Ovalbumin Peptide–Selenium Nanoparticles Alleviate Immune Suppression in Cyclophosphamide-Induced Mice: A Combined Transcriptomic and Proteomic Approach to Reveal the Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. OP-SeNPs Preparation
2.3. Animal Experiment
2.3.1. Animal Treatment
2.3.2. Immune Organ Indices
2.3.3. Histopathological Analysis
2.3.4. Biochemical Assay
2.3.5. Splenic Lymphocyte Proliferation Assay
2.3.6. Hematological Analysis
2.4. Transcriptomic Studies
2.5. Proteomic Studies
2.6. Statistical Analysis
3. Results and Discussion
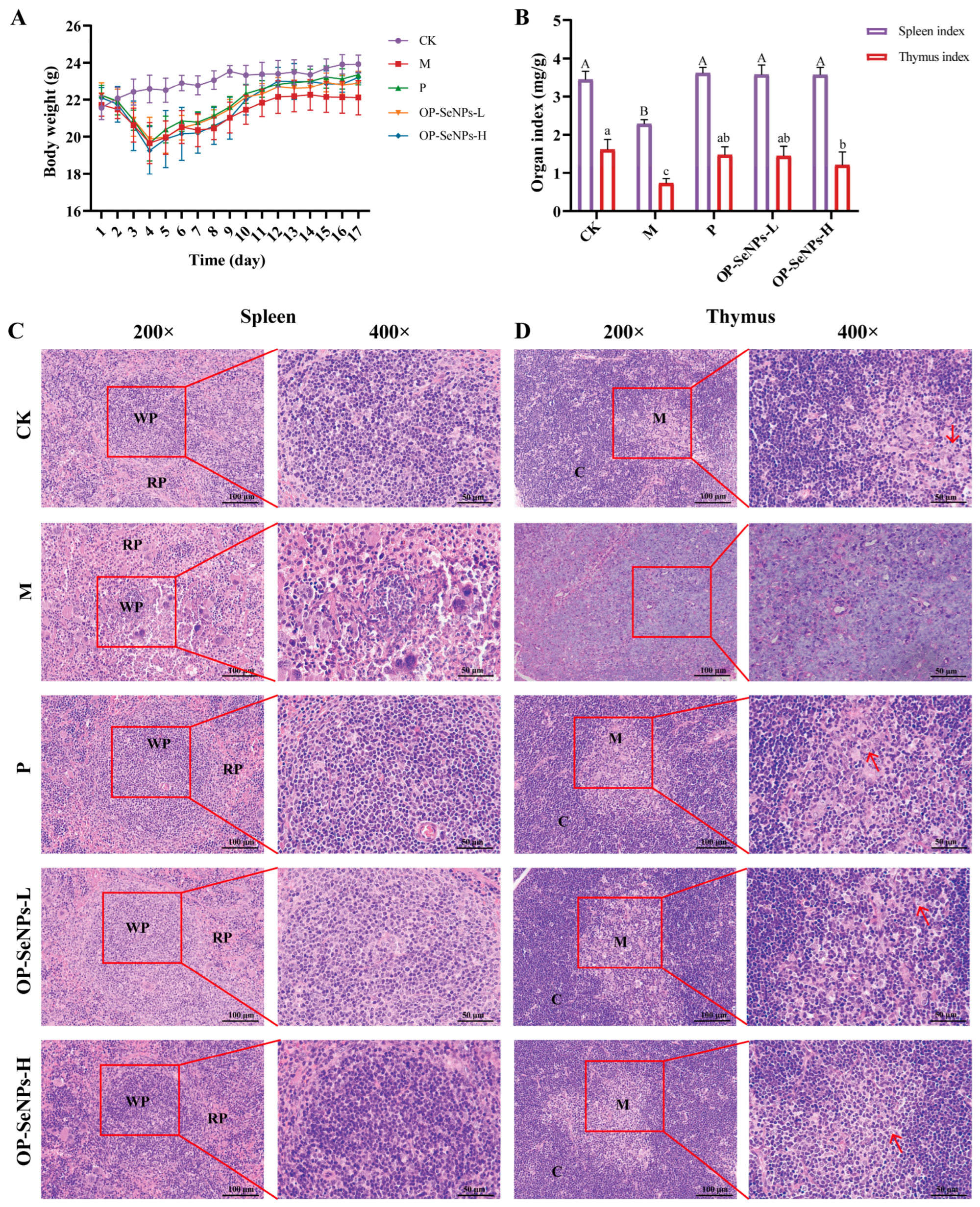
3.1. OP-SeNPs Repaired Damage to the Immune Organs
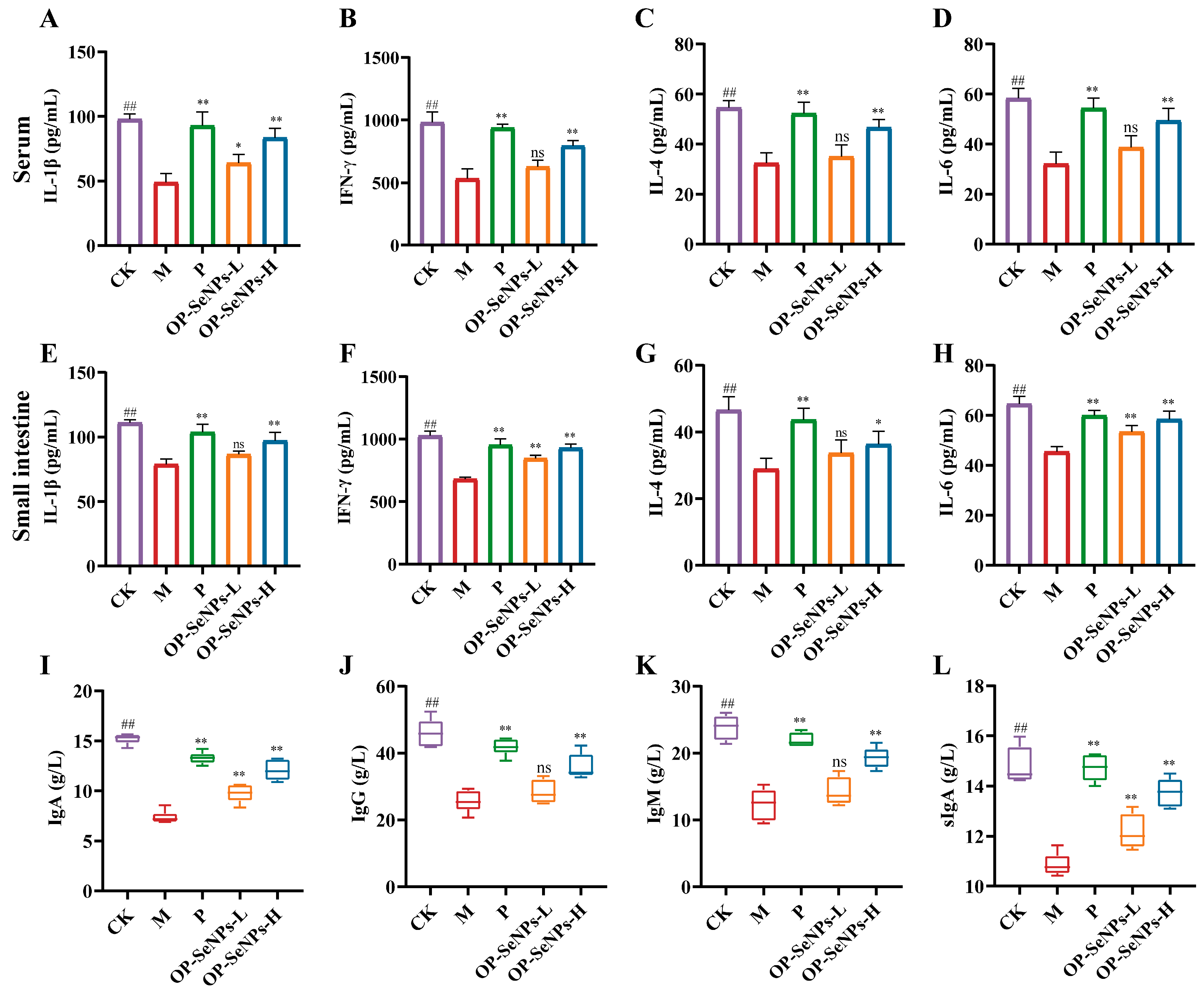
3.2. OP-SeNPs Regulated the Cytokine and Immunoglobulin Secretion
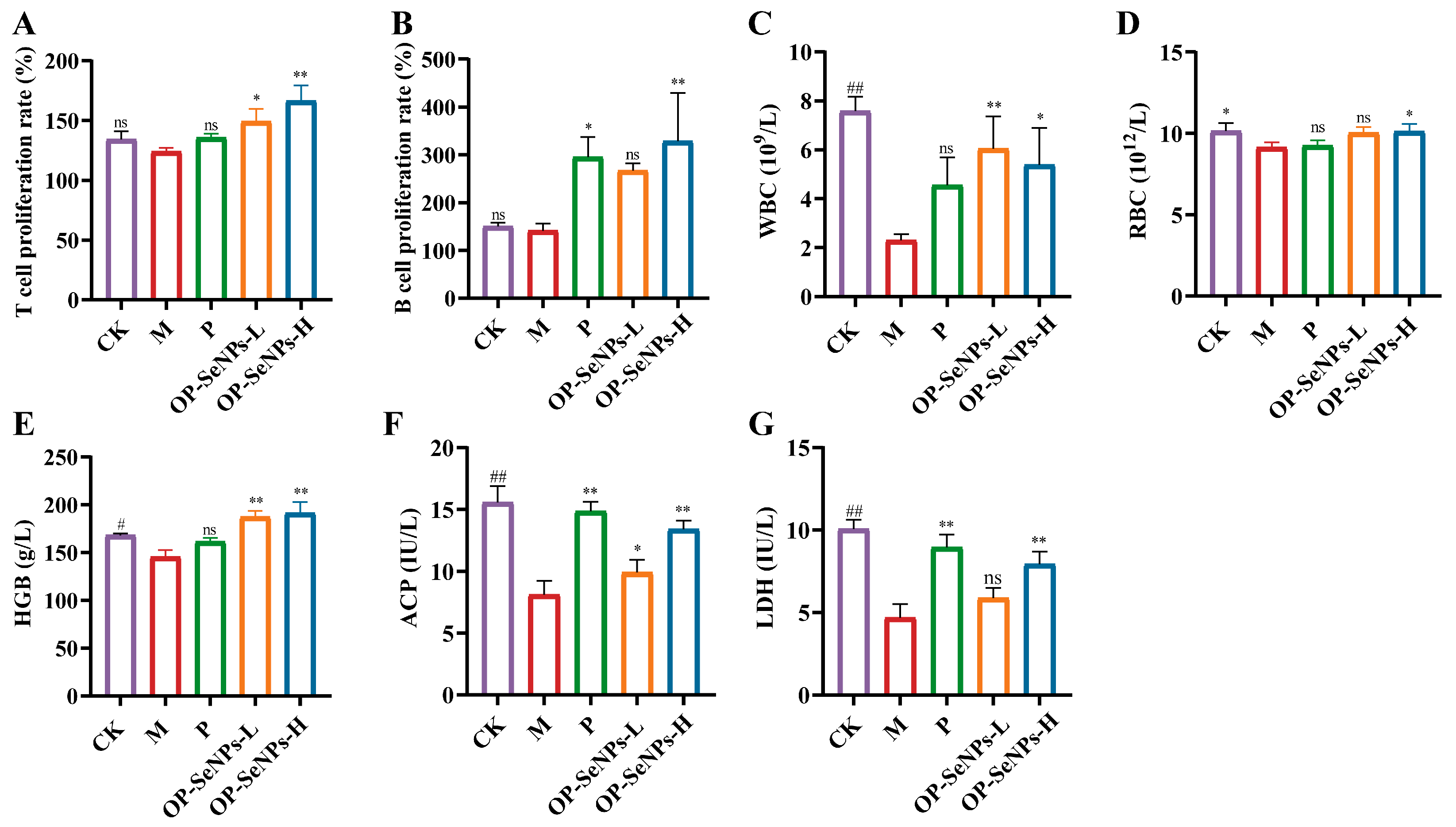
3.3. OP-SeNPs Modulated Immune Cell Function
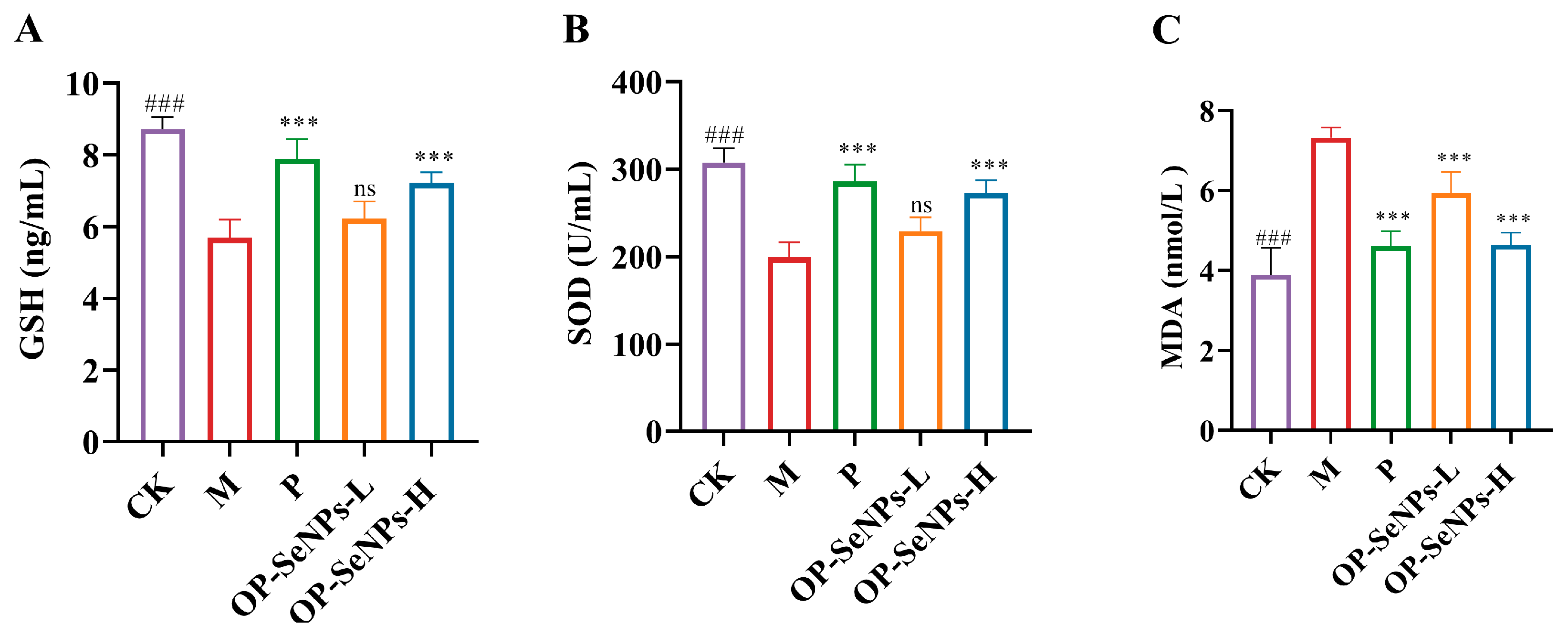
3.4. OP-SeNPs Enhanced Antioxidant Activity
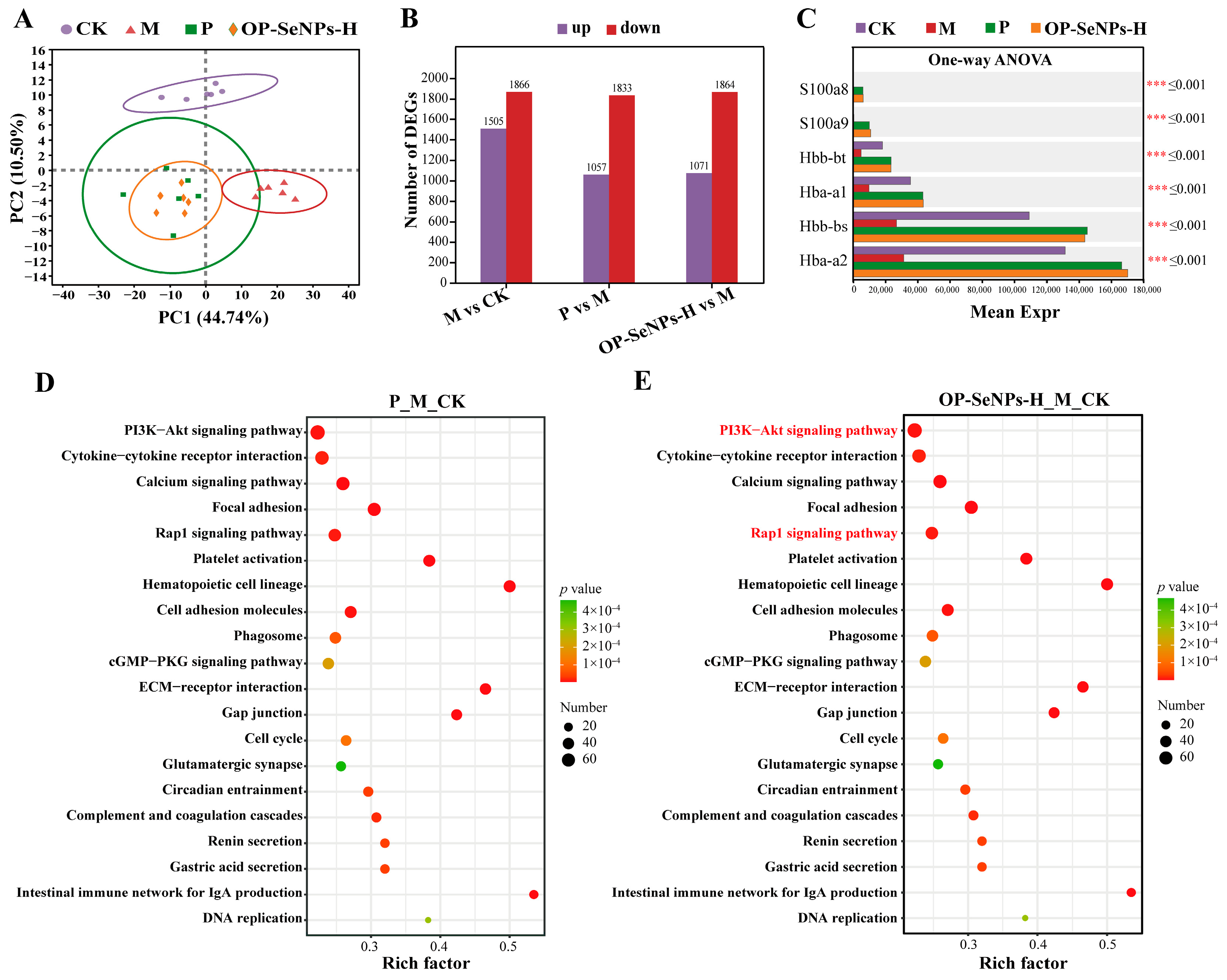
3.5. Transcriptomic Analysis of the Spleen
3.5.1. Transcriptomic Principal Component Analysis
3.5.2. Analysis of Differentially Expressed Genes
3.5.3. KEGG Enrichment Analysis of Differentially Expressed Genes
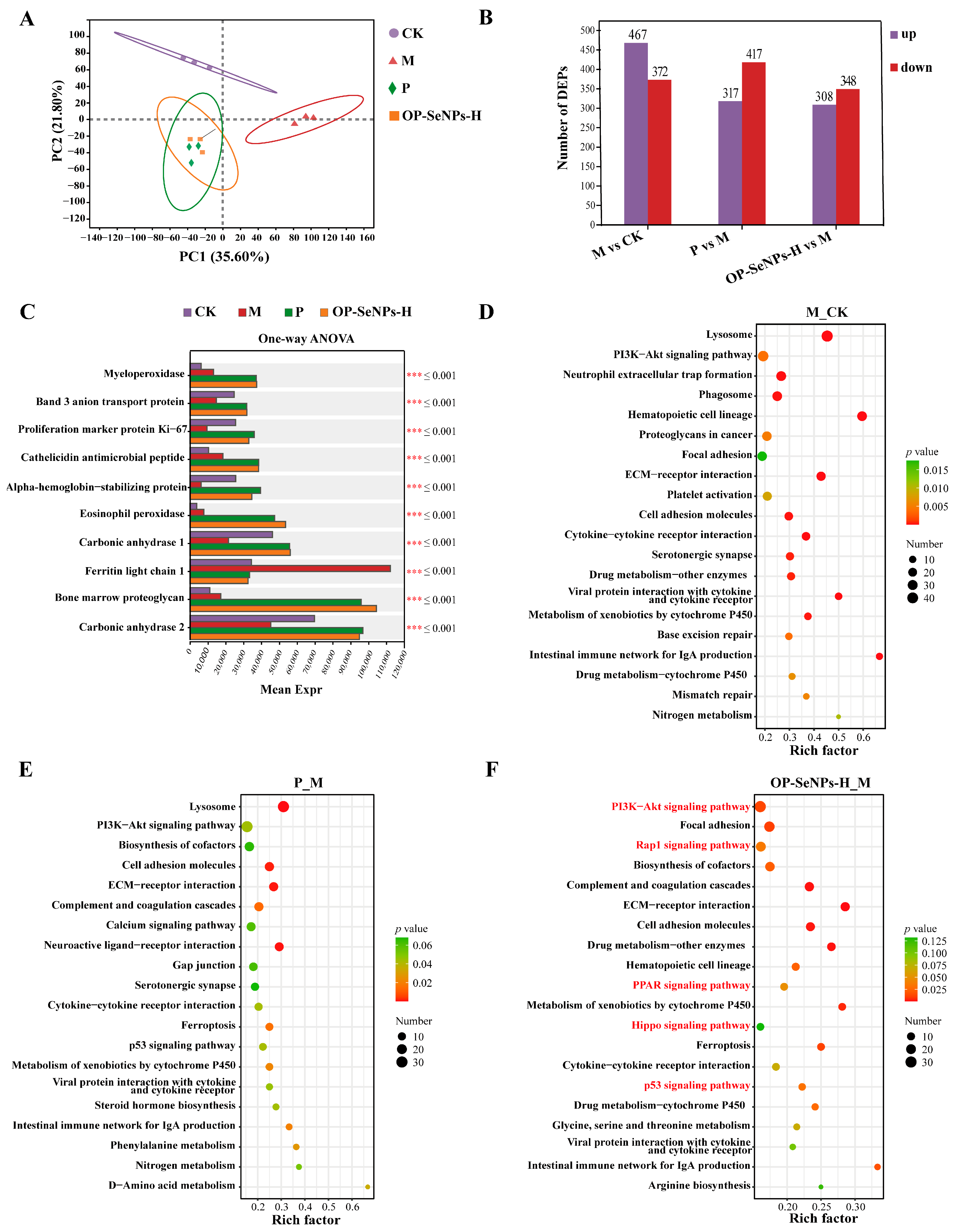
3.6. Proteomic Analysis of the Spleen
3.6.1. Proteomic Principal Component Analysis
3.6.2. Analysis of Differentially Expressed Proteins
3.6.3. KEGG Enrichment Analysis of Differentially Expressed Proteins
3.6.4. Analysis of Key Signaling Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azoulay, E.; Soares, M.; Benoit, D. Focus on immunocompromised patients. Intens. Care Med. 2016, 42, 463–465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lasselin, J.; Alvarez-Salas, E.; Grigoleit, J.S. Well-being and immune response: A multi-system perspective. Curr. Opin. Pharmacol. 2016, 29, 34–41. [Google Scholar] [CrossRef]
- Agrati, C.; Bartolini, B.; Bordoni, V.; Locatelli, F.; Capobianchi, M.R.; Di Caro, A.; Castilletti, C.; Ippolito, G. Emerging viral infections in immunocompromised patients: A great challenge to better define the role of immune response. Front. Immunol. 2023, 14, 1147871. [Google Scholar] [CrossRef]
