Enhancing Product Quality, Nutrition, Antioxidant Capacity, and Sensory Quality of Chicken Sausages by Replacing Fats with Agaricus bisporus and Soybean Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Agaricus bisporus (Ab) Powder Preparation and Pretreatment of Chicken and Pork Back Fat
2.3. Manufacture of Sausages
2.4. Composition and Energy Value
2.5. Cooking Yield (CY), Color and pH
2.6. Texture Profile Analysis (TPA)
2.7. Lipid Oxidation (TBARS)
2.8. Microstructure
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Properties and Composition of Ab Powder and Sausage
3.2. Amino Acids (AA) Profile
3.3. Fatty Acid Profile of Sausage
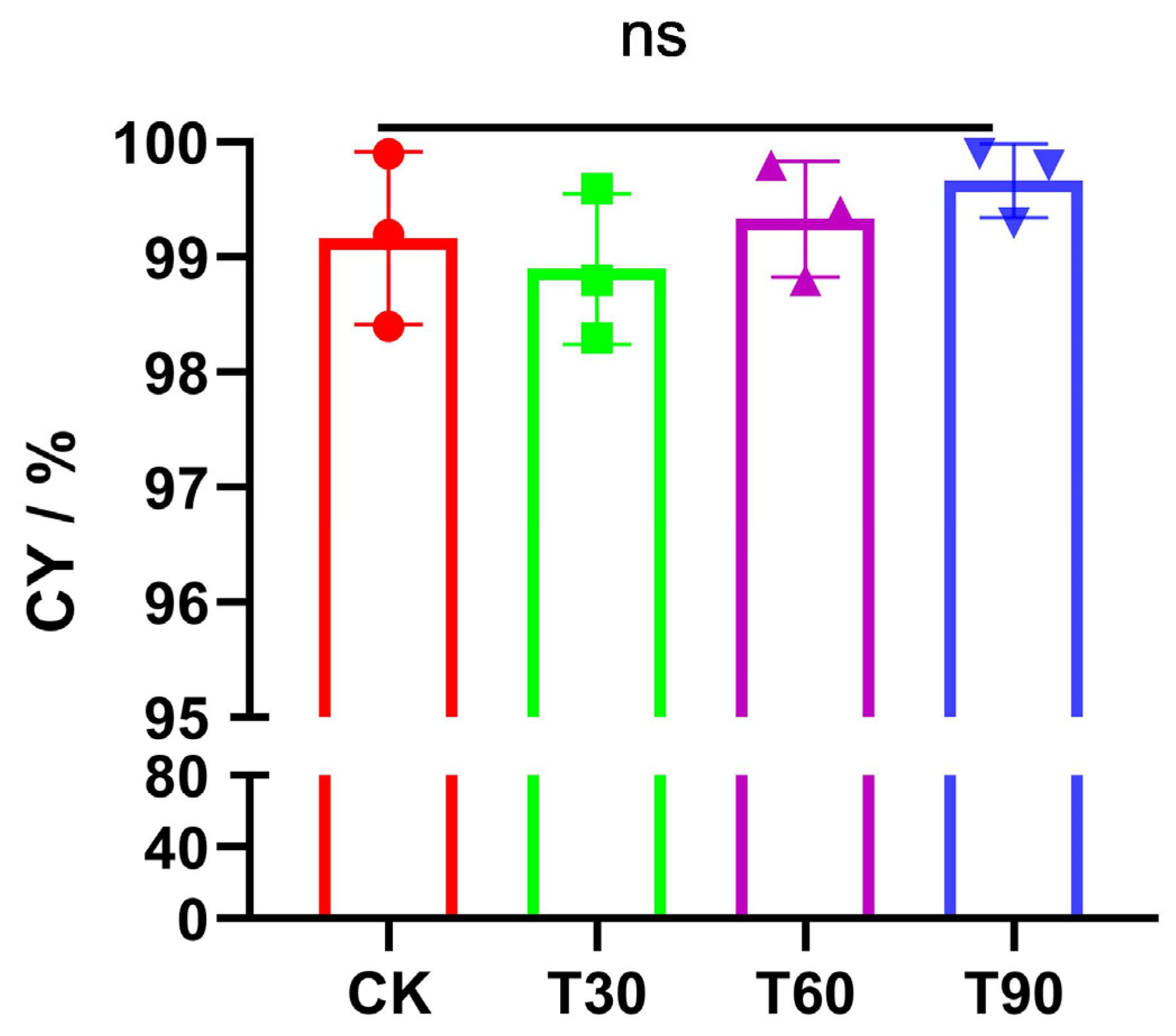
3.4. Cooking Yield (CY)
3.5. Color
3.6. TPA
3.7. TBARS Value
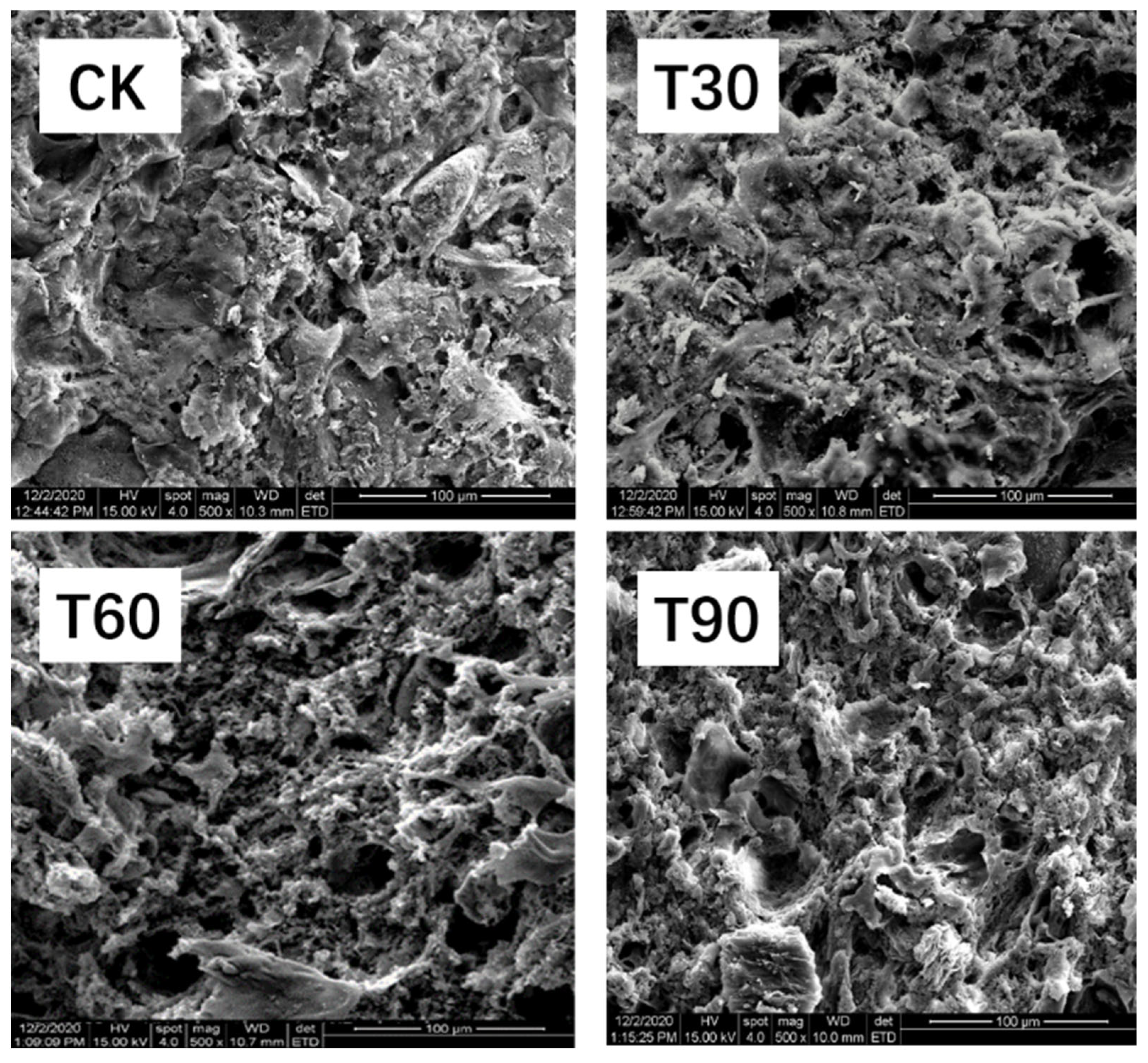
3.8. Microstructure
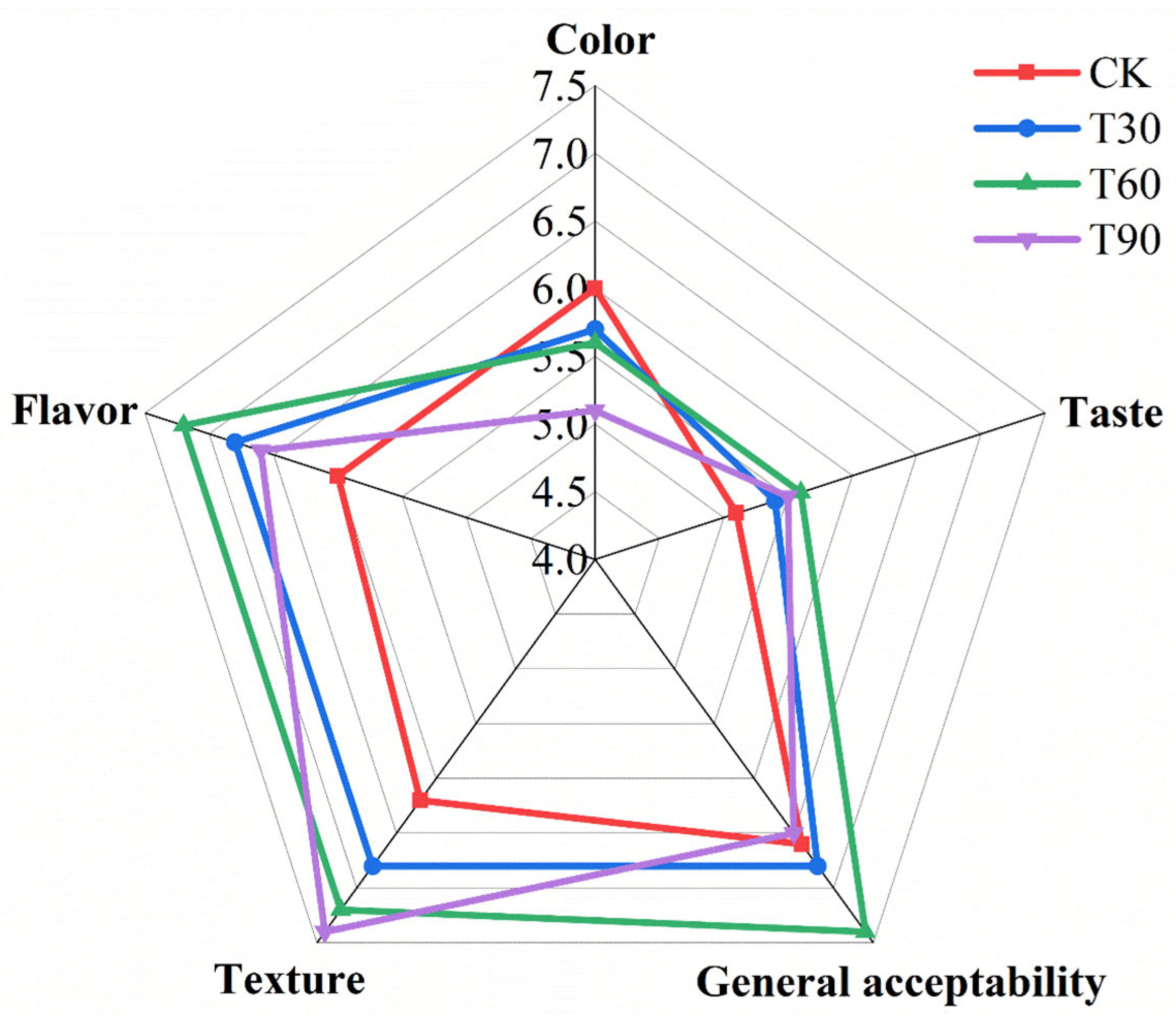
3.9. Sensory Evalution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Çapan, B.; Bağdatli, A. Investigation of physicochemical, microbiological and sensorial properties for organic and conventional retail chicken meat. Food Sci. Hum. Wellness 2021, 10, 183–190. [Google Scholar] [CrossRef]
- de Carvalho, F.A.L.; Munekata, P.E.; Pateiro, M.; Campagnol, P.C.B.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Effect of replacing backfat with vegetable oils during the shelf-life of cooked lamb sausages. LWT 2020, 122, 109052. [Google Scholar] [CrossRef]
- Varga-Visi, É.; Toxanbayeva, B. Application of fat replacers and their effect on quality of comminuted meat products with low lipid content: A review. Acta Aliment. 2017, 46, 181–186. [Google Scholar] [CrossRef]
- Kaynakci, E.; KiliÇ, B. Effect of replacing beef fat with safflower oil on physicochemical, nutritional and oxidative stability characteristics of wieners. Food Sci. Technol. 2021, 41, 52–59. [Google Scholar] [CrossRef]
- Kumar, Y. Development of Low-Fat/Reduced-Fat Processed Meat Products using Fat Replacers and Analogues. Food Rev. Int. 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Jairath, J.; Sharma, D.P.; Dabur, R.S.; Singh, P.K.; Bishnoi, S. Standardization of corn starch as a fat replacer in buffalo calf meat sausages and its effect on the quality attributes. Indian J. Anim. Res. 2017, 52, 1521–1525. [Google Scholar] [CrossRef]
- Montoya, L.; Quintero, N.; Ortiz, S.; Lopera, J.; Millán, P.; Rodríguez-Stouvenel, A. Inulin as a Fat-Reduction Ingredient in Pork and Chicken Meatballs: Its Effects on Physicochemical Characteristics and Consumer Perceptions. Foods 2022, 11, 1066. [Google Scholar] [CrossRef]
- Utama, D.T.; Jeong, H.S.; Kim, J.; Barido, F.H.; Lee, S.K. Fatty acid composition and quality properties of chicken sausage formulated with pre-emulsified perilla-canola oil as an animal fat replacer. Poult. Sci. 2019, 98, 3059–3066. [Google Scholar] [CrossRef]
