Impact of Fatty Acid Types and Microwave Post-Treatment on the Physicochemical Properties of Water Caltrop Starch–Lipid Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Water Caltrop Starch
2.3. Preparation of Water Caltrop Starch–Lipid Complex
2.4. Polarized Light Microscopy
2.5. Scanning Electron Microscopy (SEM)
2.6. Complex Index
2.7. X-Ray Diffraction (XRD) Analysis
2.8. Fourier-Transform Infrared Spectroscopy (FTIR)
2.9. Differential Scanning Calorimetry (DSC)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Polarized Light Microscopic Features
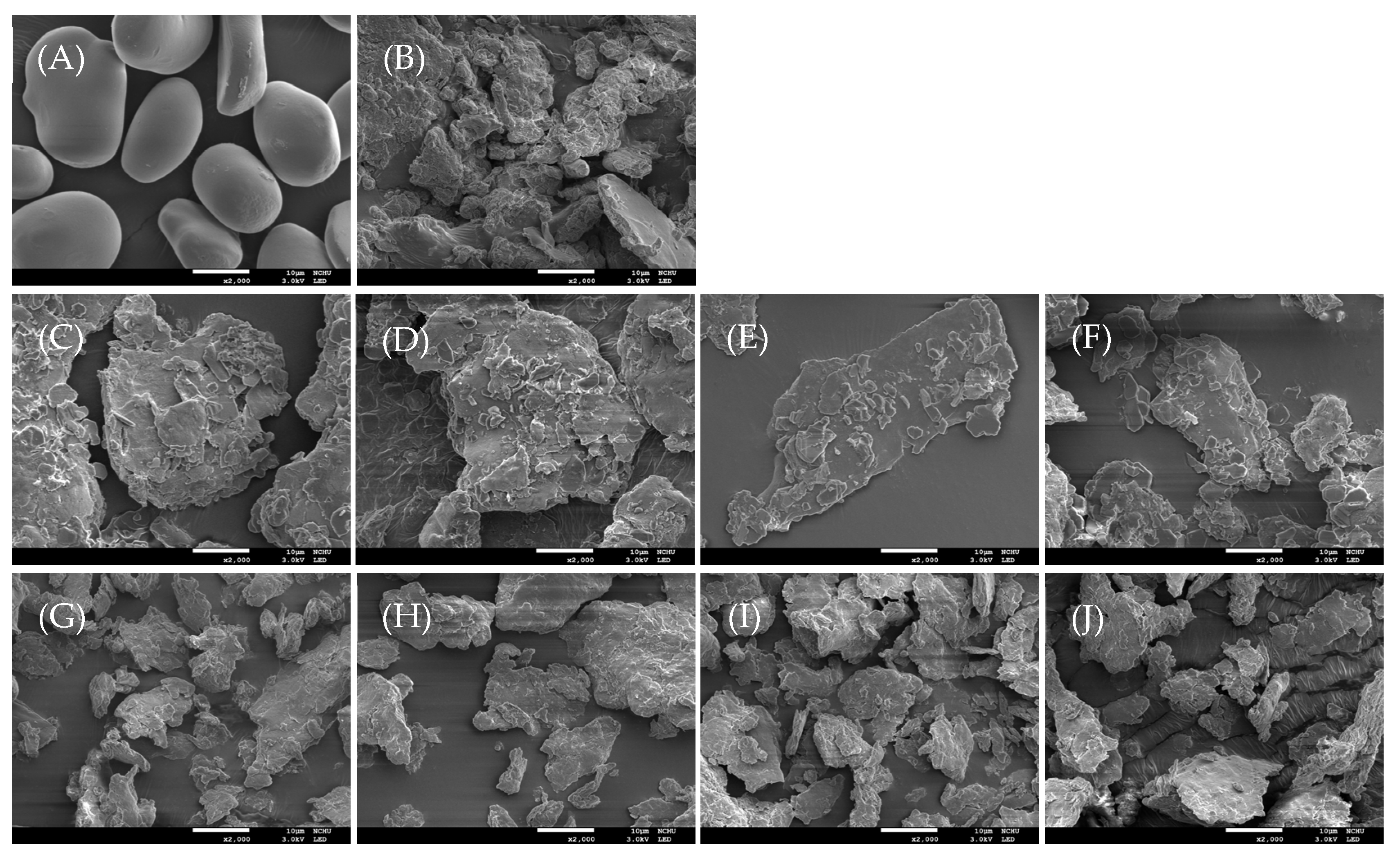
3.2. Scanning Electron Microscopic (SEM) Features
3.3. Complexation Effectiveness by Complex Index Evaluation
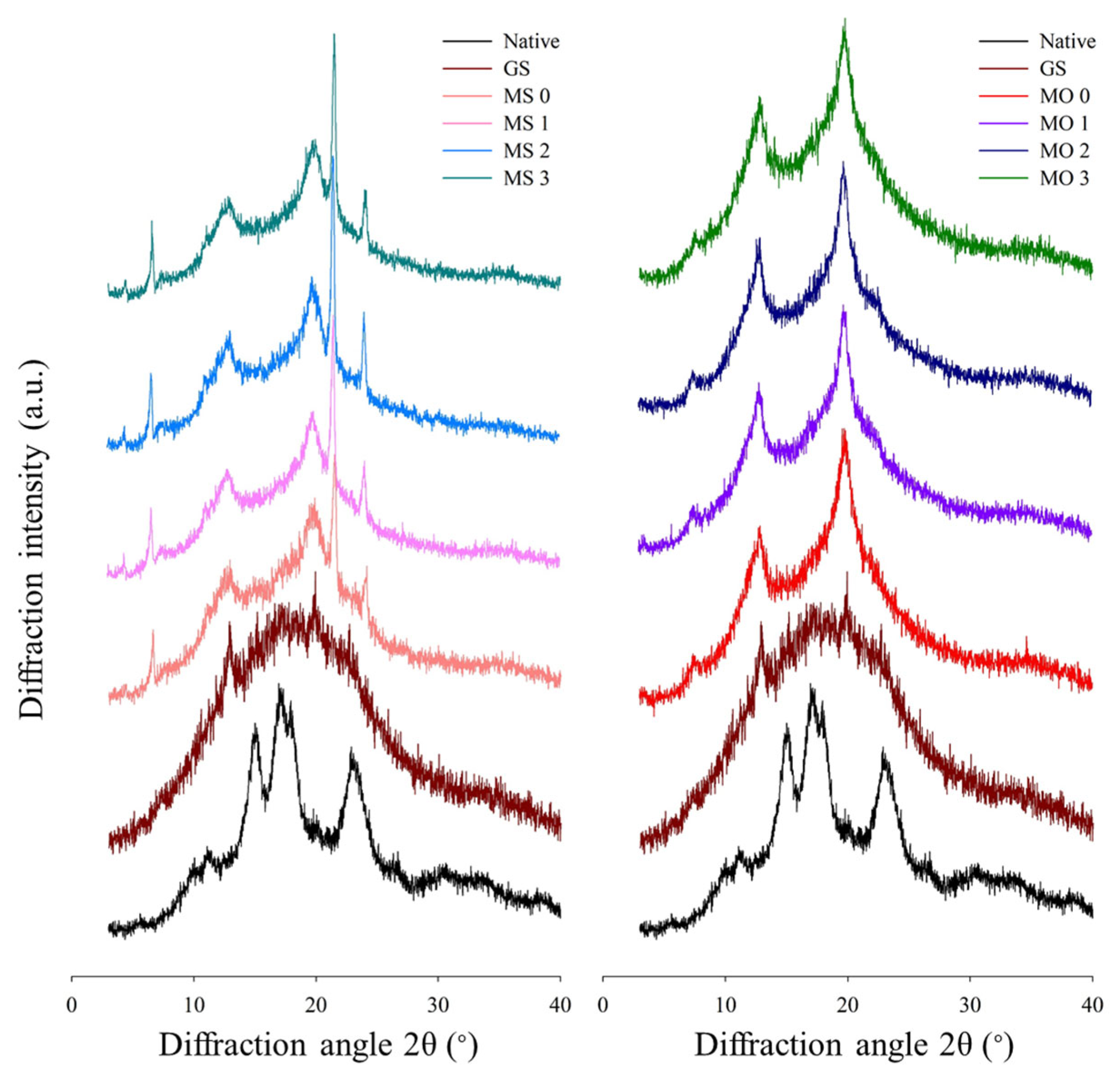
3.4. Long-Range Ordered Structure by X-Ray Diffraction (XRD) Analysis
3.5. Short-Range Ordered Structure by Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.6. Thermal Properties by Differential Scanning Calorimetry (DSC) Analysis

3.7. Mechanism of Microwave Post-Treatment on the Construction of Starch–Lipid Complexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiang, P.Y.; Li, P.H.; Huang, C.C.; Wang, C.R. Chemical composition and physical characteristics of water caltrop during growth. J. Sci. Food Agric. 2009, 89, 1298–1306. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Lai, L.-S. In vitro starch digestibility, rheological, and physicochemical properties of water caltrop starch modified with cycled heat-moisture treatment. Foods 2021, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Hasjim, J.; Lee, S.O.; Hendrich, S.; Setiawan, S.; Ai, Y.; Jane, J.L. Characterization of a novel resistant-starch and its effects on postprandial plasma-glucose and insulin responses. Cereal Chem. 2010, 87, 257–262. [Google Scholar] [CrossRef]
- Chang, R.; Tian, Y.; Lu, H.; Sun, C.; Jin, Z. Effects of fractionation and heat-moisture treatment on structural changes and digestibility of debranched waxy maize starch. Food Hydrocoll. 2020, 101, 105488. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Tovar, J. Update of the concept of type 5 resistant starch (RS5): Self-assembled starch V-type complexes. Trends Food Sci. Technol. 2021, 109, 711–724. [Google Scholar] [CrossRef]
