Isolation and Characterizations of Histamine- and Tyramine-Producing Strains Isolated from Fermented Soybean Food: Soy Sauce and Soybean Paste
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
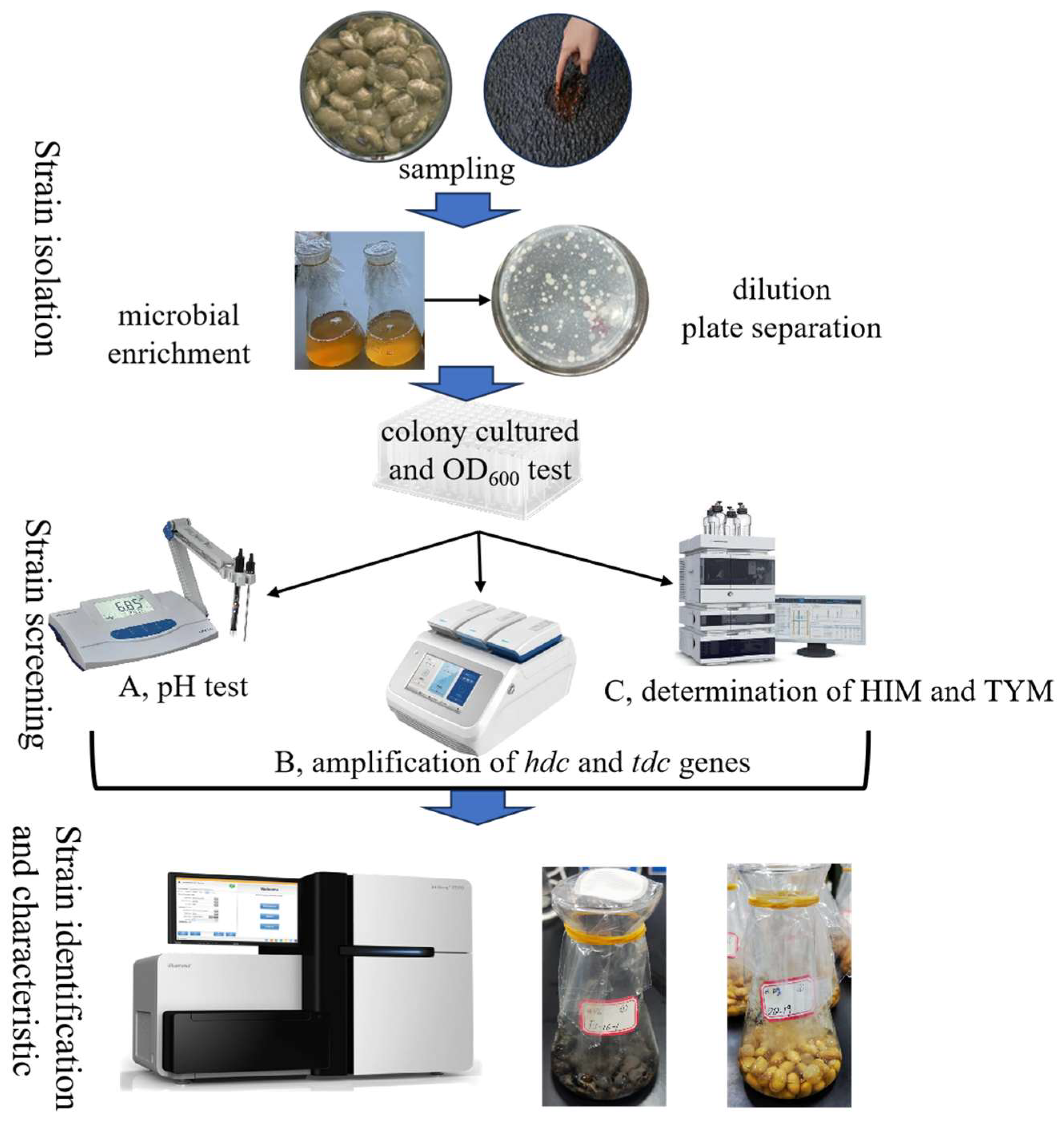
2.2. Strain Isolation
2.3. Isolation and Preliminary Screening of BAs-Producing Strains
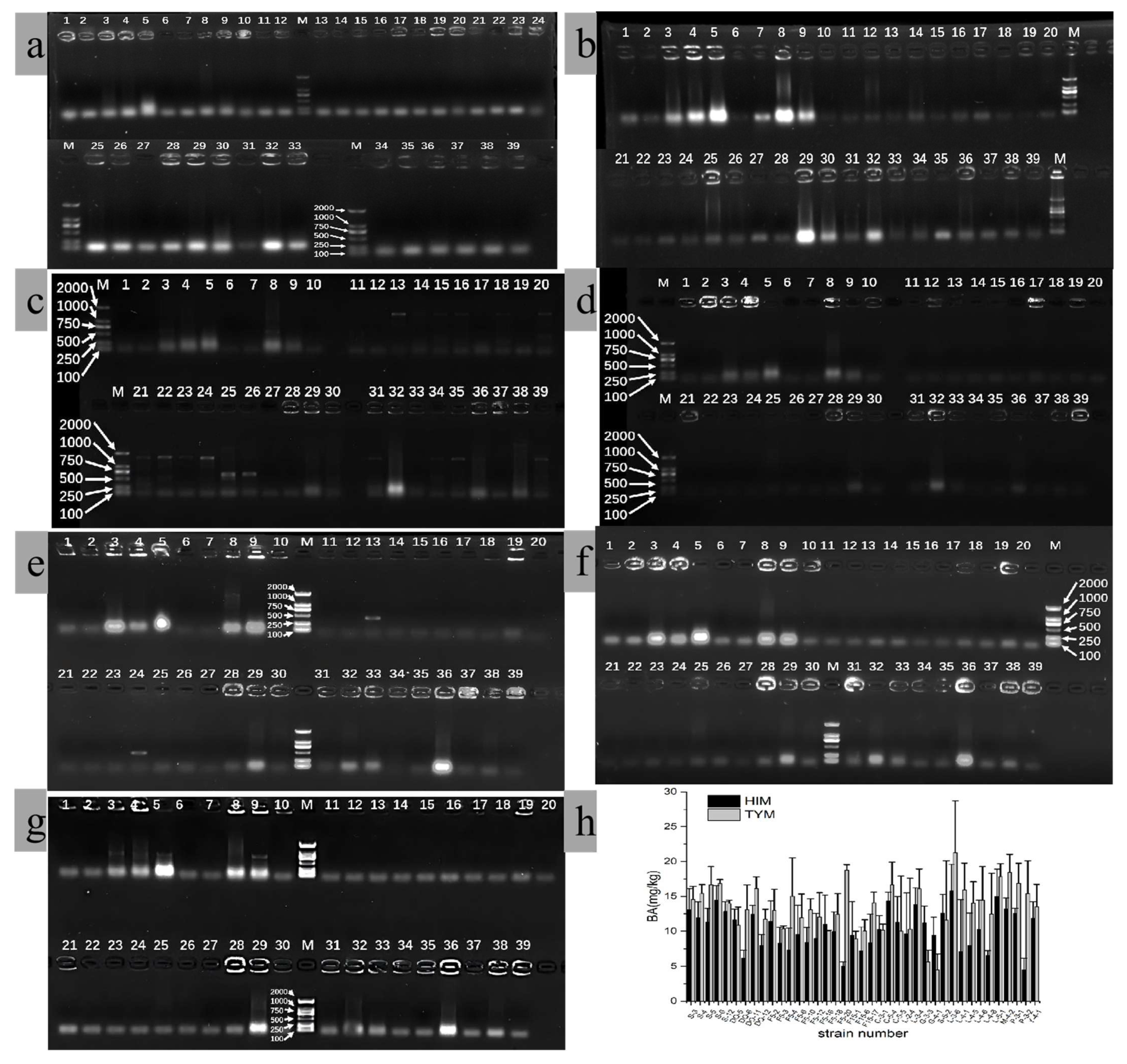
2.4. Identification of Tyrosine and Histidine Decarboxylase Genes
2.5. Secondary Screening and Isolate Identification
2.6. Simulated Fermentation with the Screened Isolates
2.7. Characteristics of HIM and TYM Formation by the Screened Isolates
2.8. Determination of BAs
2.9. Statistical Analysis
3. Results and Discussion
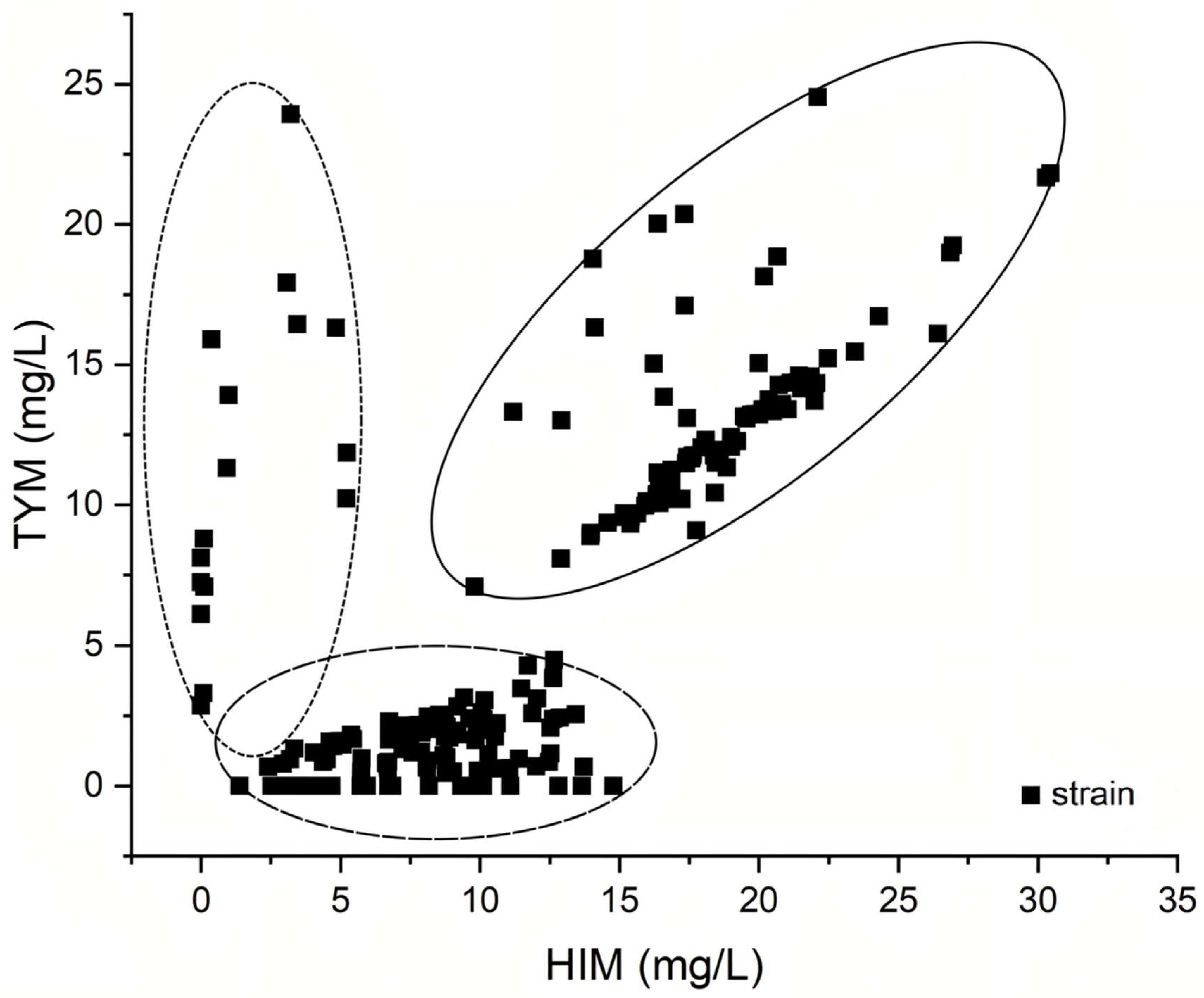
3.1. Comparison of Screening Methods for HIM- and TYM-Producing Strains
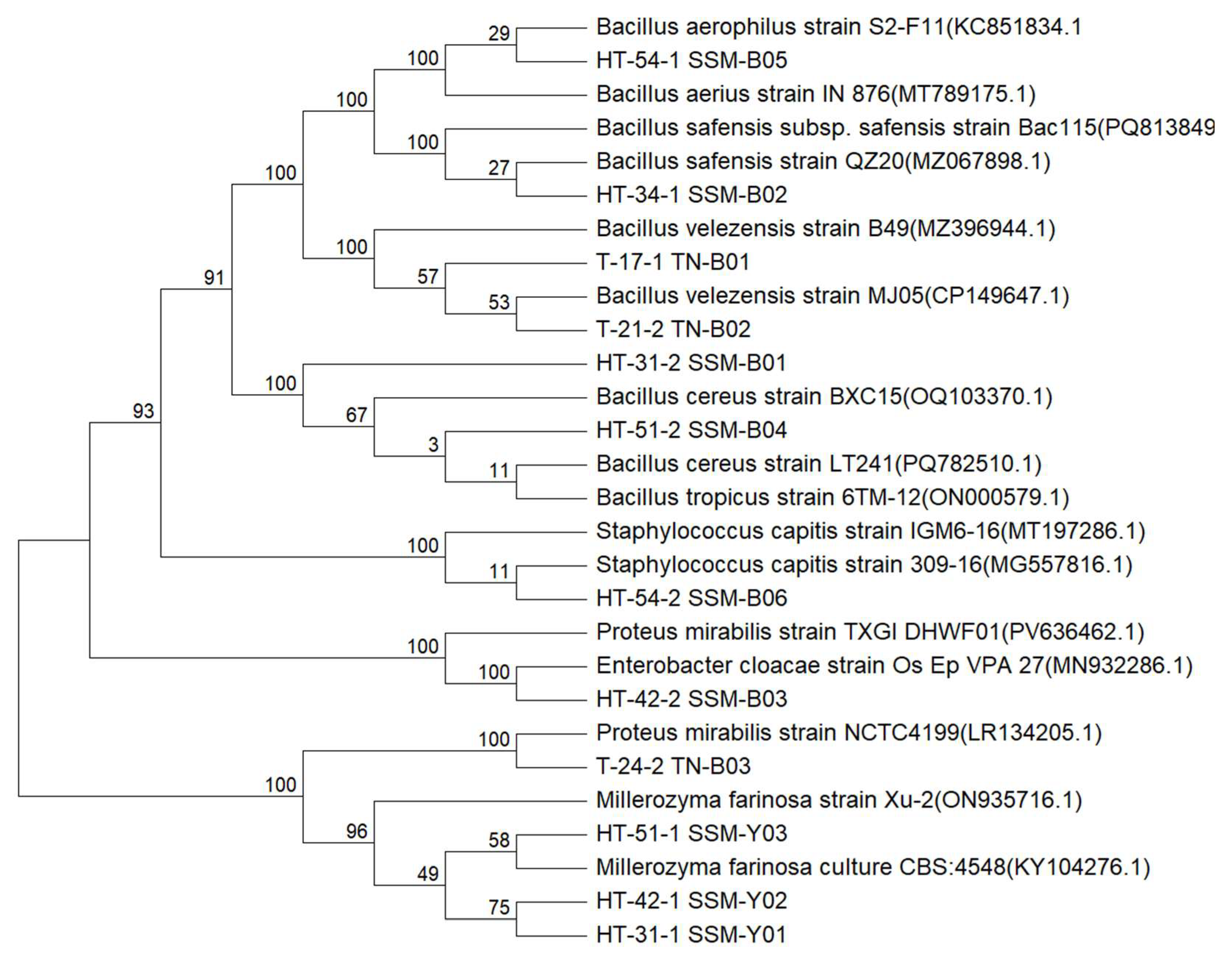
3.2. Identification of HIM- and TYM-Producing Strains
3.3. HIM- and TYM-Forming Abilities of Screened Strains
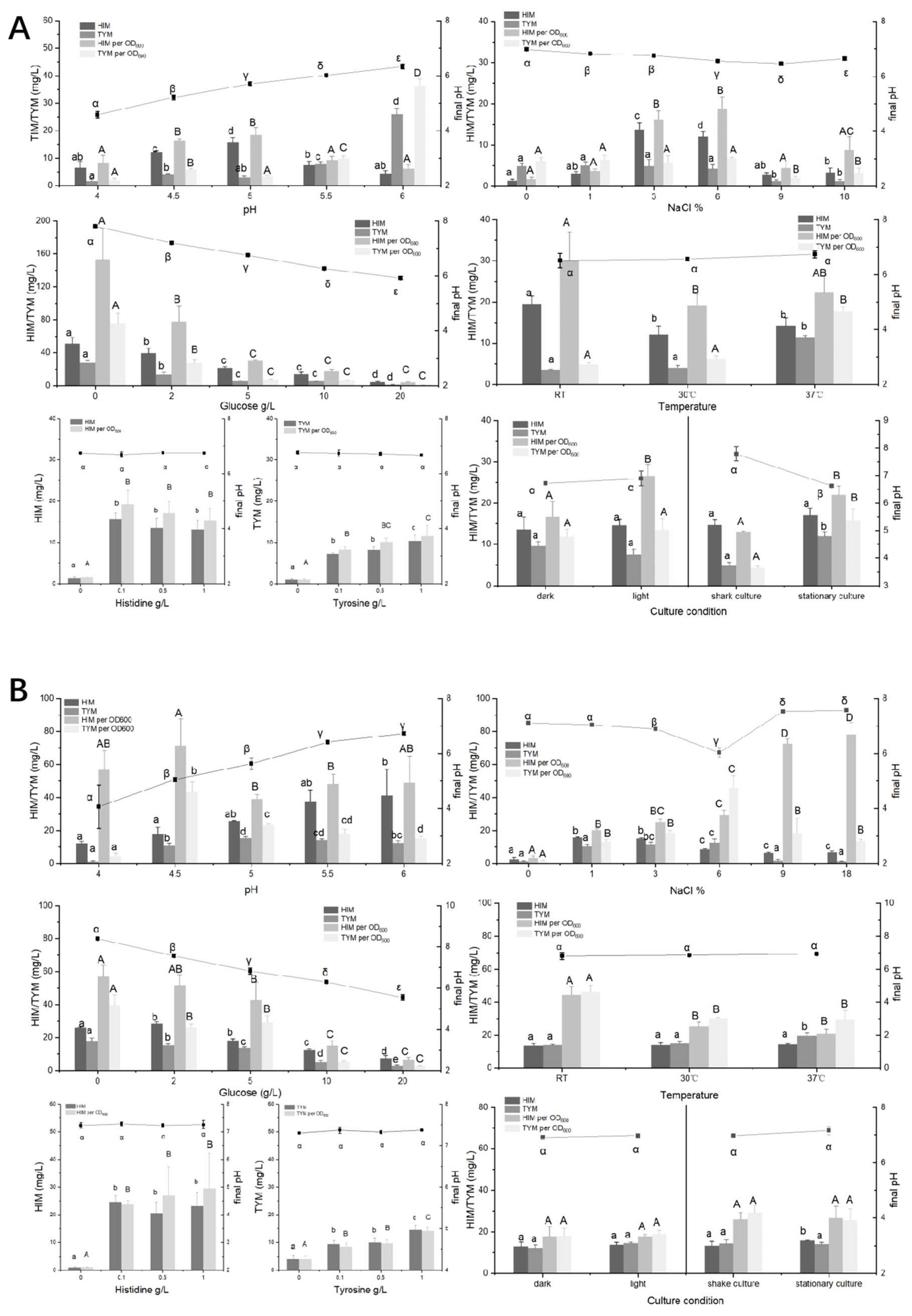
3.4. Characteristics of HIM and TYM Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, H.; Xiao, K. Modified QuEChERS combined with ultra high performance liquid chromatography tandem mass spectrometry to determine seven biogenic amines in Chinese traditional condiment soy sauce. Food Chem. 2017, 229, 502–508. [Google Scholar] [CrossRef]
- Finberg, J.P.M.; Gillman, K. Selective inhibitors of monoamine oxidase type B and the “cheese effect”. Int. Rev. Neurobiol. 2011, 100, 169–190. [Google Scholar]
- Flockhart, D.A. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: An update. Prim. Care Companion CNS Disord. 2012, 14 (Suppl. S1), 26257. [Google Scholar] [CrossRef]
