Dietary Trace Elements and Arsenic Species in Rice: A Study of Samples from Croatian Supermarkets
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sample Preparation for as Speciation and Quantification
2.3. Multielement Analysis—Sample Digestion and Quantification
2.4. Statistical Analysis
3. Results
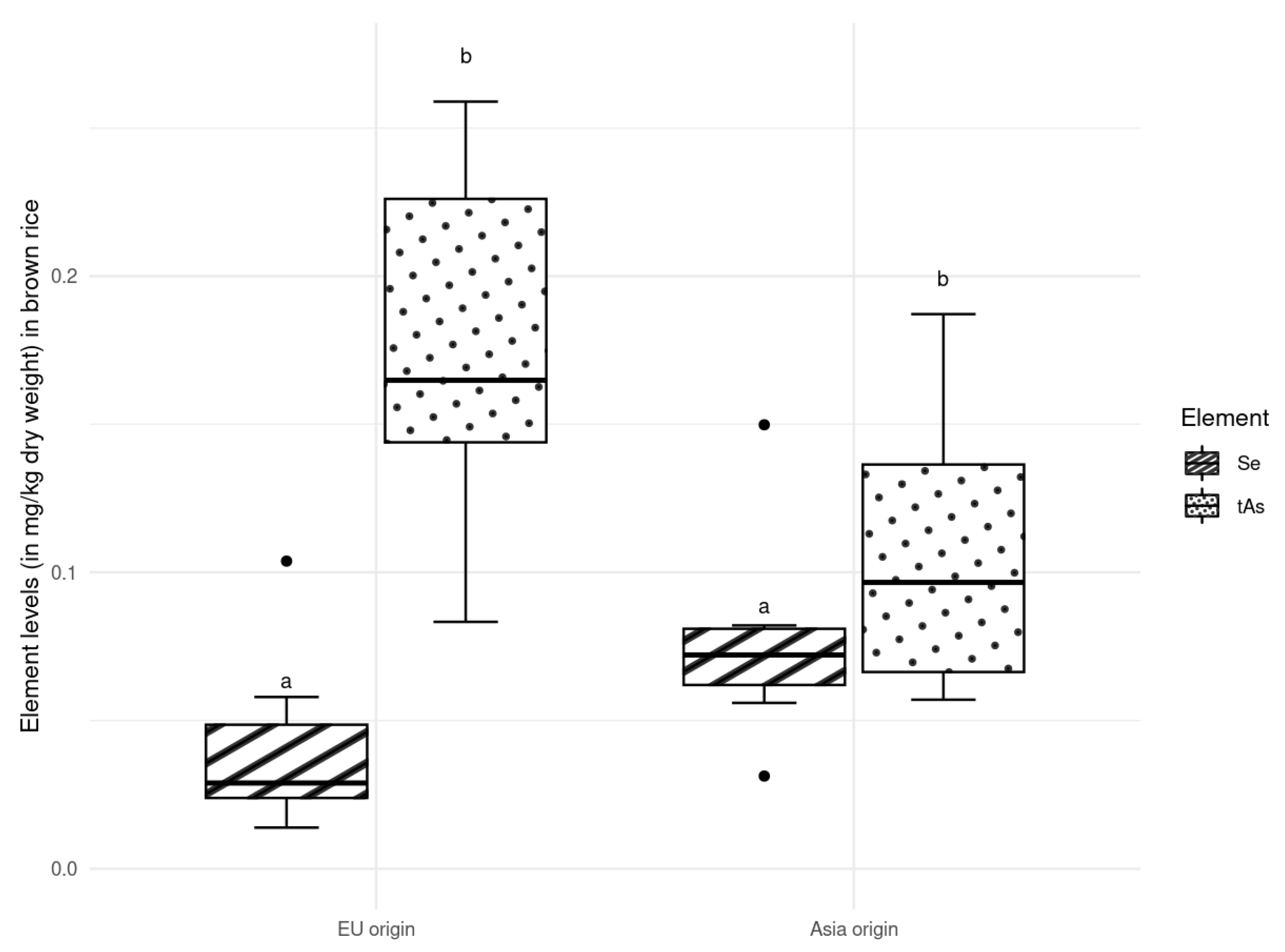
3.1. Arsenic in Rice
| Arsenic (As) (µg/kg d.wt.) | All Rice (n = 58) | White Rice (n = 31) | Parboiled Rice (n = 7) | Brown Rice (n = 20) | SRM 1568b (n = 4) |
|---|---|---|---|---|---|
| Mean ± SD (range) | Mean ± SD (range) | Mean ± SD (range) | Mean ± SD (range) | Mean ± SD [Mean ± 95% CI] | |
| Total As | 142.2 ± 57.1 (36.7–259.0) | 132.3 ± 55.1 (36.7–237.6) | 164.7 ± 49.6 (116.1–245.3) | 150.3 ± 61.2 (57.0–259.0) | 283 ± 1 [285 ± 14] |
| Inorganic As | |||||
| AsIII | 55.5 ± 26.1 (6.6–161.0) | 49.6 ± 22.5 (6.6–85.8) | 63.8 ± 11.4 (48.1–80.2) | 61.7 ± 32.1 (7.0–161.0) | 41.6 ± 6.5 / |
| AsV | 33.9 ± 24.0 (<LoD–120.1) | 22.9 ± 13.4 a (<LoD–52.3) | 54.5 ± 35.7 b (24.0–120.1) | 44.3 ± 25.0 b (7.3–83.8) | 58.4 ± 10.1 / |