- Rutschmann, M.; Solms, J. Formation of inclusion complexes of strach with different organic compounds. II, Study of ligand binding in binary model systems with decanal, 1-naphthol, monostearate and monopalmitate. Lebensm.-Wiss.+Technol. 1990, 23, 70–79. [Google Scholar]
- Duan, H.; Wang, L.; Huangfu, M.; Li, H. The impact of microbiota-derived short-chain fatty acids on macrophage activities in disease: Mechanisms and therapeutic potentials. Biomed. Pharmacother. 2023, 165, 115276. [Google Scholar] [CrossRef]
- Li, X.; Luo, S.; Hou, Y.; Liu, Y.; Hu, X.; Liu, C. Effect of triglyceride on complexation between starch and fatty acid. Int. J. Biol. Macromol. 2020, 155, 1069–1074. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Yang, Y.; Yu, X.; Xu, L.; Jiao, A.; Jin, Z. Structure, physicochemical properties and in vitro digestibility of extruded starch-lauric acid complexes with different amylose contents. Food Hydrocoll. 2023, 136, 108239. [Google Scholar] [CrossRef]
- Sun, S.; Hong, Y.; Gu, Z.; Cheng, L.; Ban, X.; Li, Z.; Li, C. Impacts of fatty acid type on binding state, fine structure, and in vitro digestion of debranched starch-fatty acid complexes with different debranching degrees. Carbohydr. Polym. 2023, 318, 121107. [Google Scholar] [CrossRef]
- Tu, D.; Ou, Y.; Zheng, Y.; Zhang, Y.; Zheng, B.; Zeng, H. Effects of freeze-thaw treatment and pullulanase debranching on the structural properties and digestibility of lotus seed starch-glycerin monostearin complexes. Int. J. Biol. Macromol. 2021, 177, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chao, C.; Niu, B.; Copeland, L.; Yu, J.; Wang, S.; Wang, S. New insight into the interactions among starch, lipid and protein in model systems with different starches. Food Hydrocoll. 2021, 112, 106323. [Google Scholar] [CrossRef]
