Seasonal Variation in Nutritional Value and Technical Quality of Lionfish (Pterois miles) from the Ionian and Aegean Seas
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling, Somatometric Indices and Proximate Composition
2.2. Fatty Acid Composition and Nutritional Indices
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albins, M.A.; Hixon, M.A. Worst Case Scenario: Potential Long-Term Effects of Invasive Predatory Lionfish (Pterois volitans) on Atlantic and Caribbean Coral-Reef Communities. Environ. Biol. Fishes 2013, 96, 1151–1157. [Google Scholar] [CrossRef]
- Côté, I.M.; Green, S.J.; Hixon, M.A. Predatory Fish Invaders: Insights from Indo-Pacific Lionfish in the Western Atlantic and Caribbean. Biol. Conserv. 2013, 164, 50–61. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Galanidi, M.; Zenetos, A.; Corsini-Foka, M.; Giovos, I.; Karachle, P.K.; Fournari-Konstantinidoy, I.; Kytinou, E.; Issaris, Y.; Azzurro, E.; et al. Updating the Occurrences of Pterois miles in the Mediterranean Sea, with Considerations on Thermal Boundaries and Future Range Expansion. Mediterr. Mar. Sci. 2020, 21, 62–69. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Bennett, C.; Brotherton, P.N.M.; Butchart, S.H.M.; Butterworth, H.M.; Clarke, S.J.; Esmail, N.; Fleishman, E.; Gaston, K.J.; Herbert-Read, J.E.; et al. A Horizon Scan of Global Biological Conservation Issues for 2024. Trends Ecol. Evol. 2024, 39, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Albins, M.A. Invasive Pacific Lionfish Pterois volitans Reduce Abundance And Species Richness Of Native Bahamian Coral-Reef Fishes. Mar. Ecol. Prog. Ser. 2015, 522, 231–243. [Google Scholar] [CrossRef]
- Côté, I.M.; Maljković, A. Predation Rates of Indo-Pacific Lionfish on Bahamian Coral Reefs. Mar. Ecol. Prog. Ser. 2010, 404, 219–225. [Google Scholar] [CrossRef]
- Green, S.J.; Akins, J.L.; Maljkovic, A.; Côté, I.M. Invasive Lionfish Drive Atlantic Coral Reef Fish Declines. PLoS ONE 2012, 7, e32596. [Google Scholar] [CrossRef]
- Lesser, M.P.; Slattery, M. Phase Shift to Algal Dominated Communities at Mesophotic Depths Associated with Lionfish (Pterois volitans) Invasion on a Bahamian Coral Reef. Biol. Invasions 2011, 13, 1855–1868. [Google Scholar] [CrossRef]
- Morris, J.A.; Akins, J.L. Feeding Ecology of Invasive Lionfish (Pterois volitans) in the Bahamian Archipelago. Environ. Biol. Fishes 2009, 86, 389–398. [Google Scholar] [CrossRef]
- Hixon, M.A.; Green, S.J.; Albins, M.A.; Akins, J.L.; Morris, J.A. Lionfish: A Major Marine Invasion. Mar. Ecol. Prog. Ser. 2016, 558, 161–165. [Google Scholar] [CrossRef]
- Morris, J.A.; Thomas, A.; Rhyne, A.L.; Breen, N.; Akins, L.; Nash, B. Nutritional Properties of the Invasive Lionfish: A Delicious and Nutritious Approach for Controlling the Invasion. AACL Bioflux 2011, 4, 21–26. [Google Scholar]
