Physicochemical and Sensory Evaluation of Romanian Monofloral Honeys from Different Supply Chains
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Classification
2.2. Reagents and Materials
2.3. Identification of Floral Origin of Honey Samples
2.4. Determination of pH and Titratable Acidity in Honey Samples
2.5. Determination of Moisture Content and Total Solids
2.6. Determination of Hydroxymethyl Furfural (HMF) Content
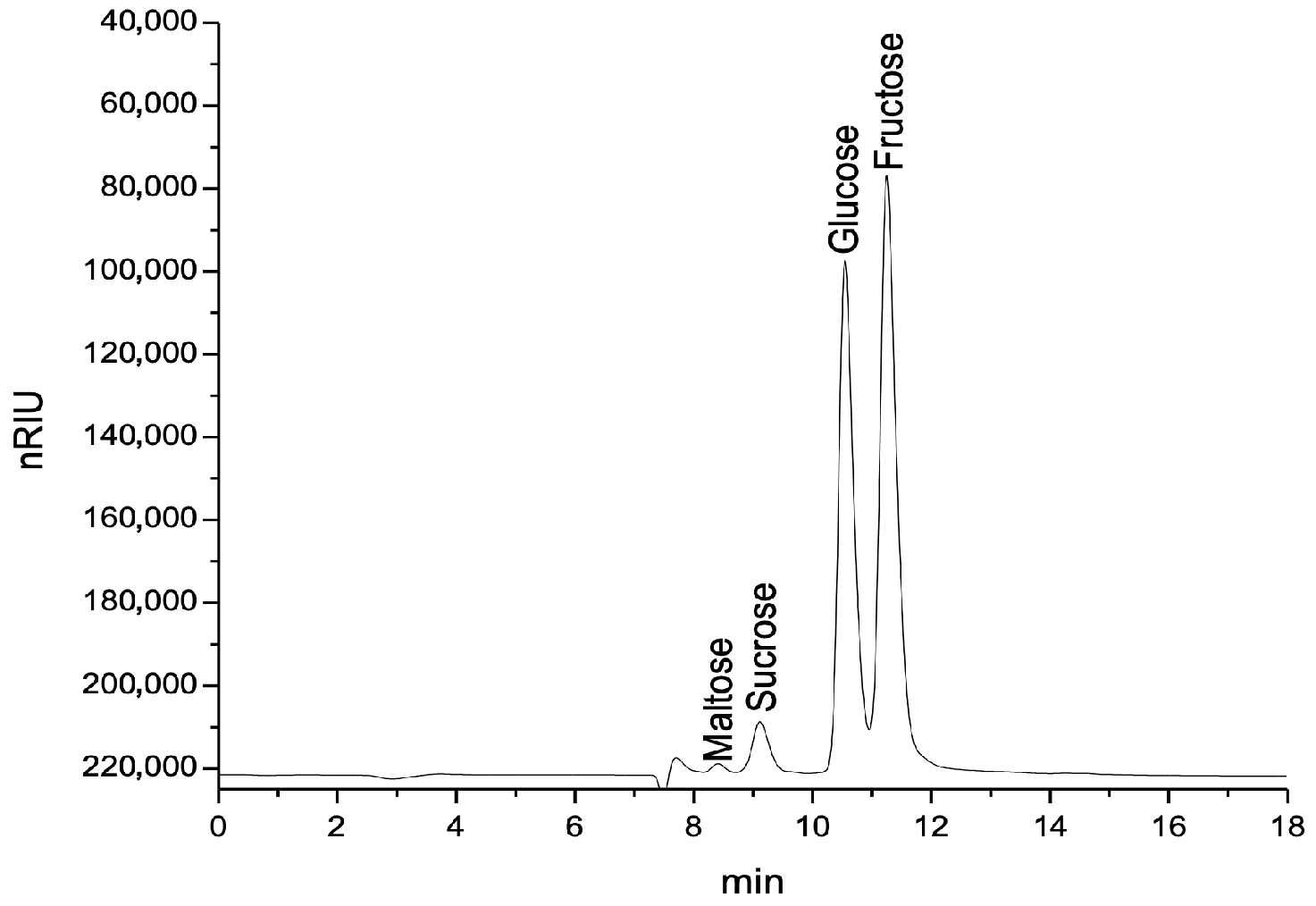
2.7. Determination of Carbohydrates by HPLC-RID
2.7.1. Sample Preparation and Carbohydrate Extraction
2.7.2. Chromatographic Conditions for Carbohydrate Analysis
2.8. Estimation of Glycemic Index
2.9. Determination of Proline Content
2.10. Sensory Evaluation
2.11. Statistical and Correlation Analysis
3. Results
3.1. Microscopic Characterization of Pollen Morphology
3.2. Acidity and pH of Honey
| Honey Sample | Acidity mEq/kg | pH |
|---|---|---|
| AH_P | 7.73 ± 0.03 a | 3.83 ± 0.05 ab |
| AH_C | 7.84 ± 0.06 a | 3.85 ± 0.06 ab |
| RH_P | 21.21 ± 0.02 e | 3.72 ± 0.08 a |
| RH_C | 19.73 ± 0.12 d | 4.05 ± 0.06 bc |
| TLH_P | 17.43 ± 0.11 c | 5.06 ± 0.14 d |
| TLH_C | 15.71 ± 0.10 b | 4.20 ± 0.10 c |
| LH_P | 21.40 ± 0.11 e | 3.69 ± 0.10 a |
| TH_P | 21.39 ± 0.02 e | 3.81 ± 0.10 ab |
3.3. Moisture and Total Solids
| Honey Sample | Refractive Index nD 20 °C | Water Content (g/100 g) |
|---|---|---|
| AH_P | 1.4946 d | 16.80 ± 1.1 ab |
| AH_C | 1.4925 e | 17.60 ± 0.35 bc |
| RH_P | 1.4875 a | 19.60 ± 0.3 e |
| RH_C | 1.4946 e | 16.80 ± 0.6 ab |
| TLH_P | 1.4951 e | 16.60 ± 0.1 ab |
| TLH_C | 1.4910 c | 18.20 ± 0.55 cd |