| AsIII+AsV | 89.3 ± 41.0 (8.1–200.0) | 72.2 ± 29.5 a (8.1–119.0) | 118.3 ± 45.7 b (72.1–200.0) | 106.0 ± 44.5 b (38.1–184.0) | 100 ± 5 [92 ± 10] |
| Organic As | |||||
| MMA | <LoD | <LoD | <LoD | <LoD | 12.0 ± 1.5 [11.6 ± 3.5] |
| DMA | 33.0 ± 28.1 (3.5–170.0) | 38.7 ± 35.2 (3.5–170.0) | 29.2 ± 17.1 (3.5–54.0) | 25.6 ± 14.0 (3.5–52.8) | 175 ± 17 [180 ± 12] |
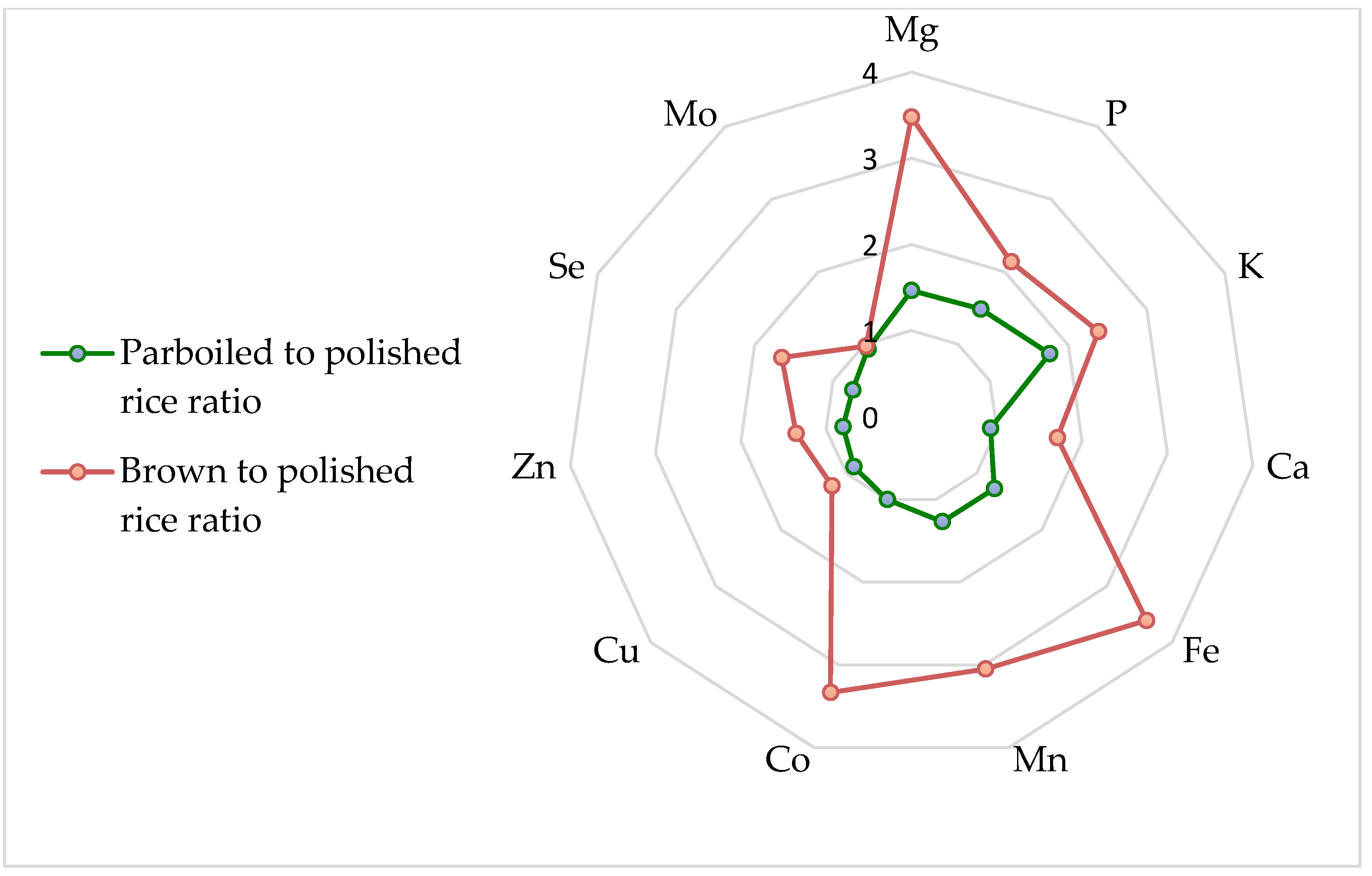
3.2. Multielement Analysis—Macro and Trace Elements in Rice
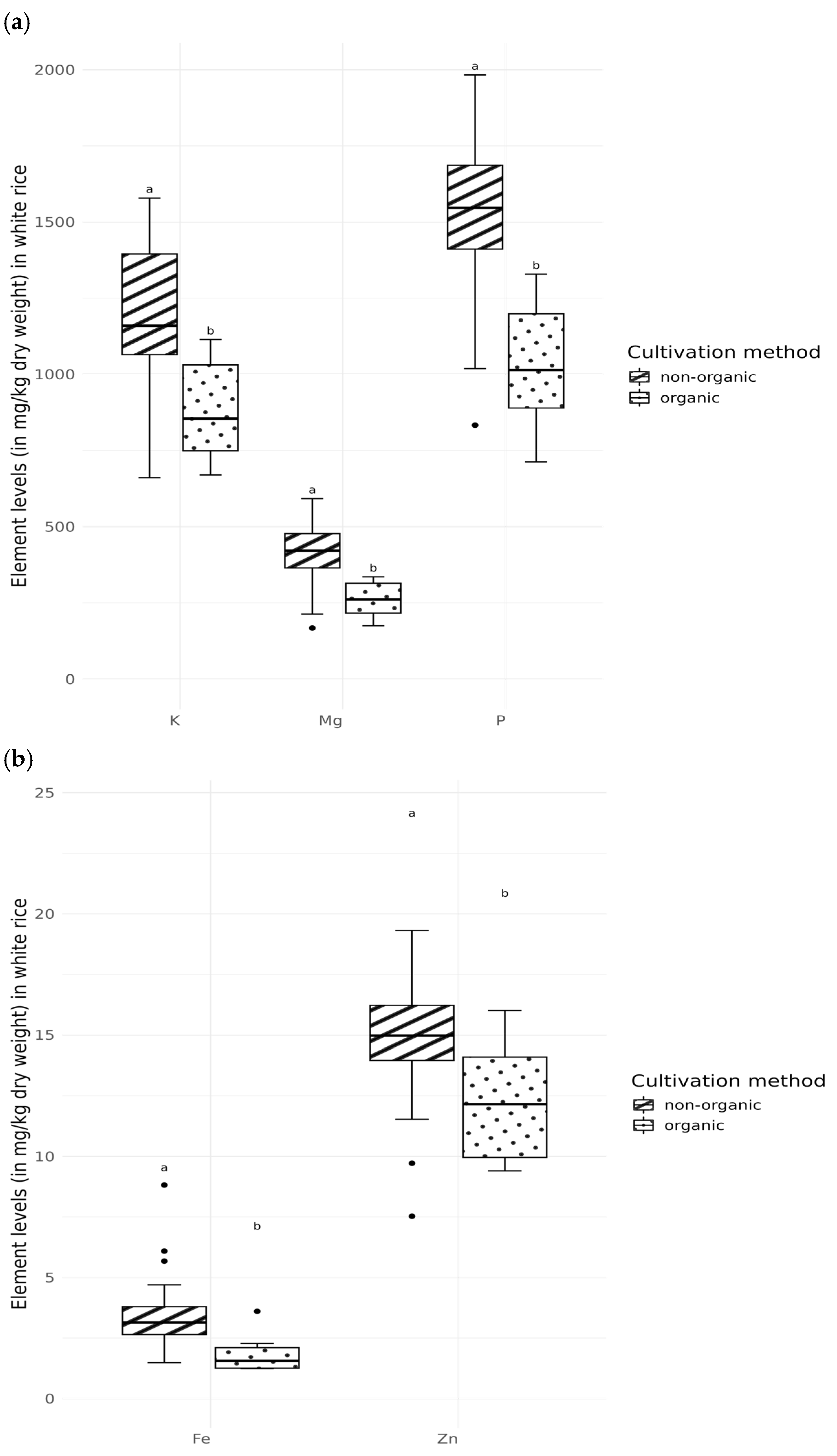
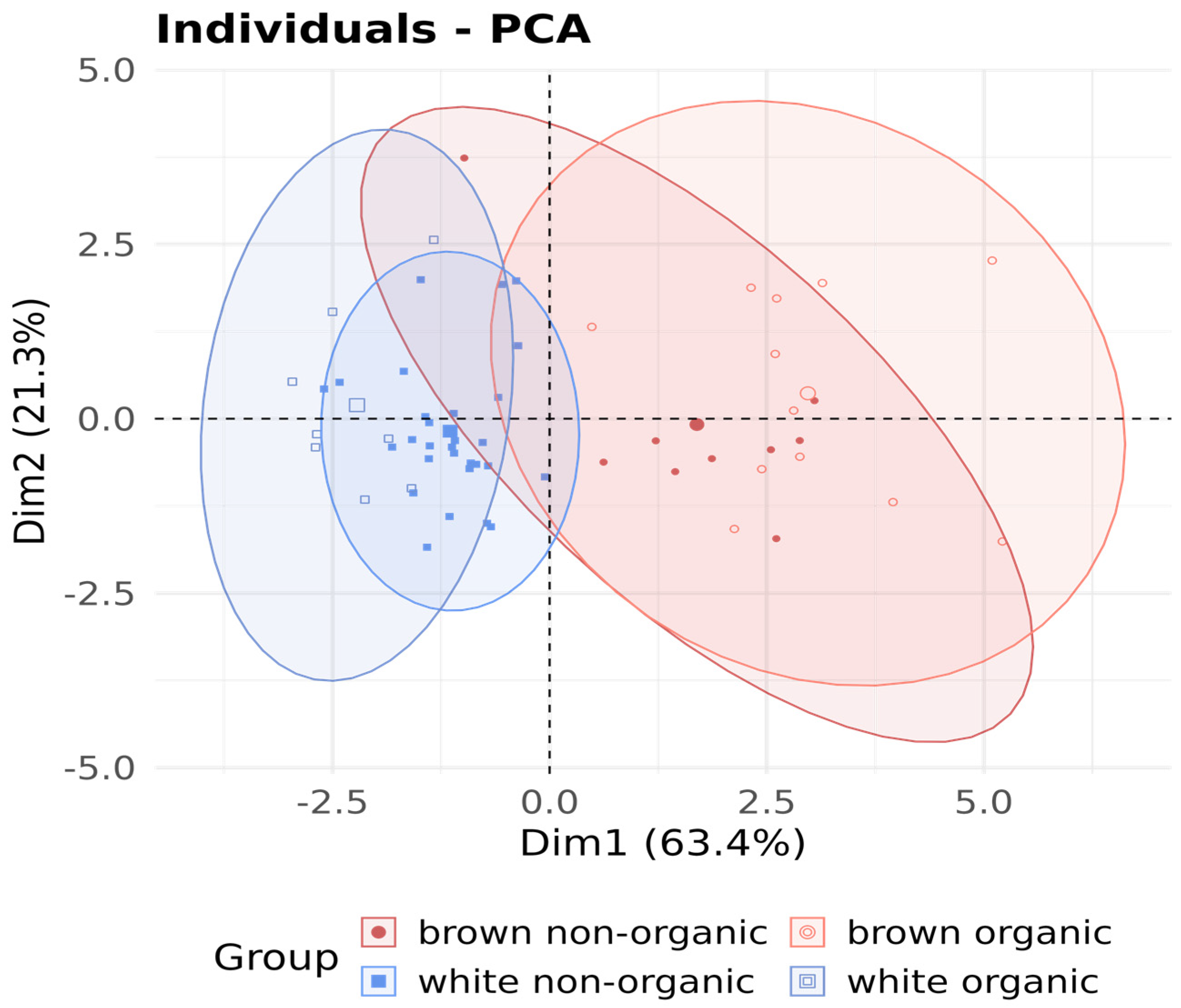
3.3. Composition of the Elements Depending on the Type of Cultivation
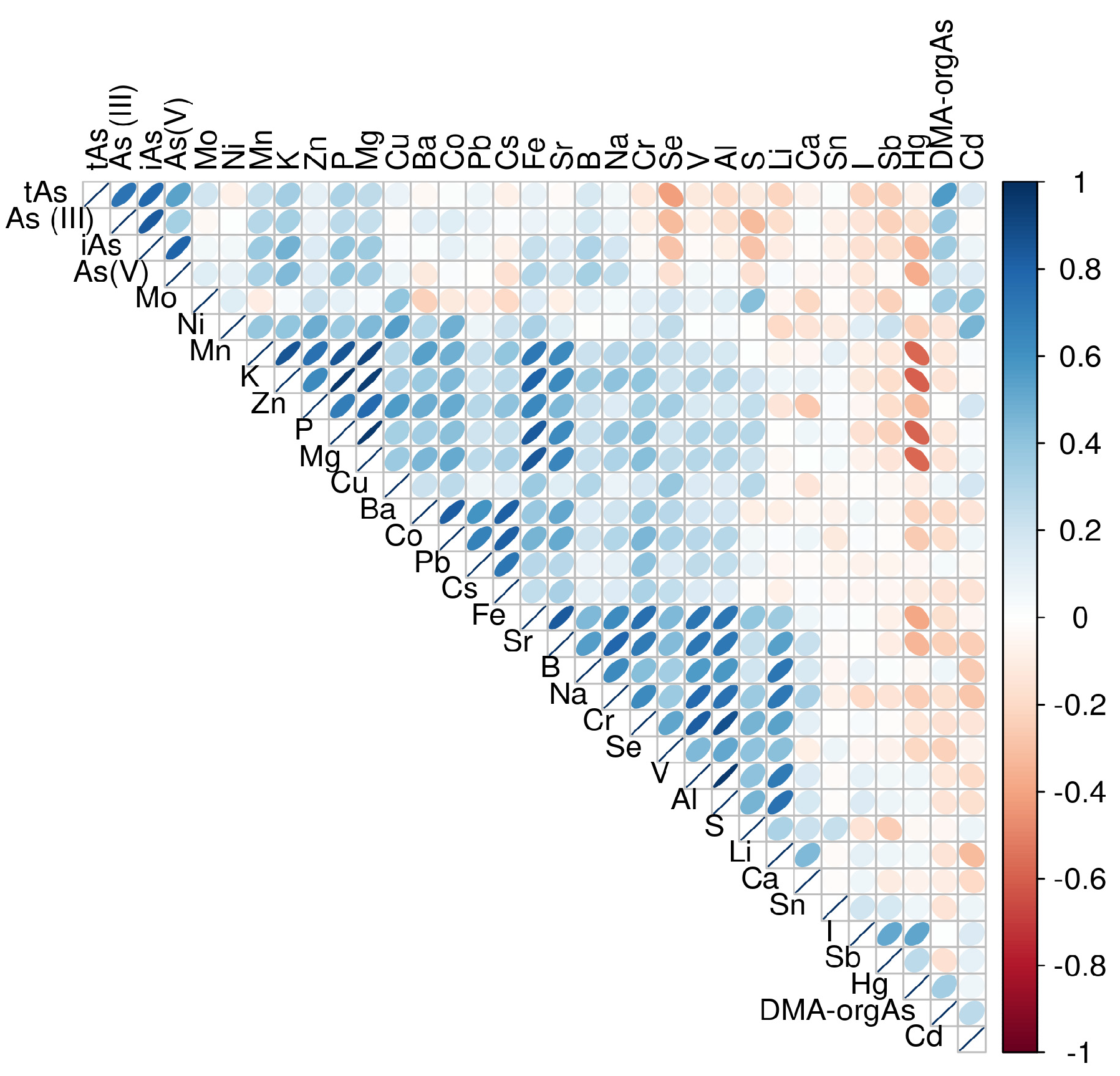
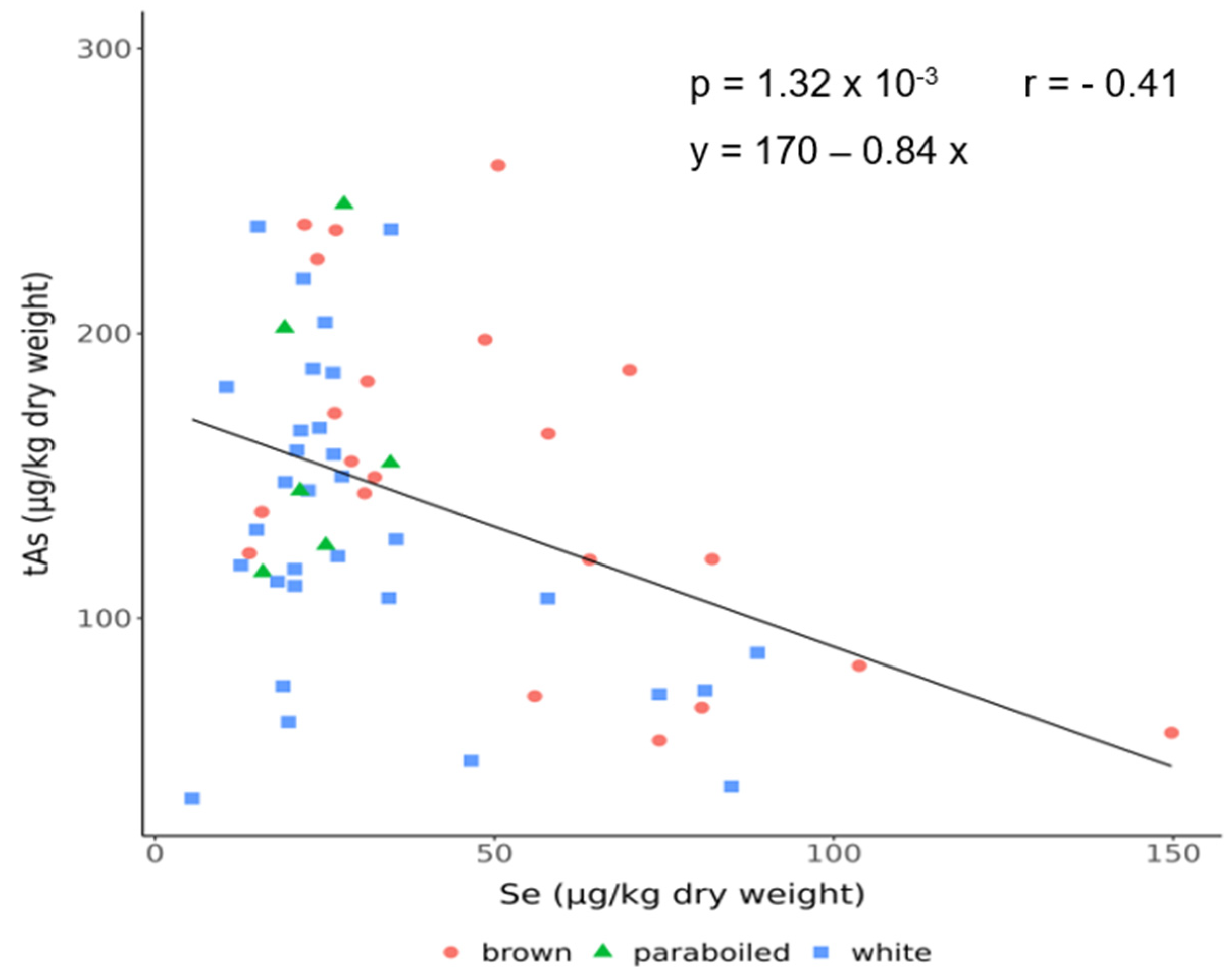
3.4. Correlation Between Arsenic and Selenium
4. Discussion
4.1. Arsenic in Rice
4.2. Dietary Macro and Trace Elements in Rice
4.3. Composition of the Elements Depending on the Type of Cultivation
4.4. Correlation Between Arsenic and Selenium
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICP-MS | inductively coupled plasma mass spectrometry |
| HPLC | high-performance liquid chromatography |
| MMA | monomethylarsonic acid |
