Characterization of the Major Odor-Active Compounds in Fresh Rhizomes and Leaves of Houttuynia cordata by Comparative Aroma Extract Dilution Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reference Odorants
2.3. Organic Solvents
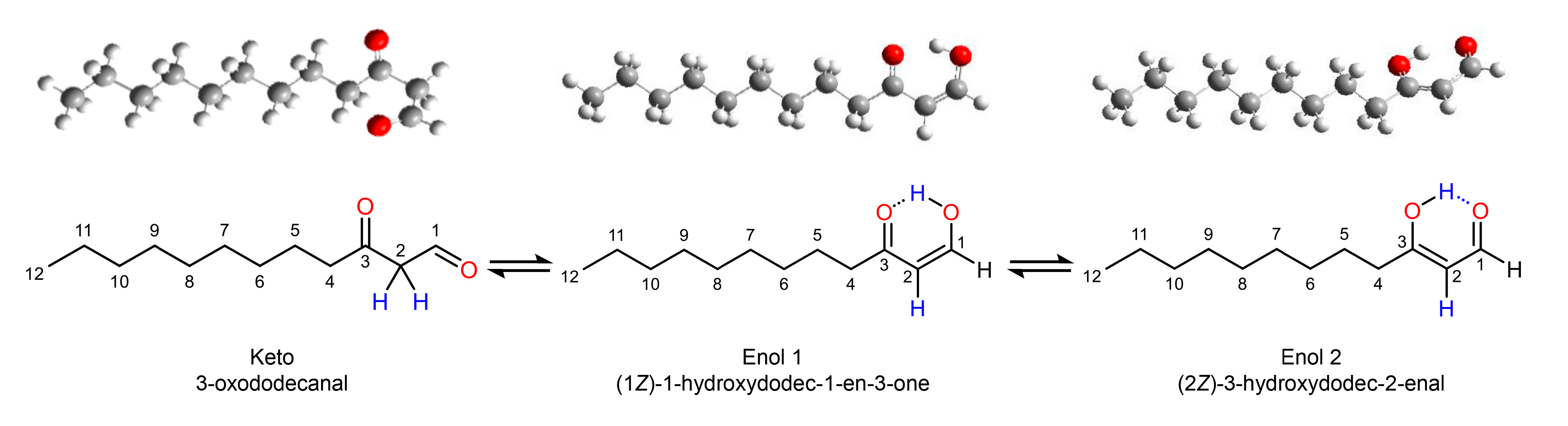
2.4. Synthesis of 3-Oxododecanal (36)
2.5. Gas Chromatography
2.6. Comparative Aroma Extract Dilution Analysis (cAEDA)
2.7. Quantitative Olfactory Profile Analyses
2.8. Quantum Calculations
3. Results and Discussion
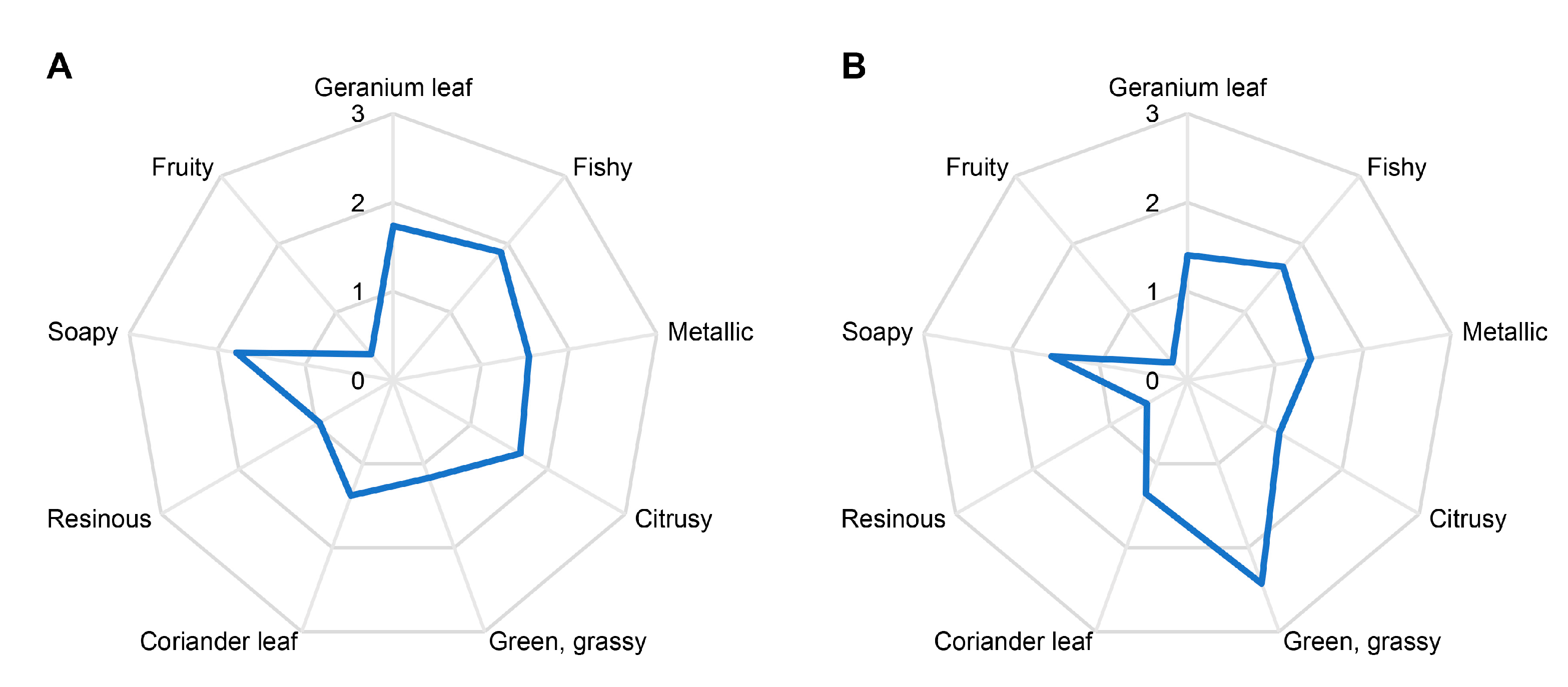
3.1. Quantitative Olfactory Profiles
3.2. Odorant Screening
3.3. Structure Assignment
3.4. Tautomeric Distribution in 36
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Nomenclature
| aSAFE | Automated solvent-assisted flavor evaporation |
| borneol | 1,7,7-trimethylbicyclo[2.2.1]heptan-2-ol |
| bornyl acetate | 1,7,7-trimethylbicyclo[2.2.1]heptan-2-yl acetate |
| cAEDA | Comparative aroma extract dilution analysis |
| (R)-carvone | (5R)-2-methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-one |
| CI | Chemical ionization |
| (E)-β-damascenone | (2E)-1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one |
| γ-decalactone | 5-hexyloxolan-2-one |
| EI | Electron ionization |
| trans-4,5-epoxy-(2E)-dec-2-enal | (2E)-3-[(2R,3R)/(2S,3S)-3-pentyloxiran-2-yl]prop-2-enal |
| eugenol | 2-methoxy-4-(prop-2-en-1-yl)phenol |
| FD | Flavor dilution |
| FFAP | Free fatty acid phase |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| GC–O | Gas chromatography–olfactometry |
| geraniol | (2E)-3,7-dimethylocta-2,6-dien-1-ol |
| geranyl acetate | (2E)-3,7-dimethylocta-2,6-dien-1-yl acetate |
| HPLC | High-performance liquid chromatography |
| HR | High resolution |
| HS | Headspace |
| i.d. | Inner diameter |
| 3-isobutyl-2-methoxypyrazine | 2-methoxy-3-(2-methylpropyl)pyrazine |
| trans-isoeugenol | 2-methoxy-4-[(1E)-prop-1-en-1-yl]phenol |
| 3-isopropyl-2-methoxypyrazine | 2-methoxy-3-(propan-2-yl)pyrazine |
| limonene | 1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene |
| linalool | 3,7-dimethylocta-1,6-dien-3-ol |
| MS | Mass spectrometry |
| myrcene | 7-methyl-3-methylideneocta-1,6-diene |
| NMR | Nuclear magnetic resonance |
| (Z)-β-ocimene | (3Z)-3,7-dimethylocta-1,3,6-triene |