- Mameli, C.; Pasinato, A.; Picca, M.; Bedogni, G.; Pisanelli, S.; Zuccotti, G.V.; Grp, A.-W. Pidotimod for the prevention of acute respiratory infections in healthy children entering into daycare: A double blind randomized placebo-controlled study. Pharmacol. Res. 2015, 97, 79–83. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.L.; Wu, J.P. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Y.; Zheng, L.; Huang, M.T.; Zhao, M.M. Collagen derived Gly-Pro-type DPP-IV inhibitory peptides: Structure-activity relationship, inhibition kinetics and inhibition mechanism. Food Chem. 2024, 441, 138370. [Google Scholar] [CrossRef]
- Li, Z.Y.; Abou-Elsoud, M.; Chen, H.; Shu, D.W.; Ren, S.Z.; Ahn, D.U.; Huang, X. Identification and Molecular Mechanism of Novel Two-Way Immunomodulatory Peptides from Ovalbumin: In Vitro Cell Experiments, De Novo Sequencing, and Molecular Docking. J. Agric. Food Chem. 2024, 72, 9856–9866. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.Z.; Li, X.L.; Zhu, H.G.; Sun, H.X. Ovalbumin-digested peptides can reverse cyclophosphamide-induced immune dysfunction in mice. Food Biosci. 2024, 58, 103642. [Google Scholar] [CrossRef]
- Tang, W.Q.; Luo, X.Q.; Fan, F.J.; Sun, X.Y.; Jiang, X.Y.; Li, P.; Ding, J.; Lin, Q.L.; Zhao, S.M.; Cheng, Y.H.; et al. Zein and gum arabic nanoparticles: Potential enhancers of immunomodulatory functional activity of selenium-containing peptides. Food Funct. 2024, 15, 9972–9982. [Google Scholar] [CrossRef]
- Zhang, T.; Qi, M.; Wu, Q.; Xiang, P.; Tang, D.J.; Li, Q. Recent research progress on the synthesis and biological effects of selenium nanoparticles. Front. Nutr. 2023, 10, 1183487. [Google Scholar] [CrossRef]
- Chen, G.S.; Yang, F.; Fan, S.H.; Jin, H.; Liao, K.S.; Li, X.M.; Liu, G.B.; Liang, J.; Zhang, J.A.; Xu, J.F.; et al. Immunomodulatory roles of selenium nanoparticles: Novel arts for potential immunotherapy strategy development. Front. Immunol. 2022, 13, 956181. