The Effects of UV-LED Technology on the Quality of Ready-to-Eat Pomegranates: Epigenetic Indicators and Metabolomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Instruments
2.2. Sample Preparation
2.3. Measurement of Physical and Chemical Indicators
2.3.1. Determination of Polyphenol Content
2.3.2. Determination of Total Antioxidant Capacity (T-AOC)
2.3.3. Determination of Anthocyanin Content
2.3.4. Determination of Ascorbic Acid Content
2.3.5. Determination of Microbiological Indicators
2.4. Metabolomic Analysis
2.4.1. Liquid Chromatography Conditions
2.4.2. Mass Spectrum Analysis
2.4.3. Data Preprocessing
2.4.4. Pathway Analysis
2.5. Statistical Analysis
3. Results
3.1. Identification and Analysis of Epigenetic Indicators
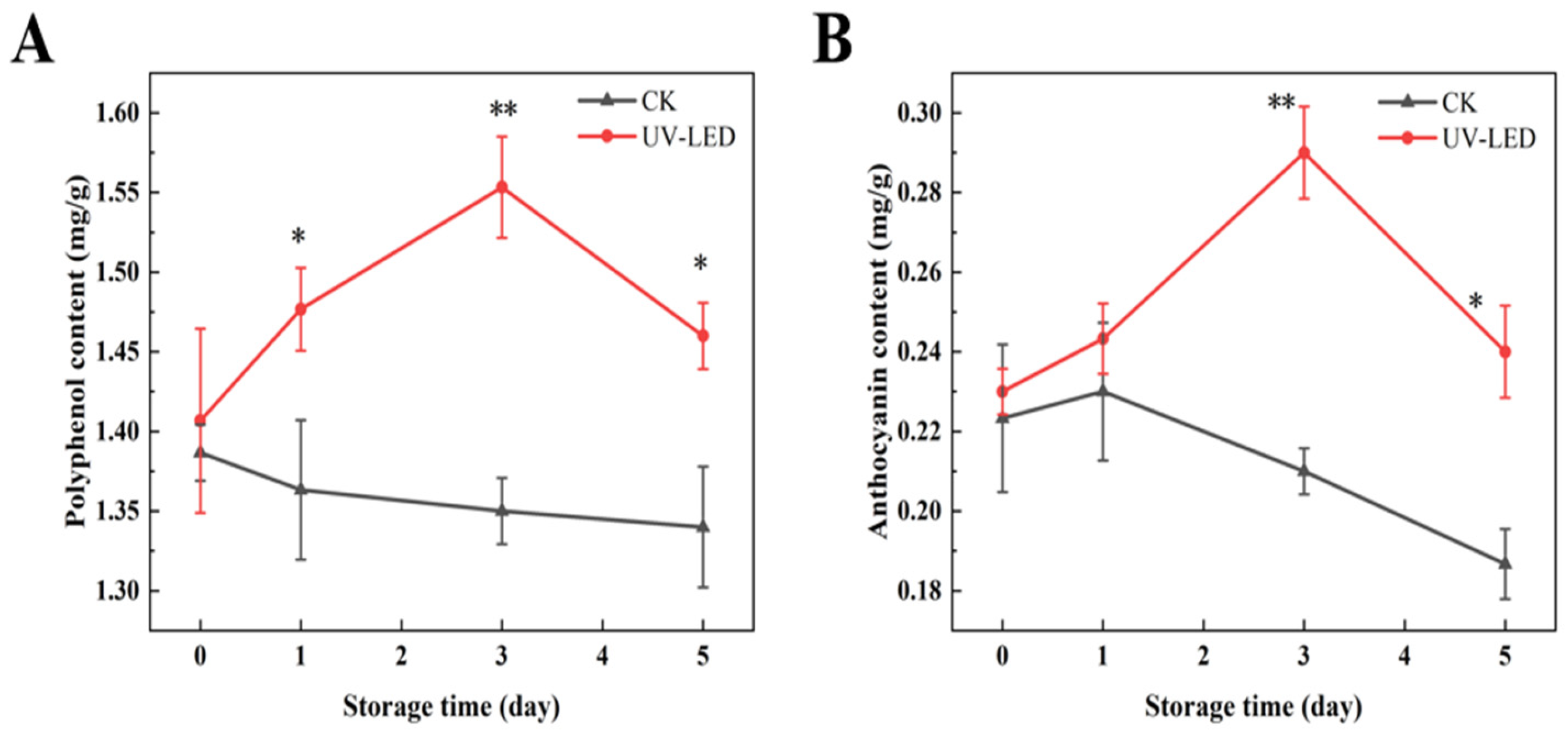
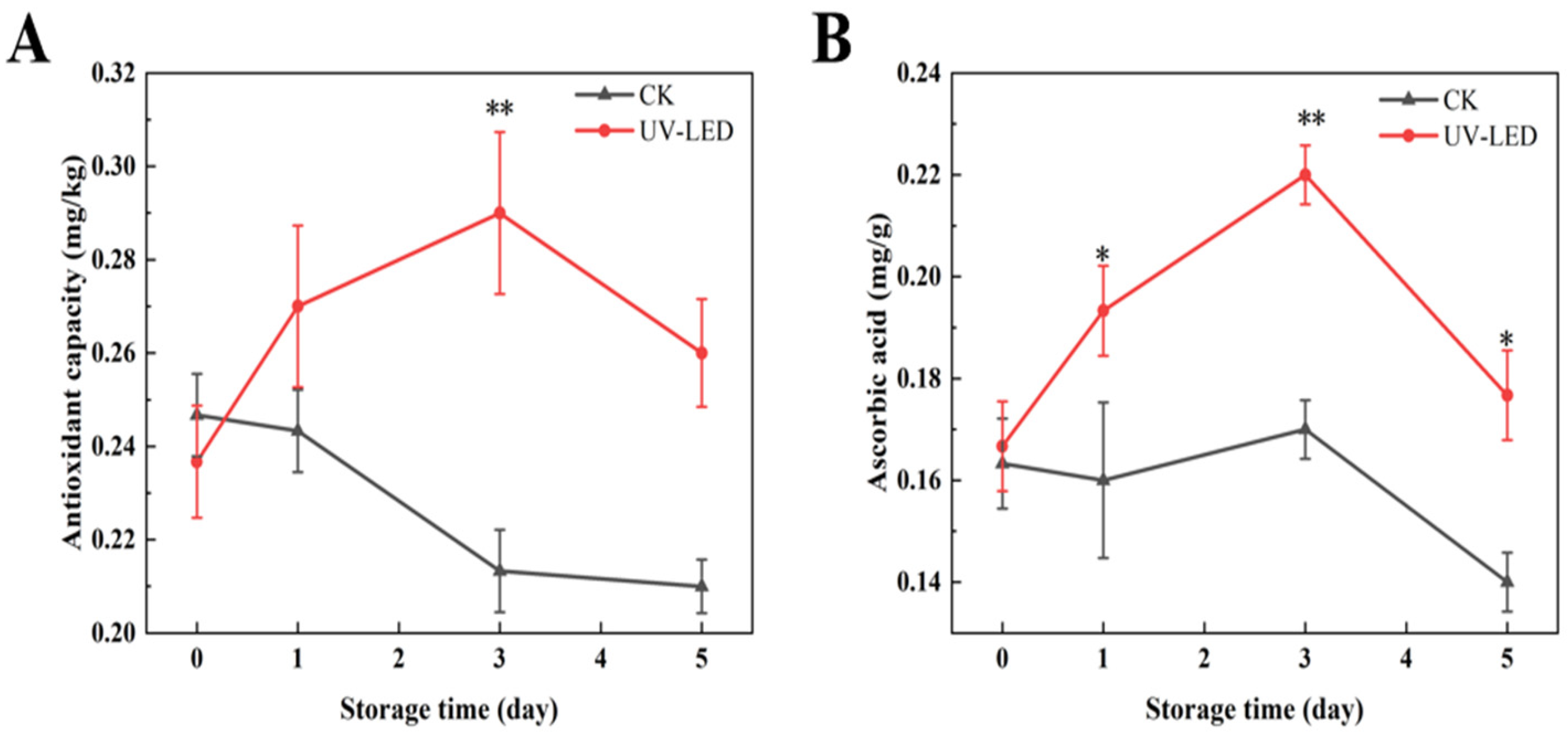
3.1.1. The Impact of UV-LED Technology on Nutrient Composition of Ready-to-Eat Pomegranate
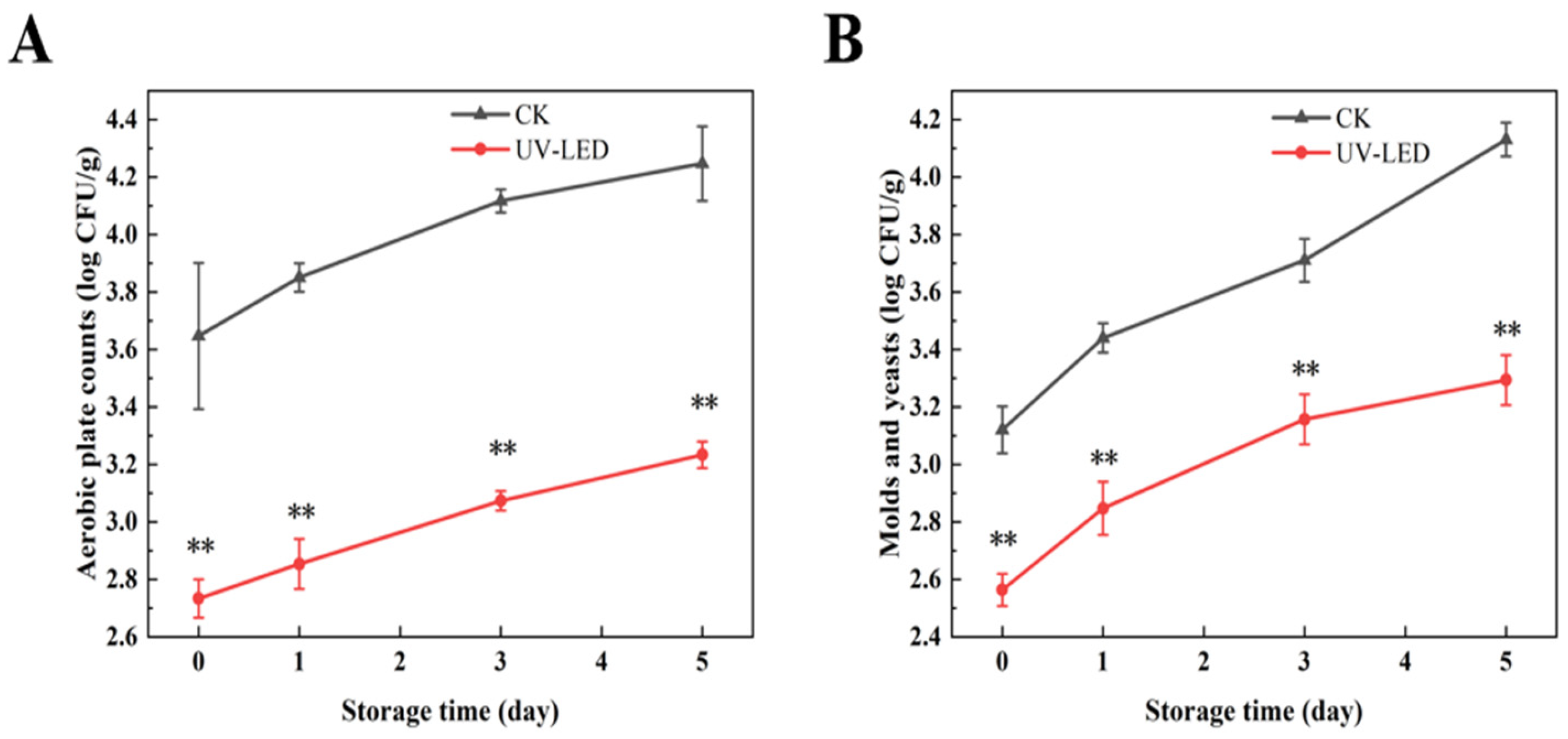
3.1.2. The Impact of UV-LED Technology on Microbial Indicators of Ready-to-Eat Pomegranate
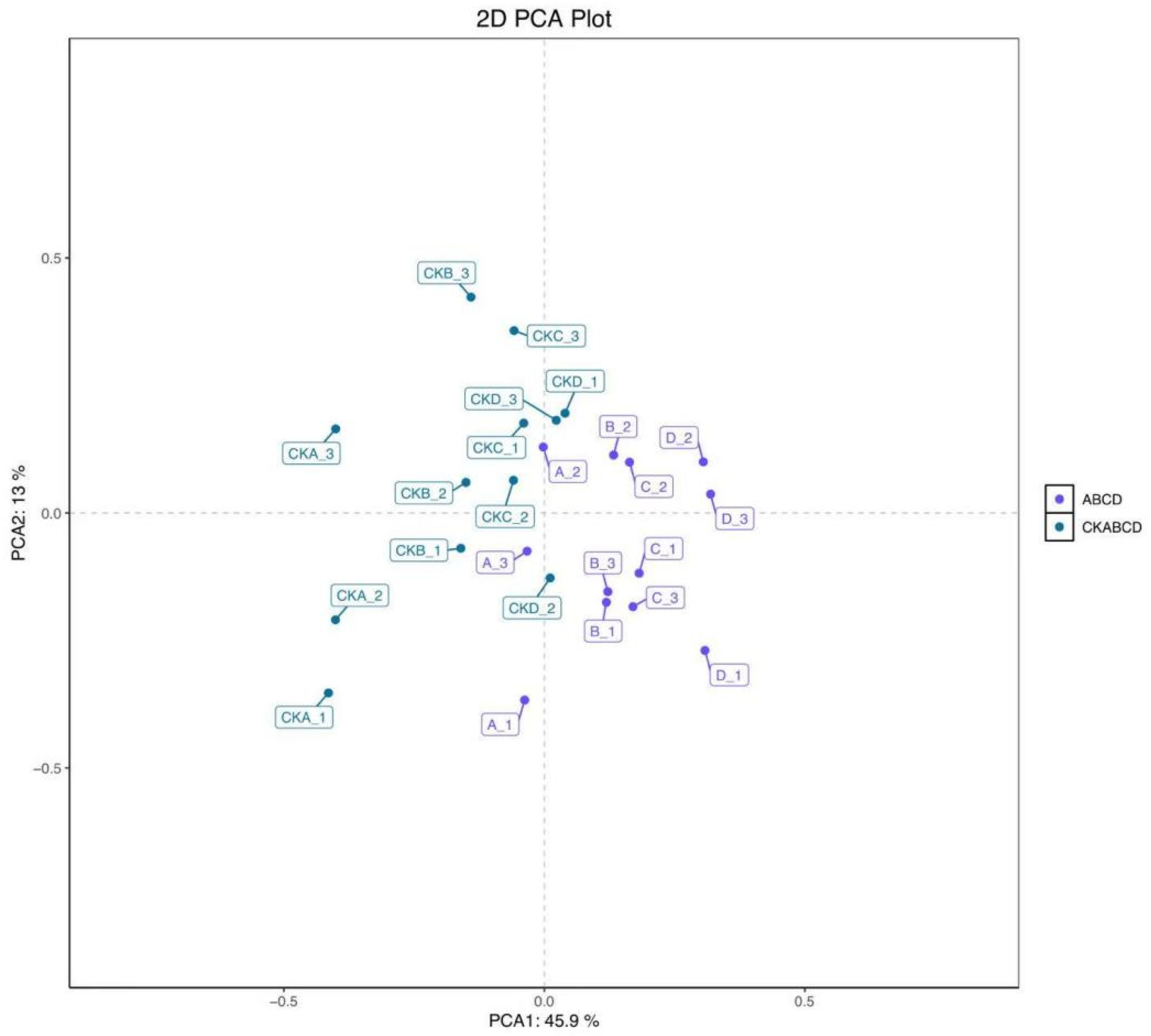
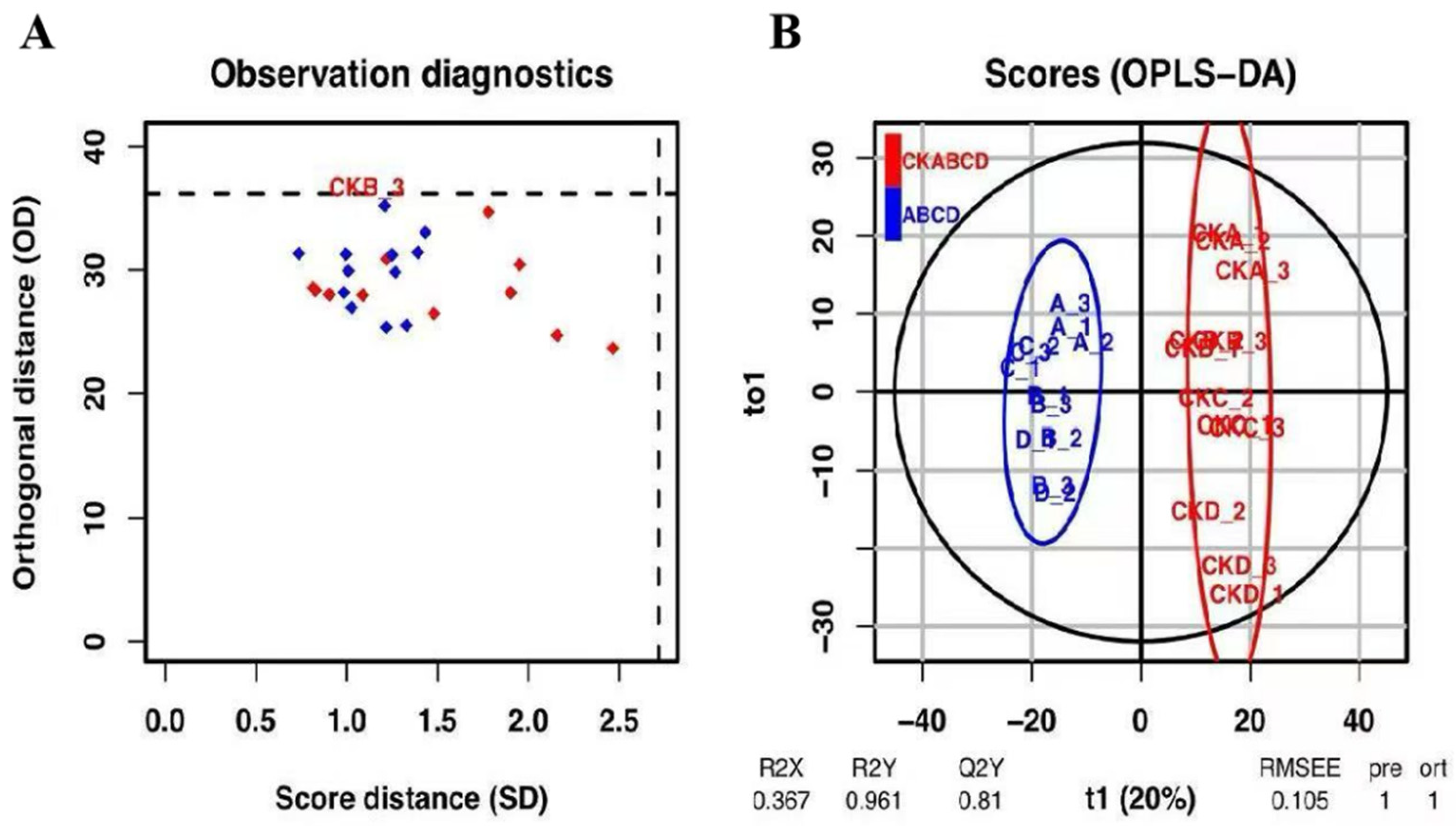
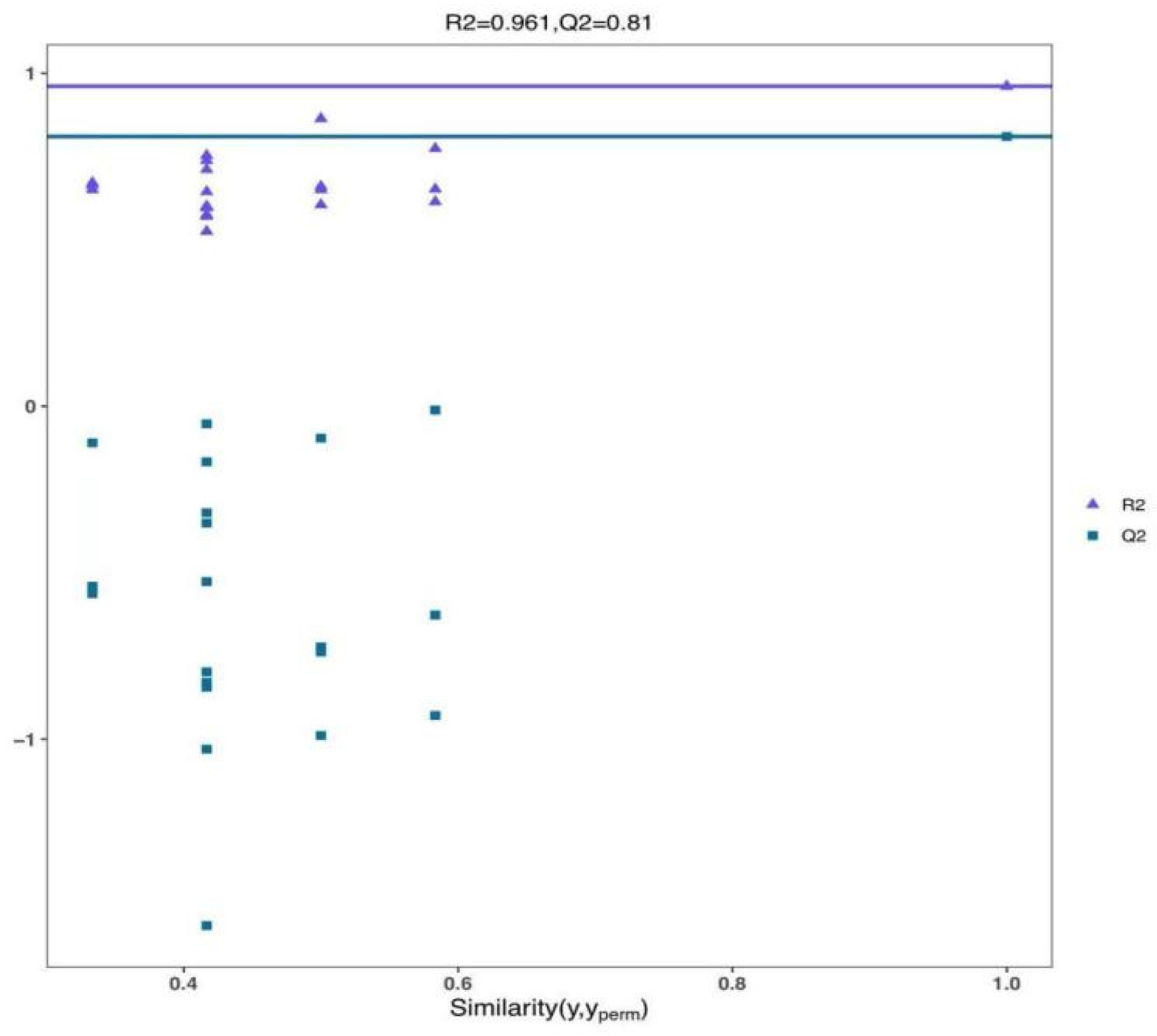
3.2. PCA and OPLS-DA Analysis
3.3. Enrichment Analysis of KEGG Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Niu, Z.; Wang, X.; Lu, X.; Sun, J.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J.; Xiao, J.; Liu, C.; et al. The Nutritional and Bioactive Components, Potential Health Function and Comprehensive Utilization of Pomegranate: A Review. Food Rev. Int. 2023, 39, 6420–6446. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Gendi, H.; El-Fakharany, E.M.; El-Ziney, M.G.; El-Yazbi, A.F.; Al Meqbaali, F.T.; Ibrahim, W.H. Antioxidant, Anti-Inflammatory, Antimicrobial, and Anticancer Activities of Pomegranate Juice Concentrate. Nutrients 2023, 15, 2709. [Google Scholar] [CrossRef] [PubMed]
- Kandylis, P.; Kokkinomagoulos, E. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Isas, A.S.; Balcells, M.F.; Galdeano, C.M.; Palomo, I.; Rodriguez, L.; Fuentes, E.; Pizarro, P.L.; Briz, R.M.; Mozzi, F.; Van Nieuwenhove, C. Fermented pomegranate juice enriched with pomegranate seed oil ameliorates metabolic disorders associated with a high-fat diet in C57BL/6 mice. Food Chem. 2025, 436, 141434. [Google Scholar] [CrossRef]