- Golani, D.; Sonin, O. New Records of the Red Sea Fishes, Pterois miles (Scorpaenidae) and Pteragogus pelycus (Labridae) from the Eastern Mediterranean Sea Daniel. Jpn. J. Ichthyol. 1992, 39, 167–169. [Google Scholar] [CrossRef]
- Bariche, M.; Torres, M.; Azzurro, E. The Presence of the Invasive Lionfish Pterois miles in the Mediterranean Sea. Mediterr. Mar. Sci. 2013, 14, 292–294. [Google Scholar] [CrossRef]
- Kletou, D.; Hall-Spencer, J.M.; Kleitou, P. A Lionfish (Pterois miles) Invasion Has Begun in the Mediterranean Sea. Mar. Biodivers. Rec. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Zenetos, A.; Karachle, P.K.; Corsini-Foka, M.; Gerovasileiou, V.; Simboura, N.; Xentidis, N.J.; Tsiamis, K. Is the Trend in New Introductions of Marine Non-Indigenous Species a Reliable Criterion for Assessing Good Environmental Status? The Case Study of Greece. Mediterr. Mar. Sci. 2020, 21, 775–793. [Google Scholar] [CrossRef]
- Savva, I.; Chartosia, N.; Antoniou, C.; Kleitou, P.; Georgiou, A.; Stern, N.; Hadjioannou, L.; Jimenez, C.; Andreou, V.; Hall-Spencer, J.M.; et al. They Are Here to Stay: The Biology and Ecology of Lionfish (Pterois miles) in the Mediterranean Sea. J. Fish Biol. 2020, 97, 148–162. [Google Scholar] [CrossRef]
- Azzurro, E.; Stancanelli, B.; Di Martino, V.; Bariche, M. Range Expansion of the Common Lionfish Pterois miles (Bennett, 1828) in the Mediterranean Sea: An Unwanted New Guest for Italian Waters. BioInvasions Rec. 2017, 6, 95–98. [Google Scholar] [CrossRef]
- Bottacini, D.; Pollux, B.J.A.; Nijland, R.; Jansen, P.A.; Naguib, M.; Kotrschal, A. Lionfish (Pterois miles) in the Mediterranean Sea: A Review of the Available Knowledge with an Update on the Invasion Front. NeoBiota 2024, 92, 233–257. [Google Scholar] [CrossRef]
- Poursanidis, D.; Kalogirou, S.; Azzurro, E.; Parravicini, V.; Bariche, M.; zu Dohna, H. Habitat Suitability, Niche Unfilling and the Potential Spread of Pterois miles in the Mediterranean Sea. Mar. Pollut. Bull. 2020, 154, 111054. [Google Scholar] [CrossRef]
- Aranda-González, I.; Aguilar-Perera, A.; Chel-Guerrero, L.; Gallegos-Tintoré, S.; Betancur-Ancona, D. Chemical Composition, Amino Acid and Fatty Acid Profiles of Lionfish Pterois volitans from the Alacranes Reef, Southern Gulf of Mexico. Indian J. Anim. Res. 2020, 54, 1246–1250. [Google Scholar] [CrossRef]
- Ayas, D.; Agılkaya, G.S.; Kosker, A.R.; Durmus, M.; Ucar, Y.; Bakan, M. The Chemical Composition of the Lionfish (Pterois miles, Bennett 1828), the New Invasive Species of the Mediterranean Sea. NESciences 2018, 3, 103–115. [Google Scholar] [CrossRef]
- Castro-González, M.I.; Caballero-Vázquez, J.A.; Guerra-Infante, F.M.; López-Hurtado, M. Analysis of the Chemical Composition of the Lionfish Pterois volitans as a Food Strategy for Its Control. Lat. Am. J. Aquat. Res. 2019, 47, 841–844. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaitherburg, MD, USA, 2005; pp. 4–5. [Google Scholar]