| β-phellandrene | 3-methylidene-6-(propan-2-yl)cyclohex-1-ene |
| α-pinene | 2,6,6-trimethylbicyclo[3.1.1]hept-2-ene |
| β-pinene | 6,6-dimethyl-2-methylidenebicyclo[3.1.1]heptane |
| RI | Retention index |
| SDE | Simultaneous distillation extraction |
| sotolon | 3-hydroxy-4,5-dimethylfuran-2(5H)-one |
| SPME | Solid phase microextraction |
| γ-terpinene | 1-methyl-4-(propan-2-yl)cyclohexa-1,4-diene |
| terpinen-4-ol | 4-methyl-1-(propan-2-yl)cyclohex-3-en-1-ol |
| TOF | Time-of-flight |
| vanillin | 4-hydroxy-3-methoxybenzaldehyde |
References
- Fu, J.; Dai, L.; Lin, Z.; Lu, H. Houttuynia cordata Thunb: A review of phytochemistry and pharmacology and quality control. Chin. Med. 2013, 4, 101–123. [Google Scholar] [CrossRef]
- Pradhan, S.; Rituparna, S.; Dehury, H.; Dhall, M.; Singh, Y.D. Nutritional profile and pharmacological aspect of Houttuynia cordata Thunb. and their therapeutic applications. Pharmacol. Res.-Mod. Chin. Med. 2023, 9, 100311. [Google Scholar] [CrossRef]
- Khamsan, S.; Intecha, N.; Inpeng, P.; Wongwan, W.; Phintakul, S.; Jitmanee, C. Chemical compositions and anticancer activity of essential oil from Houttuynia cordata Thunb. NU. Int. J. Sci. 2020, 17, 23–31. [Google Scholar]
- Gupta, S.; Bharalee, R. Genetic diversity and population structure of a medicinal herb Houttuynia cordata Thunb. of north-east India. Plant Mol. Biol. Rep. 2021, 39, 434–442. [Google Scholar] [CrossRef]
- Xu, Y.W.; Liu, L.; Zhao, D.; Zou, Y.T.; Zeng, J.W.; Wu, W. Aliphatic aldehyde rich volatile constituents of Houttuyania cordata from southwest China. J. Med. Plant Res. 2011, 5, 5844–5847. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Feng, X.; Li, X.; Wang, D.; Sun, G.; Peng, H. Vegetable Houttuynia cordata Thunb. as an important human mercury exposure route in Kaiyang county, Guizhou province, SW China. Ecotoxicol. Environ. Saf. 2020, 197, 110575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Z.; Feng, X.; Wang, A.; Li, X.; Wang, D.; Fan, L. Mercury accumulation in vegetable Houttuynia cordata Thunb. from two different geological areas in southwest China and implications for human consumption. Sci. Rep. 2021, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, R.; Wary, S.; Mili, C.; Roy, S.; Tayung, K. Antimicrobial secondary metabolites obtained from endophytic fungi inhabiting healthy leaf tissues of Houttuynia cordata Thunb., an ethnomedicinal plant of northeast India. J. Appl. Pharm. Sci. 2020, 10, 99–106. [Google Scholar] [CrossRef]