- Raza, H.; Liang, Q.; Ameer, K.; Ma, H.; Ren, X. Dual-frequency power ultrasound effects on the complexing index, physicochemical properties, and digestion mechanism of arrowhead starch-lipid complexes. Ultrason. Sonochem. 2022, 84, 105978. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Ramírez, J.E.; Cabrera-Ramirez, A.H.; Morales-Sánchez, E.; Rodriguez-García, M.E.; de la Luz Reyes-Vega, M.; Ramírez-Jiménez, A.K.; Contreras-Jiménez, B.L.; Gaytán-Martínez, M. Amylose-lipid complex formation from extruded maize starch mixed with fatty acids. Carbohydr. Polym. 2020, 246, 116555. [Google Scholar] [CrossRef]
- Ozel, B.; Dag, D.; Kilercioglu, M.; Sumnu, S.G.; Oztop, M.H. NMR relaxometry as a tool to understand the effect of microwave heating on starch-water interactions and gelatinization behavior. LWT Food Sci. Technol. 2017, 83, 10–17. [Google Scholar] [CrossRef]
- Yang, Q.; Qi, L.; Luo, Z.; Kong, X.; Xiao, Z.; Wang, P.; Peng, X. Effect of microwave irradiation on internal molecular structure and physical properties of waxy maize starch. Food Hydrocoll. 2017, 69, 473–482. [Google Scholar] [CrossRef]
- Hung, S.-H.; Lai, L.-S. Changes in the pasting and rheological properties of wheat, corn, water caltrop and lotus rhizome starches by the addition of Annona montana mucilage. Int. J. Biol. Macromol. 2024, 265, 131009. [Google Scholar] [CrossRef]
- Chang, F.; He, X.; Huang, Q. The physicochemical properties of swelled maize starch granules complexed with lauric acid. Food Hydrocoll. 2013, 32, 365–372. [Google Scholar] [CrossRef]
- Zaliha, O.; Chong, C.; Cheow, C.; Norizzah, A.; Kellens, M. Crystallization properties of palm oil by dry fractionation. Food Chem. 2004, 86, 245–250. [Google Scholar] [CrossRef]
- Chen, B.; Zeng, S.; Zeng, H.; Guo, Z.; Zhang, Y.; Zheng, B. Properties of lotus seed starch–glycerin monostearin complexes formed by high pressure homogenization. Food Chem. 2017, 226, 119–127. [Google Scholar] [CrossRef]
- Kang, X.; Jia, S.; Gao, W.; Wang, B.; Zhang, X.; Tian, Y.; Sun, Q.; Atef, M.; Cui, B.; Abd El-Aty, A. The formation of starch-lipid complexes by microwave heating. Food Chem. 2022, 382, 132319. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, Y.; Wang, X.; Fang, Y.; Lin, Q.; Ding, Y. Structural and in vitro starch digestion properties of starch-fatty acid nanocomplexes: Effect of chain lengths and degree of unsaturation of fatty acids. J. Sci. Food Agric. 2022, 102, 7239–7248. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.C.; Copeland, L. Analysis of complexes between lipids and wheat starch. Carbohydr. Polym. 2007, 67, 80–85. [Google Scholar] [CrossRef]
