fatty acids and phenolic compounds composition of anise seed. J. Food Process. Preserv. 2022, 46, e15872. [Google Scholar] [CrossRef]
- Li, Y.x.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.-T.; Feng, Y.-X.; Zhang, D.; Guo, S.-S.; Pang, X.; Geng, Z.-F.; Xi, C.; Du, S.-S. Comparative evaluation of the chemical composition and bioactivities of essential oils from four spice plants (Lauraceae) against stored-product insects. Ind. Crops Prod. 2019, 140, 111640. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, Y.; Zhang, J.; Li, M.; Yan, L.; Zhao, G.; Liu, Y.; Zhang, Y. The inhibitory effects of spice essential oils and rapidly prediction on the growth of Clostridium perfringens in cooked chicken breast. Food Control 2020, 113, 106978. [Google Scholar] [CrossRef]
- Khan, S.; Sahar, A.; Tariq, T.; Sameen, A.; Tariq, F. Essential oils in plants: Plant physiology, the chemical composition of the oil, and natural variation of the oils (chemotaxonomy and environmental effects, etc.). In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–36. [Google Scholar]
- Kumar Pandey, V.; Shams, R.; Singh, R.; Dar, A.H.; Pandiselvam, R.; Rusu, A.V.; Trif, M. A comprehensive review on clove (Caryophyllus aromaticus L.) essential oil and its significance in the formulation of edible coatings for potential food applications. Front. Nutr. 2022, 9, 987674. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Salehi, S.; Craker, L. Effect of drying methods on qualitative and quantitative properties of essential oil from the aerial parts of coriander. J. Appl. Res. Med. Aromat. Plants 2017, 4, 35–40. [Google Scholar] [CrossRef]
- Goswami, A.; Ballabh, L.; Debasmita, D.; Mitra, A. Spectral lights influence growth and metabolic efficiency leading to enhanced phytochemical contents of Coriandrum sativum L. Environ. Exp. Bot. 2024, 228, 106021. [Google Scholar] [CrossRef]
- Rawat, A.; Kholiya, S.; Chauhan, A.; Kumar, D.; Venkatesha, K.T.; Upadhyay, R.K.; Padalia, R.C. Chemical composition of the essential oil from different plant parts of Zingiber zerumbet Sm. grown in the foothills of Uttarakhand. Biochem. Syst. Ecol. 2023, 108, 104627. [Google Scholar] [CrossRef]
- Gatabazi, A.; Marais, D.; Steyn, M.; Araya, H.; du Plooy, C.; Ncube, B.; Mokgehle, S. Effect of water regimes and harvest times on yield and phytochemical accumulation of two ginger species. Sci. Hortic. 2022, 304, 111353. [Google Scholar] [CrossRef]
- Rasool, N.; Saeed, Z.; Pervaiz, M.; Ali, F.; Younas, U.; Bashir, R.; Bukhari, S.M.; Mahmood Khan, R.R.; Jelani, S.; Sikandar, R. Evaluation of essential oil extracted from ginger, cinnamon and lemon for therapeutic and biological activities. Biocatal. Agric. Biotechnol. 2022, 44, 102470. [Google Scholar] [CrossRef]
- Praveen, A.; Hitlamani, V.; Nagarajan, S.; Matche, R.S.; Chaudhari, S.R. Enrichment of Peanut butter using Curcuma Longa (turmeric) industrial byproducts and its impact on shelf life. Food Chem. 2024, 461, 140839. [Google Scholar] [CrossRef]
- Kita, A.; Rune, C.J.B.; Giacalone, D.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A.; Nemś, A.; Kolniak-Ostek, J.; Michalska-Ciechanowska, A.; Mora, M.; Romeo-Arroyo, E.; et al. Understanding consumer perception of a new product including apple pomace: The role of health, sustainability, and culinary engagement. Int. J. Gastron. Food Sci. 2024, 38, 101037. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, Y.; Xiao, H.; Mariga, A.M.; Chen, Y.; Zhang, X.; Wang, L.; Li, D.; Li, L.; Luo, Z. Rethinking of botanical volatile organic compounds applied in food preservation: Challenges in acquisition, application, microbial inhibition and stimulation. Trends Food Sci. Technol. 2022, 125, 166–184. [Google Scholar] [CrossRef]
- Basdeki, E.; Vasilaki, S.E.; Sensi, M.; Flemetakis, E.; Biscarini, F.; Power, D.; Tsironi, T. Reviewing the Correlation of Fish Quality Alteration and In-Package Headspace Composition: Evidence From a pH Freshness Indicator Case Study. Int. J. Food Sci. 2025, 2025, 3576183. [Google Scholar] [CrossRef]
- Guddi, K.; Vijay Chithra, G.; Bhavani, R.; Naik, S.; Sarkar, A. Pigments and paints from wastes. In Processing of Biomass Waste; Elsevier: Amsterdam, The Netherlands, 2024; pp. 233–243. [Google Scholar]
- Sharma, R.; Ghoshal, G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; Regueiro, J.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P.F. Increasing the Added-Value of Onions as a Source of Antioxidant Flavonoids: A Critical Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1050–1062. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Nile, S.H.; Lee, E.T.; Lee, Y.R. Economical and environmentally-friendly approaches for usage of onion (Allium cepa L.) waste. Food Funct. 2016, 7, 3354–3369. [Google Scholar] [CrossRef]
- Abdian, S.; Fakhri, S.; Moradi, S.Z.; Khirehgesh, M.R.; Echeverría, J. Saffron and its major constituents against neurodegenerative diseases: A mechanistic review. Phytomedicine 2024, 135, 156097. [Google Scholar] [CrossRef]
- Chouaibi, M.; Rezig, L.; Hamdi, S.; Ferrari, G. Chemical characteristics and compositions of red pepper seed oils extracted by different methods. Ind. Crops Prod. 2019, 128, 363–370. [Google Scholar] [CrossRef]
- Tehrani, M.; Navayee, T. Suitability of dyes from cinnamon bark on wool fibers using metal and bio-mordants. Environ. Sci. Pollut. Res. 2024, 31, 31414–31423. [Google Scholar] [CrossRef]
- Moulick, S.P.; Jahan, F.; Mamun, M.Z.U.A.; Hossain, M.I.S.; Waliullah, M.; Sathee, R.A. Analysis of indigenous spices widely consumed in Bangladesh: An assessment to explore its proximate contents, minerals, phytochemical compositions, and antioxidant activities. J. Agric. Food Res. 2023, 14, 100720. [Google Scholar] [CrossRef]
- Cvetković, T.; Ranilović, J.; Jokić, S. Quality of Pepper Seed By-Products: A Review. Foods 2022, 11, 748. [Google Scholar] [CrossRef]
- Mondal, P.; Ramasamy, S.; Amalraj, R.; Anthoniraj, C.J.; Gokila, S.; Meeran, S.M. Physicochemical characteristics of dietary fiber polysaccharides extracted from Murraya koenigii leaves and their functional role on gut homeostasis. Int. J. Biol. Macromol. 2025, 293, 139198. [Google Scholar] [CrossRef]
- Huang, W.; He, X.; Wu, J.; Ma, X.; Han, J.; Wang, L.; Wang, Y. The evaluation of deep eutectic solvents and ionic liquids as cosolvents system for improving cellulase properties. Ind. Crops Prod. 2023, 197, 116555. [Google Scholar] [CrossRef]
- Liaqat, F.; Xu, L.; Khazi, M.I.; Ali, S.; Rahman, M.U.; Zhu, D. Extraction, purification, and applications of vanillin: A review of recent advances and challenges. Ind. Crops Prod. 2023, 204, 117372. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. In Green Chemistry; Oxford University PressOxford: Oxford, UK, 2000; pp. 29–56. [Google Scholar]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Negi, T.; Kumar, A.; Sharma, S.K.; Rawat, N.; Saini, D.; Sirohi, R.; Prakash, O.; Dubey, A.; Dutta, A.; Shahi, N.C. Deep eutectic solvents: Preparation, properties, and food applications. Heliyon 2024, 10, e28784. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Cheng, H.-W.; Chang, K.-W.; Shiue, J.; Wang, J.-K.; Wang, Y.-L.; Huang, N.-T. A particle-based microfluidic molecular separation integrating surface-enhanced Raman scattering sensing for purine derivatives analysis. Microfluid. Nanofluidics 2019, 23, 1–11. [Google Scholar] [CrossRef]
- Xu, Y.; Jiao, Y.; Luo, J.; He, Z.; Zeng, M.; Shen, Q.; Chen, J.; Quan, W. The Influence of Deep Eutectic Solvents Extract from Ginger on the Formation of Heterocyclic Amines and Advanced Glycation End Products in Roast Beef Patties. Foods 2022, 11, 3161. [Google Scholar] [CrossRef]
- Julshahril, N.H.; Phuah, E.T.; Rambli, M.M. Deep eutectic solvents in the extraction of bioactive compounds in agri-food industry. Food Humanit. 2025, 4, 100468. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Flavourings and Processing Aids (CEF). Scientific opinion on recent developments in the risk assessment of chemicals in food and their potential impact on the safety assessment of substances used in food contact materials. EFSA J. 2016, 14, 4357. [Google Scholar] [CrossRef]
- Committee, E.S. Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements. EFSA J. 2009, 7, 1249. [Google Scholar] [CrossRef]
- ISO 15161:2001; Guidelines on the Application of ISO 9001:2000 for the Food and Drink Industry. International Organization for Standardization: Geneva, Switzerland, 2001.