- Baune, M.-C.; Schroeder, S.; Witte, F.; Heinz, V.; Bindrich, U.; Weiss, J.; Terjung, N. Analysis of protein-network formation of different vegetable proteins during emulsification to produce solid fat substitutes. J. Food Meas. Charact. 2021, 15, 2399–2416. [Google Scholar] [CrossRef]
- Kılıç, B.; Özer, C.O. Effects of replacement of beef fat with interesterified palm kernel oil on the quality characteristics of Turkish dry-fermented sausage. Meat Sci. 2017, 131, 18–24. [Google Scholar] [CrossRef]
- Mouta-Afif, F.; Kırkyol, M.; Akköse, A. Effects of Animal Fat Replacement with Argan Oil on the Quality Properties of Bologna-type Chicken Sausages. ACS Food Sci. Technol. 2024, 5, 234–240. [Google Scholar] [CrossRef]
- Serdaroğlu, M.; Nacak, B.; Karabıyıkoğlu, M.; Tepe, M.; Baykara, I.; Kökmen, Y. Effects of replacing beef fat with pre-emulsified pumpkin seed oil on some quality characteristics of model system chicken meat emulsions. In Proceedings of the 59th International Meat Industry Conference MEATCON2017, Zlatibor, Serbia, 1–4 October 2017; IOP Publishing Ltd.: Bristol, UK; p. 012045. [Google Scholar]
- Wang, X.; Xie, Y.; Li, X.; Liu, Y.; Yan, W. Effects of partial replacement of pork back fat by a camellia oil gel on certain quality characteristics of a cooked style Harbin sausage. Meat Sci. 2018, 146, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Botella-Martínez, C.; Viuda-Martos, M.; Pérez-Álvarez, J.A.; Fernández-López, J. Total and Partial Fat Replacement by Gelled Emulsion (Hemp Oil and Buckwheat Flour) and Its Impact on the Chemical, Technological and Sensory Properties of Frankfurters. Foods 2021, 10, 1681. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Wu, Z.; Wang, X.; Zhang, K.; Li, Y. O/W Pickering emulsions stabilized by Flammulina velutipes polysaccharide nanoparticles as a fat substitute: The effects of phase separation on emulsified sausage’s techno-functional and sensory quality. J. Sci. Food Agric. 2019, 100, 268–276. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Zeng, X.; Zhao, X.; Xu, X. Recent progress of fat reduction strategies for emulsion type meat products. Food Mater. Res. 2022, 2, 10. [Google Scholar] [CrossRef]
- Nan, H.; Zhou, H.; Li, B.; Stepanova, T.; Kondratiuk, N. Effects of Agaricus bisporus alone or in combination with soybean oil or water as fat substitutes on gel properties, rheology, water distribution, and microstructure of chicken batters. Food Sci. Technol. 2022, 42, e116121. [Google Scholar] [CrossRef]