- Lepage, G.; Roy, C.C. Improved Recovery of Fatty Acid through Direct Transesterification without Prior Extraction or Purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Alexi, N.; Kogiannou, D.; Oikonomopoulou, I.; Kalogeropoulos, N.; Byrne, D.V.; Grigorakis, K. Culinary Preparation Effects on Lipid and Sensory Quality of Farmed Gilthead Seabream (Sparus aurata) and Meagre (Argyrosomus regius): An Inter-Species Comparison. Food Chem. 2019, 301, 125263. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of Genotype, Feeding System and Slaughter Weight on the Quality of Light Lambs: II. Fatty Acid Composition of Meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Albert, O.T. Biology and Ecology of Norway Pout (Trisoptems esmarki, Nilsson 1855) in the Norwegian Deep. ICES J. Mar. Sci. 1994, 51, 45–61. [Google Scholar] [CrossRef]
- Garcia-Franquesa, E.; Molinero, A.; Valero, J.; Flos, R. Influence of Sex, Age and Season on the Feeding Habits of the Flatfish Solea senegalensis. Environ. Biol. Fishes 1996, 47, 289–298. [Google Scholar] [CrossRef]
- Karachle, P.K.; Stergiou, K.I. The Effect of Season and Sex on Trophic Levels of Marine Fishes. J. Fish Biol. 2008, 72, 1463–1487. [Google Scholar] [CrossRef]
- Morato, T.; Santos, R.S.; Andrade, J.P. Feeding Habits, Seasonal and Ontogenetic Diet Shift of Blacktail Comber, Serranus atricauda (Pisces: Serranidae), from the Azores, North-Eastern Atlantic. Fish. Res. 2000, 49, 51–59. [Google Scholar] [CrossRef]
- Rumolo, P.; Bonanno, A.; Barra, M.; Fanelli, E.; Calabrò, M.; Genovese, S.; Ferreri, R.; Mazzola, S.; Basilone, G. Spatial Variations in Feeding Habits and Trophic Levels of Two Small Pelagic Fish Species in the Central Mediterranean Sea. Mar. Environ. Res. 2016, 115, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Vagenas, G.; Karachle, P.K.; Dogrammatzi, A.; Tsikliras, A.C. Age, Growth and Feeding Habits of Boarfish (Capros aper) in the Aegean and Ionian Seas (Greece). Mar. Biol. Res. 2020, 16, 585–592. [Google Scholar] [CrossRef]
- Grigorakis, K. Fillet Proximate Composition, Lipid Quality, Yields, and Organoleptic Quality of Mediterranean-Farmed Marine Fish: A Review with Emphasis on New Species. Crit. Rev. Food Sci. Nutr. 2017, 57, 2956–2969. [Google Scholar] [CrossRef] [PubMed]
- Borderías, A.J.; Sánchez-alonso, I. First Processing Steps and the Quality of Wild and Farmed Fish. J. Food Sci. 2011, 76, 1–5. [Google Scholar] [CrossRef]