- Spano, G.; Russo, P. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Fernandez, M.; Martin, M.C.; Ruas-Madiedo, P.; Alvarez, M.A. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 2017, 218, 249–255. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.N.; Feng, W.; Cheng, H.; Muhammad, A.L.; Ye, X.Q.; Zhi, Z.J. Comparison of Biogenic Amines in Chinese Commercial Soy Sauces. Molecules 2019, 24, 1522. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, X.; Huang, G.D.; Hongsibsong, S.; Hao, G.; Li, J.Y.; Yang, J.Y.; Xu, Z.L. Formation of biogenic amines in soy sauce and reduction via simple phytochemical addition. LWT-Food Sci. Technol. 2023, 176, 114542. [Google Scholar] [CrossRef]
- Zhang, X.; Dokuta, S.; Wongta, A.; Sutan, K.; Xu, Z.L.; Zhou, K.; Hongsibsong, S. Monitoring and risk assessment of biogenic amines in commercial Thua-Nao, a traditional Northern Thai fermented soybean paste. J. Food Compos. Anal. 2024, 133, 106470. [Google Scholar] [CrossRef]
- Wang, X.H.; Ren, H.Y.; Wang, W.; Bai, T.; Li, J.X. Evaluation of key factors influencing histamine formation and accumulation in fermented sausages. J. Food Saf. 2015, 35, 395–402. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef]
- Park, Y.K.; Lee, J.H.; Mah, J.H. Occurrence and reduction of biogenic amines in traditional Asian fermented soybean foods: A review. Food Chem. 2019, 278, 1–9. [Google Scholar] [CrossRef]
- Lim, E.-S. Influence of bacteriocin-producing Bacillus strains on quality characteristics of fermented soybean product with biogenic amine-forming lactic acid bacteria. Appl. Biol. Chem. 2022, 65, 5. [Google Scholar] [CrossRef]
- Zhang, R.F.; Chen, G.J.; Yang, W.M.; Huang, Y.H.; Cheng, X.D.; Zeng, F.Y.; Kan, J.Q. Evaluation of biogenic amines produced by mucor and aspergillus oryzae in douchi fermentation process. Food Ferment. Ind. 2017, 43, 15–21. [Google Scholar]