- Ceron-Guevara, M.I.; Rangel-Vargas, E.; Lorenzo, J.M.; Bermudez, R.; Pateiro, M.; Rodriguez, J.A.; Sanchez-Ortega, I.; Santos, E.M. Reduction of Salt and Fat in Frankfurter Sausages by Addition of Agaricus bisporus and Pleurotus ostreatus Flour. Foods 2020, 9, 760. [Google Scholar] [CrossRef]
- Gasparetto, H.; de Castilhos, F.; Paula Gonçalves Salau, N. Recent advances in green soybean oil extraction: A review. J. Mol. Liq. 2022, 361, 119684. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Liu, X.; Jiang, G.; Li, C.; Li, X.; Li, Y. Roles of Lentinula edodes as the pork lean meat replacer in production of the sausage. Meat Sci. 2019, 156, 44–51. [Google Scholar] [CrossRef]
- Nan, H.; Stepanova, T.M.; Kondratiuk, N.V.; Nie, Y.; Li, B. Effects of Agaricus bisporus on gel properties of chicken myofibrillar protein. Int. J. Food Sci. Technol. 2022, 57, 5532–5541. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis; Association of Official Analytical Chemistry: Rockville, MD, USA, 2005. [Google Scholar]
- GB 5009.124-2016; Chinese National Food Safety Standard—Determination of Amino Acids in Foods. National Food and Drug Administration: Beijing, China, 2016.
- GB 5009.168-2016; Chinese National Food Safety Standard—Determination of Fatty Acids in Foods. National Food and Drug Administration: Beijing, China, 2016.
- Nagy, M.; Semeniuc, C.A.; Socaci, S.A.; Pop, C.R.; Rotar, A.M.; Sălăgean, C.D.; Tofană, M. Utilization of brewer’s spent grain and mushrooms in fortification of smoked sausages. Food Sci. Technol. 2017, 37, 315–320. [Google Scholar] [CrossRef]
- Qin, X.; Samilyk, M.; Luo, Y.; Sokolenko, V. Influence of sesame flour on physicochemical properties of sour milk drinks. East.-Eur. J. Enterp. Technol. 2021, 3, 6–16. [Google Scholar] [CrossRef]
- Mousavi, S.M.R.; Rafe, A.; Yeganehzad, S. Textural, mechanical, and microstructural properties of restructured pimiento alginate-guar gels. J. Texture Stud. 2019, 50, 155–164. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, D.-W.; Pu, H.; Xie, A.; Han, Z.; Luo, M. Non-destructive prediction of thiobarbituricacid reactive substances (TBARS) value for freshness evaluation of chicken meat using hyperspectral imaging. Food Chem. 2015, 179, 175–181. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Ren, L.; Guo, H.; Li, Y. Production of Pork Sausages Using Pleaurotus eryngii with Different Treatments as Replacements for Pork Back Fat. J. Food Sci. 2019, 84, 3091–3098. [Google Scholar] [CrossRef]
- Cofrades, S.; Benedí, J.; Garcimartin, A.; Sánchez-Muniz, F.J.; Jimenez-Colmenero, F. A comprehensive approach to formulation of seaweed-enriched meat products: From technological development to assessment of healthy properties. Food Res. Int. 2017, 99, 1084–1094. [Google Scholar] [CrossRef]