- Lou, W.; Huang, Z.; Shao, Q.; Shan, Y.; Shi, D.; Chen, Z.; Zhang, J.; Yu, W.; Wang, J.; Yang, H.; et al. Recent advances in active packaging: Insights into novel functional elements, response strategies and applications for food preservation. Food Packag. Shelf Life 2025, 49, 101489. [Google Scholar] [CrossRef]
- CEN/TS 14234:2002; Materials and Articles in Contact with Foodstuffs—Polymeric Coatings on Paper and Board—Guide to the Selection of Conditions and Test Methods for Overall Migration. European Committee for Standardization: Brussels, Belgium, 2002.
- Gooderham, N.J.; Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Rosol, T.J.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS assessment of natural flavor complexes: Clove, cinnamon leaf and West Indian bay leaf-derived flavoring ingredients. Food Chem. Toxicol. 2020, 145, 111585. [Google Scholar] [CrossRef] [PubMed]
- Sulejmanović, M.; Panić, M.; Redovniković, I.R.; Milić, N.; Drljača, J.; Damjanović, A.; Vidović, S. Sustainable isolation of ginger (Zingiber officinale) herbal dust bioactive compounds with favorable toxicological profile employing natural deep eutectic solvents (NADES). Food Chem. 2025, 464, 141545. [Google Scholar] [CrossRef] [PubMed]
- Tzani, A.; Kalafateli, S.; Tatsis, G.; Bairaktari, M.; Kostopoulou, I.; Pontillo, A.R.N.; Detsi, A. Natural Deep Eutectic Solvents (NaDESs) as Alternative Green Extraction Media for Ginger (Zingiber officinale Roscoe). Sustain. Chem. 2021, 2, 576–598. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Gürkan, R. Preparation and application of alcohol based deep eutectic solvents for extraction of curcumin in food samples prior to its spectrophotometric determination. Food Chem. 2020, 310, 125933. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, Y.; He, Q. Cocktail enzyme-assisted natural deep eutectic solvent for enhanced extraction of capsaicin from chili peppers: Mechanism exploration based on multi-experiments and molecular dynamic simulation. Food Chem. 2025, 465, 141959. [Google Scholar] [CrossRef] [PubMed]
- Sulejmanović, M.; Jerković, I.; Zloh, M.; Nastić, N.; Milić, N.; Drljača, J.; Jokić, S.; Aladić, K.; Vidović, S. Supercritical fluid extraction of ginger herbal dust bioactives with an estimation of pharmacological potential using in silico and in vitro analysis. Food Biosci. 2024, 59, 104074. [Google Scholar] [CrossRef]
- Barlow, S.M. Risk assessment of food-contact materials: Past experience and future challenges. Food Addit. Contam. Part. 2009, 26, 1526–1533. [Google Scholar] [CrossRef]
- Shawky, E.; Zhu, W.; Tian, J. A review of innovative extraction technologies for protein recovery from plant-based by-products: A step toward zero-waste processing. Int. J. Biol. Macromol. 2025, 315, 144301. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Barros, L.; Rocchetti, G.; Lucini, L.; Tanase, C.; Mocan, A.; Bunea, C.I.; Crișan, G. Bioaccessibility and bioactive potential of different phytochemical classes from nutraceuticals and functional foods. Front. Nutr. 2023, 10, 1184535. [Google Scholar] [CrossRef]
- Li, H.; Nunekpeku, X.; Adade, S.Y.-S.S.; Sheng, W.; Kwadzokpui, B.A.; Ahlivia, E.B.; Chen, Q. Phenolic compounds detection and quantification in whole grains: A comprehensive review of recent advancements in analytical methods. TrAC Trends Anal. Chem. 2025, 187, 118215. [Google Scholar] [CrossRef]
- Pedro, A.C.; Maciel, G.M.; Lima, N.P.; Lima, N.F.; Ribeiro, I.S.; Pinheiro, D.F.; Haminiuk, C.W.I. Valorization of bioactive compounds from juice industry waste: Applications, challenges, and future prospects. Trends Food Sci. Technol. 2024, 152, 104693. [Google Scholar] [CrossRef]
- Klojdová, I.; Milota, T.; Smetanová, J.; Stathopoulos, C. Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds? Pharmaceuticals 2023, 16, 362. [Google Scholar] [CrossRef]
- Álvarez, C.; Pando, D. Encapsulation Technologies Applied to Food Processing. In Food Formulation; Wiley: Hoboken, NJ, USA, 2021; pp. 121–145. [Google Scholar]
- Nsairat, H.; Alshaer, W.; Odeh, F.; Esawi, E.; Khater, D.; Bawab, A.A.; El-Tanani, M.; Awidi, A.; Mubarak, M.S. Recent advances in using liposomes for delivery of nucleic acid-based therapeutics. OpenNano 2023, 11, 100132. [Google Scholar] [CrossRef]
- Shen, X.; He, L.; Cui, Y.; Lin, Z.; Jafari, S.M.; Tan, C. Co-encapsulation of bioactive compounds in liposomal delivery systems for synergistic effects. Food Biosci. 2025, 68, 106306. [Google Scholar] [CrossRef]
- Samuel, M.; Prabhat, T.S.; Emisha, L.; Ganesan, J.J.; Issac, R.; Basavegowda, N.; Baek, K.-H.; Haldar, D. Biodegradable composite films of barley fibers for food packaging applications: A review. Int. J. Biol. Macromol. 2025, 295, 139611. [Google Scholar] [CrossRef]
- Khan, S.; Hashim, S.B.H.; Arslan, M.; Zhang, K.; Bilal, M.; Zhiyang, C.; Zhihua, L.; Tahir, H.E.; Zhai, X.; Shishir, M.R.I.; et al. Berry wax improves the physico-mechanical, thermal, water barrier properties and biodegradable potential of chitosan food packaging film. Int. J. Biol. Macromol. 2024, 261, 129821. [Google Scholar] [CrossRef]
- Lv, Y.; Yue, H.; Tan, C.; Liao, H. Characterization of a novel biodegradable active film with rose polyphenol extract and its application in edible oil packaging. Food Biosci. 2025, 63, 105784. [Google Scholar] [CrossRef]
- Shi, X.; Yang, Y.; Miao, W.; Duan, Q.; Huang, Y.; Xiao, H.; Li, C. Active biodegradable bacterial cellulose films with potential to minimize the plastic pollution: Preparation, antibacterial application, and mechanism. Food Chem. 2025, 464, 141852. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Shi, Y.; Sun, J.; Lu, X.; Zhang, H.; Li, Y.; Fu, Y.; Guo, J.; Wang, Q.; Liu, H.; et al. Sawdust-derived cellulose nanofibrils with high biosafety for potential bioprinting. Ind. Crops Prod. 2024, 209, 118025. [Google Scholar] [CrossRef]