- do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented soy products and their potential health benefits: A review. Microorganisms 2022, 10, 1606. [Google Scholar] [CrossRef]
- Chin, X.H.; Elhalis, H.; Chow, Y.; Liu, S.Q. Enhancing food safety in soybean fermentation through strategic implementation of starter cultures. Heliyon 2024, 10, e25007. [Google Scholar] [CrossRef]
- Chukeatirote, E. Thua nao: Thai fermented soybean. J. Ethn. Foods 2015, 2, 115–118. [Google Scholar] [CrossRef]
- Kim, B.; Byun, B.Y.; Mah, J.H. Biogenic amine formation and bacterial contribution in Natto products. Food Chem. 2012, 135, 2005–2011. [Google Scholar] [CrossRef]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the microbial community of Japanese koji and miso: A review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef]
- Ma, J.J.; Zhang, J.J.; Zhang, L.J.; Nie, Y.; Xu, Y. Systematic analysis of key fermentation parameters influencing biogenic amines production in spontaneous fermentation of soy sauce. Food Biosci. 2023, 52, 102484. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A narrative review on biogenic amines in fermented fish and meat products. J. Food Sci. Technol. 2021, 58, 1623–1639. [Google Scholar] [CrossRef]
- Mah, J.H.; Park, Y.K.; Jin, Y.H.; Lee, J.H.; Hwang, H.J. Bacterial production and control of biogenic amines in Asian fermented soybean foods. Foods 2019, 8, 85. [Google Scholar] [CrossRef]
- Kim, K.H.; Chun, B.H.; Kim, J.; Jeon, C.O. Identification of biogenic amine-producing microbes during fermentation of ganjang, a Korean traditional soy sauce, through metagenomic and metatranscriptomic analyses. Food Control 2021, 121, 107681. [Google Scholar] [CrossRef]