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.D.; Tian, Y.Q.; Wu, J.L.; Wang, S.Y. Synthesis, characterization, and biological activity of selenium nanoparticles conjugated with polysaccharides. Crit. Rev. Food Sci. 2021, 61, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Umar, A.; Mehta, S.K. Selenium nanomaterials: An overview of recent developments in synthesis, properties and potential applications. Prog. Mater. Sci. 2016, 83, 270–329. [Google Scholar] [CrossRef]
- Zeng, Y.; Lyu, S.; Yang, Q.; Du, Z.; Liu, X.; Shang, X.; Xu, M.; Liu, J.; Zhang, T. Preparation, physicochemical characterization, and immunomodulatory activity of ovalbumin peptide–selenium nanoparticles. Food Chem. 2025, 472, 142852. [Google Scholar] [CrossRef]
- Xia, I.F.; Kong, H.-K.; Wu, M.M.H.; Lu, Y.; Wong, K.-H.; Kwok, K.W.H. Selenium Nanoparticles (SeNPs) Immunomodulation Is More Than Redox Improvement: Serum Proteomics and Transcriptomic Analyses. Antioxidants 2022, 11, 964. [Google Scholar] [CrossRef]
- Jiang, W.Y.; He, S.; Su, D.X.; Ye, M.J.; Zeng, Q.Z.; Yuan, Y. Synthesis, characterization of tuna polypeptide selenium nanoparticle, and its immunomodulatory and antioxidant effects. Food Chem. 2022, 383, 132405. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.H.; Chen, W.M.; Wang, Y.; Wang, X.M.; Shao, Y.H.; Liu, J.; Tu, Z.C. Serum metabolism-transcriptomics investigated into the immunity of whey protein isolate-galacto-oligosaccharide conjugates after dynamic high-pressure microfluidics pretreatment. Food Res. Int. 2024, 196, 115121. [Google Scholar] [CrossRef]
- Dai, Z.Q.; Shang, L.J.; Wei, Y.S.; Li, Z.Q.; Zeng, X.F.; Chen, M.X.; Wang, X.Y.; Li, S.Y.; Qiao, S.Y.; Yu, H.T. Immunomodulatory Effects of Microcin C7 in Cyclophosphamide-Induced Immunosuppressed Mice. J. Agric. Food Chem. 2023, 71, 12700–12714. [Google Scholar] [CrossRef]
- Li, Q.; Chen, G.Y.; Chen, H.; Zhang, W.J.; Ding, Y.Y.; Yu, P.; Zhao, T.; Mao, G.H.; Feng, W.W.; Yang, L.Q.; et al. Se-enriched G. frondosa polysaccharide protects against immunosuppression in cyclophosphamide-induced mice MAPKs signal transduction pathway. Carbohyd. Polym. 2018, 196, 445–456. [Google Scholar] [CrossRef]