| DMA | dimethylarsinic acid |
| LoD | limit of detection |
| EFSA | European Food Safety Authority |
| WHO | World Health Organization |
| EU | European Union |
| DRV | Dietary Reference Value |
| PCA | Principal Component Analysis |
Appendix A
| Element | All Rice | White Rice | Parboiled Rice | Brown Rice |
|---|---|---|---|---|
| (mg/kg) | (n = 58) | (n = 31) | (n = 7) | (n = 20) |
| Mean ± SD (Range) | Mean ± SD (Range) | Mean ± SD (Range) | Mean ± SD (Range) | |
| Li | 0.013 ± 0.024 (0.003–0.111) | 0.012 ± 0.003 (0.003–0.075) | 0.014 ± 0.024 (0.003–0.063) | 0.014 ± 0.030 (0.003–0.111) |
| B | 0.887 ± 0.363 (0.397–2.514) | 0.806 ± 0.272 (0.495–1.697) | 0.966 ± 0.332 (0.702–1.599) | 0.983 ± 0.465 (0.397–2.514) |
| Na | 12.14 ± 9.35 (3.30 -59.11) | 8.44 ± 7.45 a (3.30–29.35) | 16.85± 5.30 b (12.36–25.81) | 16.26 ± 10.62 a (8.27–59.11) |
| Al | 1.719 ± 2.905 (0.2–18.291) | 1.083 ± 1.848 a (0.2–9.126) | 0.789 ± 0.868 a (0.2–2.491) | 2.922 ± 4.042 b (0.2–18.291) |
| S | 1128.48 ± 202.79 (725.88–1622.56) | 1128.81 ± 202.0 (725.88–1584.58) | 1122.73 ± 61.45 (1067.76–1242.12) | 1129.80 ± 234.62 (751.27–1622.56) |