- Marti-Quijal, F.J.; Zamuz, S.; Tomašević, I.; Rocchetti, G.; Lucini, L.; Marszałek, K.; Barba, F.J.; Lorenzo, J.M. A chemometric approach to evaluate the impact of pulses, Chlorella and Spirulina on proximate composition, amino acid, and physicochemical properties of turkey burgers. J. Sci. Food Agric. 2019, 99, 3672–3680. [Google Scholar] [CrossRef]
- National Institute of Nutrition and Health, Chinese Center for Disease Control and Prevention. China Food Composition Table, 6th ed.; Yang, Y., Zhu, W., He, M., Pan, X., Eds.; Peking University Medical Press: Beijing, China, 2019; Volume 2, p. 201. [Google Scholar]
- Bach, F.; Helm, C.V.; Bellettini, M.B.; Maciel, G.M.; Haminiuk, C.W.I. Edible mushrooms: A potential source of essential amino acids, glucans and minerals. Int. J. Food Sci. Technol. 2017, 52, 2382–2392. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Choi, J.-H.; Han, D.-J.; Kim, H.-Y.; Lee, M.-A.; Jeong, J.-Y.; Chung, H.-J.; Kim, C.-J. Effects of replacing pork back fat with vegetable oils and rice bran fiber on the quality of reduced-fat frankfurters. Meat Sci. 2010, 84, 557–563. [Google Scholar] [CrossRef]
- Dorni, C.; Sharma, P.; Saikia, G.; Longvah, T. Fatty acid profile of edible oils and fats consumed in India. Food Chem. 2018, 238, 9–15. [Google Scholar] [CrossRef]
- World Health Organization. Healthy Diet; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B. Dietary nutrients in edible mushroom, Agaricus bisporus and their radical scavenging, antibacterial, and antifungal effects. Process Biochem. 2022, 121, 10–17. [Google Scholar] [CrossRef]
- Kumar, Y.; Kumar, A.; Vishwakarma, R.K.; Kumar Singh, R. The combined effects of multiple emulsion, plant gel, and fibers from pea pods on the characteristics of low-fat meat batter. J. Food Process. Preserv. 2021, 45, e15298. [Google Scholar] [CrossRef]
- Munshi, P.; Stanley, C.B.; Ghimire-Rijal, S.; Lu, X.; Myles, D.A.; Cuneo, M.J. Molecular details of ligand selectivity determinants in a promiscuous β-glucan periplasmic binding protein. BMC Struct. Biol. 2013, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dobruchowska, J.M.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P. Structural investigation of water-soluble polysaccharides extracted from the fruit bodies of Coprinus comatus. Carbohydr. Polym. 2013, 91, 314–321. [Google Scholar] [CrossRef]
- Lei, J.; Zhang, Y.; Guo, D.; Meng, J.; Feng, C.; Xu, L.; Cheng, Y.; Liu, R.; Chang, M.; Geng, X. Extraction optimization, structural characterization of soluble dietary fiber from Morchella importuna, and its in vitro fermentation impact on gut microbiota and short-chain fatty acids. CyTA–J. Food 2022, 20, 128–142. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, T.; Wang, X.; Zou, Y.; Wang, D.; Xu, W. Effects of the structure and gel properties of myofibrillar protein on chicken breast quality treated with ultrasound-assisted potassium alginate. Food Chem. 2021, 358, 129873. [Google Scholar] [CrossRef]