| LH_P | 1.4885 b | 19.20 ± 0.1 de |
| TH_P | 1.5054 f | 16.25 ± 0.05 a |
3.4. Hydroxymethyl Furfural (HMF) Content
3.5. Carbohydrate Composition, HPLC-RID Method
3.6. Proline Content
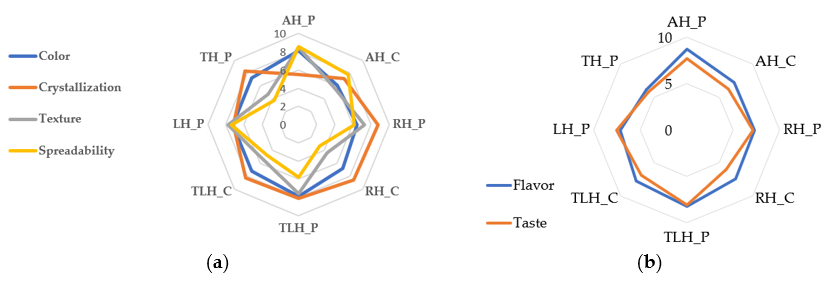
3.7. Sensory Evaluation
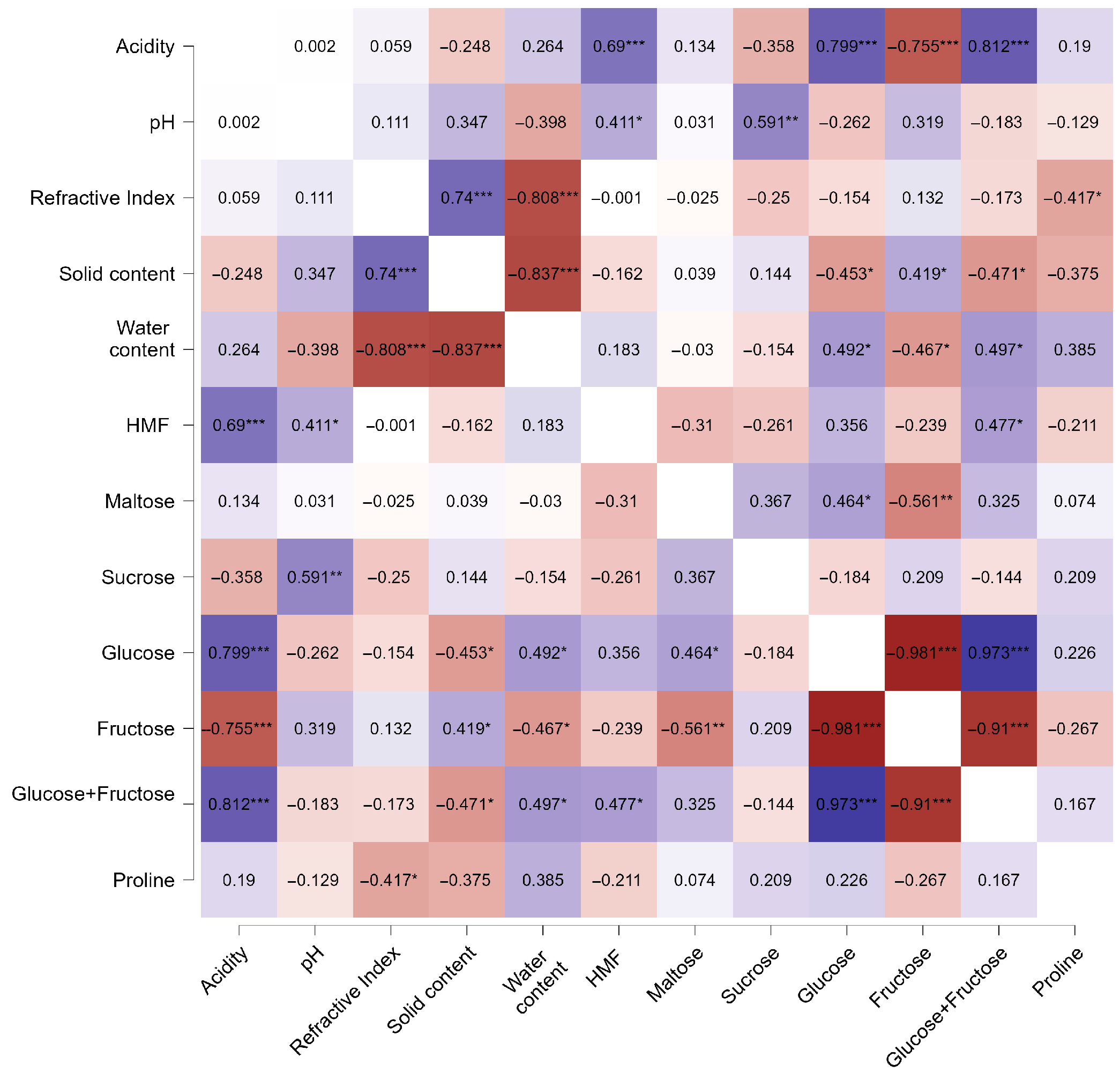
3.8. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AH | Acacia honey (Robinia pseudoacacia L.) |
| AH_C | Acacia honey commercial |
| AH_P | Acacia honey producer |
| C | Commercial |
| eGI | Estimated glycemic index |
| EU | European Union |
| F | Fructose |
| G | Glucose |
| GI | Glycemic index |
| HMF | Hydroxymethyl furfural |
| HPLC-RID | High-Performance Liquid Chromatography with Refractive Index Detection |
| H2SO4 | Sulfuric acid |
| IHC | International Honey Commission |
| LH | Lavender honey (Lavandula angustifolia L.) |
| LH_P | Producer lavender honey |
| M | Maltose |
| mEq | Milliequivalent |
| mg | Milligram |
| mL | Milliliter |
| P | Producer |
| PCA | Principal Component Analysis |
| RH | Rapeseed honey (Brassica napus L.) |
| RH_C | Commercial rapeseed honey |
| RH_P | Producer rapeseed honey |
| S | Sucrose |
| SD | Standard deviation |
| TA | Titratable acidity |
| TH | Breckland Thyme honey (Thymus serpyllum L.) |
| TH_C | Commercial Breckland Thyme honey |
| TH_P | Producer Breckland Thyme honey |
| TLH | Linden honey (Tilia spp.) |
| TLH_C | Commercial linden honey |
| TLH_P | Producer linden honey |
| TS | Total solids |
| USD | United States dollar |
| UV-vis | Ultraviolet–visible |
References