| V | 0.005 ± 0.006 (0.0023–0.0445) | 0.0034 ± 0.0028 a (0.0023–0.0143) | 0.0039 ± 0.0025 a (0.0023–0.0073) | 0.0076 ± 0.0095 b (0.0023–0.0445) |
| Cr | 0.035 ± 0.049 (0.0045–0.2431) | 0.0203 ± 0.0325 a (0.0045–0.1785) | 0.0215 ± 0.0122 ab (0.0085–0.0444) | 0.0616 ± 0.0648 b (0.014–0.2431) |
| Ni | 0.373 ± 0.289 (0.083–1.402) | 0.286 ± 0.221 a (0.083–0.977) | 0.208 ± 0.123 a (0.112–0.428) | 0.550 ± 0.331 b (0.172–1.402) |
| Sr | 0.272 ± 0.211 (0.062–1.146) | 0.154 ± 0.126 a (0.062–0.558) | 0.185 ± 0.073 a (0.117–0.319) | 0.471 ± 0.191 b (0.214–1.146) |
| Sn | 0.038 ± 0.169 (0.0027–1.03584) | 0.0344 ± 0.1426 0.0027–0.7887) | 0.0027 ± 0.0 (0.0027–0.0027) | 0.0531 ± 0.2252 (0.0027–1.0358) |
| Sb | 0.006 ± 0.011 (0.001–0.068) | 0.007 ± 0.014 (0.001–0.068) | 0.002 ± 0.001 (0.001–0.004) | 0.005 ± 0.005 (0.001–0.019) |
| I | 0.145 ± 0.089 (0.03–0.347) | 0.158 ± 00.101 (0.03–0.347) | 0.077 ± 0.075 (0.03–0.201) | 0.146 ± 0.064 (0.03–0.217) |
| Cs | 0.015 ± 0.057 (0.00003–0.3486) | 0.001 ± 0.0021 a (0.00003–0.0112) | 0.005 ± 0.012 a b (0.00028–0.0294) | 0.040 ± 0.091 b (0.0003–0.3486) |
