Fortification of Cereal-Based Food with Lactobacillus rhamnosus GG and Bacillus coagulans GBI-30 and Their Survival During Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Preparation of Pasta and Noodles
2.3. Determination of Water Absorption and Cooking Loss
2.4. Microbiological Analysis
2.5. Statistical Analysis
3. Results
3.1. Impact of Pasta and Noodle Production on Bacterial Survival
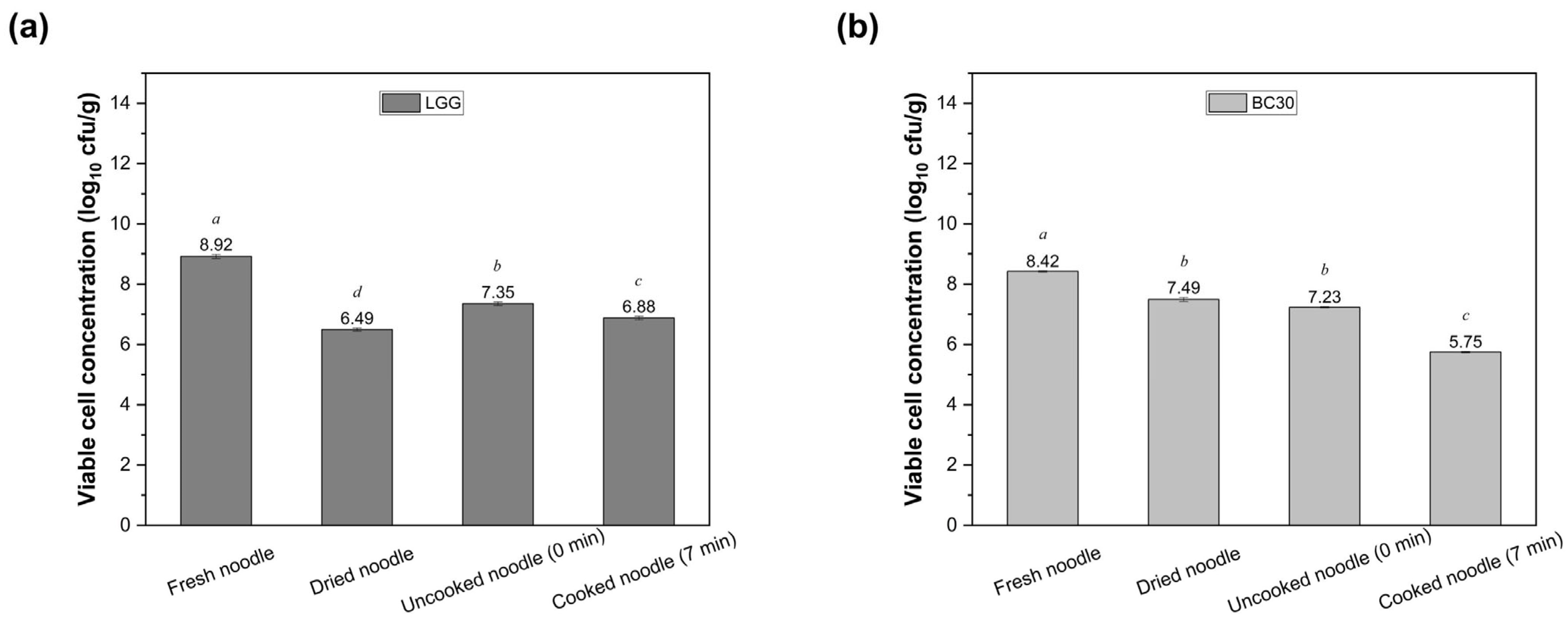
3.2. Survival of LGG and BC30 During Noodle Production and Cooking
3.3. Cooking Behaviours of Noodles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-forming Unit |
| LAB | Lactic Acid Bacteria |
| GRAS | Generally Recognized As Safe |
| LGG | Lactobacillus rhamnosus GG |
| BC30 | Bacillus coagulans GBI-30 |
| w/v | Weight by Volume |
| MRS | de Man Rogosa Sharpe |
| SD | Standard Deviation |
| ANOVA | Analysis of Variance |
References
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Hardy, G. Nutraceuticals and functional foods: Introduction and meaning. Nutrition 2000, 16, 688–689. [Google Scholar] [CrossRef] [PubMed]
- Kwak, N.-S.; Jukes, D.J. Functional foods. Part 1: The development of a regulatory concept. Food Control 2001, 12, 99–107. [Google Scholar] [CrossRef]
- Caplice, E.; Fitzgerald, G.F. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef]
- Shahani, K.M.; Chandan, R.C. Nutritional and healthful aspects of cultured and culture-containing dairy foods. J. Dairy Sci. 1979, 62, 1685–1694. [Google Scholar] [CrossRef]
- Menrad, K. Market and marketing of functional food in Europe. J. Food Eng. 2003, 56, 181–188. [Google Scholar] [CrossRef]
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-dairy probiotic food products: An emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 2626–2641. [Google Scholar] [CrossRef]
- Pinto, D.; Castro, I.; Vicente, A.; Bourbon, A.I.; Cerqueira, M.Â. Functional bakery Products: An overview and future perspectives. In Bakery Products Science and Technology, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 431–452. [Google Scholar]
- Capozzi, V.; Russo, P.; Dueñas, M.T.; López, P.; Spano, G. Lactic acid bacteria producing B-group vitamins: A great potential for functional cereals products. Appl. Microbiol. Biotechnol. 2012, 96, 1383–1394. [Google Scholar] [CrossRef]
- Carvalho-Wells, A.L.; Helmolz, K.; Nodet, C.; Molzer, C.; Leonard, C.; McKevith, B.; Thielecke, F.; Jackson, K.G.; Tuohy, K.M. Determination of the in vivo prebiotic potential of a maize-based whole grain breakfast cereal: A human feeding study. Br. J. Nutr. 2010, 104, 1353–1356. [Google Scholar] [CrossRef]
- Costabile, A.; Klinder, A.; Fava, F.; Napolitano, A.; Fogliano, V.; Leonard, C.; Gibson, G.R.; Tuohy, K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008, 99, 110–120. [Google Scholar] [CrossRef]
- IDF 163:1992; General Standard of Identity for Fermented Milks. International Dairy Federation: Brussels, Belgium, 1992.