- Vică, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Heghedűş-Mîndru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungău, S. The Antimicrobial Activity of Honey and Propolis Extracts from the Central Region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Bungau, S.G.; Popa, V.C. Between Religion and Science: Some Aspects: Concerning Illness and Healing in Antiquity. Transylvanian Rev. 2015, 24, 3–19. [Google Scholar]
- Honey Statistics 2025 by Taste, Quality, Production. Available online: https://media.market.us/honey-statistics/ (accessed on 25 May 2025).
- Honey Industry 2025. Available online: https://www.reportlinker.com/market-report/Processed-Food/505104/Honey?term=honeymarket&matchtype=p&loc_interest=&loc_physical=1011795&utm_group=standard&utm_term=honeymarket&utm_campaign=ppc&utm_source=google_ads&utm_medium=paid_ads&utm_content=transac (accessed on 25 May 2025).
- Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. Available online: https://eur-lex.europa.eu/eli/dir/2001/110/oj/eng (accessed on 25 May 2025).
- Codex Alimentarius. International Food Standards. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 25 May 2025).
- The Food and Drink (Miscellaneous Amendments Relating to Food and Wine Composition, Information and Labelling) Regulations 2021. Available online: https://www.legislation.gov.uk/uksi/2021/632/made (accessed on 25 May 2025).
- Romanian Honey Production Has Decreased Drastically Due to Excessive Temperatures. Available online: https://www.agroberichtenbuitenland.nl/actueel/nieuws/2024/09/04/romanian-honey-production-has-decreased-drastically-due-to-excessive-temperatures (accessed on 25 May 2025).