- Biton-Porsmoguer, S.; Bou, R.; Lloret, E.; Alcaide, M.; Lloret, J. Research Article Fatty Acid Composition and Parasitism of European Sardine (Sardina pilchardus) and Anchovy (Engraulis encrasicolus) Populations in the Northern Catalan Sea in the Context of Changing Environmental Conditions. Conserv. Physiol. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Vila-Belmonte, M.; Bou, R.; Lloret, E.; Lloret, J. Fatty Acid Content and Profile of Round Sardinella (Sardinella aurita), an Expanding Thermophilic Species in the NW Mediterranean. Mediterr. Mar. Sci. 2024, 25, 300–310. [Google Scholar] [CrossRef]
- Gardner, P.G.; Frazer, T.K.; Jacoby, C.A.; Yanong, R.P.E. Reproductive Biology of Invasive Lionfish (Pterois spp.). Front. Mar. Sci. 2015, 2, 7. [Google Scholar] [CrossRef]
- Mouchlianitis, F.A.; Kalaitzi, G.; Kleitou, P.; Savva, I.; Kletou, D.; Ganias, K. Reproductive Dynamics of the Invasive Lionfish (Pterois miles) in the Eastern Mediterranean Sea. J. Fish Biol. 2022, 100, 574–581. [Google Scholar] [CrossRef]
- Calder, P.C. Dietary Arachidonic Acid: Harmful, Harmless or Helpful? Br. J. Nutr. 2007, 98, 451–453. [Google Scholar] [CrossRef]
- Dannemiller, N.G.; Christiansen, E.F.; Harms, C.A.; Minter, L.J.; Ange-van Heugten, K.D. Comparison of Whole Blood Fatty Acid Profiles between Lionfish (Pterois spp.) in Wild and Managed Care Environments. J. Zool. Bot. Gard. 2022, 3, 357–365. [Google Scholar] [CrossRef]
- Batjakas, I.E.; Evangelopoulos, A.; Giannou, M.; Pappou, S.; Papanikola, E.; Atsikvasi, M.; Poursanidis, D.; Gubili, C. Lionfish Diet Composition at Three Study Sites in the Aegean Sea: An Invasive Generalist? Fishes 2023, 8, 314. [Google Scholar] [CrossRef]
- Monroig, Ó.; Tocher, D.R.; Navarro, J.C. Biosynthesis of Polyunsaturated Fatty Acids in Marine Invertebrates: Recent Advances in Molecular Mechanisms. Mar. Drugs 2013, 11, 3998–4018. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Çiçek, E.; Polat, A.; Kuley, E. Fat Content and Fatty Acid Compositions of 34 Marine Water Fish Species from the Mediterranean Sea. Int. J. Food Sci. Nutr. 2009, 60, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Saglik, S.; Alpasian, M.; Gezgin, T.; Cetinturk, K.; Tekinay, A.; Güven, K.C. Fatty Acid Composition of Wild and Cultivated Gilthead Seabream (Sparus aurata) and Sea Bass (Dicentrarchus labrax). Eur. J. Lipid Sci. Technol. 2003, 105, 104–107. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Monteiro, M.; Matos, E.; Ramos, R.; Campos, I.; Valente, L.M.P. A Blend of Land Animal Fats Can Replace up to 75% Fish Oil without Affecting Growth and Nutrient Utilization of European Seabass. Aquaculture 2018, 487, 22–31. [Google Scholar] [CrossRef]
- Yurchenko, S.; Sats, A.; Tatar, V.; Kaart, T.; Mootse, H.; Jõudu, I. Fatty Acid Profile of Milk from Saanen and Swedish Landrace Goats. Food Chem. 2018, 254, 326–332. [Google Scholar] [CrossRef]