- Zhang, T.; Li, S.L.; Yang, M.; Li, Y.J.; Ma, S.T.; Zhang, H.; Li, L.X.; Liu, X.T.; Liu, J.B.; Du, Z.Y. The influence of unique interfacial networks based on egg white proteins for the stabilization of high internal phase Pickering emulsions: Physical stability and free fatty acid release kinetics. Food Chem. 2024, 442, 138448. [Google Scholar] [CrossRef]
- Ge, H.F.; Yang, Q.; Lyu, S.; Du, Z.Y.; Liu, X.T.; Shang, X.M.; Xu, M.L.; Liu, J.B.; Zhang, T. Egg White Peptides Accelerating the Wound Healing Process Through Modulating the PI3K-AKT Pathway: A Joint Analysis of Transcriptomic and Proteomic. J. Agric. Food Chem. 2024, 72, 4100–4115. [Google Scholar] [CrossRef] [PubMed]
- Lou, R.H.; Shui, W.Q. Acquisition and Analysis of DIA-Based Proteomic Data: A Comprehensive Survey in 2023. Mol. Cell. Proteom. 2024, 23, 100712. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Xiu, W.; Yang, M.; Yu, S.; Ma, Y. Selenium nanoparticles stabilized by sweet corncob polysaccharide inhibit hypoglycemia in vitro and alleviate symptoms in type 2 diabetes mice. J. Funct. Foods 2024, 112, 105920. [Google Scholar] [CrossRef]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, C.; Zhang, T. Selenium transformation and selenium-rich foods. Food Biosci. 2021, 40, 100875. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; Mcardle, H.J.; Pelaez, C.; et al. Scientific opinion on the tolerable upper intake level for selenium. EFSA J. 2023, 21, e07704. [Google Scholar] [PubMed]
- Cui, L.N.; Chen, L.; Yang, G.; Li, Y.K.; Qiao, Z.H.; Liu, Y.; Meng, Y.; Zhou, Y.F.; Sun, L. Structural characterization and immunomodulatory activity of a heterogalactan from Panax ginseng flowers. Food Res. Int. 2021, 140, 109859. [Google Scholar] [CrossRef]
- Xie, Z.Y.; Bai, Y.X.; Chen, G.J.; Dong, W.; Peng, Y.J.; Xu, W.Q.; Sun, Y.; Zeng, X.X.; Liu, Z.H. Immunomodulatory activity of polysaccharides from the mycelium of Aspergillus cristatus, isolated from Fuzhuan brick tea, associated with the regulation of intestinal barrier function and gut microbiota. Food Res. Int. 2022, 152, 110901. [Google Scholar] [CrossRef]