- Sun, S.; Jin, Y.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Effects of fatty acids with various chain lengths and degrees of unsaturation on the structure, physicochemical properties and digestibility of maize starch-fatty acid complexes. Food Hydrocoll. 2021, 110, 106224. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Akintayo, O.A.; Adebo, O.A.; Kayitesi, E.; Njobeh, P.B. A review on the physicochemical properties of starches modified by microwave alone and in combination with other methods. Int. J. Biol. Macromol. 2021, 176, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Creek, J.A.; Ziegler, G.R.; Runt, J. Amylose Crystallization from Concentrated Aqueous Solution. Biomacromolecules 2006, 7, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, J.; Huang, Y.; Luo, Y.; Li, R.; He, Y.; He, C.; Peng, Q.; Wang, M. Preparation of starch-palmitic acid complexes by three different starches: A comparative study using the method of heating treatment and autoclaving treatment. Int. J. Biol. Macromol. 2024, 262, 130009. [Google Scholar] [CrossRef]
- Tao, Y.; Yan, B.; Fan, D.; Zhang, N.; Ma, S.; Wang, L.; Wu, Y.; Wang, M.; Zhao, J.; Zhang, H. Structural changes of starch subjected to microwave heating: A review from the perspective of dielectric properties. Trends Food Sci. Technol. 2020, 99, 593–607. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, H.R.; Choi, S.J.; Park, C.S.; Moon, T.W. Complexation of amylosucrase-modified waxy corn starch with fatty acids: Determination of their physicochemical properties and digestibilities. J. Food Sci. 2019, 84, 1362–1370. [Google Scholar] [CrossRef]
- Chung, J.-C.; Lai, L.-S. Effects of Continuous and Cycled Annealing on the Physicochemical Properties and Digestibility of Water Caltrop Starch. Foods 2023, 12, 3551. [Google Scholar] [CrossRef]