- Murtaza, S.; Sanabil; Shah, F.-u.H.; Shahbaz, M.; Murtaza, M.A.; Farooq, U.; Ma, Y. Starch-nanostructured-based active packaging for food applications. In Starch Based Nanomaterials for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2024; pp. 103–160. [Google Scholar]
- Serrano, L.; Marín, F.; Gonzalo, A.; Labidi, J. Integral use of pepper stems. Ind. Crops Prod. 2012, 40, 110–115. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rhim, J.-W. Extraction and Characterization of Cellulose Microfibers from Agricultural Wastes of Onion and Garlic. J. Nat. Fibers 2018, 15, 465–473. [Google Scholar] [CrossRef]
- Yeganeh, F.; Chiewchan, N.; Chonkaew, W. Hydrothermal pretreatment of biomass-waste-garlic skins in the cellulose nanofiber production process. Cellulose 2022, 29, 2333–2349. [Google Scholar] [CrossRef]
- Jaiswal, D.; Devnani, G.L.; Rajeshkumar, G.; Sanjay, M.R.; Siengchin, S. Review on extraction, characterization, surface treatment and thermal degradation analysis of new cellulosic fibers as sustainable reinforcement in polymer composites. Curr. Res. Green. Sustain. Chem. 2022, 5, 100271. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Zhang, T.; Zhu, Y.; Qiu, F. Fabrication of recyclable magnetic double-base aerogel with waste bioresource bagasse as the source of fiber for the enhanced removal of chromium ions from aqueous solution. Food Bioprod. Process. 2020, 119, 257–267. [Google Scholar] [CrossRef]
- Bellesia, T.; Carullo, D.; Fachin, A.; Caneva, E.; Farris, S. A soft processing technology for the extraction of cellulose from plant residues and agri-food wastes. Food Biosci. 2024, 62, 105141. [Google Scholar] [CrossRef]
- Saedi, S.; Kim, J.T.; Lee, E.H.; Kumar, A.; Shin, G.H. Fully transparent and flexible antibacterial packaging films based on regenerated cellulose extracted from ginger pulp. Ind. Crops Prod. 2023, 197, 116554. [Google Scholar] [CrossRef]
- Li, J.; Yu, Z.; Ma, X.; Zhang, X.; Yue, W. Biomass-derived heteroatom-doped carbons: Unlocking the potential of cellulose, hemicellulose, and lignin co-pyrolysis with ammonium polyphosphate for high-performance supercapacitors. J. Ind. Eng. Chem. 2024, 146, 238–251. [Google Scholar] [CrossRef]
- Menossi, M.; Misra, M.; Mohanty, A.K. Biodegradable cellulose ester blends: Studies, compatibilization, biodegradable behavior, and applications. A review. Prog. Polym. Sci. 2024, 160, 101919. [Google Scholar] [CrossRef]
- Kallel, F.; Driss, D.; Chaari, F.; Belghith, L.; Bouaziz, F.; Ghorbel, R.; Chaabouni, S.E. Garlic (Allium sativum L.) husk waste as a potential source of phenolic compounds: Influence of extracting solvents on its antimicrobial and antioxidant properties. Ind. Crops Prod. 2014, 62, 34–41. [Google Scholar] [CrossRef]
- Salim, M.H.; Kassab, Z.; Abdellaoui, Y.; García-Cruz, A.; Soumare, A.; Ablouh, E.h.; El Achaby, M. Exploration of multifunctional properties of garlic skin derived cellulose nanocrystals and extracts incorporated chitosan biocomposite films for active packaging application. Int. J. Biol. Macromol. 2022, 210, 639–653. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Y.; Zhong, Y.; Wang, D.; Deng, Y.; Meng, F.; Li, Y.; Zhang, M.; Zhang, C. Preparation of garlic stem cellulose nanocrystal/leaf extract/chitosan film for black garlic preservation by electrostatic spraying. Int. J. Biol. Macromol. 2023, 225, 518–525. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Pratama, A.B.; Fajri, N.; Sapuan, S.M.; Ilyas, R.A. Transparent and antimicrobial cellulose film from ginger nanofiber. Food Hydrocoll. 2020, 98, 105266. [Google Scholar] [CrossRef]
- Zendrato, H.M.; Masruchin, N.; Nikmatin, S.; Wistara, N.J. Effective cellulose isolation from torch ginger stem by alkaline hydrogen peroxide–Peracetic acid system. J. Ind. Eng. Chem. 2024, 131, 376–387. [Google Scholar] [CrossRef]
- Jacob, J.; Peter, G.; Thomas, S.; Haponiuk, J.T.; Gopi, S. Chitosan and polyvinyl alcohol nanocomposites with cellulose nanofibers from ginger rhizomes and its antimicrobial activities. Int. J. Biol. Macromol. 2019, 129, 370–376. [Google Scholar] [CrossRef]
- Sembada, A.A. Delignification of Cinnamon Bark (Cinnamomum verum) with Pre-treatment by NaOH to Increase Cellulose and Hemicellulose Recovery. Quagga J. Pendidik. Dan. Biol. 2022, 14, 73–76. [Google Scholar] [CrossRef]
- Moaveni, R.; Ghane, M.; Soltani, P.; Zamani, A.; Ramamoorthy, S.K. Production of Polymeric Films from Orange and Ginger Waste for Packaging Application and Investigation of Mechanical and Thermal Characteristics of Biofilms. Appl. Sci. 2024, 14, 4670. [Google Scholar] [CrossRef]
- Yue, P.; Hu, Y.; Yang, Z.; Peng, F.; Yang, L. Renewable and functional composite film from epoxidized Eucommia ulmoides gum and industrial lignin. Ind. Crops Prod. 2023, 194, 116381. [Google Scholar] [CrossRef]
- Pei, J.; Palanisamy, C.P.; Srinivasan, G.P.; Panagal, M.; Kumar, S.S.D.; Mironescu, M. A comprehensive review on starch-based sustainable edible films loaded with bioactive components for food packaging. Int. J. Biol. Macromol. 2024, 274, 133332. [Google Scholar] [CrossRef]
- Abdalla, G.; Mussagy, C.U.; Sant’Ana Pegorin Brasil, G.; Scontri, M.; da Silva Sasaki, J.C.; Su, Y.; Bebber, C.; Rocha, R.R.; de Sousa Abreu, A.P.; Goncalves, R.P.; et al. Eco-sustainable coatings based on chitosan, pectin, and lemon essential oil nanoemulsion and their effect on strawberry preservation. Int. J. Biol. Macromol. 2023, 249, 126016. [Google Scholar] [CrossRef]