- Hu, M.; Dong, J.; Tan, G.L.; Li, X.Y.; Zheng, Z.Y.; Li, M. Metagenomic insights into the bacteria responsible for producing biogenic amines in sufu. Food Microbiol. 2021, 98, 103762. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xiong, S.J.; Du, T.H.; Zhao, M.W.; Huang, G.D.; Guan, Q.Q.; Xiong, T.; Xie, M.Y. Exploring the core microorganisms and mechanisms responsible for biogenic amines production during soy sauce fermentation. Food Biosci. 2024, 59, 104236. [Google Scholar] [CrossRef]
- Singracha, P.; Niamsiri, N.; Visessanguan, W.; Lertsiri, S.; Assavanig, A. Application of lactic acid bacteria and yeasts as starter cultures for reduced-salt soy sauce (moromi) fermentation. LWT 2017, 78, 181–188. [Google Scholar] [CrossRef]
- Chen, C.M.; Wei, C.I.; Koburger, J.A.; Marshall, M.R. Comparison of Four Agar Media for Detection of Histamine-Producing Bacteria in Tuna1. J. Food Prot. 1989, 52, 808–813. [Google Scholar] [CrossRef]
- Bjornsdottir, K.; Bolton, G.E.; Green, P.D.M.; Jaykus, L.A.; Green, D.P. Detection of gram-negative histamine-producing bacteria in fish: A comparative study. J. Food Prot. 2009, 72, 1987–1991. [Google Scholar] [CrossRef]
- Zhou, K.; Siroli, L.; Patrignani, F.; Sun, Y.M.; Lanciotti, R.; Xu, Z.L. Formation of Ethyl Carbamate during the Production Process of Cantonese Soy Sauce. Molecules 2019, 24, 1474. [Google Scholar] [CrossRef]
- Liu, Y. Gene Cloning and Functional Characteristics of a L-Histidine Decarboxylase HDC1 from Enterobacter aerogenes sp. GXE3. Master’s Thesis, Guangxi University, Nanning, China, 2014. [Google Scholar]