- Barbut, A.G.S. Effect of Chloride Salts on Protein Extraction and Interfacial Protein Film Formation in Meat Batters. J. Sci. Food Agric. 1992, 58, 227–238. [Google Scholar]
- Mleczek, M.; Budka, A.; Siwulski, M.; Mleczek, P.; Gąsecka, M.; Jasińska, A.; Kalač, P.; Sobieralski, K.; Niedzielski, P.; Proch, J.; et al. Investigation of differentiation of metal contents of Agaricus bisporus, Lentinula edodes and Pleurotus ostreatus sold commercially in Poland between 2009 and 2017. J. Food Compos. Anal. 2020, 90, 103488. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Zhang, Y.; Luo, X.; Sun, J. Improving myofibrillar proteins solubility and thermostability in low-ionic strength solution: A review. Meat Sci. 2022, 189, 108822. [Google Scholar] [CrossRef]
- Alnoumani, H.; Ataman, Z.A.; Were, L. Lipid and protein antioxidant capacity of dried Agaricus bisporus in salted cooked ground beef. Meat Sci. 2017, 129, 9–19. [Google Scholar] [CrossRef]
- Papastergiadis, A.; Mubiru, E.; Van Langenhove, H.; De Meulenaer, B. Malondialdehyde measurement in oxidized foods: Evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. J. Agric. Food Chem. 2012, 60, 9589–9594. [Google Scholar] [CrossRef]
- Li, X.-L.; Meng, R.; Xu, B.-C.; Zhang, B.; Cui, B.; Wu, Z.-Z. Function emulsion gels prepared with carrageenan and zein/carboxymethyl dextrin stabilized emulsion as a new fat replacer in sausages. Food Chem. 2022, 389, 133005. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Li, L.; Wen, J.; Gu, Q.; Wu, J.; Yu, Y.; Xu, Y.; Fu, M.; Lin, X. Evaluation of the Structural, Physicochemical and Functional Properties of Dietary Fiber Extracted from Newhall Navel Orange By-Products. Foods 2021, 10, 2772. [Google Scholar] [CrossRef] [PubMed]
- Van der Sman, R.G.M.; Paudel, E.; Voda, A.; Khalloufi, S. Hydration properties of vegetable foods explained by Flory–Rehner theory. Food Res. Int. 2013, 54, 804–811. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Q.; Xiao, N.; Du, Y.; Feng, Q.; Shi, W. Changes in Gel Structure and Chemical Interactions of Hypophthalmichthys molitrix Surimi Gels: Effect of Setting Process and Different Starch Addition. Foods 2021, 10, 1681. [Google Scholar] [CrossRef]
| Raw Material/Ingredients | Formula (g) | |||
|---|---|---|---|---|
| CK | T30 | T60 | T90 | |
| Chicken batter | ||||
| Chicken | 60 | 60 | 60 | 60 |
| Pork back fat | 20 | 14 | 8 | 2 |