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar]
- Abdel-Daim, M.M.; Abo-EL-Sooud, K.; Aleya, L.; Bungau, S.G.; Najda, A.; Saluja, R. Alleviation of Drugs and Chemicals Toxicity: Biomedical Value of Antioxidants. Oxid. Med. Cell Longev. 2018, 2018, 6276438. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, S.; Jia, W.; Guo, T.; Wang, F.; Li, J.; Yao, Z. Natural Antimicrobials from Plants: Recent Advances and Future Prospects. Food Chem. 2024, 432, 137231. [Google Scholar] [CrossRef] [PubMed]
- Olah, N.; Petrescu, S.; Marian, E.; Tünde, J.; Marc, F.; Dobjanschi, L.; Honiges, A.; Kiss, R.; Bechir, E.; Curt-Mola, F.; et al. The Study of Antioxidant Capacity in Extracts from Vegetal Sources with Hypoglycaemic Action. Rev. Chim. 2019, 70, 102–106. [Google Scholar] [CrossRef]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Miraldi, E.; Cappellucci, G.; Del Casino, C.; Giordano, E.; Guarnieri, M.; Nepi, M.; Biagi, M.; Baini, G. Eudermic Properties and Chemical-Physical Characterization of Honeys of Different Botanical Origin. Nutrients 2024, 16, 3647. [Google Scholar] [CrossRef]
- Baloš, M.; Popov, N.; Prodanov-Radulovic, J.; Stojanov, I.; Jakšić, S. Sugar Profile of Different Floral Origin Honeys from Serbia. J. Apic. Res. 2020, 59, 398–405. [Google Scholar] [CrossRef]
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of Honey Criteria and Standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Dobrinas, S.; Soceanu, A.; Birghila, S.; Birghila, C.; Matei, N.; Popescu, V.; Constanda, L.M. Chemical Analysis and Quality Assessment of Honey Obtained from Different Sources. Processes 2022, 10, 2554. [Google Scholar] [CrossRef]
- Vîjan, L.E.; Mazilu, I.C.; Enache, C.; Enache, S.; Topală, C.M. Botanical Origin Influence on Some Honey Physicochemical Characteristics and Antioxidant Properties. Foods 2023, 12, 2134. [Google Scholar] [CrossRef] [PubMed]
- Mongi, R.J. Influence of Botanical Origin and Geographical Zones on Physicochemical Properties, Mineral Contents and Consumer Acceptance of Honey in Tanzania. Food Chem. Adv. 2024, 4, 100731. [Google Scholar] [CrossRef]
- Luca, L.; Pauliuc, D.; Ursachi, F.; Oroian, M. Physicochemical Parameters, Microbiological Quality, and Antibacterial Activity of Honey from the Bucovina Region of Romania. Sci. Rep. 2025, 15, 4358. [Google Scholar] [CrossRef]
- Kamal, M.M.; Rashid, M.H.U.; Mondal, S.C.; El Taj, H.F.; Jung, C. Physicochemical and Microbiological Characteristics of Honey Obtained through Sugar Feeding of Bees. J. Food Sci. Technol. 2019, 56, 2267–2277. [Google Scholar] [CrossRef]
- Parviz, M.; Karimi, F.; Rezaei, M.; Javanmard, M.R.; Javadzadeh, M.; Allahdadi, G. Assessment of the Physicochemical Quality of Iranian Honey. Qual. Assur. Saf. Crop Foods 2015, 1, 629–634. [Google Scholar] [CrossRef]
- Hajian-Tilaki, A.; Kenari, R.E.; Farahmandfar, R.; Razavi, R. Comparative Study of Physiochemical Properties in Iranian Multi-Floral Honeys: Local vs. Commercial Varieties. Heliyon 2024, 10, e37550. [Google Scholar] [CrossRef]
- Ntakoulas, D.D.; Tsagkaris, A.S.; Raptis, S.; Pasias, I.N.; Raptopoulou, K.G.; Kharoshka, A.; Schulzova, V.; Proestos, C. Study of Authenticity, Quality Characteristics and Bioactivity in Honey Samples from Different Botanical Origins and Countries. J. Food Compos. Anal. 2024, 136, 106716. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Ferreira-Santos, P.; Genisheva, Z.; El Ghouizi, A.; Aboulghazi, A.; Teixeira, J.A.; Lyoussi, B. Protective Effect of Honey and Propolis against Gentamicin-Induced Oxidative Stress and Hepatorenal Damages. Oxid. Med. Cell Longev. 2021, 2021, 9719906. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Maruska, A.; Kornysova, O.; Charczun, N.; Ligor, M.; Buszewski, B. Quantitative and Qualitative Determination of Phenolic Compounds in Honey. CHEMINĖ Technol. 2009, 352, 74–80. [Google Scholar]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International Tables of Glycemic Index and Glycemic Load Values 2021: A Systematic Review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Arcot, J.; Brand-Miller, J. A Preliminary Assessment of the Glycemic Index of Honey—A Report for the Rural Industries Research and Development Corporation; RIRDC: Kingston, Australia, 2005; ISBN 1 74151 126 7. [Google Scholar]
- Alqarni, A.S.; Owayss, A.A.; Mahmoud, A.A. Physicochemical Characteristics, Total Phenols and Pigments of National and International Honeys in Saudi Arabia. Arab. J. Chem. 2016, 9, 114–120. [Google Scholar] [CrossRef]
- Shambaugh, P.; Worthington, V.; Herbert, J.H. Differential Effects of Honey, Sucrose, and Fructose on Blood Sugar Levels. J. Manipulative Physiol. Ther. 1990, 13, 322–325. [Google Scholar] [PubMed]
- da Costa, I.F.; Toro, M.J.U. Evaluation of the Antioxidant Capacity of Bioactive Compounds and Determination of Proline in Honeys from Pará. J. Food Sci. Technol. 2021, 58, 1900–1908. [Google Scholar] [CrossRef]
- Amin, P.; Riyadi, P.; Kurniasih, R.; Husni, A. Utilization of κ-Carrageenan as Stabilizer and Thickener of Honey Pineapple (Ananas comosus [L. Merr]) Jam. Food Res. 2022, 6, 93–98. [Google Scholar] [CrossRef]
- Haș, I.M.; Vodnar, D.-C.; Bungau, A.F.; Tarce, A.G.; Tit, D.M.; Teleky, B.-E. Enhanced Elderberry Snack Bars: A Sensory, Nutritional, and Rheological Evaluation. Foods 2023, 12, 3544. [Google Scholar] [CrossRef]
- European Pharmacopoeia 11th Edition. Available online: https://pheur.edqm.eu/home (accessed on 15 May 2025).
