Evolving Dynamics of Fermented Food Microbiota and the Gut Microenvironment: Strategic Pathways to Enhance Human Health
Abstract
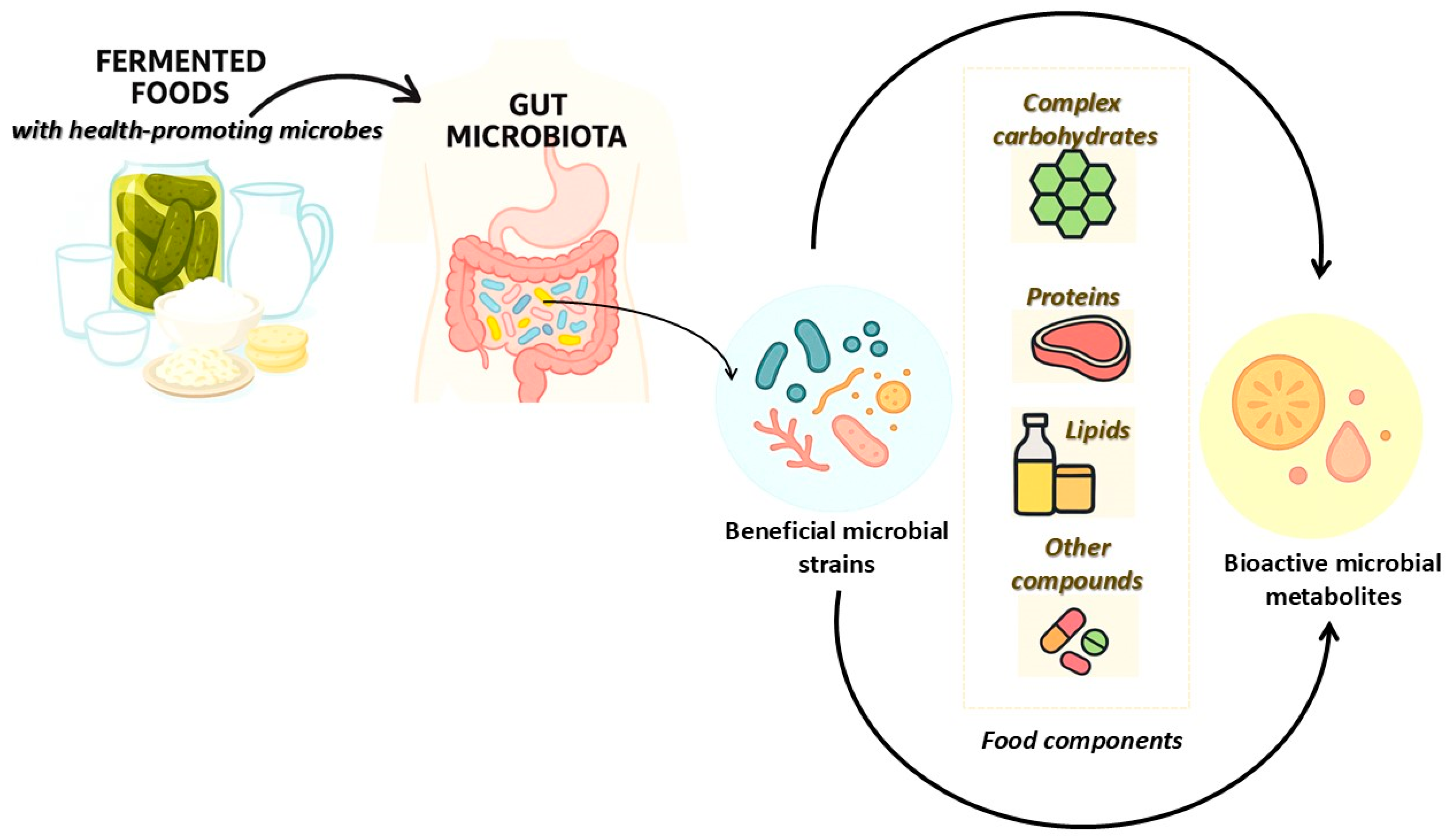
1. The Dynamic Interaction Between Consumption of Fermented Foods and Gut Microbiota
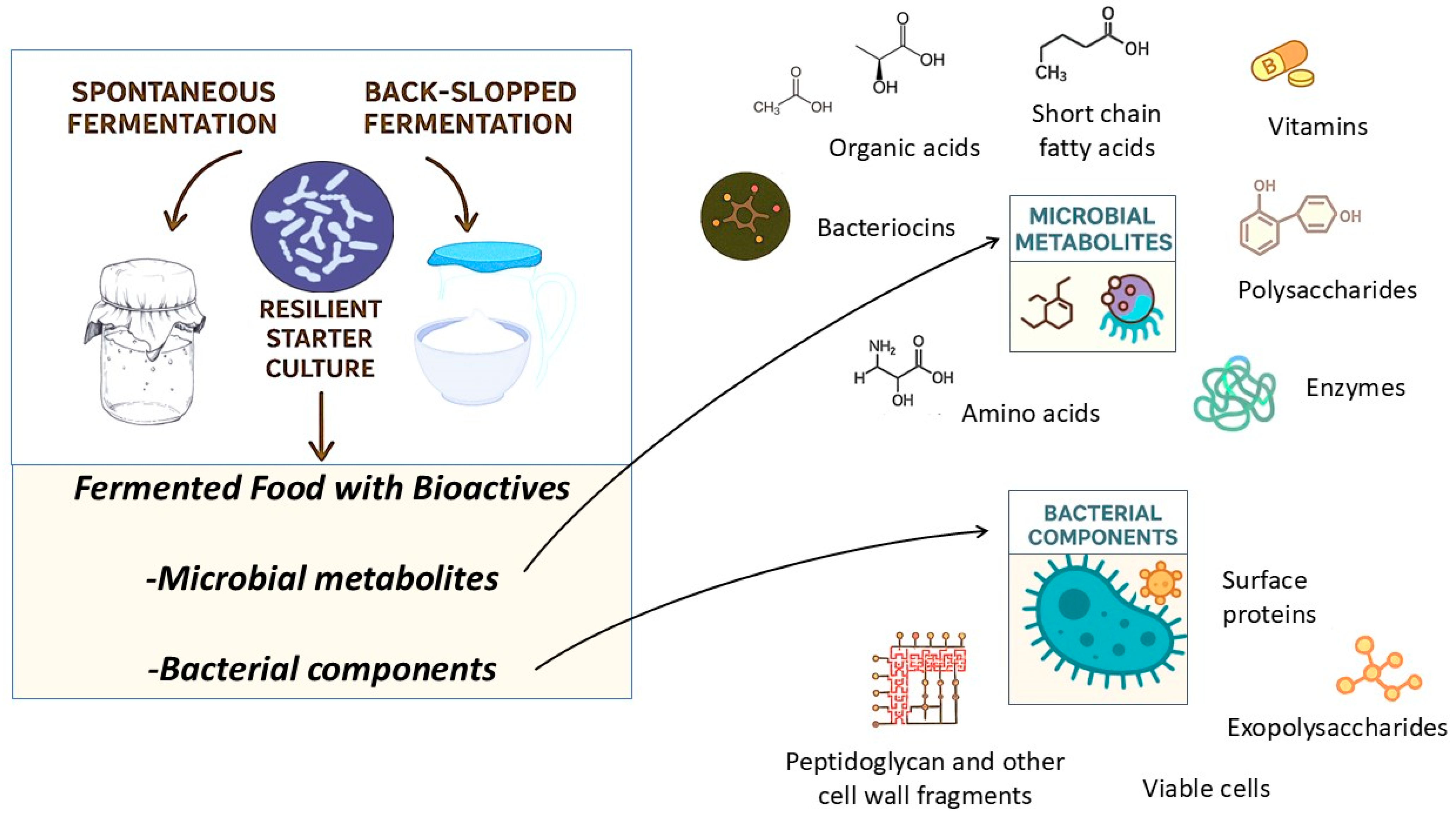
2. Fermentative Bioprocesses and Microbial Transformation of Food Substrates
3. Beneficial Microbes for Fermented Foods and Their Bioactive Metabolites
4. Fermentation-Driven Bioactive Compounds for Functional Food Applications
4.1. Bacteria and Yeasts for Fermentation
4.2. Microbially Derived Health-Promoting Metabolites in Fermented Foods
5. Impact of Diet on Sustaining Diversity in Gut Microbiota
6. Contribution of Dietary Microbiota in Alleviating Diseases
7. Limitations and Controversies in the Fermented Food–Gut Axis
8. Concluding Remarks and Future Strategies
Author Contributions
Funding
Conflicts of Interest
References
- Terpou, A. Selected Ethnic Fermented foods of Greece. In Fermented Food Products, 1st ed.; Sankaranarayanan, A., Dhanasekaran, N.A.D., Eds.; CRC Press: Boca Raton, FL, USA, 2020; p. 10. [Google Scholar] [CrossRef]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Terpou, A.; Rai, A.K. 2—Microbial transformation for improving food functionality. In Current Developments in Biotechnology and Bioengineering; Rai, A.K., Singh, S.P., Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 31–45. [Google Scholar] [CrossRef]
- Sahu, S.; Parija, T.; Panda, S.K. Chapter 25—Starter cultures: An insight into specific applications in flavoring and health promotion. In Indigenous Fermented Foods for the Tropics; Adebo, O.A., Chinma, C.E., Obadina, A.O., Soares, A.G., Panda, S.K., Gan, R.-Y., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 409–418. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. Use of Characterized Microorganisms in Fermentation of Non-Dairy-Based Substrates to Produce Probiotic Food for Gut-Health and Nutrition. Fermentation 2023, 9, 1. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Nunez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Kovtonyuk, L.V.; McCoy, K.D. Microbial metabolites and immunotherapy: Basic rationale and clinical indications. Semin. Immunol. 2023, 67, 101755. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Garavaglia, B.; Vallino, L.; Amoruso, A.; Pane, M.; Ferraresi, A.; Isidoro, C. The role of gut microbiota, immune system, and autophagy in the pathogenesis of inflammatory bowel disease: Molecular mechanisms and therapeutic approaches. Asp. Mol. Med. 2024, 4, 100056. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Schneider, E.; Gunnigle, E.; Cotter, P.D.; Cryan, J.F. Fermented foods: Harnessing their potential to modulate the microbiota-gut-brain axis for mental health. Neurosci. Biobehav. Rev. 2024, 158, 105562. [Google Scholar] [CrossRef]
- Hernández-Velázquez, R.; Flörl, L.; Lavrinienko, A.; Sebechlebská, Z.; Merk, L.; Greppi, A.; Bokulich, N.A. The future is fermented: Microbial biodiversity of fermented foods is a critical resource for food innovation and human health. Trends Food Sci. Technol. 2024, 150, 104569. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.S.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Mantzourani, I.; Alexopoulos, A.; Alexandri, M.; Kopsahelis, N.; Adamopoulou, V.; Bekatorou, A. Nutritional Improvements of Sourdough Breads Made with Freeze-Dried Functional Adjuncts Based on Probiotic Lactiplantibacillus plantarum subsp. plantarum and Pomegranate Juice. Antioxidants 2023, 12, 1113. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Portincasa, P.; Montemurro, M.; Di Palo, D.M.; Lorusso, M.P.; De Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough Fermented Breads are More Digestible than Those Started with Baker’s Yeast Alone: An In Vivo Challenge Dissecting Distinct Gastrointestinal Responses. Nutrients 2019, 11, 2954. [Google Scholar] [CrossRef]
- Plessas, S. Innovations in Sourdough Bread Making. Fermentation 2021, 7, 29. [Google Scholar] [CrossRef]
- Yilmaz, B.; Sharma, H.; Melekoglu, E.; Ozogul, F. Recent developments in dairy kefir-derived lactic acid bacteria and their health benefits. Food Biosci. 2022, 46, 101592. [Google Scholar] [CrossRef]
- Rafique, N.; Jan, S.Y.; Dar, A.H.; Dash, K.K.; Sarkar, A.; Shams, R.; Pandey, V.K.; Khan, S.A.; Amin, Q.A.; Hussain, S.Z. Promising bioactivities of postbiotics: A comprehensive review. J. Agric. Food Res. 2023, 14, 100708. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Wang, H.; Zhang, H.; Chen, W.; Lu, W. Lactic acid bacteria-derived exopolysaccharide: Formation, immunomodulatory ability, health effects, and structure-function relationship. Microbiol. Res. 2023, 274, 127432. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeong, J.Y.; Hwang, I.M.; Lee, J.-H. Modulation of fermentation dynamics in kimchi using Leuconostoc mesenteroides starter. Food Biosci. 2025, 66, 106317. [Google Scholar] [CrossRef]
- Chang, H.C. Healthy and safe Korean traditional fermented foods: Kimchi and chongkukjang. J. Ethn. Foods 2018, 5, 161–166. [Google Scholar] [CrossRef]