- Huang, K.-W.; Lai, L.-S. Impact of Ultrasonic-Assisted Preparation of Water Caltrop Starch–Lipid Complex: Structural and Physicochemical Properties. Foods 2025, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, R.; Bereka, T.Y.; Jin, Z.; Zhou, X. Physiochemical properties of V-type cold water swelling tapioca starch prepared by ethanolic extrusion. Int. J. Biol. Macromol. 2025, 306, 141435. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Yang, J.; Ge, S.; Chang, R.; Xiong, L.; Sun, Q. Preparation and characterization of size-controlled starch nanoparticles based on short linear chains from debranched waxy corn starch. LWT 2016, 74, 303–310. [Google Scholar] [CrossRef]
- Le, C.-A.-K.; Choisnard, L.; Wouessidjewe, D.; Putaux, J.-L. Polymorphism of crystalline complexes of V-amylose with fatty acids. Int. J. Biol. Macromol. 2018, 119, 555–564. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, W.; Shan, J.; Zhang, S.; Dong, F. Physical properties and loading capacity of gelatinized granular starches. Ind. Crops Prod. 2014, 53, 323–329. [Google Scholar] [CrossRef]
- Wu, F.; Chi, B.; Xu, R.; Liao, H.; Xu, X.; Tan, X. Changes in structures and digestibility of amylose-oleic acid complexes following microwave heat-moisture treatment. Int. J. Biol. Macromol. 2022, 214, 439–445. [Google Scholar] [CrossRef]
- Nie, M.; Li, J.; Lin, R.; Gong, X.; Dang, B.; Zhang, W.; Yang, X.; Wang, L.; Wang, F.; Tong, L.-T. The role of C18 fatty acids in improving the digestion and retrogradation properties of highland barley starch. Food Res. Int. 2024, 186, 114355. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, B.; Guo, Z.; Zhang, Y.; Zheng, B.; Zeng, S.; Zeng, H. Properties of lotus seed starch-glycerin monostearin V-complexes after long-term retrogradation. Food Chem. 2020, 311, 125887. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, J.; Gladden, I.; Contreras, A.; Kong, L. Complexation ability and physicochemical properties of starch inclusion complexes with C18 fatty acids. Food Hydrocoll. 2022, 123, 107175. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Fu, X.; Zhang, B.; Huang, Q. Complexation of rice starch/flour and maize oil through heat moisture treatment: Structural, in vitro digestion and physicochemical properties. Int. J. Biol. Macromol. 2017, 98, 557–564. [Google Scholar] [CrossRef]