- PalDat—A Palynological Database. Available online: https://www.paldat.org/ (accessed on 28 June 2025).
- Crăciun, M.E.; Pârvulescu, O.C.; Donise, A.C.; Dobre, T.; Stanciu, D.R. Characterization and Classification of Romanian Acacia Honey Based on Its Physicochemical Parameters and Chemometrics. Sci. Rep. 2020, 10, 20690. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Quality Evaluation of Light- and Dark-Colored Hungarian Honeys, Focusing on Botanical Origin, Antioxidant Capacity and Mineral Content. Molecules 2021, 26, 2825. [Google Scholar] [CrossRef]
- Sipos, L.; Végh, R.; Bodor, Z.; Zaukuu, J.-L.Z.; Hitka, G.; Bázár, G.; Kovacs, Z. Classification of Bee Pollen and Prediction of Sensory and Colorimetric Attributes—A Sensometric Fusion Approach by e-Nose, e-Tongue and NIR. Sensors 2020, 20, 6768. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Feás, X.; Iglesias, A.; Estevinho, L.M. Study of Organic Honey from the Northeast Portugal. Molecules 2011, 16, 5374–5386. [Google Scholar] [CrossRef] [PubMed]
- Directive2014/63/EU of the European Parliament and of the Council of 15 May 2014 Amending Council Directive 2001/110/EC Relating to Honey. Available online: https://eur-lex.europa.eu/eli/dir/2014/63/oj (accessed on 28 June 2025).
- Escuredo, O.; Rodríguez-Flores, M.S.; Rojo-Martínez, S.; Seijo, M.C. Contribution to the Chromatic Characterization of Unifloral Honeys from Galicia (NW Spain). Foods 2019, 8, 233. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Tasić, A.; Pezo, L.; Lončar, B.; Pešić, M.B.; Tešić, Ž.; Kalaba, M. Assessing the Impact of Botanical Origins, Harvest Years, and Geographical Variability on the Physicochemical Quality of Serbian Honey. Foods 2024, 13, 1530. [Google Scholar] [CrossRef]
- Pașca, C.; Mărghitaș, L.A.; Matei, I.A.; Bonta, V.; Mărgăoan, R.; Copaciu, F.; Bobiș, O.; Campos, M.G.; Dezmirean, D.S. Screening of Some Romanian Raw Honeys and Their Probiotic Potential Evaluation. Appl. Sci. 2021, 11, 5816. [Google Scholar] [CrossRef]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Physicochemical Characterization and Antioxidant Activity of Palestinian Honey Samples. Food Sci. Nutr. 2018, 6, 2056–2065. [Google Scholar] [CrossRef]
- Gašić, U.; Kečkeš, S.; Dabić, D.; Trifkovic, J.; Milojković-Opsenica, D.; Natić, M.; Tesic, Z. Phenolic Profile and Antioxidant Activity of Serbian Polyfloral Honeys. Food Chem. 2014, 145, 599–607. [Google Scholar] [CrossRef]
- Melaku, M.; Tefera, W. Physicochemical Properties, Mineral and Heavy Metal Contents of Honey in Eastern Amhara Region, Ethiopia. J. Food Compos. Anal. 2022, 114, 104829. [Google Scholar] [CrossRef]