- Blaiotta, G.; De Sena, M.; De Girolamo, F.; Aponte, M.; Romano, R. Probiotic bacilli incorporation in foods: Is really so easy? Food Microbiol. 2023, 115, 104342. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Kumar, B.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards. Scientific Opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update). EFSA J. 2013, 11, 3449. [Google Scholar]
- Konuray, G.; Erginkaya, Z. Potential use of Bacillus coagulans in the food industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef]
- Burgess, S.A.; Lindsay, D.; Flint, S.H. Thermophilic bacilli and their importance in dairy processing. Int. J. Food Microbiol. 2010, 144, 215–225. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Majeed, M.; Natarajan, S.; Sivakumar, A.; Ali, F.; Pande, A.; Majeed, S.; Karri, S. Evaluation of anti-diarrhoeal activity of Bacillus coagulans MTCC 5856 and its effect on gastrointestinal motility in Wistar rats. Int. J. Pharma Bio Sci. 2016, 7, 311–316. [Google Scholar]
- Fares, C.; Menga, V.; Martina, A.; Pellegrini, N.; Scazzina, F.; Torriani, S. Nutritional profile and cooking quality of a new functional pasta naturally enriched in phenolic acids, added with β-glucan and Bacillus coagulans GBI-30, 6086. J. Cereal Sci. 2015, 65, 260–266. [Google Scholar] [CrossRef]
- Rajam, R.; Kumar, S.B.; Prabhasankar, P.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum MTCC 5422 in fructooligosaccharide and whey protein wall systems and its impact on noodle quality. J. Food Sci. Technol. 2015, 52, 4029–4041. [Google Scholar] [CrossRef]
- Jia, B.; Devkota, L.; Sissons, M.; Dhital, S. Degradation of starch in pasta induced by extrusion below gelatinization temperature. Food Chem. 2023, 426, 136524. [Google Scholar] [CrossRef]
- Ermis, E. A review of drying methods for improving the quality of probiotic powders and characterization. Dry. Technol. 2021, 40, 2199–2216. [Google Scholar] [CrossRef]
- Setlow, P. Spore germination. Curr. Opin. Microbiol. 2003, 6, 550–556. [Google Scholar] [CrossRef]
- Cho, W.-I.; Chung, M.-S. Bacillus spores: A review of their properties and inactivation processing technologies. Food Sci. Biotechnol. 2020, 29, 1447–1461. [Google Scholar] [CrossRef]
- Luu, S.; Cruz-Mora, J.; Setlow, B.; Feeherry, F.E.; Doona, C.J.; Setlow, P. The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins. Appl. Environ. Microbiol. 2015, 81, 2927–2938. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegria, A.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef]
- Kalkan, S.; Mustafa, O.; Köksal, E.İ.; Bozkurt, N.Ş. Production of functional Turkish noodle (Erişte) supplementary probiotic and determining of some quality properties. Food Health 2020, 6, 140–150. [Google Scholar] [CrossRef]
- Martina, A.; Salvetti, E.; Felis, G.; Torriani, S. Introducing probiotics with the diet: The development of a new functional pasta enriched with Bacillus coagulans. In Proceedings of the International Scientific Conference on Probiotics and Prebiotics—IPC2014, Budapest, Hungary, 24–26 June 2014; p. 126. [Google Scholar]
- Del Nobile, M.A.; Baiano, A.; Conte, A.; Mocci, G. Influence of protein content on spaghetti cooking quality. J. Cereal Sci. 2005, 41, 347–356. [Google Scholar] [CrossRef]