- Fernandes, C.E.; da Vasconcelos, M.A.S.; de Almeida Ribeiro, M.; Sarubbo, L.A.; Andrade, S.A.C.; de Filho, A.B.M. Nutritional and Lipid Profiles in Marine Fish Species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Minasidis, V.; Doumpas, N.; Giovos, I.; Kleitou, P.; Kaminas, A.; Moutopoulos, D.K. Assessing consumer attitude towards marine non-indigenous fish species: A case study from Greece (Eastern Mediterranean Sea). Thalassas 2023, 39, 35–53. [Google Scholar]
- Sidiropoulou, N.; Mente, E.; Katsanevakis, S.; Tzanatos, E.; Lefkaditou, E.; Minasidis, V. Investigating Consumer Attitudes and Market Trading of Edible Marine Invasive Alien Species in the Greek Seafood Market. Sustainability 2024, 16, 2486. [Google Scholar] [CrossRef]
- Smith, N.; Burgess, K.; Clements, K.R.; Burgess, J.C.; Lavoie, A.; Solomon, J,N. Serving Conservation from Reef to Plate: Barriers and Opportunities for Invasive Lionfish Consumption in Restaurants. Biol. Conserv. 2023, 283, 110093. [Google Scholar] [CrossRef]
| Aegean Sea | |||||
|---|---|---|---|---|---|
| Autumn | Winter | Spring | Summer | Mean Value | |
| CI | 1.39 ± 0.19 | - | 1.40 ± 0.25 | 1.40 ± 0.13 | 1.40 ± 0.00 |
| DY (%) | 87.7 ± 3.6 a | - | 89.9 ± 4.0 b | 86.6 ± 2.9 a | 88.1 ± 1.7 |
| FY (%) | 30.0 ± 2.6 | - | 29.7 ± 1.4 | 29.7 ± 1.9 | 29.8 ± 0.1 |
| VFI (%) | 3.81 ± 1.35 | 4.28 ± 2.08 | 4.1 ± 1.07 | 4.13 ± 0.28 | |
| Ionian Sea | |||||
| CI | 1.53 ± 0.17 | 1.54 ± 0.18 | 1.41 ± 0.17 | 1.35 ± 0.24 | 1.46 ± 0.09 |
| DY (%) | 86.8 ± 3.8 a | 89.9 ± 1.6 ab | 89.6 ± 2.1 b | 88.2 ± 2.8 ab | 88.6 ± 1.4 |
| FY (%) | 28 ± 1 | 28.1 ± 1.7 | 27.1 ± 2.2 | 26.5 ± 1.4 | 27.4 ± 0.8 |
| VFI (%) | 3.87 ± 1.41 | 5.30 ± 1.03 | 4.12 ± 1.80 | 3.49 ± 1.55 | 4.20 ± 0.78 |
| Aegean Sea | |||||||
|---|---|---|---|---|---|---|---|
| Autumn | Winter | Spring | Summer | Mean Value | Female | Male | |
| Protein | 19.6 ± 0.6 | - | 19.2 ± 0.7 | 19.4 ± 1.7 | 19.4 ± 0.2 | 19.2 ± 0.6 | 19.6 ± 0.7 |
| Fat | 1.70 ± 1.12 | - | 2.23 ± 1.36 | 1.95 ± 1.18 | 1.96 ± 0.27 | 2.29 ± 0.67 | 1.63 ± 0.36 |
| Ash | 1.37 ± 0.10 a | - | 1.20 ± 0.00 b | 1.35 ± 0.11 a | 1.31 ± 0.09 | 1.29 ± 0.05 | 1.35 ± 0.11 |
| Moisture | 76.8 ± 1.4 | - | 77.6 ± 1.7 | 77.4 ± 1.8 | 77.3 ± 0.4 | 77.3 ± 0.5 | 77.3 ± 1.0 |
| Ionian Sea | |||||||
| Protein | 20.0 ± 1.2 | 19.3 ± 1.2 | 18.9 ± 1 | 18.9 ± 1 | 19.3 ± 0.5 | 19.4 ± 0.9 | 19.1 ± 0.8 |
| Fat | 2.30 ± 0.35 ab | 2.68 ± 0.57 a | 1.54 ± 0.46 b | 1.55 ± 0.54 b | 2.02 ± 0.57 | 1.89 ± 0.74 | 2.22 ± 0.37 |
| Ash | 1.44 ± 0.11 | 1.25 ± 0.10 | 1.40 ± 0.10 | 1.42 ± 0.10 | 1.38 ± 0.09 | 1.37 ± 0.05 | 1.38 ± 0.11 |
| Moisture | 76.5 ± 1.3 | 77 ± 1.4 | 78.1 ± 1 | 77.5 ± 1.1 | 77.3 ± 0.7 | 77.2 ± 1.0 | 77.5 ± 1.2 |
| Autumn | Spring | Summer | Mean Value | |
|---|---|---|---|---|
| 12:00 | 0.5 ± 0.5 | 0.8 ± 0.5 | 0.7 ± 0.5 | 0.7 ± 0.5 |
| 14:00 | 66.2 ± 52.3 | 90.7 ± 74.6 | 66.0 ± 52.0 | 74.3 ± 58.1 |
| 14:01 | 1.2 ± 1.1 | 1.4 ± 1.1 | 1.3 ± 1.3 | 1.3 ± 1.1 |
| 15:00 | 11.3 ± 7.4 | 18.8 ± 14.7 | 14.8 ± 10.7 | 15.0 ± 11.1 |