- Osaili, T.M.; Odeh, W.A.M.B.; Al Sallagi, M.S.; Al Ali, A.A.S.A.; Obaid, R.S.; Garimella, V.; Bakhit, F.S.B.; Hasan, H.; Holley, R.; El Darra, N. Quality of Honey Imported into the United Arab Emirates. Foods 2023, 12, 729. [Google Scholar] [CrossRef]
- Althaiban, M.A. Investigation of Hydroxymethylfurfural Levels in Commercial Acacia Honey for Quality Control: A Systematic Review. Discov. Appl. Sci. 2024, 6, 515. [Google Scholar] [CrossRef]
- Ligor, M.; Kowalkowski, T.; Buszewski, B. Comparative Study of the Potentially Toxic Elements and Essential Microelements in Honey Depending on the Geographic Origin. Molecules 2022, 27, 5474. [Google Scholar] [CrossRef]
- Mongi, R.J.; Ruhembe, C.C. Sugar Profile and Sensory Properties of Honey from Different Geographical Zones and Botanical Origins in Tanzania. Heliyon 2024, 10, e38094. [Google Scholar] [CrossRef]
- Czipa, N.; Phillips, C.J.C.; Kovács, B. Composition of Acacia Honeys Following Processing, Storage and Adulteration. J. Food Sci. Technol. 2019, 56, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, R.; Bera, M.; Nanda, V. Chemometric Classification of Northern India Unifloral Honey. Acta Aliment. 2013, 42, 540–551. [Google Scholar] [CrossRef]
- Tsagkaris, A.; Koulis, G.; Danezis, G.; Martakos, I.C.; Dasenaki, M.; Georgiou, C.; Thomaidis, N. Honey Authenticity: Analytical Techniques, State of the Art and Challenges. RSC Adv. 2021, 11, 11273–11294. [Google Scholar] [CrossRef] [PubMed]
- Alaerjani, W.M.A.; Mohammed, M.E.A. Impact of Floral and Geographical Origins on Honey Quality Parameters in Saudi Arabian Regions. Sci. Rep. 2024, 14, 8720. [Google Scholar] [CrossRef]
- Palomo-de León, B.A.; Castro, H.; Sánchez-Murillo, M.E.; de la Garza, A.L.; Rodríguez-Romero, B.A.; Alonzo-Macías, M.; Ramírez-Jiménez, A.K.; Cardador-Martínez, A.; Hernández-Salazar, M. Glycemic and Satiety Response to Three Mexican Honey Varieties. Foods 2023, 12, 3670. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Cui, Z.; Wang, T.; Liu, T.; Liu, G. Analysis of Free Amino Acid Composition and Honey Plant Species in Seven Honey Species in China. Foods 2024, 13, 1065. [Google Scholar] [CrossRef]
- Damto, T.; Birhanu, T.; Zewdu, A. Physicochemical and Antioxidant Characterization of Commercially Available Honey Sample from Addis Ababa Market, Ethiopia. Heliyon 2023, 9, e20830. [Google Scholar] [CrossRef]
- Mello Dos Santos, M.; Khan, N.; Lim, L.Y.; Locher, C. Antioxidant Activity, Physicochemical and Sensory Properties of Stingless Bee Honey from Australia. Foods 2024, 13, 1657. [Google Scholar] [CrossRef] [PubMed]
- Ayton, J.; Groves, L.; Berry, J. Chemical Composition and Quality Assessment of Apis Mellifera Honey in Australia. Food Sci. Nutr. 2025, 13, e70160. [Google Scholar] [CrossRef] [PubMed]