- Gupta, D.; Lall, A.; Kumar, S.; Patil, T.D.; Gaikwad, K.K. Plant-based edible films and coatings for food-packaging applications: Recent advances, applications, and trends. Sustain. Food Technol. 2024, 2, 1428–1455. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants: A comprehensive review. Arch Toxicol 2025, 99, 1893–1997. [Google Scholar] [CrossRef]
- Nain, N.; Katoch, G.K.; Kaur, S.; Rasane, P. Recent Developments in Edible Coatings for Fresh Fruits and Vegetables. J. Hortic. Res. 2021, 29, 127–140. [Google Scholar] [CrossRef]
- Ungureanu, C.; Tihan, G.; Zgârian, R.; Pandelea, G. Bio-Coatings for Preservation of Fresh Fruits and Vegetables. Coatings 2023, 13, 1420. [Google Scholar] [CrossRef]
- Chidambaram, R.; Bedi, J. Bioedible coating of meat using garlic, cinnamon and turmeric. Eur. J. Exp. Biol. 2012, 2, 1439–1443. [Google Scholar]
- Saavedra, S.C.; Ventura-Aguilar, R.I.; Bautista-Baños, S.; Barrera-Necha, L.L. Biodegradable chitosan coating for improving quality and controlling Alternaria alternata growth in figs. World J. Adv. Res. Rev. 2020, 2020, 2581–9615. [Google Scholar] [CrossRef]
- Ding, F.; Long, S.; Huang, X.; Shi, J.; Povey, M.; Zou, X. Emerging Pickering emulsion films for bio-based food packaging applications. Food Packag. Shelf Life 2024, 42, 101242. [Google Scholar] [CrossRef]
- Ding, J.-w.; Zhou, E.-m.; Wang, X.; Jiang, H.; Su, H.-f.; Gao, Q.; Guo, L.-n.; Fu, Y.-s.; Li, M.-c.; Li, D.-q.; et al. Cellulose nanocrystals-based Pickering emulsion with enhanced foliar adhesion and pH responsiveness for intelligent delivery of pesticides. Int. J. Biol. Macromol. 2025, 286, 138192. [Google Scholar] [CrossRef]
- Ramos, G.V.C.; Ramírez-López, S.; Pinho, S.C.d.; Ditchfield, C.; Moraes, I.C.F. Starch-Based Pickering Emulsions for Bioactive Compound Encapsulation: Production, Properties, and Applications. Processes 2025, 13, 342. [Google Scholar] [CrossRef]
- Yu, X.; Li, X.; Ma, S.; Wang, Y.; Zhu, W.; Wang, H. Biomass-Based, Interface Tunable, and Dual-Responsive Pickering Emulsions for Smart Release of Pesticides. Adv. Funct. Mater. 2023, 33, 2214911. [Google Scholar] [CrossRef]
- Liu, L.; Ode Boni, B.O.; Ullah, M.W.; Qi, F.; Li, X.; Shi, Z.; Yang, G. Cellulose: A promising and versatile Pickering emulsifier for healthy foods. Food Rev. Int. 2023, 39, 7081–7111. [Google Scholar] [CrossRef]
- Yupanqui-Mendoza, S.L.; Dias, I.K.; dos Santos, J.C.; Arantes, V. A novel approach for producing stable cellulose nanocrystal colloidal suspensions via hydrodynamic cavitation. Chem. Eng. Process. Process Intensif. 2025, 209, 110189. [Google Scholar] [CrossRef]
- Song, L.; Hou, F.; Yi, F.; Zhan, S.; Chen, X.; Han, X.; Zhang, R.; Liu, Z. Sustained-release film prepared by incorporation of cinnamon essential oil: Physicochemical properties and application in the preservation of mushroom (Agaricus bisporus). J. Stored Prod. Res. 2024, 105, 102253. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Hunyadi, A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef]
- Nid Ahmed, M.; Abourat, K.; Gagour, J.; Sakar, E.H.; Majourhat, K.; Koubachi, J.; Gharby, S. Valorization of saffron (Crocus sativus L.) stigma as a potential natural antioxidant for soybean (Glycine max L.) oil stabilization. Heliyon 2024, 10, e25875. [Google Scholar] [CrossRef]
- de Oliveira, I.; Santos-Buelga, C.; Aquino, Y.; Barros, L.; Heleno, S.A. New frontiers in the exploration of phenolic compounds and other bioactives as natural preservatives. Food Biosci. 2025, 68, 106571. [Google Scholar] [CrossRef]
- Nurul, M.; Hawin, N.; Poncojari, W.; Wahyu, P. Phenolic compound and antioxidant activity of Indonesian ginger leaves (Zingiber officinale Roscoe var Roscoe). GSC Biol. Pharm. Sci. 2023, 22, 371–375. [Google Scholar] [CrossRef]
- Hiemori-Kondo, M.; Ueta, R.; Nagao, K. Improving deer meat palatability by treatment with ginger and fermented foods: A deer meat heating study. Int. J. Gastron. Food Sci. 2022, 29, 100577. [Google Scholar] [CrossRef]
- Oluba, O.M.; Edeh, D.A.; Ojeaburu, S.I.; Bayo-Olorunmeke, O.A.; Josiah, S.J. Physicochemical and thermal characterization and antioxidant property of chicken feather keratin and ginger starch hybrid nanocomposite film. Carbohydr. Polym. Technol. Appl. 2023, 6, 100368. [Google Scholar] [CrossRef]
- Thivya, P.; Bhosale, Y.K.; Anandakumar, S.; Hema, V.; Sinija, V.R. Study on the characteristics of gluten/alginate-cellulose/onion waste extracts composite film and its food packaging application. Food Chem. 2022, 390. [Google Scholar] [CrossRef]
- Elattar, M.M.; Darwish, R.S.; Hammoda, H.M.; Dawood, H.M. An ethnopharmacological, phytochemical, and pharmacological overview of onion (Allium cepa L.). J. Ethnopharmacol. 2024, 324, 117779. [Google Scholar] [CrossRef]
- Pirsa, S.; Bener, M.; Şen, F.B. Biodegradable film of carboxymethyl cellulose modified with red onion peel powder waste and boron nitride nanoparticles: Investigation of physicochemical properties and release of active substances. Food Chem. 2024, 445, 138721. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Olgun, E.O.; Canli, O.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Optimization of antioxidants recovery from wild thyme (Thymus serpyllum L.) by ultrasound-assisted extraction: Multi-response approach. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100333. [Google Scholar] [CrossRef]