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W. Health Benefits of Kimchi (Korean Fermented Vegetables) as a Probiotic Food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A. Chapter 4—Importance of fermented foods on human health. In Gut Microbiota in Neurologic and Visceral Diseases; Farooqui, T., Farooqui, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 69–86. [Google Scholar] [CrossRef]
- Harahap, I.A.; Suliburska, J.; Karaca, A.C.; Capanoglu, E.; Esatbeyoglu, T. Fermented soy products: A review of bioactives for health from fermentation to functionality. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70080. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.; Tian, W.; Han, Y.; Xu, X.; Ren, T.; Tian, C.; Chen, C. Natto: A medicinal and edible food with health function. Chin. Herb. Med. 2023, 15, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Łepecka, A.; Szymański, P.; Okoń, A.; Zielińska, D. Antioxidant activity of environmental lactic acid bacteria strains isolated from organic raw fermented meat products. LWT 2023, 174, 114440. [Google Scholar] [CrossRef]
- Phong, H.X.; Viet, L.Q.; Chau, L.M.; Long, B.H.D.; Thanh, N.N.; Phat, D.T.; Truong, L.D. Isolation and Selection of Lactic Acid Bacteria with the Capacity of Producing γ-aminobutyric Acid (GABA) and Antimicrobial Activity: Its Application in Fermented Meat Product. Curr. Nutr. Food Sci. 2023, 19, 831–837. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, P.; Chen, X. Bioactive peptides derived from fermented foods: Preparation and biological activities. J. Funct. Foods 2023, 101, 105422. [Google Scholar] [CrossRef]
- Hill, C.; Tancredi, D.J.; Cifelli, C.J.; Slavin, J.L.; Gahche, J.; Marco, M.L.; Hutkins, R.; Fulgoni, V.L.; Merenstein, D.; Sanders, M.E. Positive Health Outcomes Associated with Live Microbe Intake from Foods, Including Fermented Foods, Assessed using the NHANES Database. J. Nutr. 2023, 153, 1143–1149. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Nutrition and Health through the Use of Probiotic Strains in Fermentation to Produce Non-Dairy Functional Beverage Products Supporting Gut Microbiota. Foods 2022, 11, 2760. [Google Scholar] [CrossRef]
- Sharma, R. Bioactive food components for managing cellular senescence in aging and disease: A critical appraisal and perspectives. PharmaNutrition 2021, 18, 100281. [Google Scholar] [CrossRef]
- Naseem, Z.; Mir, S.A.; Wani, S.M.; Rouf, M.A.; Bashir, I.; Zehra, A. Probiotic-fortified fruit juices: Health benefits, challenges, and future perspective. Nutrition 2023, 115, 112154. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kim, H.; Seo, K.H. Modern perspectives on the health benefits of kefir in next generation sequencing era: Improvement of the host gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cutini, M.A.; Almeida, S.A.; Nascimento, A.M.; Abreu, G.R.; Bissoli, N.S.; Lenz, D.; Endringer, D.C.; Brasil, G.A.; Lima, E.M.; Biancardi, V.C.; et al. Long-term treatment with kefir probiotics ameliorates cardiac function in spontaneously hypertensive rats. J. Nutr. Biochem. 2019, 66, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Iwatani, S.; Yamamoto, N. Functional food products in Japan: A review. Food Sci. Hum. Wellness 2019, 8, 96–101. [Google Scholar] [CrossRef]
- Nascimento da Silva, K.; Favero, A.G.; Ribeiro, W.; Ferreira, C.M.; Sartorelli, P.; Cardili, L.; Bogsan, C.S.; Bertaglia Pereira, J.N.; de Cassia Sinigaglia, R.; Cristina de Moraes Malinverni, A.; et al. Effects of kefir fermented milk beverage on sodium dextran sulfate (DSS)-induced colitis in rats. Heliyon 2023, 9, e12707. [Google Scholar] [CrossRef]
- Santos, A.; San Mauro, M.; Sanchez, A.; Torres, J.M.; Marquina, D. The antimicrobial properties of different strains of Lactobacillus spp. isolated from kefir. Syst. Appl. Microbiol. 2003, 26, 434–437. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Z.; Tian, X.; Guo, Y.; Zhang, H. Yeast β-d-glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens. Int. J. Biol. Macromol. 2016, 85, 573–584. [Google Scholar] [CrossRef]
- Hossain, M.I.; Sadekuzzaman, M.; Ha, S.-D. Probiotics as potential alternative biocontrol agents in the agriculture and food industries: A review. Food Res. Int. 2017, 100, 63–73. [Google Scholar] [CrossRef]
- Chifiriuc, M.C.; Cioaca, A.B.; Lazar, V. In vitro assay of the antimicrobial activity of kephir against bacterial and fungal strains. Anaerobe 2011, 17, 433–435. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Walker, J.W.; Diaz, H.; Madsen, K.L. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J. Nutr. 2006, 136, 1483–1487. [Google Scholar] [CrossRef]
- Mobasherpour, P.; Yavarmanesh, M.; Edalatian Dovom, M.R. Antitumor properties of traditional lactic acid bacteria: Short-chain fatty acid production and interleukin 12 induction. Heliyon 2024, 10, e36183. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, M.; Huang, Z.; Jiang, S.; Zhao, J.; Stanton, C.; Ross, R.P.; Chen, W.; Yang, B. Bifidobacterium longum subsp. Infantis modulates intestinal immunity in growing mice in a strain-specific manner. Food Biosci. 2025, 68, 106392. [Google Scholar] [CrossRef]