- Lu, H.; Wu, X.; Liang, Y.; Zhang, J. Variation in chemical composition and antibacterial activities of essential oils from two species of Houttuynia THUNB. Chem. Pharm. Bull. 2006, 54, 936–940. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Shi, C.; Wan, J.; Deng, L.; Fang, J. Determination of four volatile compounds with anti-inflammatory biological activity in Houttuynia chordata Thunb. by gas chromatography and gas chromatography-mass spectrometry. Anal. Lett. 2014, 47, 730–741. [Google Scholar] [CrossRef]
- Lee, S.G.; Kang, H. Ameliorative effect of Houttuynia cordata Thunb (Saururaceae) leaf extract in loperamide-induced constipation in rats. Trop. J. Pharm. Res. 2019, 18, 1727–1732. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.G. Bioactive components and functional properties of Hottuynia cordata and its applications. Pharm. Biol. 2009, 47, 1154–1161. [Google Scholar] [CrossRef]
- Chiow, K.H.; Phoon, M.C.; Putti, T.; Tan, B.K.H.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Kumar, A.; Iqbal, H.; Verma, R.K.; Chanda, D.; Chauhan, A.; et al. Chemical composition and allelopathic, antibacterial, antifungal, and antiacetylcholinesterase activity of fish-mint (Houttuynia cordata Thunb.) from India. Chem. Biodivers. 2017, 14, e1700189. [Google Scholar] [CrossRef]
- Yang, L.; Ji, W.; Zhong, H.; Wang, L.; Zhu, X.; Zhu, J. Anti-tumor effect of volatile oil from Houttuynia cordata Thunb. on HepG2 cells and HepG2 tumor-bearing mice. RSC Adv. 2019, 9, 31517–31526. [Google Scholar] [CrossRef]
- Ju, L.; Zhang, J.; Wang, F.; Zhu, D.; Pei, T.; He, Z.; Han, Z.; Wang, M.; Ma, Y.; Xiao, W. Chemical profiling of Houttuynia cordata Thunb. by UPLC-Q-TOF-MS and analysis of its antioxidant activity in C2C12 cells. J. Pharm. Biomed. Anal. 2021, 204, 114271. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Deng, X.; Hu, Q.; Xiao, X.; Jiang, J.; Ma, X.; Wu, M. Houttuynia cordata Thunb: An ethnopharmacological review. Front. Pharmacol. 2021, 12, 714694. [Google Scholar] [CrossRef]