- Liang, Q.X.; Dong, J.; Wang, S.Y.; Shao, W.J.; Ahmed, A.F.; Zhang, Y.; Kang, W.Y. Immunomodulatory effects of Nigella sativa seed polysaccharides by gut microbial and proteomic technologies. Int. J. Biol. Macromol. 2021, 184, 483–496. [Google Scholar] [CrossRef]
- Tadayon, S.; Dunkel, J.; Takeda, A.; Halle, O.; Karikoski, M.; Gerke, H.; Rantakari, P.; Virtakoivu, R.; Pabst, O.; Salmi, M.; et al. Clever-1 contributes to lymphocyte entry into the spleen via the red pulp. Sci. Immunol. 2019, 4, eaat0297. [Google Scholar] [CrossRef]
- Liang, Q.X.; Zhao, Q.C.; Hao, X.T.; Wang, J.M.; Ma, C.Y.; Xi, X.F.; Kang, W.Y. The Effect of Polysaccharide on Immunization Analyzed by Intestinal Flora and Proteomics. Front. Nutr. 2022, 9, 841230. [Google Scholar] [CrossRef]
- Ren, Z.X.; Yang, F.; Yao, S.J.; Bi, L.J.; Jiang, G.Q.; Huang, J.; Tang, Y.P. Effects of low molecular weight peptides from monkfish (Lophius litulon) roe on immune response in immunosuppressed mice. Front. Nutr. 2022, 9, 929105. [Google Scholar] [CrossRef]
- Jie, D.; Gao, T.T.; Shan, Z.S.; Song, J.Y.; Zhang, M.; Kurskaya, O.; Sharshov, K.; We, L.X.; Bi, H.T. Immunostimulating effect of polysaccharides isolated from Ma-Nuo-Xi decoction in cyclophosphamide-immunosuppressed mice. Int. J. Biol. Macromol. 2020, 146, 45–52. [Google Scholar] [CrossRef]
- Ma, T.T.; Li, C.; Zhao, F.Q.; Cao, J.; Zhang, X.Y.; Shen, X.R. Effects of co-fermented collagen peptide-jackfruit juice on the immune response and gut microbiota in immunosuppressed mice. Food Chem. 2021, 365, 130487. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Bi, M.Y.; Yang, J.; Cai, J.Z.; Zhang, H.R.; Zhu, Y.; Zheng, Y.Y.; Liu, Q.; Shi, G.L.; Zhang, Z.W. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-ΚB pathway in swine small intestine. J. Hazard. Mater. 2022, 421, 126704. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.F.; Huang, H.H. Surface morphology and protective effect of Hericium erinaceus polysaccharide on cyclophosphamide-induced immunosuppression in mice. Carbohydr. Polym. 2021, 251, 116930. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, C.P.; Zhang, Y.; Li, Y.; Sun, J.R. Immunomodulatory and antioxidant effects of pomegranate peel polysaccharides on immunosuppressed mice. Int. J. Biol. Macromol. 2019, 137, 504–511. [Google Scholar] [CrossRef]