- Curutchet, A.; Dellacassa, E.; Ringuelet, J.A.; Chaves, A.R.; Vina, S.Z. Nutritional and sensory quality during refrigerated storage of fresh-cut mints (Mentha × piperita and M. spicata). Food Chem. 2014, 143, 231–238. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Yang, Y.; Mulati, A.; Hong, J.; Wang, J. The Effects of Cold-Plasma Technology on the Quality Properties of Fresh-Cut Produce: A Review. Foods 2025, 14, 149. [Google Scholar] [CrossRef]
- Wang, J.; Ma, S.; Zhou, N.; Yang, X.; Xing, J.; Hong, J. Using ultrasonic washing combined with UV-LEDs as a novel chemical-free method to disinfect fresh ready-to-eat produce. Ultrason. Sonochem. 2024, 107, 106926. [Google Scholar] [CrossRef]
- Abadias, M.; Alegre, I.; Oliveira, M.; Altisent, R.; Vinas, I. Growth potential of Escherichia coli O157:H7 on fresh-cut fruits (melon and pineapple) and vegetables (carrot and escarole) stored under different conditions. Food Control 2012, 27, 37–44. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L.J.; Rupasinghe, H.P.V. Effect of thermal and non-thermal pasteurisation on the microbial inactivation and phenolic degradation in fruit juice: A mini-review. J. Sci. Food Agric. 2013, 93, 981–986. [Google Scholar] [CrossRef]
- Xiong, T.; Rahman, F.U.; Wang, X.; Gong, H.; Zheng, X.; Zhu, X.; Liu, X. The effect of ultraviolet-C on the senescence of bitter gourd fruit and the key factors analyzed by transcriptomic and metabolomic analyses. Food Chem. 2025, 465, 142015. [Google Scholar] [CrossRef]
- Adhikari, A.; Syamaladevi, R.M.; Killinger, K.; Sablani, S.S. Ultraviolet-C light inactivation of Escherichia coli O157:H7 and Listeria monocytogenes on organic fruit surfaces. Int. J. Food Microbiol. 2015, 210, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Syamaladevi, R.M.; Lu, X.; Sablani, S.S.; Insan, S.K.; Adhikari, A.; Killinger, K.; Rasco, B.; Dhingra, A.; Bandyopadhyay, A.; Annapure, U. Inactivation of Escherichia coli Population on Fruit Surfaces Using Ultraviolet-C Light: Influence of Fruit Surface Characteristics. Food Bioprocess Technol. 2013, 6, 2959–2973. [Google Scholar] [CrossRef]