- Lin, C.H.; Chao, L.K.; Lin, L.Y.; Wu, C.S.; Chu, L.P.; Huang, C.H.; Chen, H.C. Analysis of volatile compounds from different parts of Houttuynia cordata Thunb. Mol. 2022, 27, 8893. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, S.; Ma, J.; Wu, D.; Hong, L.; Yu, Z. GC-MS analyses of the volatiles of Houttuynia cordata Thunb. Pak. J. Pharm. Sci. 2016, 29, 1591–1600. [Google Scholar]
- Asakawa, Y.; Tomiyama, K.; Sakurai, K.; Kawakami, Y.; Yaguchi, Y. Volatile compounds from the different organs of Houttuynia cordata and Litsea cubeba (L. citriodora). J. Oleo Sci. 2017, 66, 889–895. [Google Scholar] [CrossRef]
- Liang, M.; Qi, M.; Zhang, C.; Zhou, S.; Fu, R.; Huang, J. Gas chromatography-mass spectrometry analysis of volatile compounds from Houttuynia cordata Thunb after extraction by solid-phase microextraction, flash evaporation and steam distillation. Anal. Chim. Acta 2005, 531, 97–104. [Google Scholar] [CrossRef]
- Qi, S.; Zha, L.; Peng, Y.; Luo, W.; Chen, K.; Li, X.; Huang, D.; Yin, D. Quality and metabolomics analysis of Houttuynia cordata based on HS-SPME/GC-MS. Mol. 2022, 27, 3921. [Google Scholar] [CrossRef] [PubMed]
- Ch, M.I.; Wen, Y.F.; Cheng, Y. Gas chromatographic/mass spectrometric analysis of the essential oil of Houttuynia cordata Thunb by using on-column methylation with tetramethylammonium acetate. J. AOAC Int. 2007, 90, 60–67. [Google Scholar] [CrossRef]
- Pan, X.; Li, H.; Chen, D.; Zheng, J.; Yin, L.; Zou, J.; Zhang, Y.; Deng, K.; Xiao, M.; Meng, L.; et al. Comparison of essential oils of Houttuynia cordata Thunb. from different processing methods and harvest seasons based on GC-MS and chemometric analysis. Int. J. Anal. Chem. 2021, 2021, 8324169. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Leung, K.S.; Jiang, Z.; Dong, X.; Zhao, Z. Establishment of GC-MS fingerprint of fresh Houttuynia cordata. Chem. Pharm. Bull. 2005, 53, 1484–1489. [Google Scholar] [CrossRef]
- Kwon, H.D.; Cha, I.H.; Lee, W.K.; Song, J.H.; Park, I.H. Antibacterial activity of volatile flavor components from Houttuynia cordata Thunb. J. Food Sci. Nutr. 1996, 1, 208–213. [Google Scholar]
- Zhou, J.; Fan, Q.; Zhang, Y.; Castillo, R.; Xiao, M.; Liu, H.; Zhu, Z.; Liu, Y.; Yang, Y.; Zhou, Y.; et al. Novel mathematical model for the assessment of similarity of chromatographic fingerprints of volatile oil from Houttuynia cordata. Pharmacogn. Mag. 2021, 17, 154–162. [Google Scholar] [CrossRef]