| 16:00 | 517.9 ± 341.5 | 628.0 ± 416.1 | 538.9 ± 342.2 | 561.6 ± 349.4 |
| 16:1n9 | 12.7 ± 10.1 | 16.0 ± 10.3 | 15.3 ± 11.3 | 14.7 ± 10.0 |
| 16:1n7 | 102.6 ± 74.9 | 146.3 ± 109.9 | 113.7 ± 83.6 | 120.9 ± 87.3 |
| 16:1n11 | - | 5.3 ± 3.2 | 4.9 ± 2.4 | 5.1 ± 2.7 |
| 17:00 | 19.1 ± 12.0 | 27.8 ± 18.0 | 25.6 ± 16.4 | 24.1 ± 15.2 |
| 17:1n7 | 6.4 ± 4.2 | 11.7 ± 7.6 | 11.2 ± 7.2 | 9.8 ± 6.6 |
| 18:00 | 160.3 ± 95.1 | 199.3 ± 111.7 | 188.2 ± 103.7 | 182.6 ± 98.9 |
| 18:1n9 | 309.0 ± 213.6 | 353.0 ± 207.5 | 304.1 ± 182.0 | 322.0 ± 190.6 |
| 18:1n7 | 41.0 ± 25.8 a | 5.6 ± 3.2 b | 5.1 ± 3.2 b | 17.2 ± 22.4 |
| 18:2n9 | 9.0 ± 7.5 | 9.0 ± 4.9 | 10.0 ± 6.6 | 9.3 ± 6.0 |
| 18:2n6 | 22.0 ± 14.0 | 31.6 ± 18.2 | 25.1 ± 14.9 | 26.3 ± 15.4 |
| 18:3n6 | 1.3 ± 0.9 | 3.7 ± 2.3 | 3.5 ± 2.5 | 2.8 ± 2.2 |
| 18:3n3 | 4.5 ± 3.1 | 9.3 ± 6.2 | 6.7 ± 4.2 | 6.8 ± 4.9 |
| 20:00 | 5.8 ± 3.6 | 9.8 ± 5.9 | 8.3 ± 4.6 | 8.0 ± 4.9 |
| 20:1n11 | 2.1 ± 1.7 | 4.7 ± 2.6 | 6.0 ± 5.7 | 4.3 ± 3.9 |
| 20:1n9 | 21.8 ± 15.8 | 45.8 ± 38.7 | 26.8 ± 22.5 | 31.5 ± 27.8 |
| 20:1n7 | 2.0 ± 1.4 | 5.5 ± 3.4 | 5.0 ± 3.7 | 4.2 ± 3.2 |
| 20:2n9 | 6.6 ± 8.1 | 6.3 ± 2.7 | 7.0 ± 5.2 | 6.6 ± 5.4 |
| 20:2n6 | 4.3 ± 2.7 | 9.1 ± 5.2 | 8.0 ± 4.2 | 7.1 ± 4.4 |
| 20:3n6 | 2.0 ± 1.2 a | 7.2 ± 2.5 b | 8.5 ± 4.0 b | 5.9 ± 3.9 |
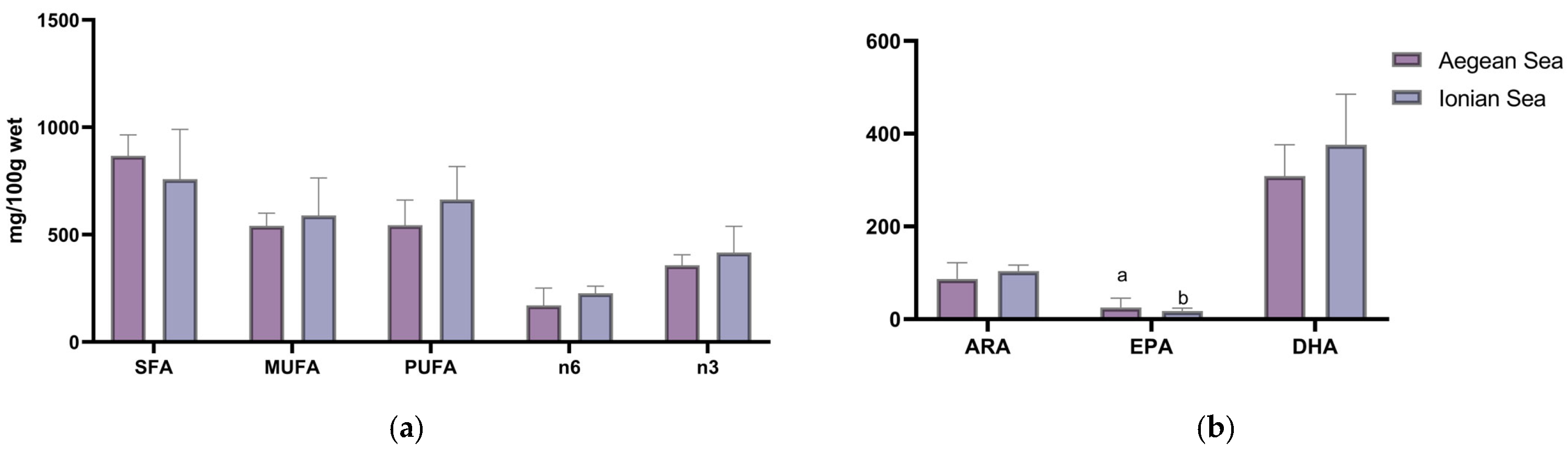
| 20:4n6 (ARA) | 48.0 ± 24.4 | 94.9 ± 40.9 | 117.0 ± 76.6 | 86.6 ± 57.2 |
| 20:3n3 | 1.1 ± 0.7 a | 5.5 ± 2.1 b | 6.6 ± 2.9 b | 4.4 ± 3.1 |
| 20:5n3 (EPA) | 48.4 ± 30.2 a | 15.2 ± 10.3 b | 12.2 ± 7.2 b | 25.3 ± 24.5 |
| 22:4n6 | 2.3 ± 1.4 a | 27.6 ± 14.2 ab | 36.8 ± 33.3 b | 22.2 ± 24.7 |
| 22:5n6 | 2.4 ± 1.8 a | 31.7 ± 16.8 b | 28.8 ± 16.4 b | 21.0 ± 18.6 |
| 22:5n3 | 16.8 ± 10.1 | 3.0 ± 1.4 | 15.8 ± 32.8 | 11.9 ± 19.7 |
| 22:6n3 (DHA) | 246.1 ± 161.6 | 379.9 ± 228.6 | 300.7 ± 183.9 | 308.9 ± 190.2 |
| 24:1n9 | 8.5 ± 3.4 a | 27.4 ± 12.5 b | 22.3 ± 13.2 ab | 19.4 ± 13.0 |
| SFA | 783.2 ± 512.5 | 975.2 ± 640.6 | 842.5 ± 528.6 | 867.0 ± 535.7 |
| MUFA | 509.7 ± 346.5 | 609.5 ± 388.1 | 503.2 ± 315.4 | 540.8 ± 333.7 |
| PUFA | 410.2 ± 258.0 | 634.0 ± 335.8 | 586.6 ± 328.3 | 543.6 ± 307.0 |
| n6 | 77.7 ± 42.2 | 205.8 ± 92.2 | 227.7 ± 142.7 | 170.4 ± 116.9 |
| n3 | 316.9 ± 204.9 | 412.8 ± 248.4 | 342.0 ± 207.6 | 357.2 ± 211.9 |
| EPA + DHA | 294.5 ± 191.3 | 395.1 ± 238.9 | 312.9 ± 190.8 | 334.1 ± 200.7 |
| n3/n6 | 4.0 ± 1.1 a | 2.0 ± 0.6 b | 1.7 ± 0.7 b | 2.6 ± 1.3 |
| AI | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 |
| TI | 0.6 ± 0.0 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| HH | 1.2 ± 0.1 a | 1.3 ± 0.1 ab | 1.4 ± 0.2 b | 1.3 ± 0.2 |
| Autumn | Winter | Spring | Summer | Mean Value | |
|---|---|---|---|---|---|
| 12:00 | 1.3 ± 0.4 | 1.1 ± 0.3 | 0.6 ± 0.1 | 0.8 ± 0.4 | 0.9 ± 0.4 |
| 14:00 | 90.2 ± 17.0 ab | 119.7 ± 39.5 a | 48.7 ± 14.1 b | 52.2 ± 24.8 b | 74.3 ± 36.6 |
| 14:01 | 1.8 ± 0.5 | 2.1 ± 1.0 | 1.1 ± 0.3 | 1.2 ± 0.6 | 1.5 ± 0.7 |