- Manzocco, L.; Da Pieve, S.; Bertolini, A.; Bartolomeoli, I.; Maifreni, M.; Vianello, A.; Nicoli, M.C. Surface decontamination of fresh-cut apple by UV-C light exposure: Effects on structure, colour and sensory properties. Postharvest Biol. Technol. 2011, 61, 165–171. [Google Scholar] [CrossRef]
- Benito Martinez-Hernandez, G.; Huertas, J.-P.; Navarro-Rico, J.; Gomez, P.A.; Artes, F.; Palop, A.; Artes-Hernandez, F. Inactivation kinetics of foodborne pathogens by UV-C radiation and its subsequent growth in fresh-cut kailan-hybrid broccoli. Food Microbiol. 2015, 46, 263–271. [Google Scholar] [CrossRef]
- Soro, A.B.; Shokri, S.; Nicolau-Lapena, I.; Ekhlas, D.; Burgess, C.M.; Whyte, P.; Bolton, D.J.; Bourke, P.; Tiwari, B.K. Current challenges in the application of the UV-LED technology for food decontamination. Trends Food Sci. Technol. 2023, 131, 264–276. [Google Scholar] [CrossRef]
- Haley, O.C.; Pliakoni, E.D.; Rivard, C.; Nwadike, L.; Bhullar, M. The Attenuation of Microbial Reduction in Blueberry Fruit Following UV-LED Treatment. J. Food Protect. 2023, 86, 100056. [Google Scholar] [CrossRef]
- Guersu, H. Reusable Smart Lids for Improving Food Safety at Household Level with Programmable UV-C Technology. Sustainability 2024, 16, 5370. [Google Scholar] [CrossRef]
- Sato, M.; Ramarathnam, N.; Suzuki, Y.; Ohkubo, T.; Takeuchi, M.; Ochi, H. Varietal Differences in the Phenolic Content and Superoxide Radical Scavenging Potential of Wines from Different Sources. J. Agric. Food Chem. 1996, 44, 37–41. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Yin, M.; Gao, S.; Xu, M.; Du, G. PyWRKY40 negatively regulates anthocyanin synthesis in pear fruit. Plant Sci. 2025, 351, 112323. [Google Scholar] [CrossRef]