| Ab mushroom | 0 | 2 | 4 | 6 |
| Soybean oil | 0 | 4 | 8 | 12 |
| Ice water | 20 | 20 | 20 | 20 |
| Others | ||||
| Salt | 1.4 | 1.4 | 1.4 | 1.4 |
| Sodium tripolyphosphate | 0.3 | 0.3 | 0.3 | 0.3 |
| Chicken essence | 0.1 | 0.1 | 0.1 | 0.1 |
| Sugar | 0.65 | 0.65 | 0.65 | 0.65 |
| White pepper | 0.15 | 0.15 | 0.15 | 0.15 |
| Potato starch | 5 | 5 | 5 | 5 |
| The total sum | 107.6 | 107.6 | 107.6 | 107.6 |
| Composition (%) | Color and pH | ||
|---|---|---|---|
| Moisture | 6.96 ± 0.11 | L* | 51.26 ± 0.49 |
| Ash | 10.15 ± 0.34 | a* | 1.91 ± 0.09 |
| Protein | 22.14 ± 0.53 | b* | 14.70 ± 0.13 |
| Fat | 2.53 ± 0.05 | pH | 6.53 ± 0.05 |
| Carbohydrate | 58.22 ± 0.73 | ||
| Essential amino acid (%) | Non-essential Amino acid (%) | ||
| Val | 1.19 ± 0.10 | ASP | 1.89 ± 0.11 |
| Met | 0.27 ± 0.01 | Glu | 5.02 ± 0.24 |
| Ile | 1.02 ± 0.03 | Ser | 0.83 ± 0.02 |
| Leu | 1.44 ± 0.09 | Gly | 1.11 ± 0.08 |
| Phe | 0.97 ± 0.02 | Pro | 1.01 ± 0.05 |
| His | 0.41 ± 0.01 | Ala | 1.79 ± 0.02 |
| Lys | 1.21 ± 0.10 | Cyst | 0.06 ± 0.01 |
| Thr | 1.03 ± 0.08 | Tyr | 0.77 ± 0.02 |
| Arg | 0.98 ± 0.02 | ||
| Items | CK | T30 | T60 | T90 |
|---|---|---|---|---|
| Moisture (g/100 g) | 61.06 ± 1.32 a | 64.58 ± 2.22 a | 61.85 ± 1.18 a | 62.71 ± 1.25 a |
| Ash (g/100 g) | 0.73 ± 0.07 c | 0.76 ± 0.05 c | 1.54 ± 0.01 b | 1.92 ± 0.06 a |
| Protein (g/100 g) | 15.38 ± 1.40 a | 14.36 ± 0.99 a | 12.51 ± 1.48 ab | 11.90 ± 0.94 b |
| Fat (g/100 g) | 18.56 ± 0.31 a | 16.74 ± 0.24 b | 14.80 ± 0.34 c | 12.90 ± 0.21 d |
| Carbohydrate (g/100 g) | 4.27 ± 0.37 c | 3.56 ± 0.35 c | 9.30 ± 0.34 b | 10.57 ± 0.35 a |
| Energy (kcal/100 g) | 245.64 ± 9.87 a | 222.34 ± 7.52 b | 220.44 ± 10.34 b | 205.98 ± 7.05 b |
| Amino Acids | CK | T30 | T60 | T90 |
|---|---|---|---|---|
| Essential | ||||
| Val | 0.77 ± 0.01 b | 0.83 ± 0.02 a | 0.82 ± 0.01 a | 0.81 ± 0.01 a |
| Met | 0.34 ± 0.01 c | 0.43 ± 0.02 a | 0.38 ± 0.02 b | 0.41 ± 0.02 ab |
| Ile | 0.69 ± 0.02 c | 0.84 ± 0.03 a | 0.77 ± 0.01 b | 0.75 ± 0.01 b |
| Leu | 1.10 ± 0.05 a | 1.32 ± 0.21 a | 1.21 ± 0.10 a | 1.19 ± 0.08 a |
| Phe | 0.61 ± 0.02 c | 0.72 ± 0.02 a | 0.66 ± 0.01 b | 0.65 ± 0.01 b |
| His | 0.34 ± 0.01 a | 0.35 ± 0.02 a | 0.34 ± 0.01 a | 0.35 ± 0.02 a |
| Lys | 1.18 ± 0.08 b | 1.37 ± 0.09 a | 1.26 ± 0.07 ab | 1.32 ± 0.08 ab |
| The | 0.69 ± 0.02 b | 0.77 ± 0.01 a | 0.73 ± 0.01 a | 0.74 ± 0.02 a |
| Arg | 1.04 ± 0.08 a | 1.02 ± 0.05 a | 1.02 ± 0.04 a | 1.03 ± 0.03 a |
| Non-essential | ||||
| ASP | 1.23 ± 0.06 b | 1.38 ± 0.03 a | 1.34 ± 0.05 ab | 1.38 ± 0.04 a |
| Glu | 2.13 ± 0.15 a | 2.29 ± 0.05 a | 2.33 ± 0.06 a | 2.35 ± 0.07 a |
| Ser | 0.41 ± 0.01 c | 0.49 ± 0.01 a | 0.45 ± 0.02 b | 0.50 ± 0.01 a |
| Gly | 0.71 ± 0.01 d | 0.82 ± 0.01 b | 0.76 ± 0.02 c | 0.95 ± 0.01 a |