| 15:00 | 17.7 ± 4.2 | 24.0 ± 7.0 | 10.6 ± 3.9 | 10.5 ± 4.1 | 15.0 ± 7.0 |
| 16:00 | 527.8 ± 80.1 ab | 630.4 ± 149.9 a | 348.7 ± 98.1 b | 357.4 ± 135.6 b | 452.4 ± 160.0 |
| 16:1n9 | 18.7 ± 3.3 | 22.1 ± 7.2 | 13.1 ± 3.6 | 12.8 ± 5.9 | 16.2 ± 6.1 |
| 16:1n7 | 161.6 ± 29.6 ab | 184.8 ± 62.5 a | 91.4 ± 28.5 b | 102.2 ± 44.5 b | 130.9 ± 55.0 |
| 16:1n11 | 10.7 ± 1.8 | 12.7 ± 2.8 | 4.5 ± 1.5 | 6.1 ± 2.5 | 8.1 ± 3.9 |
| 17:00 | 25.9 ± 5.9 | 35.1 ± 6.9 | 17.4 ± 5.6 | 17.0 ± 6.4 | 22.9 ± 9.2 |
| 17:1n7 | 12.9 ± 2.6 | 16.3 ± 4.1 | 8.7 ± 3.1 | 9.1 ± 3.3 | 11.4 ± 4.3 |
| 18:00 | 188.4 ± 34.6 ab | 214.4 ± 50.9 a | 128.9 ± 37.8 a | 126.5 ± 40.0 b | 160.2 ± 53.2 |
| 18:1n9 | 384.1 ± 58.7 ab | 443.9 ± 112.6 a | 269.4 ± 79.9 ab | 259.8 ± 105.0 b | 330.1 ± 114.0 |
| 18:1n7 | 8.5 ± 1.4 | 10.2 ± 2.2 | 4.4 ± 1.5 | 5.7 ± 2.4 | 7.0 ± 2.8 |
| 18:2n9 | 12.8 ± 2.5 | 14.9 ± 7.6 | 10.9 ± 3.3 | 9.1 ± 3.7 | 11.6 ± 4.6 |
| 18:2n6 | 35.0 ± 7.0 | 46.5 ± 7.9 | 20.0 ± 5.3 | 24.4 ± 9.3 | 30.4 ± 12.2 |
| 18:3n6 | 4.8 ± 1.2 | 5.0 ± 1.1 | 2.9 ± 1.3 | 3.8 ± 2.2 | 4.1 ± 1.7 |
| 18:3n3 | 12.8 ± 1.9 | 16.6 ± 3.9 | 6.5 ± 1.9 | 8.9 ± 3.8 | 10.8 ± 4.7 |
| 20:00 | 10.9 ± 2.7 | 13.0 ± 3.5 | 6.8 ± 2.2 | 7.6 ± 2.6 | 9.3 ± 3.5 |
| 20:1n11 | 6.8 ± 3.0 | 6.7 ± 0.5 | 5.1 ± 2.2 | 5.1 ± 2.7 | 5.8 ± 2.4 |
| 20:1n9 | 44.6 ± 9.1 | 52.0 ± 19.2 | 23.7 ± 7.5 | 26.5 ± 10.0 | 35.4 ± 16.0 |
| 20:1n7 | 9.5 ± 3.4 | 8.4 ± 1.3 | 5.3 ± 2.3 | 6.2 ± 2.8 | 7.2 ± 3.0 |
| 20:2n9 | 9.3 ± 2.8 | 10.0 ± 4.5 | 8.2 ± 2.5 | 7.2 ± 3.3 | 8.6 ± 3.2 |
| 20:2n6 | 15.2 ± 3.9 | 14.6 ± 1.8 | 7.8 ± 2.3 | 9.8 ± 4.1 | 11.6 ± 4.4 |
| 20:3n6 | 14.1 ± 4.2 | 13.2 ± 2.3 | 9.1 ± 3.9 | 9.5 ± 3.4 | 11.3 ± 4.0 |
| 20:4n6 (ARA) | 122.5 ± 39.5 | 97.3 ± 25.7 | 93.6 ± 35.8 | 102.6 ± 45.1 | 104.3 ± 36.9 |
| 20:3n3 | 7.9 ± 1.1 | 8.7 ± 1.9 | 4.4 ± 1.6 | 5.2 ± 2.1 | 6.4 ± 2.4 |
| 20:5n3 (EPA) | 22.3 ± 4.4 | 23.4 ± 7.5 | 12.1 ± 3.2 | 12.6 ± 4.0 | 17.0 ± 6.9 |
| 22:4n6 | 39.8 ± 15.5 | 29.7 ± 11.6 | 30.9 ± 12.5 | 30.2 ± 14.4 | 32.7 ± 13.3 |
| 22:5n6 | 34.0 ± 5.6 | 37.4 ± 5.9 | 28.1 ± 10.4 | 27.1 ± 9.1 | 31.1 ± 8.6 |
| 22:5n3 | 5.9 ± 2.5 | 5.9 ± 1.3 | 2.4 ± 0.7 | 3.7 ± 1.4 | 4.4 ± 2.1 |
| 22:6n3 (DHA) | 420.9 ± 63.5 ab | 508.6 ± 145.1 a | 304.1 ± 91.8 b | 271.7 ± 61.6 b | 364.5 ± 125.8 |
| 24:1n9 | 37.3 ± 6.4 | 48.7 ± 14.6 | 22.6 ± 7.0 | 23.8 ± 6.6 | 31.9 ± 13.2 |
| SFA | 862.2 ± 139.2 ab | 1037.6 ± 256.6 a | 561.7 ± 159.1 b | 572.1 ± 211.7 b | 735.1 ± 267.3 |
| MUFA | 681.9 ± 110.7 ab | 789.5 ± 211.9 a | 439.6 ± 130.9 b | 448.1 ± 179.5 b | 572.7 ± 210.6 |
| PUFA | 757.5 ± 117.9 ab | 831.7 ± 186.1 a | 541.1 ± 160.8 ab | 525.8 ± 152.9 b | 648.7 ± 194.9 |
| n6 | 265.5 ± 73.8 | 243.6 ± 39.1 | 192.4 ± 68.6 | 207.4 ± 85.4 | 225.4 ± 72.0 |
| n3 | 469.8 ± 70.0 ab | 563.2 ± 154.3 a | 329.6 ± 98.5 b | 302.1 ± 71.8 b | 403.1 ± 139.3 |