- Warren, C.R.; O’Sullivan, J.F.; Friesen, M.; Becker, C.E.; Zhang, X.; Liu, P.; Wakabayashi, Y.; Morningstar, J.E.; Shi, X.; Choi, J.; et al. Induced Pluripotent Stem Cell Differentiation Enables Functional Validation of GWAS Variants in Metabolic Disease. Cell Stem Cell 2017, 20, 547–557. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a Robust and Repeatable UPLC-MS Method for the Long-Term Metabolomic Study of Human Serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.A.; Villumsen, K.R.; Ernst, M.; Hansen, M.; Forberg, T.; Gopalakrishnan, S.; Gilbert, M.T.P.; Bojesen, A.M.; Kristiansen, K.; Limborg, M.T. A multi-omics approach unravels metagenomic and metabolic alterations of a probiotic and synbiotic additive in rainbow trout (Oncrhynchus mykiss). Microbiome 2022, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Reig, M.; Jaumot, J.; Garcia-Reiriz, A.; Tauler, R. Evaluation of changes induced in rice metabolome by Cd and Cu exposure using LC-MS with XCMS and MCR-ALS data analysis strategies. Anal. Bioanal. Chem. 2015, 407, 8835–8847. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomic Data Exploration and Analysis with the Human Metabolome Database. In Computational Methods and Data Analysis for Metabolomics; Li, S., Ed.; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 2104, pp. 165–184. [Google Scholar]
- Horai, H.; Arita, M.; Kanaya, S.; Niei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007, 35, D527–D532. [Google Scholar] [CrossRef]
- Abdelrazig, S.; Safo, L.; Rance, G.A.; Fay, M.W.; Theodosiou, E.; Topham, P.D.; Kim, D.-H.; Fernandez-Castane, A. Metabolic characterisation of Magnetospirillum gryphiswaldense MSR-1 using LC-MS-based metabolite profiling. Rsc. Adv. 2020, 10, 32548–32560. [Google Scholar] [CrossRef]
- Thevenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome. Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Xi, W.; Fang, B.; Zhao, Q.; Jiao, B.; Zhou, Z. Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chem. 2014, 161, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Zhang, Y.; Sun, R.; Xia, M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E975–E988. [Google Scholar] [CrossRef] [PubMed]