- Cubadda, R.E.; Carcea, M.; Marconi, E.; Trivisonno, M.C. Influence of gluten proteins and drying temperature on the cooking quality of durum wheat pasta. Cereal Chem. 2007, 84, 48–55. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Quality evaluation of probiotic pasta produced with Bacillus coagulans GBI-30. Innov. Food Sci. Emerg. Technol. 2020, 66, 102489. [Google Scholar] [CrossRef]
- Manthey, F.A.; Sinha, S.; Wolf-Hall, C.E.; Hall, C.A., III. Effect of flaxseed flour and packaging on shelf life of refrigerated pasta. J. Food Process. Preserv. 2008, 32, 75–87. [Google Scholar] [CrossRef]
- Fu, B.X. Asian noodles: History, classification, raw materials, and processing. Food Res. Int. 2008, 41, 888–902. [Google Scholar] [CrossRef]
- Petitot, M.; Barron, C.; Morel, M.-H.; Micard, V. Impact of legume flour addition on pasta structure: Consequences on its in vitro starch digestibility. Food Biophys. 2010, 5, 284–299. [Google Scholar] [CrossRef]
- Oh, N.; Seib, P.; Deyoe, C.; Ward, A. Noodles II. The surface firmness of cooked noodles from soft and hard wheat flours. Cereal Chem. 1985, 62, 431–436. [Google Scholar]


| Sample | Water Absorption (%) | Cooking Loss (%) |
|---|---|---|
| Pasta | ||
| Fresh | ||
| Control Pasta | 64.36 ± 2.15 c | 18.79 ± 0.63 a |
| LGG Pasta | 59.38 ± 0.38 d | 10.60 ± 0.09 e |
| BC30 Pasta | 47.31 ± 0.24 e | 17.24 ± 0.11 b |
| Dried | ||
| Control Pasta | 91.15 ± 7.04 a | 19.05 ± 0.20 a |
| LGG Pasta | 83.80 ± 0.35 b | 13.99 ± 0.32 d |
| BC30 Pasta | 60.07 ± 0.38 d | 15.84 ± 0.59 c |
| Noodle | ||
| Fresh | ||
| Control Noodle | 65.94 ± 0.28 b | 4.04 ± 0.49 a |
| LGG Noodle | 55.82 ± 2.27 c,d | 2.98 ± 0.16 d |
| BC30 Noodle | 52.05 ± 2.81 d | 3.67 ± 0.26 a,b |
| Dried | ||
| Control Noodle | 69.44 ± 0.32 a | 3.45 ± 0.07 b |
| LGG Noodle | 60.97 ± 4.60 b,c | 2.40 ± 0.24 e |
| BC30 Noodle | 56.50 ± 2.11 c,d | 3.03 ± 0.13 c,d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wu, P.; Chen, X.D.; Yu, A.; Dhital, S. Fortification of Cereal-Based Food with Lactobacillus rhamnosus GG and Bacillus coagulans GBI-30 and Their Survival During Processing. Foods 2025, 14, 2250. https://doi.org/10.3390/foods14132250
Wang J, Wu P, Chen XD, Yu A, Dhital S. Fortification of Cereal-Based Food with Lactobacillus rhamnosus GG and Bacillus coagulans GBI-30 and Their Survival During Processing. Foods. 2025; 14(13):2250. https://doi.org/10.3390/foods14132250
Chicago/Turabian StyleWang, Junyan, Peng Wu, Xiao Dong Chen, Aibing Yu, and Sushil Dhital. 2025. "Fortification of Cereal-Based Food with Lactobacillus rhamnosus GG and Bacillus coagulans GBI-30 and Their Survival During Processing" Foods 14, no. 13: 2250. https://doi.org/10.3390/foods14132250
APA StyleWang, J., Wu, P., Chen, X. D., Yu, A., & Dhital, S. (2025). Fortification of Cereal-Based Food with Lactobacillus rhamnosus GG and Bacillus coagulans GBI-30 and Their Survival During Processing. Foods, 14(13), 2250. https://doi.org/10.3390/foods14132250