- Chen, M.W.; Zhang, F.; Su, Y.J.; Chang, C.H.; Li, J.H.; Gu, L.P.; Yang, Y.J. Immunomodulatory effects of egg white peptides on immunosuppressed mice and sequence identification of immunomodulatory peptides. Food Biosci. 2022, 49, 101873. [Google Scholar] [CrossRef]
- Li, M.Z.; Huang, X.J.; Hu, J.L.; Cui, S.W.; Xie, M.Y.; Nie, S.P. The protective effects against cyclophosphamide (CTX)-induced immunosuppression of three glucomannans. Food Hydrocoll. 2020, 100, 105445. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Meng, M.; Sun, H.Q.; Li, Y.; Ren, Y.Y.; Zhang, Y.M. Immunostimulatory activity of glycopeptides from under normal and cyclophosphamide induced immunosuppressive conditions in mice models. Food Funct. 2016, 7, 3566–3576. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zong, Z.M.; Chen, S.L.; Chen, A.H.; Wei, X.Y. Ameliorative effect of Trametes orientalis polysaccharide against immunosuppression and oxidative stress in cyclophosphamide-treated mice. Int. J. Biol. Macromol. 2017, 95, 1216–1222. [Google Scholar] [CrossRef]
- Adamiak, M.; Suszynska, M.; Abdel-Latif, A.; Abdelbaset-Ismail, A.; Ratajczak, J.; Kucia, M.; Ratajczak, M.Z. Novel Evidence That the Mannan-Binding Lectin (MBL) Pathway of Complement Activation Plays a Pivotal Role in Triggering Mobilization of Hematopoietic Stem/Progenitor Cells By Activation of Both the Complement and Coagulation Cascades. Blood 2016, 128, 3371. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.D.; Jin, Y.Y.; Xiao, Z.P.; Lin, Y.Y.; Chen, H.H.; Wang, M.Q.; Yaqoob, M.U. Galactooligosaccharides as a protective agent for intestinal barrier and its regulatory functions for intestinal microbiota. Food Res. Int. 2022, 155, 111003. [Google Scholar] [CrossRef]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef]