| Ba | 0.309 ± 0.589 (0.023–2.970) | 0.095 ± 0.160 a (0.023–0.891) | 0.081 ± 0.021 a (0.061–0.108) | 0.690 ± 0.844 b (0.107–2.970) |
| Hg | 0.013 ± 0.008 (0.002–0.031) | 0.017 ± 0.006 a (0.008–0.031) | 0.008 ± 0.002 b (0.007–0.10) | 0.007 ± 0.006 b (0.002–0.026) |
| Pb | 0.006 ± 0.012 (0.003–0.090) | 0.003 ± 0.005 (0.003–0.003) | 0.003 ± 0.0 (0.003–0.030) | 0.009 ± 0.019 (0.003–0.090) |
| Cd | 0.037 ± 0.035 (0.002–0.208) | 0.040 ± 0.033 (0.002–0.152) | 0.014 ± 0.009 (0.002–0.029) | 0.039 ± 0.042 (0.002–0.208) |

References
- Food and Agriculture Organization of the United Nations (FAO). Food Outlook—Biannual Report on Global Food Markets; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- da Silva, I.J.S.; Paim, A.P.S.; da Silva, M.J. Composition and estimate of daily mineral intake from samples of Brazilian rice. Microchem. J. 2018, 137, 131–138. [Google Scholar] [CrossRef]
- Shahbandeh, J.; Top Countries Based on Production of Milled Rice 2022/23. Statista. 2024. Available online: https://www.statista.com/statistics/255945/top-countries-of-destination-for-us-rice-exports-2011/ (accessed on 10 January 2025).