| Pro | 0.55 ± 0.01 d | 0.64 ± 0.01 b | 0.59 ± 0.01 c | 0.69 ± 0.01 a |
| Ala | 0.80 ± 0.02 c | 0.91 ± 0.01 a | 0.85 ± 0.02 b | 0.90 ± 0.03 ab |
| Cyst | 0.10 ± 0.01 b | 0.13 ± 0.01 a | 0.11 ± 0.01 ab | 0.11 ± 0.01 ab |
| Tyr | 0.51 ± 0.02 ab | 0.53 ± 0.01 a | 0.49 ± 0.02 b | 0.52 ± 0.02 ab |
| TAA | 13.19 ± 0.59 b | 14.85 ± 0.61 a | 14.12 ± 0.49 ab | 14.63 ± 0.48 ab |
| ∑EAA | 6.76 ± 0.30 b | 7.65 ± 0.47 a | 7.19 ± 0.28 ab | 7.25 ± 0.28 ab |
| ∑NEAA | 6.44 ± 0.29 b | 7.19 ± 0.14 ab | 6.92 ± 0.21 b | 7.40 ± 0.20 a |
| EAA/NEAA | 1.05 ± 0.02 b | 1.06 ± 0.01 a | 1.04 ± 0.02 ab | 0.98 ± 0.02 b |
| Fatty Acid | CK | T30 | T60 | T90 |
|---|---|---|---|---|
| C14:0 | 1.10 ± 0.23 a | 0.92 ± 0.14 a | 0.61 ± 0.12 b | 0.26 ± 0.21 c |
| C15:0 | 0.05 ± 0.00 a | 0.05 ± 0.00 a | - | - |
| C16:0 | 23.70 ± 2.45 a | 20.81 ± 0.91 a | 17.73 ± 1.02 b | 13.54 ± 1.23 c |
| C17:0 | 0.27 ± 0.02 a | 0.24 ± 0.01 a | 0.19 ± 0.01 b | - |
| C18:0 | 10.91 ± 2.34 a | 9.43 ± 1.96 ab | 7.52 ± 1.55 ab | 5.17 ± 1.02 b |
| C20:0 | - | - | 0.44 ± 0.01 b | 0.58 ± 0.02 a |
| C22:0 | 0.10 ± 0.02 d | 0.19 ± 0.02 c | 0.27 ± 0.04 b | 0.37 ± 0.03 a |
| ∑SFA | 36.13 ± 2.39 a | 31.64 ± 2.08 b | 26.76 ± 2.34 c | 19.92 ± 2.06 d |
| C16:1 | 2.20 ± 0.54 a | 1.93 ± 0.21 a | 1.29 ± 0.11 b | 0.59 ± 0.35 c |
| C17:1 | 0.29 ± 0.01 a | 0.26 ± 0.02 a | 0.17 ± 0.04 b | 0.09 ± 0.00 c |
| C20:1 | 0.98 ± 0.07 a | 0.85 ± 0.16 a | 0.59 ± 0.09 b | 0.36 ± 0.05 c |
| C18:1n9t | - | - | - | - |
| C18:1n9c | 45.51 ± 3.15 a | 39.81 ± 2.01 b | 34.63 ± 2.16 c | 26.71 ± 3.04 d |
| C22:1n9 | 0.32 ± 0.03 a | 0.35 ± 0.02 a | 0.31 ± 0.04 a | 0.30 ± 0.01 a |
| C24:1 | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 0.09 ± 0.01 a | 0.09 ± 0.01 a |
| ∑MUFA | 49.41 ± 3.12 a | 43.3 ± 2.45 b | 37.09 ± 2.46 c | 28.14 ± 2.37 d |
| C18:2n6 | 13.00 ± 1.04 d | 22.35 ± 2.36 c | 32.81 ± 2.41 b | 47.03 ± 2.17 a |
| C18:3n6 | 0.27 ± 0.08 a | 0.38 ± 0.04 a | - | - |
| C18:3n3 | 0.60 ± 0.04 d | 1.82 ± 0.11 c | 3.05 ± 0.63 b | 4.78 ± 1.04 a |
| C20:2 | 0.53 ± 0.08 a | 0.45 ± 0.07 a | 0.29 ± 0.02 b | 0.13 ± 0.01 c |
| C20:3n3 | 0.06 ± 0.01 a | 0.06 ± 0.00 a | - | - |
| ∑PUFA | 14.47 ± 2.31 d | 25.06 ± 3.05 c | 36.15 ± 3.14 b | 51.94 ± 3.48 a |
| PUFA/SFA | 0.40 ± 0.02 d | 0.79 ± 0.07 c | 1.15 ± 0.09 b | 2.61 ± 1.01 a |
| PUFA/MUFA | 0.29 ± 0.02 d | 0.58 ± 0.11 c | 0.97 ± 0.14 b | 1.85 ± 0.19 a |
| MUFA/SFA | 1.37 ± 0.03 a | 1.37 ± 0.09 a | 1.39 ± 0.08 a | 1.41 ± 0.14 a |
| (MUFA+PUFA)/SFA | 1.77 ± 0.09 d | 2.16 ± 0.11 c | 2.74 ± 0.24 b | 4.02 ± 0.85 a |
| Omega6/Omega3 | 21.67 | 12.28 | 10.76 | 9.84 |
| Items | CK | T30 | T60 | T90 |
|---|---|---|---|---|
| L* | 86.78 ± 0.06 a | 75.52 ± 0.23 b | 68.42 ± 0.39 c | 64.20 ± 0.15 d |