- Veiga, P.; Pons, N.; Agrawal, A.; Oozeer, R.; Guyonnet, D.; Brazeilles, R.; Faurie, J.M.; van Hylckama Vlieg, J.E.; Houghton, L.A.; Whorwell, P.J.; et al. Changes of the human gut microbiome induced by a fermented milk product. Sci. Rep. 2014, 4, 6328. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Park, J.S.; Kim, Y.-W.; Lee, W.; Park, K.; Kim, S.-K. Efficient surface display of single-chain variable fragments against tumor necrosis factor α on engineered probiotic Saccharomyces boulardii and its application in alleviating intestinal inflammation in vivo. New Biotechnol. 2025, 86, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Terpou, A.; Dasenaki, M.; Nigam, P.S. Current status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification. Sustain. Food Technol. 2023, 1, 500–510. [Google Scholar] [CrossRef]
- Chen, K.Y.; Luo, H.L.; Li, Y.Q.; Han, X.M.; Gao, C.C.; Wang, N.Y.; Lu, F.P.; Wang, H.K. TK1501 fermented soybeans alleviate dextran sulfate sodium-induced colitis by regulating intestinal cell function. J. Sci. Food Agric. 2023, 103, 5422–5431. [Google Scholar] [CrossRef]
- Shakerian, M.; Razavi, S.H.; Ziai, S.A.; Khodaiyan, F.; Yarmand, M.S.; Moayedi, A. Proteolytic and ACE-inhibitory activities of probiotic yogurt containing non-viable bacteria as affected by different levels of fat, inulin and starter culture. J. Food Sci. Technol. 2015, 52, 2428–2433. [Google Scholar] [CrossRef]
- Sharma, M.; Chandel, D.; Shukla, G. Antigenotoxicity and Cytotoxic Potentials of Metabiotics Extracted from Isolated Probiotic, Lactobacillus rhamnosus MD 14 on Caco-2 and HT-29 Human Colon Cancer Cells. Nutr. Cancer 2020, 72, 110–119. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Xue, J.; Yang, J.; Chen, X.; Shao, Y.; Kwok, L.Y.; Bilige, M.; Mang, L.; Zhang, H. Angiotensin-converting enzyme inhibitory activity of Lactobacillus helveticus strains from traditional fermented dairy foods and antihypertensive effect of fermented milk of strain H9. J. Dairy Sci. 2014, 97, 6680–6692. [Google Scholar] [CrossRef]
- Guo, W.; Song, Y.; Sun, Y.; Li, C.; Du, H.; You, Q.; Cai, Y.; Lang, Y.; Shao, L. Association Between Probiotic, Prebiotic, Synbiotics, and Yogurt Supplements and Diabetic Kidney Disease: The National Health and Nutrition Examination Survey 2007–2016. J. Ren. Nutr. 2025, S1051–S2276. [Google Scholar] [CrossRef]
- Lim, S.-M.; Lee, N.-K.; Kim, K.-T.; Paik, H.-D. Probiotic Lactobacillus fermentum KU200060 isolated from watery kimchi and its application in probiotic yogurt for oral health. Microb. Pathog. 2020, 147, 104430. [Google Scholar] [CrossRef]
- Uttarwar, R.G.; Mekonnen, S.A.; Van Beeck, W.; Wang, A.; Finnegan, P.; Roberts, R.F.; Merenstein, D.; Slupsky, C.M.; Marco, M.L. Effects of Bifidobacterium animalis subsp. lactis BB-12 and yogurt on mice during oral antibiotic administration. Microbiol. Res. 2024, 286, 127794. [Google Scholar] [CrossRef]
- Hata, Y.; Yamamoto, M.; Ohni, M.; Nakajima, K.; Nakamura, Y.; Takano, T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am. J. Clin. Nutr. 1996, 64, 767–771. [Google Scholar] [CrossRef]
- Mizushima, S.; Ohshige, K.; Watanabe, J.; Kimura, M.; Kadowaki, T.; Nakamura, Y.; Tochikubo, O.; Ueshima, H. Randomized controlled trial of sour milk on blood pressure in borderline hypertensive men. Am. J. Hypertens. 2004, 17, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Han, X.; Cen, S.; Duan, H.; Feng, S.; Xue, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol. Res. 2020, 233, 126409. [Google Scholar] [CrossRef] [PubMed]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of Fermented Milk Product With Probiotic Modulates Brain Activity. Gastroenterology 2013, 144, 1394–1401.e1394. [Google Scholar] [CrossRef]
- Prado, M.R.; Blandon, L.M.; Vandenberghe, L.P.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Shami, A.; Ali, M.A.; Almohawes, Z.N.; Mohammed, A.E.; Bin-Meferij, M.M. Kefir: A protective dietary supplementation against viral infection. Biomed. Pharmacother. 2021, 133, 110974. [Google Scholar] [CrossRef]
- Tu, M.-Y.; Han, K.-Y.; Chang, G.R.-L.; Lai, G.-D.; Chang, K.-Y.; Chen, C.-F.; Lai, J.-C.; Lai, C.-Y.; Chen, H.-L.; Chen, C.-M. Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice. Nutrients 2020, 12, 3432. [Google Scholar] [CrossRef]
- Tiss, M.; Souiy, Z.; Abdeljelil, N.b.; Njima, M.; Achour, L.; Hamden, K. Fermented soy milk prepared using kefir grains prevents and ameliorates obesity, type 2 diabetes, hyperlipidemia and Liver-Kidney toxicities in HFFD-rats. J. Funct. Foods 2020, 67, 103869. [Google Scholar] [CrossRef]
- Higurashi, S.; Kunieda, Y.; Matsuyama, H.; Kawakami, H. Effect of cheese consumption on the accumulation of abdominal adipose and decrease in serum adiponectin levels in rats fed a calorie dense diet. Int. Dairy J. 2007, 17, 1224–1231. [Google Scholar] [CrossRef]
- Decadt, H.; De Vuyst, L. Insights into the microbiota and defects of present-day Gouda cheese productions. Curr. Opin. Food Sci. 2023, 52, 101044. [Google Scholar] [CrossRef]
- Álvarez Ramos, L.; Arrieta Baez, D.; Dávila Ortiz, G.; Carlos Ruiz Ruiz, J.; Manuel Toledo López, V. Antioxidant and antihypertensive activity of Gouda cheese at different stages of ripening. Food Chem. X 2022, 14, 100284. [Google Scholar] [CrossRef]
- Smacchi, E.; Gobbetti, M. Peptides from several italian cheeses inhibitory to proteolytic enzymes of lactic acid bacteria, pseudomonas fluorescens ATCC 948 and to the angiotensin I-converting enzyme. Enzym. Microb. Technol. 1998, 22, 687–694. [Google Scholar] [CrossRef]