- Lee, J.G.; Chae, Y.; Shin, Y.; Kim, Y.J. Chemical composition and antioxidant capacity of black pepper pericarp. Appl. Biol. Chem. 2020, 63, 1–9. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Kim, H.-W.; Lee, M.-K.; Kim, H.-J.; Kim, J.-B.; Choe, J.-S.; Lee, Y.-M.; Jang, H.-H. Antioxidant and Anti-Inflammatory Activities in Relation to the Flavonoids Composition of Pepper (Capsicum annuum L.). Antioxidants 2020, 9, 986. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Liu, Y.; Tang, Y.; Che, Z.; Wu, T. Chemical profiles and screening of potential α-glucosidase inhibitors from Sichuan pepper using ultra-filtration combined with UHPLC-Q-TOF. Ind. Crops Prod. 2020, 143, 111874. [Google Scholar] [CrossRef]
- Eden, W.T.; Alighiri, D.; Wijayati, N.; Mursiti, S. Synthesis of Chalcone Derivative from Clove Leaf Waste as a Natural Antioxidant. Pharm. Chem. J. 2021, 55, 269–274. [Google Scholar] [CrossRef]
- Pagliari, S.; Forcella, M.; Lonati, E.; Sacco, G.; Romaniello, F.; Rovellini, P.; Fusi, P.; Palestini, P.; Campone, L.; Labra, M.; et al. Antioxidant and Anti-Inflammatory Effect of Cinnamon (Cinnamomum verum J. Presl) Bark Extract after In Vitro Digestion Simulation. Foods 2023, 12, 452. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Leyva, J.M.; Ortega-Ramírez, L.A.; Carrazco-Lugo, D.K.; Pérez-Carlón, J.J.; Melgarejo-Flores, B.G.; González-Aguilar, G.A.; Miranda, M.R.A. Pectin-cinnamon leaf oil coatings add antioxidant and antibacterial properties to fresh-cut peach. Flavour. Fragr. J. 2013, 28, 39–45. [Google Scholar] [CrossRef]
- Ferraz, M.C.; Procópio, F.R.; de Figueiredo Furtado, G.; Munhoz Moya, A.M.T.; Cazarin, C.B.B.; Hubinger, M.D. Cinnamon and paprika oleoresin emulsions: A study of physicochemical stability and antioxidant synergism. Food Res. Int. 2021, 150, 110777. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, A.; Mahmoudi, H.; Zaouali, W.; M’Rabet, Y.; Casabianca, H.; Hosni, K. Enzyme-aided release of bioactive compounds from coriander (Coriandrum sativum L.) seeds and their residue by-products and evaluation of their antioxidant activity. J. Food Sci. Technol. 2018, 55, 3065–3076. [Google Scholar] [CrossRef]
- Alkass, J.E.; Baker, I.A.; Saleh, H.H.; Alkass, J.E.; Saleh, H.H.; Baker, I.A.; Saleh, H.H. Reduction of oxidative rancidity and microbial activities of the Karadi lamb patties in freezing storage using natural antioxidant extracts of Rosemary and Ginger. Int. J. Agric. Food Res. 2013, 2, 31–42. [Google Scholar] [CrossRef]
- Yemenicioğlu, A. Recent developments shaping the future of antimicrobial edible food packaging: A review. Int. J. Food Sci. Technol. 2024, 59, 9646–9665. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Zhang, W.; Zhang, L.; Zhao, H.; Wang, S.; Huang, C. Recent Advances in Sustainable Antimicrobial Food Packaging: Insights into Release Mechanisms, Design Strategies, and Applications in the Food Industry. J. Agric. Food Chem. 2023, 71, 11806–11833. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Mulati, A.; Yang, Y.; Wang, J. Antimicrobial activity and mechanism of food-medicine homology in food preservation: A review. Food Control 2025, 178, 111507. [Google Scholar] [CrossRef]
- Chau, T.P.; Saravanan, M.; Al-Ansari, M.M.; Al- Dahmash, N.D.; Kuriakose, L.L.; Sindhu, R. Antimicrobial and biocompatibility nature of methanol extract of Lannea coromandelica bark and edible coating film preparation for fruit preservation. Environ. Res. 2024, 243, 117861. [Google Scholar] [CrossRef]
- Ru, Y.; Zhu, Y.; Wang, X.; Dong, Q.; Ma, Y. Edible antimicrobial yeast-based coating with basil essential oil for enhanced food safety. Innov. Food Sci. Emerg. Technol. 2024, 93, 103612. [Google Scholar] [CrossRef]
- Ali, H.; Dixit, S.; Almutairi, B.O.; Yadav, N. Synthesis and characterization of eco-friendly TiO2 nanoparticle from combine extract of onion and garlic peel. J. King Saud. Univ. Sci. 2023, 35, 102918. [Google Scholar] [CrossRef]
- Merlo, T.C.; Contreras-Castillo, C.J.; Saldaña, E.; Barancelli, G.V.; Dargelio, M.D.B.; Yoshida, C.M.P.; Ribeiro Junior, E.E.; Massarioli, A.; Venturini, A.C. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019, 125, 108633. [Google Scholar] [CrossRef]
- Nguyen, Q.-D.; Tran, T.T.V.; Nguyen, N.-N.; Nguyen, T.-P.; Lien, T.-N. Preparation of gelatin/carboxymethyl cellulose/guar gum edible films enriched with methanolic extracts from shallot wastes and its application in the microbiological control of raw beef. Food Packag. Shelf Life 2023, 37, 101091. [Google Scholar] [CrossRef]
- Fang, Z.; Yang, Y.; Lin, S.; Xu, L.; Chen, S.; Lv, W.; Wang, N.; Dong, S.; Lin, C.; Xie, Y.; et al. Development and antimicrobial activity of composite edible films of chitosan and nisin incorporated with perilla essential oil-glycerol monolaurate emulsions. Food Chem. 2025, 462, 141006. [Google Scholar] [CrossRef]
- Lakshan, N.D.; Senanayake, C.M.; Liyanage, T.; Lankanayaka, A. Clove essential oil emulsions-loaded arrowroot starch-beeswax-based edible coating extends the shelf life and preserves the postharvest quality of fresh tomatoes (Solanum lycopersicum L.) stored at room temperature. Sustain. Food Technol. 2024, 2, 1052–1068. [Google Scholar] [CrossRef]
- Bassey, A.P.; Cui, X.; Ibeogu, I.H.; Wang, F.; Nasiru, M.M.; Bako, H.K.; Fan, L.; Liu, X. Fabrication and characterization of gelatin/carboxymethyl chitosan composite film incorporated with carvacrol and its preservation efficacy in Chinese mitten crab (Eriocheir sinensis). Food Hydrocoll. 2025, 160, 110723. [Google Scholar] [CrossRef]
- Dadkhah, H.; Pirsa, S.; Javadi, A.; Mohtarami, F. Biodegradable film based on sodium alginate/flax seed mucilage modified with norbixin and WO3 nanoparticles: Investigation of physicochemical properties and release of active substances. Biomass Convers. Biorefinery 2024, 14, 17663–17675. [Google Scholar] [CrossRef]