- Pinto, E.; Almeida, A.; Ferreira, I.M.P.L.V.O. Essential and non-essential/toxic elements in rice available in the Portuguese and Spanish markets. J. Food Compos. Anal. 2016, 48, 81–87. [Google Scholar] [CrossRef]
- Šlejkovec, Z.; Gorše, L.; Grobler, A.; Jagodic, M.; Falnoga, I. Arsenic speciation and elemental composition of rice samples from the Slovenian market. Food Chem. 2020, 342, 128348. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Arakaki, D.G.; Melo, E.; Leite, L.; Pereira, H.; da Silva, K.R.N.; Guimarães, R.d.C.A.; Freitas, K.d.C.; Hiane, P.A.; Bogo, D.; et al. Determination of selected metals and metalloids in different types of rice by inductively coupled plasma-optical emission spectrometry (ICP-OES). Biol. Trace Elem. Res. 2025, 203, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, T.E.; Banati, R.B.; Nikagolla, C.; Violi, J.P.M.; Bishop, D.P. Concentration and distribution of toxic and essential elements in traditional rice varieties of Sri Lanka grown on an Anuradhapura district farm. Biol. Trace Elem. Res. 2024, 202, 2891–2899. [Google Scholar] [CrossRef]
- Junaid-Ur-Rahman, S.; Chughtai, M.F.J.; Khaliq, A.; Liaqat, A.; Pasha, I.; Ahsan, S.; Tanweer, S.; Saeed, K.; Siddiqa, A.; Mehmood, T.; et al. Rice: A potential vehicle for micronutrient fortification. Clin. Phytosci. 2022, 8, 14. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Some drinking-water disinfectants and contaminants, including arsenic. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2004; Volume 84. Available online: https://www.ncbi.nlm.nih.gov/books/NBK402251/ (accessed on 13 January 2004).
- European Food Safety Authority (EFSA). Scientific opinion on arsenic in food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- Luvonga, C.; Rimmer, C.A.; Yu, L.L.; Lee, S.B. Organoarsenicals in seafood: Occurrence, dietary exposure, toxicity, and risk assessment considerations—A review. J. Agric. Food Chem. 2020, 68, 943–960. [Google Scholar] [CrossRef]
- Taylor, V.; Goodale, B.; Raab, A.; Schwerdtle, T.; Reimer, K.; Conklin, S.; Karagas, M.R.; Francesconi, K.A. Human exposure to organic arsenic species from seafood. Sci. Total Environ. 2017, 580, 266–282. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, I.M.M.; Hasegawa, H. Effects of cooking on dietary exposure to arsenic from rice and vegetables: Human health risk. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 248–255. [Google Scholar] [CrossRef]
- Chowdhury, M.T.A.; Meharg, A.A.; Deacon, C.; Hossain, M.; Norton, G.J. Hydrogeochemistry and arsenic contamination of groundwater in the Haor basins of Bangladesh. Water Qual. Expo. Health 2012, 4, 67–78. [Google Scholar] [CrossRef]
- Williams, P.N.; Islam, S.; Islam, R.; Jahiruddin, M.; Adomako, E.; Soliaman, A.R.; Rahman, G.K.; Lu, Y.; Deacon, C.; Zhu, Y.G.; et al. Arsenic limits trace mineral nutrition (selenium, zinc, and nickel) in Bangladesh rice grain. Environ. Sci. Technol. 2009, 43, 8430–8436. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.N.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef]
- Marin, A.R.; Masscheleyn, P.H.; Patrick, W.H. Soil redox-pH stability of arsenic species and its influence on arsenic uptake by rice. Plant Soil 1993, 152, 245–253. [Google Scholar] [CrossRef]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Liu, W.; Chen, X.; Hu, Y.; Zhu, Y. Association of arsenic with nutrient elements in rice plants. Metallomics 2013, 5, 784–792. [Google Scholar] [CrossRef]
- Jo, G.; Todorov, T.I. Distribution of nutrient and toxic elements in brown and polished rice. Food Chem. 2019, 289, 299–307. [Google Scholar] [CrossRef]
- Rahman, H.; Carey, M.; Hossain, M.; Savage, L.; Rafiqul Islam, M.; Meharg, A.A. Modifying the parboiling of rice to remove inorganic arsenic, while fortifying with calcium. Environ. Sci. Technol. 2019, 53, 5249–5255. [Google Scholar] [CrossRef]
- Kalita, T.; Gohain, U.P.; Hazarika, J. Effect of different processing methods on the nutritional value of rice. Curr. Res. Nutr. Food Sci. 2021, 9, 683–691. [Google Scholar] [CrossRef]
- Menon, M.; Sarkar, B.; Hufton, J.; Reynolds, C.; Reina, S.V.; Young, S. Do arsenic levels in rice pose a health risk to the UK population? Ecotoxicol. Environ. Saf. 2020, 197, 110601. [Google Scholar] [CrossRef]