- Guidone, A.; Zotta, T.; Matera, A.; Ricciardi, A.; De Filippis, F.; Ercolini, D.; Parente, E. The microbiota of high-moisture mozzarella cheese produced with different acidification methods. Int. J. Food Microbiol. 2016, 216, 9–17. [Google Scholar] [CrossRef]
- Morandi, S.; Silvetti, T.; Battelli, G.; Brasca, M. Can lactic acid bacteria be an efficient tool for controlling Listeria monocytogenes contamination on cheese surface? The case of Gorgonzola cheese. Food Control 2019, 96, 499–507. [Google Scholar] [CrossRef]
- Feeney, E.L.; Barron, R.; Dible, V.; Hamilton, Z.; Power, Y.; Tanner, L.; Flynn, C.; Bouchier, P.; Beresford, T.; Noronha, N.; et al. Dairy matrix effects: Response to consumption of dairy fat differs when eaten within the cheese matrix—A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; Kim, Y.H.; Oh, S.; Lee, H.J.; Kim, J.H.; Park, S.H.; Kim, H.J.; Lee, S.J.; Chun, T. Anti-inflammatory potential of a heat-killed Lactobacillus strain isolated from Kimchi on house dust mite-induced atopic dermatitis in NC/Nga mice. J. Appl. Microbiol. 2017, 123, 535–543. [Google Scholar] [CrossRef]
- Lee, H.A.; Kim, H.; Lee, K.W.; Park, K.Y. Dietary Nanosized Lactobacillus plantarum Enhances the Anticancer Effect of Kimchi on Azoxymethane and Dextran Sulfate Sodium-Induced Colon Cancer in C57BL/6J Mice. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 147–159. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Toda, N.; Tamura, Y.; Terakado, S.; Ueno, M.; Otsuka, K.; Numabe, A.; Kawabata, Y.; Uehara, Y. Japanese traditional miso soup attenuates salt-induced hypertension and its organ damage in Dahl salt-sensitive rats. Nutrition 2012, 28, 924–931. [Google Scholar] [CrossRef]
- Cardoso Umbelino Cavallini, D.; Jovenasso Manzoni, M.S.; Bedani, R.; Roselino, M.N.; Celiberto, L.S.; Vendramini, R.C.; De Valdez, G.F.; Saes Parra Abdalla, D.; Aparecida Pinto, R.; Rosetto, D.; et al. Probiotic Soy Product Supplemented with Isoflavones Improves the Lipid Profile of Moderately Hypercholesterolemic Men: A Randomized Controlled Trial. Nutrients 2016, 8, 52. [Google Scholar] [CrossRef]
- Cha, Y.-S.; Edward, O.; Mun, E.-G. P31-011-23 Cheonggukjang Improves Obesity Related Parameters and Gut Microbiota Dysbiosis in High Fat Diet Induced Obese Mice. Curr. Dev. Nutr. 2023, 7, 101577. [Google Scholar] [CrossRef]
- Chen, H.; McGowan, E.M.; Ren, N.; Lal, S.; Nassif, N.; Shad-Kaneez, F.; Qu, X.; Lin, Y. Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomark. Insights 2018, 13, 1177271918785130. [Google Scholar] [CrossRef]
- Tubaro, F. 43—Antioxidant Activity of Beer’s Maillard Reaction Products: Features and Health Aspects. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 449–457. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Plessas, S. Advancements in the Use of Fermented Fruit Juices by Lactic Acid Bacteria as Functional Foods: Prospects and Challenges of Lactiplantibacillus (Lpb.) plantarum subsp. plantarum Application. Fermentation 2022, 8, 6. [Google Scholar] [CrossRef]
- Ojo, O.C.; Agboola, S.A. Antibacterial Effects of Palm Wine (Elaeis guineensis) on Salmonella typhi Isolated from Different Sources. Int. J. Pathog. Res. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Amoa-Awua, W.K.; Sampson, E.; Tano-Debrah, K. Growth of yeasts, lactic and acetic acid bacteria in palm wine during tapping and fermentation from felled oil palm (Elaeis guineensis) in Ghana. J. Appl. Microbiol. 2007, 102, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Prathiviraj, R.; Rajeev, R.; Jose, C.M.; Begum, A.; Selvin, J.; Kiran, G.S. Fermentation microbiome and metabolic profiles of Indian palm wine. Gene Rep. 2022, 27, 101543. [Google Scholar] [CrossRef]
- Stadnik, J.; Kęska, P. Meat and fermented meat products as a source of bioactive peptides. Acta Sci. Pol. Technol. Aliment. 2015, 14, 181–190. [Google Scholar] [CrossRef]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the microbial community of Japanese koji and miso: A review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef]
- Plessas, S. The Rendering of Traditional Fermented Foods in Human Diet: Distribution of Health Benefits and Nutritional Benefits. Fermentation 2022, 8, 751. [Google Scholar] [CrossRef]
- Basa, K.; Papanikolaou, S.; Dimopoulou, M.; Terpou, A.; Kallithraka, S.; Nychas, G.J.E. Trials of Commercial-and Wild-Type Saccharomyces cerevisiae Strains under Aerobic and Microaerophilic/Anaerobic Conditions: Ethanol Production and Must Fermentation from Grapes of Santorini (Greece) Native Varieties. Fermentation 2022, 8, 249. [Google Scholar] [CrossRef]
- Kallis, M.; Sideris, K.; Kopsahelis, N.; Bosnea, L.; Kourkoutas, Y.; Terpou, A.; Kanellaki, M. Pistacia terebinthus resin as yeast immobilization support for alcoholic fermentation. Foods 2019, 8, 127. [Google Scholar] [CrossRef]
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Plessas, S. Assessment of ready-to-use freeze-dried immobilized biocatalysts as innovative starter cultures in sourdough bread making. Foods 2019, 8, 40. [Google Scholar] [CrossRef]
- Gialleli, A.I.; Ganatsios, V.; Terpou, A.; Kanellaki, M.; Bekatorou, A.; Koutinas, A.A.; Dimitrellou, D. Technological development of brewing in domestic refrigerator using freeze-dried raw materials. Food Technol. Biotechnol. 2017, 55, 325–332. [Google Scholar] [CrossRef]