- Mohite, A.M.; Chandel, D. Formulation of edible films from fenugreek mucilage and taro starch. SN Appl. Sci. 2020, 2, 1900. [Google Scholar] [CrossRef]
- Osojnik Črnivec, I.G.; Skrt, M.; Šeremet, D.; Sterniša, M.; Farčnik, D.; Štrumbelj, E.; Poljanšek, A.; Cebin, N.; Pogačnik, L.; Smole Možina, S.; et al. Waste streams in onion production: Bioactive compounds, quercetin and use of antimicrobial and antioxidative properties. Waste Manag. 2021, 126, 476–486. [Google Scholar] [CrossRef]
- Cabassa, I.d.C.C.; Oliveira Filho, J.G.d.; Silva, B.A.d.; Barreto, H.P.M.; Silva, K.P.d.; Pauli, E.R.; Alves, V.M.; Egea, M.B. Bioactive peptides and protein hydrolysates from food proteins in biopolymer films: A comprehensive review on innovations in food preservation. Food Hydrocoll. 2025, 160, 110831. [Google Scholar] [CrossRef]
- Xia, J.; Sun, X.; Jia, P.; Li, L.; Xu, K.; Cao, Y.; Lü, X.; Wang, L. Multifunctional sustainable films of bacterial cellulose nanocrystal-based, three-phase pickering nanoemulsions: A promising active food packaging for cheese. Chem. Eng. J. 2023, 466, 143295. [Google Scholar] [CrossRef]
- Qin, Z.; Zou, Y.; Zhang, Y.; Wang, P.; Zhang, H. Electrospun pullulan nanofiber loading zanthoxylum bungeanum essential oil/β-cyclodextrin inclusion complexes for active packaging. Int. J. Biol. Macromol. 2022, 210, 465–474. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, J.; Lv, Y.; Du, J.; Rojas, O.J.; Wang, H. Controlled release of active food packaging by nanocarriers: Design, mechanism, models and applications. Food Packag. Shelf Life 2025, 49, 101524. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, Y.; Jiang, H.; Chen, L.; Shen, C.; Zhang, W.; Cao, J.; Jiang, W. Improved sustained-release properties of ginger essential oil in a Pickering emulsion system incorporated in sodium alginate film and delayed postharvest senescence of mango fruits. Food Chem. 2024, 435, 137534. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Dipti, T.T.; Islam, M.N.; Abdullah, A.T.M.; Jahan, S.; Alam, M.M.; Karim, M.R. Chemical composition, antioxidant and antibacterial activity of Piper chaba stem extracts with preservative effects on storage of raw beef patties. Saudi J. Biol. Sci. 2023, 30, 103663. [Google Scholar] [CrossRef]
- Maniglia, B.C.; De Paula, R.L.; Domingos, J.R.; Tapia-Blácido, D.R. Turmeric dye extraction residue for use in bioactive film production: Optimization of turmeric film plasticized with glycerol. LWT 2015, 64, 1187–1195. [Google Scholar] [CrossRef]
- Enidiok, E.S.; Enidiok, S.E.; Anakor, D.O.; Erifeta, G.O.; Thanikaivelan, P.; Oluba, O.M. Development and characterization of chia oil-activated ginger starch-feather keratin biocomposite for prolonged post-harvest preservation of tomato fruits. Carbohydr. Polym. Technol. Appl. 2024, 7, 100464. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, P.; Yuan, F.; Yu, Z.; Huang, S.; Lu, L. Ginger residue-derived nanocellulose as a sustainable reinforcing agent for composite films. Int. J. Biol. Macromol. 2025, 308, 142754. [Google Scholar] [CrossRef]
- Shen, B.; Yan, Z.; Yang, T.; Zhu, L.; Wang, Y.; Jiang, L. Waste citrus pectin/garlic bionanohybrids for edible food preservation. J. Food Eng. 2024, 364, 111800. [Google Scholar] [CrossRef]
- Radoor, S.; Jayakumar, A.; Karayil, J.; Kim, J.T.; Siengchin, S. Nelumbo nucifera flower extract incorporated alginate/polyvinyl alcohol films as a sustainable pH indicator for active food packaging applications. Int. J. Biol. Macromol. 2024, 273, 133170. [Google Scholar] [CrossRef]
- Chaudhary, B.U.; Lingayat, S.; Banerjee, A.N.; Kale, R.D. Development of multifunctional food packaging films based on waste Garlic peel extract and Chitosan. Int. J. Biol. Macromol. 2021, 192, 479–490. [Google Scholar] [CrossRef]
- Hernández-Varela, J.D.; Chanona-Pérez, J.J.; Resendis-Hernández, P.; Gonzalez Victoriano, L.; Méndez-Méndez, J.V.; Cárdenas-Pérez, S.; Calderón Benavides, H.A. Development and characterization of biopolymers films mechanically reinforced with garlic skin waste for fabrication of compostable dishes. Food Hydrocoll. 2022, 124, 107252. [Google Scholar] [CrossRef]
- Batool, Z.; Sameen, D.E.; Kamal, M.A.; Shen, B. Developing natural microcapsules by encapsulating peptides for preserving Zanthoxylum Bungeanum. Food Chem. 2025, 463, 141318. [Google Scholar] [CrossRef] [PubMed]
- Sutil, G.A.; Andrade, K.S.; Rebelatto, E.A.; Lanza, M. Effect of supercritical CO2 impregnation of piperine and black pepper extract on properties of poly(l-lactic acid) films. J. Supercrit. Fluids 2025, 216, 106441. [Google Scholar] [CrossRef]
- Sabbah, M.; Altamimi, M.; Di Pierro, P.; Schiraldi, C.; Cammarota, M.; Porta, R. Black edible films from protein-containing defatted cake of Nigella sativa seeds. Int. J. Mol. Sci. 2020, 21, 832. [Google Scholar] [CrossRef]
- Uitterhaegen, E.; Parinet, J.; Labonne, L.; Mérian, T.; Ballas, S.; Véronèse, T.; Merah, O.; Talou, T.; Stevens, C.V.; Chabert, F.; et al. Performance, durability and recycling of thermoplastic biocomposites reinforced with coriander straw. Compos. Part. A Appl. Sci. Manuf. 2018, 113, 254–263. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Adhikari, B.; Wang, M. Microencapsulation of Sichuan pepper essential oil in soybean protein isolate-Sichuan pepper seed soluble dietary fiber complex coacervates. Food Hydrocoll. 2022, 125, 107421. [Google Scholar] [CrossRef]