- Putseys, J.; Lamberts, L.; Delcour, J.A. Amylose-inclusion complexes: Formation, identity and physico-chemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Gelders, G.G.; Vanderstukken, T.C.; Goesaert, H.; Delcour, J.A. Amylose–lipid complexation: A new fractionation method. Carbohydr. Polym. 2004, 56, 447–458. [Google Scholar] [CrossRef]



| Sample 1 | Complex Index (%) 2 |
|---|---|
| Native | - 3 |
| GS | - 3 |
| MS 0 | 78.8 ± 0.1 c |
| MS 1 | 79.5 ± 0.1 b |
| MS 2 | 80.1 ± 0.2 a |
| MS 3 | 79.8 ± 0.2 ab |
| MO 0 | 77.3 ± 0.1 d |
| MO 1 | 77.3 ± 0.3 d |
| MO 2 | 77.8 ± 0.2 d |
| MO 3 | 75.7 ± 0.1 e |
| Sample 1 | Relative Crystallinity (%) 2 | Crystalline Structure |
|---|---|---|
| Native | 44.5 ± 0.4 a | A-type |
| GS | 4.5 ± 0.6 f | V-type |
| MS 0 | 16.8 ± 0.4 e | V-type |
| MS 1 | 19.4 ± 0.8 d | V-type |
| MS 2 | 20.2 ± 0.5 d | V-type |
| MS 3 | 27.2 ± 0.7 b | V-type |
| MO 0 | 19.5 ± 0.3 d | V-type |
| MO 1 | 23.3 ± 0.3 c | V-type |
| MO 2 | 23.4 ± 0.3 c | V-type |
| MO 3 | 24.3 ± 0.3 c | V-type |
| Sample 1 | 1047 cm−1/1022 cm−1 | 995 cm−1/1022 cm−1 |
|---|---|---|
| Native | 0.711 ± 0.002 a | 1.278 ± 0.000 a |
| GS | 0.685 ± 0.002 b | 1.143 ± 0.002 b |
| MS 0 | 0.656 ± 0.001 c | 1.095 ± 0.003 cd |
| MS 1 | 0.668 ± 0.014 bc | 1.100 ± 0.008 c |
| MS 2 | 0.674 ± 0.028 bc | 1.082 ± 0.001 d |
| MS 3 | 0.676 ± 0.006 bc | 1.102 ± 0.001 c |
| MO 0 | 0.651 ± 0.008 c | 1.108 ± 0.007 c |
| MO 1 | 0.656 ± 0.003 c | 1.101 ± 0.006 c |
| MO 2 | 0.655 ± 0.007 c | 1.098 ± 0.002 c |
| MO 3 | 0.664 ± 0.011 bc | 1.092 ± 0.014 cd |
| Type Ⅰ | Type Ⅱ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | To 2,3 (℃) | Tp 2,3 (℃) | Te 2,3 (℃) | Te − To 2,3 (℃) | ΔH 2,3 (J/g) | To 2,3 (℃) | Tp 2,3 (℃) | Te 2,3 (℃) | Te − To 2,3 (℃) | ΔH 2,3 (J/g) |
| Native | - 4 | - 4 | - 4 | - 4 | - 4 | - 4 | - 4 | - 4 | - 4 | - 4 |
| GS | - 4 | - 4 | - 4 | - 4 | - 4 | - 4 | - 4 | - 4 | - 4 | - 4 |
| MS 0 | 92.41 ± 0.11 a | 100.11 ± 0.41 a | 108.42 ± 0.06 a | 16.01 ± 0.04 a | 7.81 ± 0.07 a | 117.42 ± 2.1 a | 121.17 ± 1.7 a | 124.97 ± 0.96 a | 7.55 ± 1.14 b | 0.39 ± 0.06 b |