- Engels, W.; Düsterhöft, E.-M. Starter Cultures for Cheese Manufacture. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 352–357. [Google Scholar] [CrossRef]
- Laranjo, M.; Potes, M.E.; Elias, M. Role of Starter Cultures on the Safety of Fermented Meat Products. Front. Microbiol. 2019, 10, 853. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial terroir and food innovation: The case of yeast biodiversity in wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kathuria, D.; Singh, N. Nutritional and health promoting properties of traditional regional foods: Harnessing omics techniques for microbial and metabolite identification. J. Funct. Foods 2025, 130, 106919. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Suri, S.; Trif, M.; Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Meisel, H.; Bernard, H.; Fairweather-Tait, S.; FitzGerald, R.; Hartmann, R.; Lane, C.; McDonagh, D.; Teucher, B.; Wal, J. Nutraceutical and functional food ingredients for food and pharmaceutical applications. Br. J. Nutr. 2001, 85, 635. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, P.; Wu, X.; Wang, Y.; Rehman, T.; Yao, X.; Luo, Y.; Yang, Z. Expression of antimicrobial peptide Cecropin P1 in Saccharomyces cerevisiae and its antibacterial and antiviral activity in vitro. Electron. J. Biotechnol. 2021, 50, 16–22. [Google Scholar] [CrossRef]
- Govindarajan, D.K.; Kandaswamy, K. Antimicrobial peptides: A small molecule for sustainable healthcare applications. Med. Microecol. 2023, 18, 100090. [Google Scholar] [CrossRef]
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liu, Q.; Lei, Z.; Sun, T. Development and challenges of antimicrobial peptide delivery strategies in bacterial therapy: A review. Int. J. Biol. Macromol. 2023, 253, 126819. [Google Scholar] [CrossRef]
- Terpou, A.; Ganatsios, V.; Kanellaki, M.; Koutinas, A.A. Entrapped psychrotolerant yeast cells within pine sawdust for low temperature wine making: Impact on wine quality. Microorganisms 2020, 8, 764. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Fioriti, F.; Rifflet, A.; Gomperts Boneca, I.; Zugasti, O.; Royet, J. Bacterial peptidoglycan serves as a critical modulator of the gut-immune-brain axis in Drosophila. Brain Behav. Immun. 2024, 119, 878–897. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Kong, S.; Chen, N.; Hung, W.-L.; Zhao, W.; Zeng, Z.; Zhang, J.; Yang, Z. Lipoteichoic acid obtained from Lactobacillus paracasei via low-temperature pasteurization alleviates the macrophage inflammatory response by downregulating the NF-κB signaling pathway. J. Funct. Foods 2023, 107, 105673. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Karakuş, G. Chapter 12—The food-gut-health axis of dairy lactic acid bacteria. In Handbook of Sourdough Microbiota and Fermentation; Ozogul, F., Rocha, J.o.M., Bartkiene, E., Eds.; Academic Press: Oxford, UK, 2025; pp. 213–227. [Google Scholar] [CrossRef]
- Hajialibabaei, R.; Sayeli, F.G.; Aghadavod, E.; Poudineh, M.; Khaledi, A.; Bamneshin, K. The Beneficial Role of Probiotics and Gut Microbiota in Signaling Pathways, Immunity, Apoptosis, Autophagy, and intestinal barrier for Effective Wound Healing Post-Burn Injury. Microb. Pathog. 2025, 206, 107816. [Google Scholar] [CrossRef]
- Yi, C.; Huang, S.; Zhang, W.; Guo, L.; Xia, T.; Huang, F.; Yan, Y.; Li, H.; Yu, B. Synergistic interactions between gut microbiota and short chain fatty acids: Pioneering therapeutic frontiers in chronic disease management. Microb. Pathog. 2025, 199, 107231. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yu, Z.; Zhao, Y.; Li, L.; Liu, Y.; Liu, Y. Goat milk fermented with combined lactic acid bacterium alter microbial community structures and levels of the targeted short-chain fatty acids in the large intestine of mice. Food Res. Int. 2022, 157, 111352. [Google Scholar] [CrossRef]
- Sasaki, H.; Hayashi, K.; Imamura, M.; Hirota, Y.; Hosoki, H.; Nitta, L.; Furutani, A.; Shibata, S. Combined resistant dextrin and low-dose Mg oxide administration increases short-chain fatty acid and lactic acid production by gut microbiota. J. Nutr. Biochem. 2023, 120, 109420. [Google Scholar] [CrossRef]
- Balmori, V.; Marnpae, M.; Kamonsuwan, K.; Chusak, C.; Nungarlee, U.; Sivapornnukul, P.; Chanchaem, P.; Payungporn, S.; Charoensiddhi, S.; Suantawee, T.; et al. Comparative effects of non-fermented and Lacticaseibacillus paracasei-fermented pomelo juice on gut microbiota composition and short-chain fatty acid production: An in vitro colonic model. Food Chem. X 2024, 24, 102041. [Google Scholar] [CrossRef] [PubMed]
- Ajayeoba, T.A.; Ijabadeniyi, O.A. 9—Lactic acid bacteria for the generation of bioactive peptides. In Lactic Acid Bacteria as Cell Factories; Montet, D., Ray, R.C., De Carvalho Azevedo, V.A., Paramithiotis, S., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 165–182. [Google Scholar] [CrossRef]
- Möller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Sarikaya, B.; Kocyigit, E.; Atabilen, B.; Çelik, M.N.; Capasso, R.; Ağagündüz, D.; Budán, F. Contributions of Gamma-Aminobutyric Acid (GABA) Produced by Lactic Acid Bacteria on Food Quality and Human Health: Current Applications and Future Prospects. Foods 2024, 13, 2437. [Google Scholar] [CrossRef]