- Sommella, A.; Deacon, C.; Norton, G.; Pigna, M.; Violante, A.; Meharg, A.A. Total arsenic, inorganic arsenic, and other element concentrations in Italian rice grain varies with origin and type. Environ. Pollut. 2013, 181, 38–43. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain (CONTAM Panel); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; et al. Update of the Risk Assessment of Inorganic Arsenic in Food. EFSA J. 2024, 22, e8488. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011; 115p. [Google Scholar]
- European Food Safety Authority (EFSA). Dietary reference values for nutrients: Summary report. EFSA Support. Publ. 2017, 14, e15121. [Google Scholar] [CrossRef]
- González-Martínez, F.; Johnson-Restrepo, B.; Quiñones, L.A. Arsenic inorganic exposure, metabolism, genetic biomarkers and its impact on human health: A mini-review. Toxicol. Lett. 2024, 398, 105–117. [Google Scholar] [CrossRef]
- Yao, B.M.; Chen, P.; Sun, G.X. Distribution of elements and their correlation in bran, polished rice, and whole grain. Food Sci. Nutr. 2020, 8, 982–992. [Google Scholar] [CrossRef]
- Zavala, Y.J.; Duxbury, J.M. Arsenic in rice: I. Estimating normal levels of total arsenic in rice grain. Environ. Sci. Technol. 2008, 42, 3856–3860. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006; L191/103; European Union: Brussels, Belgium, 2023; Available online: https://eurlex.europa.eu//legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 18 March 2025).
- Munera-Picazo, S.; Cano-Lamadrid, M.; Castaño-Iglesias, M.C.; Burló, F.; Carbonell-Barrachina, A.A. Arsenic in your food: Potential health hazards from arsenic found in rice. Nutr. Diet. Suppl. 2015, 7, 1–10. [Google Scholar] [CrossRef]
- Oteiza, J.M.; Barril, P.A.; Quintero, C.E.; Savio, M.; Befani, R.; Fernandez Cirelli, A.; Echegaray, N.S.; Murad, C.; Buedo, A. Arsenic in Argentinean polished rice: Situation overview and regulatory framework. Food Control 2020, 109, 106909. [Google Scholar] [CrossRef]
- Huang, J.H.; Fecher, P.; Ilgen, G.; Hu, K.N.; Yang, J. Speciation of arsenite and arsenate in rice grain—Verification of nitric acid-based extraction method and mass sample survey. Food Chem. 2012, 130, 453–459. [Google Scholar] [CrossRef]
- Jorhem, L.; Astrand, C.; Sundstrom, B.; Baxter, M.; Stokes, P.; Lewis, J.; Grawe, K.P. Elements in rice on the Swedish market: Part 2. Chromium, copper, iron, manganese, platinum, rubidium, selenium and zinc. Food Addit. Contam. A 2008, 25, 841–850. [Google Scholar] [CrossRef]
- de Oliveira, V.F.; Busanello, C.; Viana, V.E.; Stafen, C.F.; Pedrolo, A.M.; Paniz, F.P.; Pedron, T.; Pereira, R.M.; Rosa, S.A.; Junior, A.M.d.M.; et al. Assessing mineral and toxic elements content in rice grains grown in southern Brazil. J. Food Compos. Anal. 2021, 100, 103914. [Google Scholar] [CrossRef]
- Maganti, S.; Swaminathan, R.; Parida, A. Variation in iron and zinc content in traditional rice genotypes. Agric. Res. 2020, 9, 316–328. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Zeng, J.-Y.; Ding, S.; Li, J.; Liu, X.; Guan, D.-X.; Ma, L.Q. Arsenic contents, speciation and bioaccessibility in rice grains from China: Regional and variety differences. J. Hazard. Mater. 2022, 437, 129431. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz Tuncel, N.; Andac, A.E.; Polat Kaya, H.; Tuncel, N.B. The impact of milling degree on physicochemical, nutritional, rheological, textural properties, and in vitro digestibility of rice: From brown rice to white rice. Food Chem. 2025, 464, 141795. [Google Scholar] [CrossRef]
- Abbas, S.; Murtaza, S.; Aslam, F.; Khawar, A.; Rafique, S.; Naheed, S. Effect of Processing on Nutritional Value of Rice (Oryza sativa). World J. Med. Sci. 2011, 6, 68–73. [Google Scholar]
- Heinemann, R.J.B.; Fagundes, P.L.; Pinto, E.A.; Penteado, M.V.C.; Lanfer-Marquez, U.M. Comparative study of nutrient composition of commercial brown, parboiled and milled rice from Brazil. J. Food Compos. Anal. 2005, 18, 287–296. [Google Scholar] [CrossRef]