- Farias, T.R.B.; Rodrigues, S.; Fernandes, F.A.N. Effect of dielectric barrier discharge plasma excitation frequency on the enzymatic activity, antioxidant capacity and phenolic content of apple cubes and apple juice. Food Res. Int. 2020, 136, 109617. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, S.; Staniszewska, I.; Liu, Z.-L.; Zielinska, D.; Pan, Z.; Xiao, H.-W.; Zielinska, M. Effect of cold atmospheric pressure plasma pretreatment on the drying kinetics, physicochemical properties and selected bioactive compounds of okra pods subjected to hot air impingement drying. Dry. Technol. 2023, 41, 2405–2417. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The Effects of Cold Plasma-Activated Water Treatment on the Microbial Growth and Antioxidant Properties of Fresh-Cut Pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Liu, H.-N.; Pei, M.-S.; Wei, T.-L.; Yu, Y.-H.; Guo, D.-L. Molecular cloning and expression analysis of hydrogen peroxide sensors under H2O2 and ROS inhibitor treatment in ‘Kyoho’ grape berry. Postharvest Biol. Technol. 2021, 180, 111617. [Google Scholar] [CrossRef]
- Cheng, G.; Duan, X.; Shi, J.; Lu, W.; Luo, Y.; Jiang, W.; Jiang, Y. Effects of reactive oxygen species on cellular wall disassembly of banana fruit during ripening. Food Chem. 2008, 109, 319–324. [Google Scholar] [CrossRef]
- Ozaki, K.; Uchida, A.; Takabe, T.; Shinagawa, F.; Tanaka, Y.; Takabe, T.; Hayashi, T.; Hattori, T.; Rai, A.K.; Takabe, T. Enrichment of sugar content in melon fruits by hydrogen peroxide treatment. J. Plant Physiol. 2009, 166, 569–578. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Chen, X.; Chen, Z.; Zhang, Z.; Li, T.; Qu, H.; Jiang, Y. Effects of hydrogen water treatment on antioxidant system of litchi fruit during the pericarp browning. Food Chem. 2021, 336, 127618. [Google Scholar] [CrossRef]
- Arabia, A.; Munne-Bosch, S.; Munoz, P. Ascorbic acid as a master redox regulator of fruit ripening. Postharvest Biol. Technol. 2024, 207, 112614. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Onyeaka, H.; Miri, T.; Obileke, K.; Anumudu, C.; Hart, A. A Cold Plasma Technology for Ensuring the Microbiological Safety and Quality of Foods. Food Eng. Rev. 2022, 14, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, C.; Jiang, A.; Sun, X.; Guan, Q.; Hu, W. Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innov. Food Sci. Emerg. 2020, 59, 102256. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Ferracane, A.; Manousi, N.; Zachariadis, G.; Tranchida, P.Q.; Mondello, L.; Samanidou, V.F.; Rosenberg, E.J.T. A volatilomics analytical protocol employing solid phase microextraction coupled to GC×GC-MS analysis and combined with multivariate chemometrics for the detection of pomegranate juice adulteration. Talanta 2024, 266, 9. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.-Z.; Xie, D.-Y. Biosynthesis and metabolic engineering of anthocyanins in Arabidopsis thaliana. Recent Pat. Biotechnol. 2014, 8, 47–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Gao, Y.; Xian, X.; Zhang, D.; Zhao, W.; Wang, X.; Wang, Y. Orchestrating anthocyanin biosynthesis in fruit of fruit trees: Transcriptional, post-transcriptional, and post-translational regulation. Int. J. Biol. Macromol. 2025, 307. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, Y.; Chen, Y.; Wang, X.; Phyon, J.M.; Zhou, S.; Cao, Z.; Wang, Y.; Yang, J. Light regulates SlCOP1-mediated degradation of SlJAF13, a transcription factor essential for anthocyanin biosynthesis in Aft tomato fruit. Plant Physiol. Bioch. 2025, 221, 109572. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Symposium 12: Interaction of natural colors with other ingredients—Anthocyanin pigments—Bioactivity and coloring properties. J. Food Sci. 2004, 69, C419–C421. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, W.; Hu, Z.; Yu, X.; Tu, Y.; Zhang, M.; Huang, J.; Chen, G. Metabolic and molecular analysis of nonuniform anthocyanin pigmentation in tomato fruit under high light. Hortic. Res.-Engl. 2019, 6, 56. [Google Scholar] [CrossRef]
- Yuan, Y.-W.; Rebocho, A.B.; Sagawa, J.M.; Stanley, L.E.; Bradshaw, H.D., Jr. Competition between anthocyanin and flavonol biosynthesis produces spatial pattern variation of floral pigments between Mimulus species. Proc. Natl. Acad. Sci. USA 2016, 113, 2448–2453. [Google Scholar] [CrossRef] [PubMed]