- O’Sullivan, D.J.; Fallico, V.; O’Sullivan, O.; McSweeney, P.L.H.; Sheehan, J.J.; Cotter, P.D.; Giblin, L. High-throughput DNA sequencing to survey bacterial histidine and tyrosine decarboxylases in raw milk cheeses. BMC Microbiol. 2015, 15, 266. [Google Scholar] [CrossRef]
- Marcobal, A.; De Las Rivas, B.; Moreno, A.M.V.; Muñoz, R. Multiplex PCR method for the simultaneous detection of histamine-, tyramine-, and putrescine-producing lactic acid bacteria in foods. J. Food Prot. 2005, 68, 874–878. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Moreno, A.M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Chang, M.; Chang, H.C. Development of a screening method for biogenic amine producing Bacillus spp. Int. J. Food Microbiol. 2012, 153, 269–274. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, Y.; Lan, X. Isolation, characterization, and application of biogenic amines-degrading strains from fermented food. J. Food Saf. 2020, 40, e12716. [Google Scholar] [CrossRef]
- Henríquez-Aedo, K.; Durán, D.; Garcia, A.; Hengst, M.B.; Aranda, M. Identification of biogenic amines-producing lactic acid bacteria isolated from spontaneous malolactic fermentation of chilean red wines. LWT-Food Sci. Technol. 2016, 68, 183–189. [Google Scholar] [CrossRef]
- Ma, J.; Nie, Y.; Zhang, L.J.; Xu, Y. Ratio of Histamine-Producing/Non-Histamine-Producing Subgroups of Tetragenococcus halophilus Determines the Histamine Accumulation during Spontaneous Fermentation of Soy Sauce. Appl. Environ. Microbiol. 2023, 89, e01884-22. [Google Scholar] [CrossRef]
- Han, G.H.; Cho, T.Y.; Yoo, M.S.; Kim, C.S.; Kim, J.M.; Kim, H.A.; Kim, M.O.; Kim, S.C.; Lee, S.A.; Ko, Y.S.; et al. Biogenic amines formation and content in fermented soybean paste (Cheonggukjang). Korean J. Food Sci. Technol. 2007, 39, 541–545. [Google Scholar]