- Liang, Q.; Zhou, W.; Peng, S.; Liang, Z.; Liu, Z.; Zhu, C.; Mou, H. Current status and potential of bacteriocin-producing lactic acid bacteria applied in the food industry. Curr. Res. Food Sci. 2025, 10, 100997. [Google Scholar] [CrossRef]
- Saud, S.; Xiaojuan, T.; Fahad, S. The consequences of fermentation metabolism on the qualitative qualities and biological activity of fermented fruit and vegetable juices. Food Chem. X 2024, 21, 101209. [Google Scholar] [CrossRef]
- Gawai, K.M.; Mudgal, S.P.; Prajapati, J.B. Chapter 3—Stabilizers, Colorants, and Exopolysaccharides in Yogurt. In Yogurt in Health and Disease Prevention; Shah, N.P., Ed.; Academic Press: Oxford, UK, 2017; pp. 49–68. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Cruz, A.G.; Pereira, E.; Almeida da Costa, W.K.; da Silva Rocha, R.; Targino de Souza Pedrosa, G.; Rocha, C.d.S.; Alves, J.M.; Alvarenga, V.O.; Sant’Ana, A.S.; et al. Postbiotics: An overview of concepts, inactivation technologies, health effects, and driver trends. Trends Food Sci. Technol. 2023, 138, 199–214. [Google Scholar] [CrossRef]
- Qayyum, N.; Shuxuan, W.; Yantin, Q.; Ruiling, W.; Wang, S.; Ismael, M.; Lü, X. Characterization of Short-chain fatty acid-producing and cholesterol assimilation potential probiotic Lactic acid bacteria from Chinese fermented rice. Food Biosci. 2023, 52, 102404. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. The ancestral and industrialized gut microbiota and implications for human health. Nat. Rev. Microbiol. 2019, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.R.; Davenport, E.R.; Gautam, Y.; Bhandari, D.; Tandukar, S.; Ng, K.M.; Fragiadakis, G.K.; Holmes, S.; Gautam, G.P.; Leach, J.; et al. Gut microbiome transition across a lifestyle gradient in Himalaya. PLoS Biol. 2018, 16, e2005396. [Google Scholar] [CrossRef]
- Smits, S.A.; Leach, J.; Sonnenburg, E.D.; Gonzalez, C.G.; Lichtman, J.S.; Reid, G.; Knight, R.; Manjurano, A.; Changalucha, J.; Elias, J.E.; et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017, 357, 802–806. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e4114. [Google Scholar] [CrossRef]
- Gille, D.; Schmid, A.; Walther, B.; Vergeres, G. Fermented Food and Non-Communicable Chronic Diseases: A Review. Nutrients 2018, 10, 448. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.P.; Taillandier, P.; Beaufort, S. Metabolome-microbiome signatures in the fermented beverage, Kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef]
- Taylor, B.C.; Lejzerowicz, F.; Poirel, M.; Shaffer, J.P.; Jiang, L.; Aksenov, A.; Litwin, N.; Humphrey, G.; Martino, C.; Miller-Montgomery, S.; et al. Consumption of Fermented Foods Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. mSystems 2020, 5, e00901-19. [Google Scholar] [CrossRef]
- den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef]
- Diaz-Lopez, A.; Bullo, M.; Martinez-Gonzalez, M.A.; Corella, D.; Estruch, R.; Fito, M.; Gomez-Gracia, E.; Fiol, M.; Garcia de la Corte, F.J.; Ros, E.; et al. Dairy product consumption and risk of type 2 diabetes in an elderly Spanish Mediterranean population at high cardiovascular risk. Eur. J. Nutr. 2016, 55, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, Q.; Giovannucci, E.; Mozaffarian, D.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.J.; van Dongen, M.C.; Wijckmans, N.; den Biggelaar, L.; Oude Elferink, S.J.; Singh-Povel, C.M.; Schram, M.T.; Sep, S.J.; van der Kallen, C.J.; Koster, A.; et al. Consumption of dairy foods in relation to impaired glucose metabolism and type 2 diabetes mellitus: The Maastricht Study. Br. J. Nutr. 2016, 115, 1453–1461. [Google Scholar] [CrossRef]
- Iwasa, M.; Aoi, W.; Mune, K.; Yamauchi, H.; Furuta, K.; Sasaki, S.; Takeda, K.; Harada, K.; Wada, S.; Nakamura, Y.; et al. Fermented milk improves glucose metabolism in exercise-induced muscle damage in young healthy men. Nutr. J. 2013, 12, 83. [Google Scholar] [CrossRef]
- Unno, T.; Choi, J.H.; Hur, H.G.; Sadowsky, M.J.; Ahn, Y.T.; Huh, C.S.; Kim, G.B.; Cha, C.J. Changes in human gut microbiota influenced by probiotic fermented milk ingestion. J. Dairy Sci. 2015, 98, 3568–3576. [Google Scholar] [CrossRef]
- Silva, K.R.; Rodrigues, S.A.; Filho, L.X.; Lima, A.S. Antimicrobial activity of broth fermented with kefir grains. Appl. Biochem. Biotechnol. 2009, 152, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Friques, A.G.; Arpini, C.M.; Kalil, I.C.; Gava, A.L.; Leal, M.A.; Porto, M.L.; Nogueira, B.V.; Dias, A.T.; Andrade, T.U.; Pereira, T.M.; et al. Chronic administration of the probiotic kefir improves the endothelial function in spontaneously hypertensive rats. J. Transl. Med. 2015, 13, 390. [Google Scholar] [CrossRef]
- Klippel, B.F.; Duemke, L.B.; Leal, M.A.; Friques, A.G.; Dantas, E.M.; Dalvi, R.F.; Gava, A.L.; Pereira, T.M.; Andrade, T.U.; Meyrelles, S.S.; et al. Effects of Kefir on the Cardiac Autonomic Tones and Baroreflex Sensitivity in Spontaneously Hypertensive Rats. Front. Physiol. 2016, 7, 211. [Google Scholar] [CrossRef]
- Gomez-Guzman, M.; Toral, M.; Romero, M.; Jimenez, R.; Galindo, P.; Sanchez, M.; Zarzuelo, M.J.; Olivares, M.; Galvez, J.; Duarte, J. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol. Nutr. Food Res. 2015, 59, 2326–2336. [Google Scholar] [CrossRef]