- Brugliera, F.; Tao, G.-Q.; Tems, U.; Kalc, G.; Mouradova, E.; Price, K.; Stevenson, K.; Nakamura, N.; Stacey, I.; Katsumoto, Y.; et al. Violet/Blue Chrysanthemums-Metabolic Engineering of the Anthocyanin Biosynthetic Pathway Results in Novel Petal Colors. Plant Cell Physiol. 2013, 54, 1696–1710. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Bou-Mitri, C.; Dargham, M.B.; Abou Jaoudeh, M.; Farhat, A.; El Hayek, J.; Mosleh, J.M.B.; Bou-Maroun, E. Phytochemical composition, biological activities and antioxidant potential of pomegranate fruit, juice and molasses: A review. Food Biosci. 2023, 55, 103034. [Google Scholar] [CrossRef]
- Huang, W.; Hutabarat, R.P.; Chai, Z.; Zheng, T.; Zhang, W.; Li, D. Antioxidant Blueberry Anthocyanins Induce Vasodilation via PI3K/Akt Signaling Pathway in High-Glucose-Induced Human Umbilical Vein Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 1575. [Google Scholar] [CrossRef]
- Huang, J.; Wu, J.; Liao, X.; Lao, F. Recent Progress in the Study on the Interaction between Anthocyanins and Vitamin C in Fruit and Vegetable Beverages. Food Sci. 2022, 43, 358–371. [Google Scholar]
- Roewer, N.; Broscheit, J. Delphinidin for Combating Melanoma Cells. U.S. Patent 09511047, 6 December 2016. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.; Wang, Y.; Rao, L.; Zhao, L. Health effects of fruit juices and beverages with varying degrees of processing. Food Sci. Hum. Wellness 2024, 13, 2456–2479. [Google Scholar] [CrossRef]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, Z.; Hu, J.; Li, Y.; Sun, J.; Bai, W. Optimized Synthesis and Antioxidant Activity of Anthocyanins Delphinidin-3-O-glucoside and Petunidin-3-O-glucoside. J. Agric. Food Chem. 2024, 72, 15005–15012. [Google Scholar] [CrossRef]
- Du, L.; Ding, X.; Tian, Y.; Chen, J.; Li, W. Effect of anthocyanins on metabolic syndrome through interacting with gut microbiota. Pharmacol. Res. 2024, 210, 107511. [Google Scholar] [CrossRef]