- Tigistu, T.; Worku, Z.; Mohammed, A. Evaluation of the Physicochemical Properties of Honey Produced in Doyogena and Kachabira Districts of Kembata Tambaro Zone, Southern Ethiopia. Heliyon 2021, 7, e06803. [Google Scholar] [CrossRef] [PubMed]
- Bereksi-Reguig, D.; Allali, H.; Taib, N.; Aissaoui, N.; Wlodarczyk-Stasiak, M.; Kowalski, R. Bioactive Compounds, Antioxidant Properties, and Antimicrobial Profiling of a Range of West Algerian Honeys: In Vitro Comparative Screening Prior to Therapeutic Purpose. Foods 2024, 13, 4120. [Google Scholar] [CrossRef]
- Al-Habsi, N.A.; Davis, F.J.; Niranjan, K. Development of Novel Methods to Determine Crystalline Glucose Content of Honey Based on DSC, HPLC, and Viscosity Measurements, and Their Use to Examine the Setting Propensity of Honey. J. Food Sci. 2013, 78, E845–E852. [Google Scholar] [CrossRef]
- Weber, M.; Meixner, M.; Dasbach, R.; Rozhon, W.; Dasbach, M. Analysis of Sugar Crystal Size in Honey. MethodsX 2022, 9, 101823. [Google Scholar] [CrossRef]
- Polatidou, K.; Nouska, C.; Tananaki, C.; Biliaderis, C.G.; Lazaridou, A. Physicochemical and Rheological Characteristics of Monofloral Honeys-Kinetics of Creaming-Crystallization. Foods 2025, 14, 1835. [Google Scholar] [CrossRef]
- de Sousa, J.M.B.; de Souza, E.L.; Marques, G.; de Benassi, M.T.; Gullón, B.; Pintado, M.M.; Magnani, M. Sugar Profile, Physicochemical and Sensory Aspects of Monofloral Honeys Produced by Different Stingless Bee Species in Brazilian Semi-Arid Region. LWT 2016, 65, 645–651. [Google Scholar] [CrossRef]
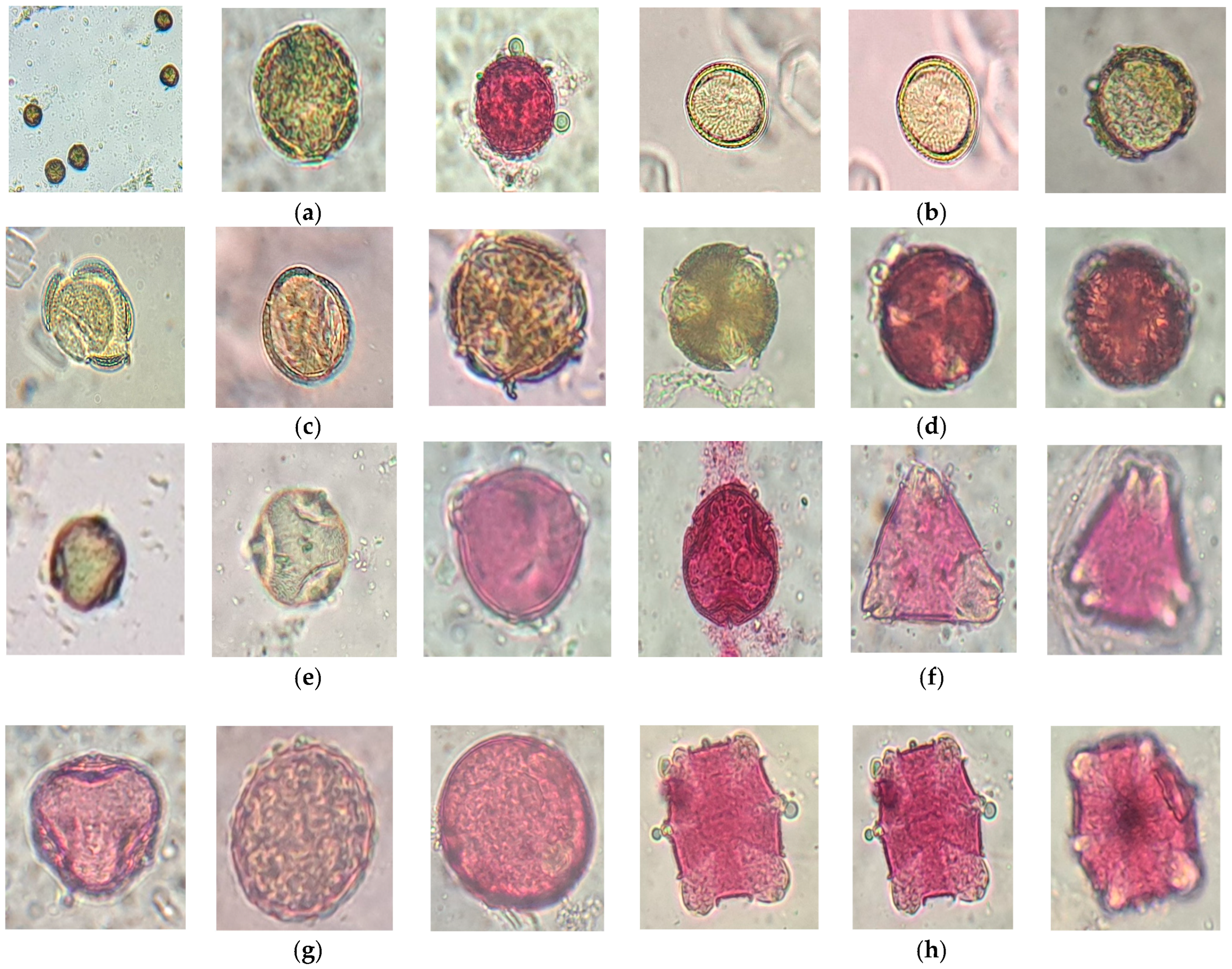





| Sample Code | Botanical Origin | Source Type | Collection Region |
|---|---|---|---|
| AH_P | Acacia (Robinia pseudoacacia L.) | Producer | Brăila County |
| AH_C | Commercial | National retail | |
| RH_P | Rapeseed (Brassica napus L.) | Producer | Buzău County |
| RH_C | Commercial | National retail | |
| TLH_P | Linden (Tilia spp.) | Producer | Buzău County |
| TLH_C | Commercial | National retail | |
| LH_P | Lavender (Lavandula angustifolia L.) | Producer | Vaslui County |
| TH_P | Thyme (Thymus spp.) | Producer | Sălaj County |
| Pk | Rt (min) | Compound | AH_P | AH_C | RH_P | RH_C | TLH_P | TLH_C | LH_P | TH_P |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.37 | Maltose | 1.423 ± 0.01 e | 0.496 ± 0.01 a | 1.445 ± 0.01 e | 1.595 ± 0.02 f | 0.929 ± 0.01 c | 1.663 ± 0.01 g | 0.616 ± 0.01 b | 1.038 ± 0.01 d |
| 2 | 9.16 | Sucrose | 2.822 ± 0.01 c | 3.103 ± 0.01 e | 3.024 ± 0.01 d | 3.217 ± 0.02 f | 3.404 ± 0.01 g | 3.107 ± 0.01 e | 1.983 ± 0.01 a | 2.121 ± 0.01 b |