- Schlumpberger, P.; Stübner, C.A.; Steinhaus, M. Development and evaluation of an automated solvent-assisted flavour evaporation (aSAFE). Eur. Food Res. Technol. 2022, 248, 2591–2602. [Google Scholar] [CrossRef]
- Steinhaus, M. Gas chromatography-olfactometry: Principles, practical aspects and applications in food analysis. In Advanced Gas Chromatography in Food Analysis; Tranchida, P.Q., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 337–399. [Google Scholar] [CrossRef]
- Kreissl, J.; Schieberle, P. Characterization of aroma-active compounds in Italian tomatoes with emphasis on new odorants. J. Agric. Food Chem. 2017, 65, 5198–5208. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, X.; Wang, L.; Wei, Z. Studies on antimicrobial and antiviral compounds—Synthesis of derivatives of decanoyl acetaldehyde. Acta Pharm. Sin. 1979, 14, 428–433. [Google Scholar]
- Guth, H.; Grosch, W. 3-Methylnonane-2,4-dione—An intense odour compound formed during flavour reversion of soya-bean oil. Lipid/Fett 1989, 91, 225–230. [Google Scholar] [CrossRef]
- Bemelmanns, J.M.H. Review of isolation and concentration techniques. In Progress in Flavour Research, Land, D.G., Nursten, H.E., Eds.; Applied Science Publishers: London, UK, 1979; pp. 79–88. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2007, 120, 215–241. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New basis set exchange: An open, up-to-date resource for the molecular sciences community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Shermo: A general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 2021, 1200, 113249. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochemistry 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Blee, E. Phytooxylipins and plant defense reactions. Prog. Lipid Res. 1998, 37, 33–72. [Google Scholar] [CrossRef]
- Kreissl, J.; Mall, V.; Steinhaus, P.; Steinhaus, M. Leibniz-LSB@TUM Odorant Database; Version 1.2; Leibniz Institute for Food Systems Biology at the Technical University of Munich: Freising, Germany, 2022; Available online: https://www.leibniz-lsb.de/en/databases/leibniz-lsbtum-odorant-database (accessed on 29 May 2025).