- Jeon, A.R.; Lee, J.H.; Mah, J.H. Biogenic amine formation and bacterial contribution in Cheonggukjang, a Korean traditional fermented soybean food. LWT 2018, 92, 282–289. [Google Scholar] [CrossRef]
- Bermúdez, R.; Lorenzo, J.M.; Fonseca, S.; Franco, I.; Carballo, J. Strains of Staphylococcus and Bacillus isolated from traditional sausages as producers of biogenic amines. Front. Microbiol. 2012, 3, 151. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Kung, H.F.; Chang, S.C.; Lee, T.M.; Wei, C.I. Histamine formation by histamine-forming bacteria in douchi, a Chinese traditional fermented soybean product. Food Chem. 2007, 103, 1305–1311. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Cachaldora, A.; Fonseca, S.; Gómez, M.; Franco, I.; Carballo, J. Production of biogenic amines “in vitro” in relation to the growth phase by Enterobacteriaceae species isolated from traditional sausages. Meat Sci. 2010, 86, 684–691. [Google Scholar] [CrossRef]
- Ahmed, O.M. Histamine and other biogenic amines formation in canned tuna fish inoculated with Morganella morganii or Proteus mirabilis in determining food safety during temperature abuse. Nutr. Food Toxicol. 2019, 3, 690–700. [Google Scholar]
- Visessanguan, W.; Benjakul, S.; Potachareon, W.; Panya, A.; Riebroy, S. Accelerated proteolysis of soy proteins during fermentation of thua-nao inoculated with Bacillus subtilis. J. Food Biochem. 2005, 29, 349–366. [Google Scholar] [CrossRef]
- Hoang, N.X.; Ferng, S.; Ting, C.H.; Chiou, R.Y.Y.; Hsu, C.K. Optimizing the initial moromi fermentation conditions to improve the quality of soy sauce. LWT 2016, 74, 242–250. [Google Scholar] [CrossRef]