| MS 1 | 92.21 ± 0.00 ab | 98.49 ± 0.1 b | 104.76 ± 0.65 b | 12.55 ± 0.65 b | 4.15 ± 0.3 b | 115.47 ± 0.72 a | 119.21 ± 0.45 ab | 122.77 ± 0.16 a | 7.3 ± 0.88 b | 0.95 ± 0.04 a |
| MS 2 | 91.96 ± 0.27 b | 98.82 ± 0.6 b | 105.43 ± 1.89 ab | 13.46 ± 1.61 ab | 4.81 ± 0.82 b | 114.21 ± 0.26 a | 118.79 ± 2.24 ab | 122.64 ± 0.87 a | 8.43 ± 0.6 b | 1.03 ± 0.21 a |
| MS 3 | 90.04 ± 0.04 c | 97.81 ± 0.12 b | 103.53 ± 0.97 b | 12.57 ± 1.02 b | 4.45 ± 0.63 b | 108.68 ± 0.49 b | 115.95 ± 1.29 b | 122.61 ± 1.83 a | 13.93 ± 1.33 a | 1.00 ± 0.00 a |
| MO 0 | 87.61 ± 0.24 A | 94.93 ± 0.74 A | 100.56 ± 0.32 B | 12.94 ± 0.07 A | 1.99 ± 0.14 A | 108.78 ± 0.42 A | 113.4 ± 0.27 B | 117.59 ± 0.38 A | 8.81 ± 0.8 B | 0.51 ± 0.04 D |
| MO 1 | 87.57 ± 0.76 A | 95.38 ± 0.12 A | 101.27 ± 0.12 AB | 13.7 ± 0.89 A | 1.86 ± 0.24 A | 107.85 ± 0.98 A | 114.67 ± 0.82 AB | 119.35 ± 0.93 A | 11.49 ± 0.04 A | 1.1 ± 0.49 B |
| MO 2 | 87.48 ± 0.76 A | 95.18 ± 0.34 A | 100.8 ± 0.38 B | 13.32 ± 0.37 A | 1.78 ± 0.06 A | 108.79 ± 1.55 A | 114.23 ± 0.7 AB | 118.8 ± 1.03 A | 10.01 ± 0.51 B | 1.24 ± 0.02 A |
| MO 3 | 88.25 ± 0.11 A | 95.44 ± 0.45 A | 101.79 ± 0.12 A | 13.54 ± 0.00 A | 2.25 ± 0.14 A | 109.95 ± 0.04 A | 115.24 ± 0.22 A | 119.3 ± 0.23 A | 9.31 ± 0.18 B | 0.81 ± 0.02 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.-C.; Lai, L.-S. Impact of Fatty Acid Types and Microwave Post-Treatment on the Physicochemical Properties of Water Caltrop Starch–Lipid Complexes. Foods 2025, 14, 2254. https://doi.org/10.3390/foods14132254
Lee P-C, Lai L-S. Impact of Fatty Acid Types and Microwave Post-Treatment on the Physicochemical Properties of Water Caltrop Starch–Lipid Complexes. Foods. 2025; 14(13):2254. https://doi.org/10.3390/foods14132254
Chicago/Turabian StyleLee, Pei-Chang, and Lih-Shiuh Lai. 2025. "Impact of Fatty Acid Types and Microwave Post-Treatment on the Physicochemical Properties of Water Caltrop Starch–Lipid Complexes" Foods 14, no. 13: 2254. https://doi.org/10.3390/foods14132254
APA StyleLee, P.-C., & Lai, L.-S. (2025). Impact of Fatty Acid Types and Microwave Post-Treatment on the Physicochemical Properties of Water Caltrop Starch–Lipid Complexes. Foods, 14(13), 2254. https://doi.org/10.3390/foods14132254