- Myszor, I.T.; Lapka, K.; Hermannsson, K.; Rekha, R.S.; Bergman, P.; Gudmundsson, G.H. Bile acid metabolites enhance expression of cathelicidin antimicrobial peptide in airway epithelium through activation of the TGR5-ERK1/2 pathway. Sci. Rep. 2024, 14, 6750. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, G.L.; Lu, J.; Li, L.J. Crosstalk between circRNAs and the PI3K/AKT signaling pathway in cancer progression. Signal Transduct. Target. Ther. 2021, 6, 400. [Google Scholar] [CrossRef]
- Li, Q.Z.; Chang, Y.Z.; He, Z.M.; Chen, L.; Zhou, X.W. Immunomodulatory activity of Ganoderma lucidum immunomodulatory protein PI3K/Akt and MAPK signaling pathways in RAW264.7 cells. J. Cell. Physiol. 2019, 234, 23337–23348. [Google Scholar] [CrossRef]
- Arimura, Y.; Shiroki, F.; Kuwahara, S.; Kato, H.; Dianzani, U.; Uchiyama, T.; Yagi, J. Akt is a neutral amplifier for Th cell differentiation. J. Biol. Chem. 2004, 279, 11408–11416. [Google Scholar] [CrossRef]
- Ma, N.; Du, H.J.; Ma, G.X.; Yang, W.J.; Han, Y.H.; Hu, Q.H.; Xiao, H. Characterization of the Immunomodulatory Mechanism of a Protein by Isobaric Tags for Relative and Absolute Quantitation Proteomics. J. Agric. Food Chem. 2020, 68, 13189–13199. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Li, D.C.; Ding, H.; Jiang, M.H.; Zhao, Y.J.; Yu, D.K.; Zhang, R.; Chen, W.; Chen, R.; Zheng, Y.X.; et al. GLIS3 mediated by the Rap1/PI3K/AKT signal pathway facilitates real-ambient PM exposure disturbed thyroid hormone homeostasis regulation. Ecotoxicol. Environ. Saf. 2022, 232, 113248. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Kodama, T.; Sato, K.; Murai, K.; Yoshioka, T.; Shigekawa, M.; Yamada, R.; Hikita, H.; Sakamori, R.; Akita, H.; et al. Dysregulation of PI3K and Hippo signaling pathways synergistically induces chronic pancreatitis via CTGF upregulation. J. Clin. Investig. 2021, 131, e143414. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Choi, S.Y.; Wen, T.M.; Chen, C.L.; Thapa, N.; Lee, J.H.; Cryns, V.L.; Anderson, R.A. A p53-phosphoinositide signalosome regulates nuclear AKT activation. Nat. Cell Biol. 2022, 24, 1099–1113. [Google Scholar] [CrossRef]
- Riaz, F.; Wei, P.; Pan, F. PPARs at the crossroads of T cell differentiation and type 1 diabetes. Front. Immunol. 2023, 14, 1292238. [Google Scholar] [CrossRef]