- Oubannin, S.; Asbbane, A.; Goh, K.W.; Singh, J.; Zafar, I.; Bouyahya, A.; Gharby, S. Green enrichment of argan oil (Argania spinosa L.) with thyme (Thymus vulgaris L.) and oregano (Origanum vulgare L.) leaves: Evaluating quality and stability improvements. Food Chem. 2024, 24, 101818. [Google Scholar] [CrossRef]
- Yu, M.; Hou, Y.; Zheng, L.; Han, Y.; Wang, D. Soy protein isolate-based active films functionalized with Zanthoxylum bungeanum by-products: Effects on barrier, mechanical, antioxidant and cherry tomato preservation performance. Int. J. Biol. Macromol. 2023, 253, 127539. [Google Scholar] [CrossRef]
- Gaur, S.; Kaur, M.; Kalra, R.; Rene, E.R.; Goel, M. Application of microbial resources in biorefineries: Current trend and future prospects. Heliyon 2024, 10, e28615. [Google Scholar] [CrossRef] [PubMed]
- Weiss, V.; Okun, Z.; Shpigelman, A. Utilization of hydrocolloids for the stabilization of pigments from natural sources. Curr. Opin. Colloid. Interface Sci. 2023, 68, 101756. [Google Scholar] [CrossRef]
- Mahmud, M.Z.A.; Mobarak, M.H.; Hossain, N. Emerging trends in biomaterials for sustainable food packaging: A comprehensive review. Heliyon 2024, 10, e24122. [Google Scholar] [CrossRef]
| Spice | Botanical Name | Waste Parts/By-Products | References |
|---|---|---|---|
Turmeric | Curcuma longa L. | Leaves, spent residue, press residue | [19,20] |
Pepper | Capsicum spp. | Seeds, defective fruits, spent residue | [21] |
Saffron | Crocus sativus L. | Leaves, petals, stamens, defective stigmas | [22] |
Coriander | Coriandrum sativum L. | Spent residue, roots, bark, seeds | [23] |
Ginger | Zingiber officinale | Skin/peel, stems, stalk, leaves, herbal dust, spent residue | [24] |
Garlic | Allium sativum | Husks/peels, straws, stem, leaves | [25] |
Onion | Allium cepa | Skin, roots, top and bottom bulbs, smashed/deformed onions | [26] |
Clove | Syzygium aromaticum | Leaves, spent residue, stem | [27] |
Szechuan Pepper | Zanthoxylum spp. | Leaves, seeds, branches | [28,29] |
Cumin | Cuminum cyminum | Oilseed cake | [30] |
| Spice | Botanical Name | By-Products | Chemical Composition (%) | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ash | Protein | Fat | Starch | Pectin | Cellulose | Sugars | Hemicellulose and Lignin | Uronic Acids | Fiber | ||||
| Ginger | Zingiber officinale | Stem and Leaves | 7.04 | 6.02 | - | - | - | 48.48 | 52.14 | 31.50 | - | - | [34] |
| Spent Residue | 9.37 | 11.74 | 1.67 | 50.79 | 2.09 | 3.84 | 4.52 | - | - | - | [34] | ||
| Pomace | - | 8.33 | - | 16.62 | - | - | 76.42 | - | 4.42 | - | [35] | ||
| Turmeric | Curcuma longa L. | Dye Extraction Residue | 5.80 | 4.20 | - | 64.00 | - | 8.30 | - | 13.10 | - | 21.00 | [36] |
| Juice Extraction Press Residue | - | 7.40 | 5.98 | - | - | - | 42.46 | - | - | 5.75 | [19] | ||
| Spent turmeric powder | 26.04 | 12.67 | - | - | - | - | 39.66 | - | - | - | [37] | ||
| Leaves | 9.4 | 6.0 | 0.5 | - | - | - | - | 44.74 | - | 34.5 | [38] | ||
| Pepper | Capsicum spp. | Seeds | 3.05 | 28.33 | 18.39 | - | - | - | - | - | - | - | [39] |
| Pomace | 5.67 | 15.52 | 10.80 | 1.78 | 6.40 | 18.25 | 9.58 | 23.55 | - | 48.27 | [40] | ||
| Coriander | Coriandrum sativum L. | Flowers | 3.52 | 11.94 | 6.19 | - | - | - | - | - | - | 8.41 | [41] |
| Saffron | Crocus sativus L. | Petals | 12.4 | 21.7 | 14.1 | - | - | - | - | - | - | 28.9 | [42] |
| Garlic | Allium sativum | Biomass | 2.92 | 13.09 | - | - | - | 25.37 | 83.99 | 47.73 | - | - | [43] |
| Straw | 10.30 | 3.32 | 4.59 | - | - | - | - | 6.68 | - | 25.80 | [44] | ||
| Peel | 7.37 | 2.61 | 0.22 | 8.5 | 26.00 | 18.62 | 7.87 | 26.93 | - | 62.10 | [45] | ||
| Spice Waste/By-Product | Extraction Conditions | Main Phenolic Compounds | Reference |
|---|---|---|---|
Saffron flowers | Solvent extraction: MeOH:H2O (7:3 v/v, 1:10 w/v), 37 °C, 24 h | 3-Hydroxytyrosol (C8H10O3) Epicatechin (C15H14O6) Vanillic Acid (C8H8O4) Rosmarinic Acid (C18H16O8) Gallic Acid (C7H6O5) Quercetin (C15H10O7) 4-Hydroxybenzoic Acid (C7H6O3) Chlorogenic Acid (C16H18O9) Myricetin (C15H10O8) Salicylic Acid (C7H6O3) | [61] |
Saffron defective stigmas |
| Coumaric Acid (C9H8O3) Rosmarinic Acid (C18H16O8) Vanillic Acid (C8H8O4) Caffeic Acid (C9H8O4) Gallic Acid (C7H6O5) Hydroxycinnamic Acids (C9H8O3) Syringic Acid (C9H10O5) Hydroxybenzoic Acid (C7H6O3) Rutin (C27H30O16) Naringin (C27H32O14) Catechin (C15H14O6) Isovanillin (C8H8O3) Epicatechin (C15H14O6) Kaempferol (C15H10O6) | [62] |
Saffron processing residue | DES extraction: L-lactic acid: Glycine (55–85% w/v), 50 °C, 150 min | Kaempferol-3-O-Sophoroside (C27H30O16) Isorhamnetin Glucosides (C22H22O12) Quercetin (C15H10O7) | [63] |
Sage residue from steam distillation | UAE: MeOH: H2O (7:3 v/v, 1:10 w/v), 30 °C, 15 min | Vicenin-2 (C27H30O15) Epigallocatechin (C15H14O7) Luteolin-7-O-Rutinoside (C27H30O16) Luteolin-7-O-Glucoside (C21H20O11) Verbascoside (C29H36O15) Isorhamnetin-3-Rutinoside (C28H32O16) Apigenin-7-O-Glucoside (C21H20O10) | [64] |
Sage dust | Soxhlet extraction: Methylene chloride/Hexane (120 mL each, 12:1 w/v), 40 °C, 6 h | Caffeic Acid (C9H8O4) Hydroxybenzoic Acid (C7H6O3) Ferulic Acid (C10H10O4) Rosmarinic Acid (C18H16O8) | [33] |
Sage dry leaves | UAE: H2O/EtOH/MeOH (1:10 w/v), 20–40 °C, 15–300 min | Ferulic Acid (C10H10O4) Ellagic Acid (C14H6O8) Caffeic Acid (C9H8O4) p-Hydroxybenzoic Acid (C7H6O3) Vanillic Acid (C8H8O4) Syringic Acid (C9H10O5) p-Coumaric Acid (C9H8O3) Chlorogenic Acid (C16H18O9) 3-Hydroxybenzoic Acid (C7H6O3) 3,4-Hydroxybenzoic Acid (C24H20O7) Luteolin (C15H10O6) Myricetin (C15H10O8) Chrysin (C15H10O4) Kaempferol (C15H10O6) Galangin (C15H10O5) | [65] |
Turmeric leaves | Solvent extraction: Distilled water (1:25 w/v), 85 °C, 150 min | Rutin (C27H30O16) Quercitrin (C21H20O11) Myricetin-3-O-rhamnoside (C21H20O12) Quercetin (C15H10O7) Diosmetin (C16H12O6) Miquelianin (C21H18O13) | [60] |