| a* | 0.43 ± 0.01 d | 1.24 ± 0.02 c | 2.40 ± 0.02 b | 2.65 ± 0.03 a |
| b* | 14.25 ± 0.15 d | 17.47 ± 0.09 a | 16.82 ± 0.08 b | 16.29 ± 0.02 c |
| △E* | - | 11.74 ± 0.18 c | 18.64 ± 0.34 b | 22.78 ± 0.16 a |
| C | 14.26 ± 0.15 d | 17.52 ± 0.09 a | 16.99 ± 0.08 b | 16.50 ± 0.04 c |
| H° | 88.27 ± 15.00 a | 85.94 ± 4.50 a | 81.87 ± 4.00 a | 80.77 ± 0.67 a |
| TPA | CK | T30 | T60 | T90 |
|---|---|---|---|---|
| Hardness/N | 42.5 ± 1.9 b | 43.3 ± 2.9 b | 57.0 ± 5.8 a | 61.8 ± 3.2 a |
| Springiness | 0.93 ± 0.00 a | 0.93 ± 0.00 a | 0.94 ± 0.01 a | 0.93 ± 0.01 a |
| Cohesiveness | 0.76 ± 0.00 a | 0.72 ± 0.02 b | 0.74 ± 0.02 ab | 0.70 ± 0.02 b |
| Chewiness/N·mm | 29.9 ± 1.0 b | 29.7 ± 0.8 b | 38.2 ± 1.5 a | 40.0 ± 0.7 a |
| Day (d) | CK | T30 | T60 | T90 |
|---|---|---|---|---|
| 1 | 0.101 ± 0.001 d,D | 0.148 ± 0.001 c,E | 0.202 ± 0.001 b,C | 0.275 ± 0.001 a,A |
| 5 | 0.147 ± 0.004 d,C | 0.156 ± 0.001 c,D | 0.185 ± 0.003 b,D | 0.261 ± 0.006 a,B |
| 10 | 0.217 ± 0.013 a,B | 0.169 ± 0.006 b,C | 0.174 ± 0.003 b,E | 0.227 ± 0.003 a,C |
| 15 | 0.291 ± 0.003 a,B | 0.181 ± 0.007 b,B,C | 0.159 ± 0.004 c,F | 0.150 ± 0.005 c,E |
| 20 | 0.295 ± 0.001 a,B | 0.189 ± 0.001 bc,B | 0.235 ± 0.006 b,B | 0.181 ± 0.001 c,D |
| 25 | 0.328 ± 0.006 a,A | 0.224 ± 0.003 c,A | 0.247 ± 0.005 b,A | 0.223 ± 0.002 c,C |
| 30 | 0.298 ± 0.006 a,B | 0.210 ± 0.013 b,A | 0.159 ± 0.006 c,F | 0.102 ± 0.008 d,G |
| 35 | 0.232 ± 0.002 a,C | 0.161 ± 0.002 b,C | 0.147 ± 0.003 c,G | 0.126 ± 0.001 d,F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, H.; Zhou, H.; Stepanova, T.M.; Zhu, Z.; Li, B. Enhancing Product Quality, Nutrition, Antioxidant Capacity, and Sensory Quality of Chicken Sausages by Replacing Fats with Agaricus bisporus and Soybean Oil. Foods 2025, 14, 2296. https://doi.org/10.3390/foods14132296
Nan H, Zhou H, Stepanova TM, Zhu Z, Li B. Enhancing Product Quality, Nutrition, Antioxidant Capacity, and Sensory Quality of Chicken Sausages by Replacing Fats with Agaricus bisporus and Soybean Oil. Foods. 2025; 14(13):2296. https://doi.org/10.3390/foods14132296
Chicago/Turabian StyleNan, Haijuan, Haixu Zhou, Tetiana M. Stepanova, Zongshuai Zhu, and Bo Li. 2025. "Enhancing Product Quality, Nutrition, Antioxidant Capacity, and Sensory Quality of Chicken Sausages by Replacing Fats with Agaricus bisporus and Soybean Oil" Foods 14, no. 13: 2296. https://doi.org/10.3390/foods14132296
APA StyleNan, H., Zhou, H., Stepanova, T. M., Zhu, Z., & Li, B. (2025). Enhancing Product Quality, Nutrition, Antioxidant Capacity, and Sensory Quality of Chicken Sausages by Replacing Fats with Agaricus bisporus and Soybean Oil. Foods, 14(13), 2296. https://doi.org/10.3390/foods14132296