- Bano, I.; Skalickova, S.; Arbab, S.; Urbankova, L.; Horky, P. Toxicological effects of nanoselenium in animals. J. Anim. Sci. Biotechnol. 2022, 13, 72. [Google Scholar] [CrossRef]
- He, Y.; Chen, S.; Liu, Z.; Cheng, C.; Li, H.; Wang, M. Toxicity of selenium nanoparticles in male Sprague–Dawley rats at supranutritional and nonlethal levels. Life Sci. 2014, 115, 44–51. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, H.; Guo, S.; Xiao, S.; Li, N.; Li, M.; Wu, Y.; Liu, H. Long-term administration of low-dose selenium nanoparticles with different sizes aggravated atherosclerotic lesions and exhibited toxicity in apolipoprotein E-deficient mice. Chem. Biol. Interact. 2021, 347, 109601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Yang, Q.; Du, Z.; Liu, X.; Shang, X.; Xu, M.; Liu, J.; Lyu, S.; Zhang, T. Ovalbumin Peptide–Selenium Nanoparticles Alleviate Immune Suppression in Cyclophosphamide-Induced Mice: A Combined Transcriptomic and Proteomic Approach to Reveal the Mechanism. Foods 2025, 14, 2295. https://doi.org/10.3390/foods14132295
Zeng Y, Yang Q, Du Z, Liu X, Shang X, Xu M, Liu J, Lyu S, Zhang T. Ovalbumin Peptide–Selenium Nanoparticles Alleviate Immune Suppression in Cyclophosphamide-Induced Mice: A Combined Transcriptomic and Proteomic Approach to Reveal the Mechanism. Foods. 2025; 14(13):2295. https://doi.org/10.3390/foods14132295
Chicago/Turabian StyleZeng, Yingnan, Qi Yang, Zhiyang Du, Xuanting Liu, Xiaomin Shang, Menglei Xu, Jingbo Liu, Siwen Lyu, and Ting Zhang. 2025. "Ovalbumin Peptide–Selenium Nanoparticles Alleviate Immune Suppression in Cyclophosphamide-Induced Mice: A Combined Transcriptomic and Proteomic Approach to Reveal the Mechanism" Foods 14, no. 13: 2295. https://doi.org/10.3390/foods14132295
APA StyleZeng, Y., Yang, Q., Du, Z., Liu, X., Shang, X., Xu, M., Liu, J., Lyu, S., & Zhang, T. (2025). Ovalbumin Peptide–Selenium Nanoparticles Alleviate Immune Suppression in Cyclophosphamide-Induced Mice: A Combined Transcriptomic and Proteomic Approach to Reveal the Mechanism. Foods, 14(13), 2295. https://doi.org/10.3390/foods14132295