- Gardini, F.; Martuscelli, M.; Caruso, M.C.; Galgano, F.; Crudele, M.A.; Favati, F.; Guerzoni, M.E.; Suzzi, G. Effects of pH, temperature and NaCl concentration on the growth kinetics, proteolytic activity and biogenic amine production of Enterococcus faecalis. Int. J. Food Microbiol. 2001, 64, 105–117. [Google Scholar] [CrossRef]
- Yang, Q.; Meng, J.; Zhang, W.; Liu, L.; He, L.; Deng, L.; Zeng, X.F.; Ye, C. Effects of amino acid decarboxylase genes and pH on the amine formation of enteric bacteria from Chinese traditional fermented fish (Suan Yu). Front. Microbiol. 2020, 11, 1130. [Google Scholar] [CrossRef]


| Target Gene | Primer | Sequence (5′-3′) | Amplicon Size (bp) | Note | Reference |
|---|---|---|---|---|---|
| hdc | JV16HC | AGATGGTATTGTTTCTTATG | 367 | G+ | [30] |
| JV17HC | AGACCATACACCATAACCTT | ||||
| HDC-f | TCHATYARYAACTGYGGTGACTGGRG | 709 | G− | ||
| HDC-r | CCCACAKCATBARWGGDGTRTGRCC | ||||
| HDC3 | GATGGTATTGTTTCKTATGA | 435 | G+ | [31] | |
| HDC4 | CAAACACCAGCATCTTC | ||||
| HIS2-f | AAYTSNTTYGAYTTYGARAARGARGT | 531 | G− | ||
| HIS2-r | TANGGNSANCCDATCATYTTRTGNCC | ||||
| tdc | TDC-P1 | CCRTARTCNGGNATAGCRAARTCNGTRTG | 1100 | ||
| TDC-P2 | GAYATNATNGGNATNGGNYTNGAYCARG | ||||
| TDC-41 | CAYGTNGAYGCNGCNTAYGGNGG | 924 | [32] | ||
| TDC-42 | AYRTANCCCATYTTRTGNGGRTC | ||||
| TD5 | CAAATGGAAGAAGAAGTAGG | 213 | |||
| TD2 | ACATAGTCAACCATRTTGAA |
| Strain | Amino Acid Medium | Moromi or Soybean Medium | |||
|---|---|---|---|---|---|
| HIM mg/kg | TYM mg/kg | HIM mg/kg | TYM mg/kg | ||
| Moromi | Millerozyma farinosa-HT-31-1 | 4.89 ± 0.35 | 9.78 ± 1.45 | ||
| Bacillus cereus-HT-31-2 | 22.10 ± 0.47 | 12.84 ± 1.90 | 43.62 ± 3.46 | 26.03 ± 3.13 | |
| Bacillus safensis-HT-34-1 | 17.56 ± 3.41 | 11.81 ± 1.83 | |||
| Millerozyma farinosa-HT-42-1 | 26.52 ± 2.42 | 11.15 ± 1.35 | 10.21 ± 1.73 | 8.27 ± 0.83 | |