- Liu, C.F.; Tung, Y.T.; Wu, C.L.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Antihypertensive effects of Lactobacillus-fermented milk orally administered to spontaneously hypertensive rats. J. Agric. Food Chem. 2011, 59, 4537–4543. [Google Scholar] [CrossRef]
- Rodriguez-Figueroa, J.C.; Gonzalez-Cordova, A.F.; Astiazaran-Garcia, H.; Vallejo-Cordoba, B. Hypotensive and heart rate-lowering effects in rats receiving milk fermented by specific Lactococcus lactis strains. Br. J. Nutr. 2013, 109, 827–833. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Lee, M.S.; Jeon, J.Y.; Ha, E.S.; Kim, T.H.; Yoon, J.Y.; Ok, C.O.; Lee, H.K.; Hwang, W.S.; Choe, S.J.; et al. Beneficial effects of fresh and fermented kimchi in prediabetic individuals. Ann. Nutr. Metab. 2013, 63, 111–119. [Google Scholar] [CrossRef]
- Han, K.; Bose, S.; Wang, J.H.; Kim, B.S.; Kim, M.J.; Kim, E.J.; Kim, H. Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Mol. Nutr. Food Res. 2015, 59, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef]
- Kanai, T.; Mikami, Y.; Hayashi, A. A breakthrough in probiotics: Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease. J. Gastroenterol. 2015, 50, 928–939. [Google Scholar] [CrossRef]
- Sheehan, D.; Moran, C.; Shanahan, F. The microbiota in inflammatory bowel disease. J. Gastroenterol. 2015, 50, 495–507. [Google Scholar] [CrossRef]
- Aden, K.; Rehman, A.; Waschina, S.; Pan, W.H.; Walker, A.; Lucio, M.; Nunez, A.M.; Bharti, R.; Zimmerman, J.; Bethge, J.; et al. Metabolic Functions of Gut Microbes Associate With Efficacy of Tumor Necrosis Factor Antagonists in Patients With Inflammatory Bowel Diseases. Gastroenterology 2019, 157, 1279–1292.e1211. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Watanabe, T.; Takaoka, M.; Suzuki, K.; Murakami, T.; Murakami, N.; Sumikawa, S. Anti-Inflammatory Effect on Colitis and Modulation of Microbiota by Fermented Plant Extract Supplementation. Fermentation 2021, 7, 55. [Google Scholar] [CrossRef]
- Martini, G.R.; Tikhonova, E.; Rosati, E.; DeCelie, M.B.; Sievers, L.K.; Tran, F.; Lessing, M.; Bergfeld, A.; Hinz, S.; Nikolaus, S.; et al. Selection of cross-reactive T cells by commensal and food-derived yeasts drives cytotoxic T(H)1 cell responses in Crohn’s disease. Nat. Med. 2023, 29, 2602–2614. [Google Scholar] [CrossRef]
- Sidhartha Ranjit Sinha, S.U. Effects of a Fermented Food-Supplemented on Patients with Ulcerative Colitis; Stanford University: Stanford, CA, USA, 2024. [Google Scholar]
- Lei, G.; Khan, A.; Budryn, G.; Grzelczyk, J. Probiotic products from laboratory to commercialization. Trends Food Sci. Technol. 2025, 155, 104807. [Google Scholar] [CrossRef]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological Exploration of Different Types of Kefir Grains. Fermentation 2017, 3, 1. [Google Scholar] [CrossRef]
- Plessas, S.; Alexopoulos, A.; Voidarou, C.; Stavropoulou, E.; Bezirtzoglou, E. Microbial ecology and quality assurance in food fermentation systems. The case of kefir grains application. Anaerobe 2011, 17, 483–485. [Google Scholar] [CrossRef]
- Ribera, C.; Sánchez-Ortí, J.V.; Clarke, G.; Marx, W.; Mörkl, S.; Balanzá-Martínez, V. Probiotic, prebiotic, synbiotic and fermented food supplementation in psychiatric disorders: A systematic review of clinical trials. Neurosci. Biobehav. Rev. 2024, 158, 105561. [Google Scholar] [CrossRef]
- SaeidiFard, N.; Djafarian, K.; Shab-Bidar, S. Fermented foods and inflammation: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 35, 30–39. [Google Scholar] [CrossRef]
- de Simone, C. The Unregulated Probiotic Market. Clinical Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef]
- FAO/WHO. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; FAO: Rome, Italy, 2001. [Google Scholar]
- Bond, J. Gut reactions. New Sci. 2022, 256, 46–49. [Google Scholar] [CrossRef]
- Cunningham, M.; Vinderola, G.; Charalampopoulos, D.; Lebeer, S.; Sanders, M.E.; Grimaldi, R. Applying probiotics and prebiotics in new delivery formats—Is the clinical evidence transferable? Trends Food Sci. Technol. 2021, 112, 495–506. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Xiao, Y.; Cai, R.; Pan, X.; Hu, Y. Effects of a new compound probiotic on growth performance, antioxidant capacity, intestinal health, gut microbiota and metabolites of broilers. Poult. Sci. 2025, 104, 105215. [Google Scholar] [CrossRef]
- Francis, D.V.; Dahiya, D.; Gokhale, T.; Nigam, P.S. Sustainable packaging materials for fermented probiotic dairy or non-dairy food and beverage products: Challenges and innovations. AIMS Microbiol. 2024, 10, 320–339. [Google Scholar] [CrossRef]
| Fermented Food | Starter Cultures Causing Possible Bioactivity | Health-Beneficial Effects | Reference |
|---|---|---|---|
| Natural Yoghurt | Lactobacillus delbrueckii subsp. bulgaricus Streptococcus thermophilus | ACE-inhibitory | [50] |
| Yoghurt with probiotics | Lactobacillus helveticus Lacticaseibacillus rhamnosus Lacticaseibacillus fermentum Bifidobacterium animalis subsp. lactis | Antiproliferative, anticancer, immunomodulatory, antimicrobial, prevention of dysbiosis-associated weight loss, reduction of systemic inflammation, decreased prevalence of diabetic kidney disease, and promotion of oral health | [51,52,53,54,55] |