Garlic husks |
| Gallic Acid (C7H6O5) Trans-ferulic Acid (C10H10O4) 4-Hydroxybenzoic Acid (C7H6O3) Caffeic Acid (C9H8O4) Hydroxybenzoic Acid (C7H6O3) | [56] |
Coriander seeds |
| Catechin (C15H14O6) 3,4-Dimethoxycinnamic Acid (C11H12O4) Coumaric Acid (C9H8O3) Daidzein (C15H10O4) Ferulic Acid (C10H10O4) Sinapic Acid (C11H12O5) Trans-ferulic Acid (C10H10O4) Chlorogenic Acid (C16H18O9) Neochlorogenic Acid (C16H18O9) Luteolin (C15H10O6) Kaempferol (C15H10O6) Quercetin (C15H10O7) | [66] |
Coriander seed cake | Solvent extraction: 100% MeOH (1:4 w/v), room temperature, 30 min | Eugenol (C10H12O2) | [67] |
Szechuan pepper leaves | MAE: 65% EtOH (1:30 w/v), 500 W, 70 °C, 4 min | Quercetin (C15H10O7) Quercetin-3-O-Glucoside (C21H20O12) Hyperoside (C21H20O12) Rutin (C27H30O16) Isoquercetin (C21H20O12) Kaempferol-3-O-Galactoside (C21H20O11) Diosmetin (C16H12O6) Apigenin (C15H10O5) Catechin (C15H14O6) Epicatechin (C15H14O6) Chlorogenic Acid (C16H18O9) Protocatechuic Acid (C7H6O4) Caffeic Acid (C9H8O4) | [68,69] |
Szechuan pepper twigs | Solvent extraction: MeOH/Dichloromethane/Ethylacetate/n-Butyl alcohol | Trans-Ferulic Acid (C10H10O4) Methyl Ferulate (C11H12O4) Vanillic Acid (C8H8O4) Methyl p-Hydroxycinnamate (C10H10O3) Eugenol (C10H12O2) Methyl Syringate (C10H12O5) | [70] |
Ginger peel | UAE: EtOH (1:11.25 w/v), room temperature, 15 min | Rutin (C27H30O16) Zingerone (C11H14O3) Quercetin (C15H10O7) Kaempferol (C15H10O6) Naringenin (C15H12O5) 6-Gingerone (C17H26O3) | [71] |
Ginger leaves | Hot-water extraction: H2O (1:10 w/v), 90 ± 2 °C, 60 min | Kaempferol (C15H10O6) Quercetin (C15H10O7) 3-O-Robinobioside-7-O-Rhamnoside (C66H128O19Si11) 3-O-Galactoside-7-O-Rhamnoside (C27H30O15) | [59] |
Onion peel | Solvent extraction: 80% MeOH (1:10 w/v), 33 ± 3 °C, 24 h | Vanillic Acid (C8H8O4) Quercetin (C15H10O7) Cyanidin (C15H11O6+) | [72] |
Clove aerial parts | Maceration: EtOH (1:1; v/v, 500 mL), room temperature, 72 h | Eugenol (C10H12O2) Eugenyl Acetate (C12H14O3) 4-(2-Propenyl)-Phenol (C9H10O) Gallic Acid (C7H6O5) | [73] |
Thyme herbal dust |
| Protocatechuic Acid (C7H6O4) Vanillic Acid (C8H8O4) Coumaroyl Hexoside (C15H18O8) Luteolin (C15H10O6) Kaempferol-3-Rutinoside (C27H30O15) | [74] |
| Spice By-Product | By-Product Bioactive Compounds | Film Preparation Method(s) and Conditions | Biocomposite Composition | Key Findings in Food Packaging Systems | Food Matrix Application | References |
|---|---|---|---|---|---|---|
| Turmeric extraction dye residue | Curcuminoids Starch Fiber | Solvent casting, Oven drying; 35 °C, 7 h | Turmeric starch Glycerol |
| N/A | [231] |
| Ginger starch residue | Starch | Solvent casting, Oven drying; 50 °C, 72 h | Keratin feather Chia seed oil Glycerol |
| Coating on tomato fruits | [232] |
| Ginger waste | Nanocellulose | Solvent casting, Oven drying; 60 °C, 24 h | Sodium Alginate (SA) Chitosan (CS) |
| N/A | [233] |
| Garlic waste | Quantum Carbon Dots (CDs) | Solvent casting, Oven drying; 60 °C, 3 h | Pectin Glycerol |
| Coating on strawberries | [234] |
| Garlic peels | Polyphenols | Solvent casting, Oven drying; 40 °C, 24 h | Alginate Polyvinyl alcohol Nelumbo nucifera (lotus) flower extract |
| Shrimp freshness | [235] |
| Garlic waste peel | Polyphenols | Solvent casting, Oven drying; 60 °C, 5 h | Chitosan |
| Red Apples | [236] |
| Garlic skin | Polyphenols CNCs | Solvent casting, Oven drying; 37 °C, 72 h | Chitosan |
| [168] | |
| Garlic skin | Polyphenols | Solvent casting, Oven drying; 60 °C, 15 h | Potato Starch Gellan Gum Glycerol |
| N/A | [237] |
| Szechuan pepper seeds | Nisin | Water-in-oil-in-water (W/O/W) microencapsulation technique, 25 °C, 15 min | Gum Arabic |
| Coating on szechuan peppers | [238] |
| Black pepper grains | Piperine | Compression molding; Cooling, 25 °C | PLA |
| N/A | [239] |
| Cumin defatted seed cake | Proteins | Solvent casting, Oven drying; 37 °C, 48 h | Glycerol Transglutaminase (TGase) |
| N/A | [240] |
| Coriander straw | Fiber | Twin-screw extrusion compounding, Injection molding; 100 mm/s, 2000 bar, 20 °C, 15 s | Polypropylene (PP) Biobased low-density polyethylene (LDPE) |
| N/A | [241] |
| Szechuan pepper seed | Soluble Dietary fiber (SDF) | Complex coacervation and Spray drying, pH 4.1, SPI-to-SDF mass ratio 6:1 | Soybean protein isolate (SPI) |
| Szechuan pepper essential oil | [242] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Ahlivia, E.B.; Bruce, B.B.; Zou, X.; Battino, M.; Savić, D.; Katona, J.; Shen, L. The Road to Re-Use of Spice By-Products: Exploring Their Bioactive Compounds and Significance in Active Packaging. Foods 2025, 14, 2445. https://doi.org/10.3390/foods14142445
Zhang D, Ahlivia EB, Bruce BB, Zou X, Battino M, Savić D, Katona J, Shen L. The Road to Re-Use of Spice By-Products: Exploring Their Bioactive Compounds and Significance in Active Packaging. Foods. 2025; 14(14):2445. https://doi.org/10.3390/foods14142445
Chicago/Turabian StyleZhang, Di, Efakor Beloved Ahlivia, Benjamin Bonsu Bruce, Xiaobo Zou, Maurizio Battino, Dragiša Savić, Jaroslav Katona, and Lingqin Shen. 2025. "The Road to Re-Use of Spice By-Products: Exploring Their Bioactive Compounds and Significance in Active Packaging" Foods 14, no. 14: 2445. https://doi.org/10.3390/foods14142445
APA StyleZhang, D., Ahlivia, E. B., Bruce, B. B., Zou, X., Battino, M., Savić, D., Katona, J., & Shen, L. (2025). The Road to Re-Use of Spice By-Products: Exploring Their Bioactive Compounds and Significance in Active Packaging. Foods, 14(14), 2445. https://doi.org/10.3390/foods14142445