| EPA + DHA | 443.1 ± 66.8 ab | 532.0 ± 151.5 a | 316.3 ± 94.8 b | 284.3 ± 65.3 b | 381.5 ± 132.1 |
| n3/n6 | 1.9 ± 0.5 | 2.3 ± 0.3 | 1.8 ± 0.5 | 1.6 ± 0.4 | 1.9 ± 0.3 |
| AI | 0.6 ± 0.0 | 0.7 ± 0.1 | 0.6 ± 0.0 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| TI | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| HH | 1.6 ± 0.1 ab | 1.6 ± 0.3 a | 1.8 ± 0.0 b | 1.7 ± 0.2 ab | 1.7 ± 0.2 |
| Season | Location | Sex | Season * Location | Season * Sex | Location * Sex | Season * Location * Sex | |
|---|---|---|---|---|---|---|---|
| DY | 4.7 | 0.4 | 11.4 | 1.8 | 0.8 | 0.3 | 1.2 |
| CI | 2.9 | 3.1 | 0.8 | 5.1 | 1.0 | 3.6 | 1.4 |
| FY | 9.5 | 33.1 | 4.5 | 1.4 | 4.8 | 2.2 | 5.2 |
| Moisture | 13.2 | 0.3 | 1.2 | 1.9 | 17.3 | 0.1 | 0.6 |
| Protein | 0.2 | 0.9 | 2.5 | 8.0 | 17.6 | 0.3 | 2.7 |
| Fat | 8.7 | 0.5 | 0.9 | 7.6 | 4.3 | 8.6 | 4.8 |
| Ash | 30.0 | 20.4 | 0.9 | 5.8 | 5.7 | 0.2 | 0.4 |
| SFA | 0.6 | 17.3 | 3.4 | 1.2 | 4.5 | 3.5 | 4.3 |
| MUFA | 0.7 | 7.7 | 3.5 | 1.4 | 4.5 | 3.6 | 5.5 |
| PUFA | 6.3 | 2.1 | 2.2 | 4.2 | 5.9 | 4.5 | 6.4 |
| n6 | 22.9 | 0.0 | 0.2 | 10.6 | 4.1 | 4.1 | 4.1 |
| n3 | 1.7 | 4.5 | 3.0 | 1.8 | 5.6 | 3.4 | 6.1 |
| ARA | 22.7 | 0.2 | 0.0 | 5.6 | 4.1 | 2.1 | 3.3 |
| EPA | 23.1 | 12.7 | 0.0 | 17.0 | 2.0 | 0.0 | 2.1 |
| DHA | 4.2 | 2.6 | 3.0 | 3.8 | 5.0 | 3.3 | 5.0 |
| EPA + DHA | 2.5 | 3.9 | 2.7 | 2.1 | 5.1 | 3.0 | 5.3 |
| n3/n6 | 33.8 | 14.3 | 1.4 | 17.9 | 0.5 | 0.0 | 0.1 |
| AI | 16.2 | 50.6 | 4.6 | 2.0 | 11.4 | 0.4 | 2.0 |
| TI | 2.3 | 47.5 | 1.6 | 0.6 | 10.1 | 0.2 | 0.5 |
| HH | 8.2 | 60.5 | 3.9 | 1.5 | 12.4 | 0.8 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsiri, M.; Kogiannou, D.; Nikoloudaki, C.; Kleidas, I.; Dogrammatzi, A.; Karachle, P.K.; Grigorakis, K. Seasonal Variation in Nutritional Value and Technical Quality of Lionfish (Pterois miles) from the Ionian and Aegean Seas. Foods 2025, 14, 2353. https://doi.org/10.3390/foods14132353
Kotsiri M, Kogiannou D, Nikoloudaki C, Kleidas I, Dogrammatzi A, Karachle PK, Grigorakis K. Seasonal Variation in Nutritional Value and Technical Quality of Lionfish (Pterois miles) from the Ionian and Aegean Seas. Foods. 2025; 14(13):2353. https://doi.org/10.3390/foods14132353
Chicago/Turabian StyleKotsiri, Mado, Dimitra Kogiannou, Chrisanthi Nikoloudaki, Ioannis Kleidas, Aikaterini Dogrammatzi, Paraskevi K. Karachle, and Kriton Grigorakis. 2025. "Seasonal Variation in Nutritional Value and Technical Quality of Lionfish (Pterois miles) from the Ionian and Aegean Seas" Foods 14, no. 13: 2353. https://doi.org/10.3390/foods14132353
APA StyleKotsiri, M., Kogiannou, D., Nikoloudaki, C., Kleidas, I., Dogrammatzi, A., Karachle, P. K., & Grigorakis, K. (2025). Seasonal Variation in Nutritional Value and Technical Quality of Lionfish (Pterois miles) from the Ionian and Aegean Seas. Foods, 14(13), 2353. https://doi.org/10.3390/foods14132353