| 3 | 10.55 | Glucose | 22.841 ± 0.1 b | 20.277 ± 0.01 a | 42.354 ± 0.2 h | 31.520 ± 0.1 e | 25.426 ± 0.1 c | 31.988 ± 0.2 f | 30.494 ± 0.1 d | 33.626 ± 0.1 g |
| 4 | 11.27 | Fructose | 47.151 ± 0.1 e | 49.507 ± 0.2 f | 37.746 ± 0.1 a | 42.696 ± 0.2 c | 47.305 ± 0.1 e | 41.589 ± 0.2 b | 43.592 ± 0.2 d | 41.736 ± 0.2 b |
| Fructose + glucose | 69.99 ± 0.14 b | 69.78 ± 0.14 a | 80.10 ± 0.22 e | 74.22 ± 0.22 d | 72.73 ± 0.14 c | 73.58 ± 0.22 d | 74.09 ± 0.22 c | 75.36 ± 0.22 d | ||
| Fructose/glucose ratio | 2.06 | 2.44 | 0.89 | 1.35 | 1.86 | 1.3 | 1.43 | 1.24 | ||
| Sample | Color | Crystallization | Spreadability | Texture | Flavor | Taste |
|---|---|---|---|---|---|---|
| AH_C | 5.9 ± 0.96 a | 7.3 ± 1.02 b | 7.77 ± 0.82 e,f | 6.03 ± 0.96 c | 7.47 ± 0.82 b,c | 6.4 ± 1.0 a,b |
| AH_P | 7.97 ± 0.85 e | 5.3 ± 1.06 a | 8.43 ± 0.63 f | 8.37 ± 0.81 e | 8.57 ± 0.68 d | 7.6 ± 1.0 c,d |
| LH_P | 7.2 ± 0.92 c,d | 7.03 ± 1.13 b | 7.63 ± 0.93 e | 7.63 ± 0.93 d,e | 7.2 ± 1.03 b | 7.43 ± 0.94 c,d |
| RH_C | 6.87 ± 0.97 b,c | 8.57 ± 0.73 c | 3.5 ± 0.86 a | 4.23 ± 0.97 a | 7.67 ± 0.88 b,c | 5.97 ± 1.3 a |
| RH_P | 6.43 ± 1.04 a,b | 8.7 ± 0.53 c | 6.57 ± 0.97 d | 7.3 ± 1.26 d | 7.1 ± 0.8 b | 7.4 ± 1.1 c,d |
| TH_P | 7.3 ± 0.95 c,d,e | 8.17 ± 0.83 c | 4.0 ± 1.05 a | 4.97 ± 0.85 a,b | 5.93 ± 1.08 a | 5.93 ± 1.11 a |
| TLH_C | 7.57 ± 0.94 c,d,e | 8.17 ± 0.83 c | 4.8 ± 1.16 b | 5.4 ± 1.16 b,c | 7.6 ± 1.07 b,c | 7.0 ± 1.02 b,c |
| TLH_P | 7.7 ± 0.95 d,e | 8.27 ± 0.83 c | 5.77 ± 1.01 c | 7.6 ± 0.89 d,e | 8.13 ± 0.82 c,d | 7.97 ± 1.03 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bratosin, E.D.; Tit, D.M.; Pasca, M.B.; Purza, A.L.; Bungau, G.; Marin, R.C.; Radu, A.F.; Gitea, D. Physicochemical and Sensory Evaluation of Romanian Monofloral Honeys from Different Supply Chains. Foods 2025, 14, 2372. https://doi.org/10.3390/foods14132372
Bratosin ED, Tit DM, Pasca MB, Purza AL, Bungau G, Marin RC, Radu AF, Gitea D. Physicochemical and Sensory Evaluation of Romanian Monofloral Honeys from Different Supply Chains. Foods. 2025; 14(13):2372. https://doi.org/10.3390/foods14132372
Chicago/Turabian StyleBratosin, Elena Daniela, Delia Mirela Tit, Manuela Bianca Pasca, Anamaria Lavinia Purza, Gabriela Bungau, Ruxandra Cristina Marin, Andrei Flavius Radu, and Daniela Gitea. 2025. "Physicochemical and Sensory Evaluation of Romanian Monofloral Honeys from Different Supply Chains" Foods 14, no. 13: 2372. https://doi.org/10.3390/foods14132372
APA StyleBratosin, E. D., Tit, D. M., Pasca, M. B., Purza, A. L., Bungau, G., Marin, R. C., Radu, A. F., & Gitea, D. (2025). Physicochemical and Sensory Evaluation of Romanian Monofloral Honeys from Different Supply Chains. Foods, 14(13), 2372. https://doi.org/10.3390/foods14132372