| Probiotic food beverages | Lactobacillus delbrueckii subsp. bulgaricus Streptococcus thermophilus, Lactobacillus casei Bifidobacterium lactis and many other species | For cognitive treatment via gut–brain signaling | [19] |
| Sour milk | Lactobacillus helveticus Levilactobacillus brevis | Antihypertensive effects, relieves anxiety, improves sleep quality | [56,57,58] |
| Fermented dairy and non-dairy products | Bifidobacterium animalis Streptococcus thermophilus Lactobacillus delbrueckii subsp. bulgaricus, Lactococcus lactis | Alleviating allergic reactions and symptoms | [59] |
| Milk-based or plant-sourced kefir | Probiotic LAB and Bifidobacteria | Antioxidant, anti-inflammatory, antihypertensive; antiviral; prevents osteoporosis | [2,60,61,62,63] |
| Gouda cheese | Lactococcus cremoris Lactococcus lactis Lc. lactis subsp. lactis biovar diacetylactis Leuconostoc spp. | Antioxidant, antihypertensive; beneficial effect on abdominal adipose | [64,65,66] |
| Mozzarella cheese | Streptococcus thermophilus, Lactobacillus bulgaricus, Lactobacillus helveticus Lactococcus lactis Leuconostoc lactic acid bacteria (LAB) Enterococcus | ACE-inhibitory | [67,68] |
| Gorgonzola cheese | Streptococcus thermophilus, Lactobacillus delbrueckii yeast Penicillium roqueforti | ACE-inhibitory, antimicrobial | [67,69] |
| Cheddar cheese | Mesophilic culture | Sustains blood lipid profile in individuals at risk of metabolic diseases | [70] |
| Kimchi | Lactobacillus brevis | Anti-inflammatory | [71] |
| Kimchi | Lactiplantibacillus plantarum | Antitumoral effects | [72] |
| Miso | Antihypertensive effects | [73] | |
| Natto (fermented soya beans) | Bacillus subtilis var. natto | Prevention of osteoporosis; antibacterial, anticancer, antioxidant | [27] |
| Fermented soy product | Lactobacillus helveticus Enterococcus faecium | Improved total cholesterol | [74] |
| Cheonggukjang (fermented soya paste) | Bacillus subtilis, Bacillus amyloliquefaciens, Rhizopus oligosporus | Improves obesity-related parameters and gut microbiota dysbiosis | [75] |
| Water kefir | L. mali | Reduction of body weight and lipid accumulation | [76] |
| Sourdough bread | LAB culture S. cerevisiae | Reduction of gastric volume; higher fullness perception and hydrogen production | [17] |
| Fermented dairy and non-dairy food | Lactic acid bacteria; probiotic yeast species | Relieves gastrointestinal tract inflammation, IBD, IBS, and induction of cancer | [77] |
| Food containing prebiotic materials and dietary fibers | Probiotic species of bacteria | Minimizing risks of IBS, IBD, colorectal cancer | [78,79] |
| Probiotic beverages made from fermented fruits, vegetables, and cereals | Several species of probiotic bacteria | Diarrhoea control; regaining lost hydration, nutrition, and stabilizing gut microbiota | [80] |
| Palm wine/Toddy/Kallu (fermented sap of palm trees) | Leuconostoc mesenteroides Lactobacillus plantarum Liquorilactobacillus nagelii Liquorilactobacillus sucicola Saccharomyces sp. Acetobacter sp. | Antibacterial; improves eyesight and gastrointestinal tract | [81,82,83] |
| Dry-cured fermented sausages (e.g., salami, chorizo, Thai naem) | Staphylococcus carnosus, Micrococcus, lactic acid bacteria | Antioxidant, antimicrobial, antihypertensive (ACE-inhibitory) | [84] |
| Category | Component or Metabolite That Provides Bioactivity | Health-Related Functions | Reference |
|---|---|---|---|
| Bacterial Components | Cell wall components (e.g., peptidoglycans, lipoteichoic acid) | Immune modulation, anti-inflammatory activity | [104,105] |
| Enzymes /Surface proteins (incl. ESPs) | Enhance gut barrier, signal host receptors | [106,107] | |
| Exopolysaccharides (EPSs) | Immunomodulation, antioxidant activity, cholesterol reduction, prebiotic effect | [21] | |
| Viable probiotic cells | Gut microbiota balance, competitive exclusion of pathogens | [5,106] | |
| Microbial Metabolites | Short-chain fatty acids (SCFAs) (e.g., acetate, propionate, butyrate) | Energy source for colon cells; anti-inflammatory; metabolic regulation | [108,109,110,111] |
| Organic acids (e.g., lactic acid) | Pathogen inhibition, pH regulation, preservation | [5,96] | |
| Amino acids/Bioactive peptides | Antihypertensive, antioxidant; immunomodulatory effects | [106,112,113] | |
| Other small molecules (e.g., GABA, B vitamins, polyamines) | Neuromodulation, coenzyme function, cellular signaling | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpou, A.; Dahiya, D.; Nigam, P.S. Evolving Dynamics of Fermented Food Microbiota and the Gut Microenvironment: Strategic Pathways to Enhance Human Health. Foods 2025, 14, 2361. https://doi.org/10.3390/foods14132361
Terpou A, Dahiya D, Nigam PS. Evolving Dynamics of Fermented Food Microbiota and the Gut Microenvironment: Strategic Pathways to Enhance Human Health. Foods. 2025; 14(13):2361. https://doi.org/10.3390/foods14132361
Chicago/Turabian StyleTerpou, Antonia, Divakar Dahiya, and Poonam Singh Nigam. 2025. "Evolving Dynamics of Fermented Food Microbiota and the Gut Microenvironment: Strategic Pathways to Enhance Human Health" Foods 14, no. 13: 2361. https://doi.org/10.3390/foods14132361
APA StyleTerpou, A., Dahiya, D., & Nigam, P. S. (2025). Evolving Dynamics of Fermented Food Microbiota and the Gut Microenvironment: Strategic Pathways to Enhance Human Health. Foods, 14(13), 2361. https://doi.org/10.3390/foods14132361