- Hunter, D.; Foster, M.; McArthur, J.O.; Ojha, R.; Petocz, P.; Samman, S. Evaluation of the micronutrient composition of plant foods produced by organic and conventional agricultural methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 571–582. [Google Scholar] [CrossRef]
- Hou, J.; Gao, X.; Entz, M.H. Enhancing zinc and iron bioavailability through crop rotation and organic farming: Insights from a long-term study. Field Crops Res. 2025, 322, 109710. [Google Scholar] [CrossRef]

| White Rice | Parboiled Rice | Brown Rice | |
|---|---|---|---|
| EI (µg/kg bw) | 0.034 | 0.055 | 0.049 |
| % RP of 0.06 µg/kg bw/day [25] | 56.1 | 91.9 | 82.3 |
| % BDML05 of 3 µg/kg bw/day [26] | 1.1 | 1.8 | 1.6 |
| Element (mg/kg) | All Rice (n = 58) | White Rice (n = 31) | Parboiled Rice (n = 7) | Brown Rice (n = 20) | SRM 1568b (n = 4) |
|---|---|---|---|---|---|
| Mean ± SD (Range) | Mean ± SD (Range) | Mean ± SD (Range) | Mean ± SD (Range) | Mean ± SD [Mean ± 95% CI] | |
| Mg | 705 ± 482 (168–1931) | 369 ± 115 a (168–592) | 450 ± 75 a (325–514) | 1274 ± 330 b (438–1931) | 539 ± 3 [559 ± 10] |
| P | 1959 ± 811 (712–4281) | 1372 ± 322 a (713–1983) | 1896 ± 245 b (1583–2116) | 2846 ± 585 c (1365–4281) | 1515 ± 5 [1530 ± 40] |
| K | 1732 ± 818 (661–3913) | 1103 ± 262 a (661–1579) | 1866 ± 186 b (1584–2078) | 2624 ± 585 c (1132–3913) | 1130 ± 6 [1282 ± 11] |
| Ca | 114.2 ± 179.4 (36.2–1144.1) | 91.9 ± 155.5 a (36.2–912.1) | 234.4 ± 445.8 a (37.4–1144.1) | 112.7 ± 25.2 b (61.1–156.7) | 112 ± 2 [118.4 ± 3.1] |
| Fe | 6.06 ± 4.91 (1.24–24.43) | 3.06 ± 1.64 a (1.24–8.82) | 3.43 ± 0.29 a (2.95–3.76) | 11.3 ± 4.53 b (4.04–24.43) | 7.05 ± 0.06 [7.42 ± 0.44] |
| Mn | 17.6 ± 11.5 (4.78–43.6) | 10.06 ± 3.10 a (4.78–17.46) | 10.39 ± 3.86 a (6.35–17.72) | 30.64 ± 8.78 b (10.75–43.64) | 18.45 ± 0.03 [19.2 ± 1.8] |
| Co | 0.011 ± 0.012 (<LoD–0.072) | 0.006 ± 0.003 a (0.001–0.016) | 0.006 ± 0.003 a (0.003–0.013) | 0.019 ± 0.017 b (0.004–0.072) | 0.0223 ± 0.0004 [0.0177 ± 0.0005] |
| Cu | 2.11 ± 0.661 (0.932–3.871) | 1.99 ± 0.723 a (0.932–3.868) | 1.79 ± 0.421 a (1.38–2.40) | 2.39 ± 0.527 b (1.35–3.24) | 2.29 ± 0.02 [2.35 ± 0.16] |
| Zn | 15.6 ± 4.10 (7.52–22.81) | 14.1 ± 2.81 a (7.53–19.32) | 10.2 ± 2.14 b (7.52–13.70) | 19.1 ± 3.06 c (11.64–22.76) | 20.09 ± 0.13 [19.42 ± 0.26] |
| Se | 0.038 ± 0.028 (<LoD–0.151) | 0.032 ± 0.022 a (<LoD–0.089) | 0.024 ± 0.007 ab (0.016–0.035) | 0.052 ± 0.034 b (0.014–0.150) | 0.335 ± 0.035 [0.365 ± 0.029] |
| Mo | 0.654 ± 0.181 (0.339–1.197) | 0.665 ± 0.203 (0.339–1.200) | 0.624 ± 0.127 (0.418–0.768) | 0.647 ± 0.164 (0.390–1.012) | 1.430 ± 0.008 [1.451 ± 0.048] |
| Element | DRV (mg/day) | Estimated Intake per Serving (% DRV) | |||
|---|---|---|---|---|---|
| All Rice | White Rice | Parboiled Rice | Brown Rice | ||
| Mg | 300–350 | 6.6 | 3.5 | 4.2 | 12.0 |
| P | 550 | 11.8 | 8.2 | 11.4 | 17.1 |
| K | 3500 | 1.6 | 1.0 | 1.8 | 2.5 |
| Ca | 1000 | 0.4 | 0.3 | 0.8 | 0.4 |
| Fe | 11 | 1.8 | 0.9 | 1.1 | 3.4 |
| Mn | 3 | 19.3 | 11.1 | 11.4 | 33.7 |
| Cu | 1.3–1.6 | 4.4 | 4.1 | 3.7 | 4.9 |
| Zn | 7.5–16.3 | 3.1 | 2.9 | 2.1 | 3.9 |
| Se | 0.07 | 1.8 | 1.5 | 1.1 | 2.5 |
| Mo | 0.065 | 33.2 | 33.8 | 31.7 | 32.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumora Samarin, I.; Sulimanec, A.; Orct, T.; Benutić, A.; Marciuš, B.; Tomljanović, K.; Jurasović, J. Dietary Trace Elements and Arsenic Species in Rice: A Study of Samples from Croatian Supermarkets. Foods 2025, 14, 2261. https://doi.org/10.3390/foods14132261
Rumora Samarin I, Sulimanec A, Orct T, Benutić A, Marciuš B, Tomljanović K, Jurasović J. Dietary Trace Elements and Arsenic Species in Rice: A Study of Samples from Croatian Supermarkets. Foods. 2025; 14(13):2261. https://doi.org/10.3390/foods14132261
Chicago/Turabian StyleRumora Samarin, Ivana, Antonija Sulimanec, Tatjana Orct, Anica Benutić, Bernardo Marciuš, Karla Tomljanović, and Jasna Jurasović. 2025. "Dietary Trace Elements and Arsenic Species in Rice: A Study of Samples from Croatian Supermarkets" Foods 14, no. 13: 2261. https://doi.org/10.3390/foods14132261
APA StyleRumora Samarin, I., Sulimanec, A., Orct, T., Benutić, A., Marciuš, B., Tomljanović, K., & Jurasović, J. (2025). Dietary Trace Elements and Arsenic Species in Rice: A Study of Samples from Croatian Supermarkets. Foods, 14(13), 2261. https://doi.org/10.3390/foods14132261