- Xue, Q.; Wang, Z.; Yin, H. Highly efficient extraction of decanoyl acetaldehyde in Houttuynia cordata thunb under steam distillation and its mass spectrometric analysis. J. Anal. Sci. 2012, 28, 28–33. [Google Scholar]
- Liu, Y.; Yang, Y.; Wang, B.; Wang, R.; Pang, J.; Jiang, Y.; Liu, Y. Development and verification of a precolumn derivatization LC-MS/MS method for the pharmacokinetic study of houttuynine of Houttuynia essential oil. Mol. 2021, 26, 2327. [Google Scholar] [CrossRef]
- Masur, M.; Griitzmacher, H.; Munster, H.; Budzikiewicz, H. Mass spectrometric fragmentation of the tautomers of 1,3-diketones—A gas chromatographic/mass spectrometric study. Org. Mass Spectrom. 1987, 22, 493–500. [Google Scholar] [CrossRef]
- Choudhury, B.H.; Baruah, A.M.; Sarmah, T.C.; Baishya, S. Nutritional and antinutritional composition of twenty five indigenous leafy vegetables of Jorhat district of Assam state, India. Asian J. Chem. 2017, 29, 65–68. [Google Scholar] [CrossRef]
- Nowroozi, A.; Jalbout, A.F.; Roohi, H.; Khalilinia, E.; Sadeghi, M.; Leon de, A.; Raissi, H. Hydrogen bonding in acetylacetaldehyde: Theoretical insights from the theory of atoms in molecules. Int. J. Quantum Chem. 2009, 109, 1505–1514. [Google Scholar] [CrossRef]

| No. 1 | Odorant 2 | Odor 3 | RI 4 | FD Factor 5 | ||
|---|---|---|---|---|---|---|
| FFAP | DB-5 | Rhizomes | Leaves | |||
| 1 | butane-2,3-dione | butter | 983 | 600 | 4 | 2 |
| 2 | α-pinene | resinous | 1024 | 936 | 256 | 128 |
| 3 | ethyl 2-methylbutanoate | fruity | 1047 | 860 | 2 | 64 |
| 4 | 2-methylpropan-1-ol | malty | 1083 | 633 | <1 | 2 |
| 5 | hex-1-en-3-one | rubber, pungent | 1091 | 788 | 1 | 2 |
| 6 | (3Z)-hex-3-enal | green, grassy | 1135 | 804 | 32 | 256 |
| 7 | myrcene | geranium leaf | 1152 | 1004 | 2048 | 2048 |
| 8 | limonene | citrusy | 1186 | 1033 | 256 | 8 |
| 9 | γ-terpinene | gasoline | 1233 | 1060 | 8 | <1 |
| 10 | octanal | citrusy, green | 1271 | 1008 | 32 | 32 |
| 11 | oct-1-en-3-one | mushroom | 1286 | 980 | 32 | 128 |
| 12 | (5Z)-octa-1,5-dien-3-one | metallic | 1351 | 984 | 512 | 1024 |
| 13 | unknown | fruity, honey | 1374 | 1109 | 2 | 8 |
| 14 | 3-isopropyl-2-methoxypyrazine 6 | earthy, pea | 1407 | 1096 | 4 | <1 |
| 15 | acetic acid | vinegar | 1422 | 638 | 4 | 2 |
| 16 | 3-(methylsulfanyl)propanal | cooked potato | 1432 | 912 | 32 | <1 |
| 17 | decanal | soapy, citrusy | 1478 | 1202 | 64 | 256 |
| 18 | 3-isobutyl-2-methoxypyrazine 6 | green bell pepper | 1500 | 1183 | 16 | <1 |
| 19 | linalool | citrusy, floral | 1511 | 1109 | 1 | 4 |
| 20 | unknown | herbaceous, clove | 1536 | 1113 | <1 | 4 |
| 21 | bornyl acetate | mountain pine | 1558 | 1287 | 16 | 2 |
| 22 | undecan-2-one | soapy, green | 1584 | 1300 | 32 | 16 |
| 23 | butanoic acid | sweaty | 1600 | 818 | 32 | 8 |
| 24 | 2-/3-methylbutanoic acid | sweaty | 1637 | 870 | 4 | 2 |
| 25 | (2Z)-2-butyloct-2-enal | citrusy, soapy | 1658 | 1386 | 8 | 16 |
| 26 | borneol | earthy, moldy | 1661 | 1170 | 4 | <1 |
| 27 | (2E,4E)-nona-2,4-dienal | fatty, green | 1692 | 1217 | 4 | 32 |