| Enterobacter cloacae-HT-42-2 | 4.30 ± 1.19 | 8.14 ± 1.49 | |||
| Millerozyma farinose-HT-51-1 | 5.79 ± 0.75 | 11.54 ± 0.79 | |||
| Bacillus cereus-HT-51-2 | 4.35 ± 1.45 | 8.43 ± 0.53 | |||
| Bacillus aerophilus-HT-54-1 | 13.41 ± 1.08 | 10.61 ± 1.04 | |||
| Staphylococcus capitis-HT-54-2 | 4.89 ± 0.74 | 8.96 ± 0.89 | |||
| Thua nao | Proteus mirabilis-T-24-2 | 22.66 ± 4.87 | 159.55 ± 6.56 | ||
| Bacillus velezensis-T-17-1 | 17.34 ± 0.42 | 40.87 ± 5.27 | |||
| Bacillus velezensis-T-21-2 | 16.44 ± 2.14 | 40.36 ± 10.93 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, S.; Liu, H.; Wongta, A.; Xu, Z.; Zhou, K.; Hongsibsong, S. Isolation and Characterizations of Histamine- and Tyramine-Producing Strains Isolated from Fermented Soybean Food: Soy Sauce and Soybean Paste. Foods 2025, 14, 2407. https://doi.org/10.3390/foods14142407
Zhang X, Li S, Liu H, Wongta A, Xu Z, Zhou K, Hongsibsong S. Isolation and Characterizations of Histamine- and Tyramine-Producing Strains Isolated from Fermented Soybean Food: Soy Sauce and Soybean Paste. Foods. 2025; 14(14):2407. https://doi.org/10.3390/foods14142407
Chicago/Turabian StyleZhang, Xiao, Sihao Li, Heng Liu, Anurak Wongta, Zhenlin Xu, Kai Zhou, and Surat Hongsibsong. 2025. "Isolation and Characterizations of Histamine- and Tyramine-Producing Strains Isolated from Fermented Soybean Food: Soy Sauce and Soybean Paste" Foods 14, no. 14: 2407. https://doi.org/10.3390/foods14142407
APA StyleZhang, X., Li, S., Liu, H., Wongta, A., Xu, Z., Zhou, K., & Hongsibsong, S. (2025). Isolation and Characterizations of Histamine- and Tyramine-Producing Strains Isolated from Fermented Soybean Food: Soy Sauce and Soybean Paste. Foods, 14(14), 2407. https://doi.org/10.3390/foods14142407