| 28 | 3-methylnonane-2,4-dione | hay, aniseed, fishy | 1700 | 1248 | 32 | 64 |
| 29 | pentanoic acid | sweaty | 1706 | 908 | <1 | 2 |
| 30 | (R)-carvone | spearmint | 1710 | 1248 | 16 | 16 |
| 31 | geranyl acetate | floral, rose | 1729 | 1381 | 32 | 64 |
| 32 | (E)-β-damascenone | cooked apple | 1789 | 1386 | 64 | 64 |
| 33 | geraniol | rose, citrusy | 1831 | 1261 | 64 | 4 |
| 34 | 2-methoxyphenol 6 | smoky, gammon | 1841 | 1088 | 8 | 4 |
| 35 | (2E)-dodec-2-enal | coriander leaf | 1854 | 1467 | 128 | 128 |
| 36 | 3-oxododecanal 7 | metallic, soapy, fishy | 1894 | 1490 | 16,384 | 4096 |
| 37 | trans-4,5-epoxy-(2E)-dec-2-enal | metallic | 1994 | 1381 | 16 | 32 |
| 38 | 4-methoxybenzaldehyde | woodruff, aniseed | 2013 | 1257 | 16 | 32 |
| 39 | unknown | metallic | 2033 | 1470 | 16 | 8 |
| 40 | 4-methylphenol | phenolic | 2053 | 1083 | 16 | 32 |
| 41 | γ-decalactone | peach, coconut | 2140 | 1467 | 2 | <1 |
| 42 | eugenol 6 | clove | 2153 | 1362 | 32 | 8 |
| 43 | sotolon | fenugreek | 2187 | 1108 | 4 | 4 |
| 44 | decanoic acid | soapy, musty | 2250 | 1381 | 4 | 8 |
| 45 | trans-isoeugenol 6 | smoky, clove | 2343 | 1452 | 64 | 32 |
| 46 | phenylacetic acid 6 | honey, beeswax | 2523 | 1274 | 4 | 4 |
| 47 | vanillin | vanilla | 2557 | 1405 | 64 | 16 |
| Solvent | Tautomer | 1 | 2 | 3 | 4 | BD 5 |
|---|---|---|---|---|---|---|
| water | enol 1 | −389,465.001 | −2.516 | −389,465.626 | 1.789 | 82.452 |
| enol 2 | −389,464.343 | −2.102 | −389,464.555 | 0.718 | 13.522 | |
| keto | −389,459.916 | −5.812 | −389,463.837 | – | 4.026 | |
| chloroform | enol 1 | −389,465.001 | −9.842 | −389,472.953 | 3.033 | 79.163 |
| enol 2 | −389,464.343 | −9.695 | −389,472.149 | 2.228 | 20.363 | |
| keto | −389,459.916 | −11.895 | −389,469.920 | – | 0.474 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Liu, J.; Kreissl, J.; Oellig, C.; Vetter, W.; Steinhaus, M.; Frank, S. Characterization of the Major Odor-Active Compounds in Fresh Rhizomes and Leaves of Houttuynia cordata by Comparative Aroma Extract Dilution Analysis. Foods 2025, 14, 2303. https://doi.org/10.3390/foods14132303
Xu Z, Liu J, Kreissl J, Oellig C, Vetter W, Steinhaus M, Frank S. Characterization of the Major Odor-Active Compounds in Fresh Rhizomes and Leaves of Houttuynia cordata by Comparative Aroma Extract Dilution Analysis. Foods. 2025; 14(13):2303. https://doi.org/10.3390/foods14132303
Chicago/Turabian StyleXu, Zhenli, Jing Liu, Johanna Kreissl, Claudia Oellig, Walter Vetter, Martin Steinhaus, and Stephanie Frank. 2025. "Characterization of the Major Odor-Active Compounds in Fresh Rhizomes and Leaves of Houttuynia cordata by Comparative Aroma Extract Dilution Analysis" Foods 14, no. 13: 2303. https://doi.org/10.3390/foods14132303
APA StyleXu, Z., Liu, J., Kreissl, J., Oellig, C., Vetter, W., Steinhaus, M., & Frank, S. (2025). Characterization of the Major Odor-Active Compounds in Fresh Rhizomes and Leaves of Houttuynia cordata by Comparative Aroma Extract Dilution Analysis. Foods, 14(13), 2303. https://doi.org/10.3390/foods14132303