- Yuan, L.; Cheng, F.; Yi, J.; Cai, S.; Liao, X.; Lao, F.; Zhou, L. Effect of high-pressure processing and thermal treatments on color and in vitro bioaccessibility of anthocyanin and antioxidants in cloudy pomegranate juice. Food Chem. 2022, 373, 131397. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Omara, T.; Kahwa, I.; Khan, U.M. Anticancer potential of delphinidin and its derivatives: Therapeutic and mechanistic insights. Med. Chem. Res. 2024, 33, 1769–1786. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Li, Q.; West, L.; West, M.; de Mejia, E.G. Anthocyanin condensed forms do not affect color or chemical stability of purple corn pericarp extracts stored under different pHs. Food Chem. 2017, 232, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.R.T.; Cairns, J.R.K.; Safitri, A.; Fatchiyah, F.J.A.I.M. Virtual Prediction of the Delphinidin-3-O-glucoside and Peonidin-3-O-glucoside as Anti-inflammatory of TNF-α Signaling. Acta Inform. Med. 2019, 27, 152–157. [Google Scholar] [CrossRef]

| Metabolite | Formula | mz | Content Change |
|---|---|---|---|
| Chrysanthemin | C21H22O11 | 449.11 | 1.24 |
| Delphinidin-3,5-O-diglucoside | C27H31O17 | 627.16 | 2.64 |
| Cyanidin-3,5-O-diglucoside | C27H31O16 | 611.16 | 1.11 |
| Leucodelphinidin 3-glucoside | C21H24O13 | 489.10 | 1.48 |
| Peonidin-3-O-rutinoside chloride | C28H33O15 | 610.18 | 46.46 |
| Delphinidin-3-caffeoylglucoside | C30H27O15 | 645.17 | 1.53 |
| Peonidine-3-O-glucoside chloride | C22H23O11 | 463.12 | 1.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aihaiti, A.; Li, Y.; Huang, X.; Yang, Y.; Mulati, A.; Wang, J. The Effects of UV-LED Technology on the Quality of Ready-to-Eat Pomegranates: Epigenetic Indicators and Metabolomic Analysis. Foods 2025, 14, 2192. https://doi.org/10.3390/foods14132192
Aihaiti A, Li Y, Huang X, Yang Y, Mulati A, Wang J. The Effects of UV-LED Technology on the Quality of Ready-to-Eat Pomegranates: Epigenetic Indicators and Metabolomic Analysis. Foods. 2025; 14(13):2192. https://doi.org/10.3390/foods14132192
Chicago/Turabian StyleAihaiti, Aihemaitijiang, Yuanpeng Li, Xinmeng Huang, Yuting Yang, Ailikemu Mulati, and Jiayi Wang. 2025. "The Effects of UV-LED Technology on the Quality of Ready-to-Eat Pomegranates: Epigenetic Indicators and Metabolomic Analysis" Foods 14, no. 13: 2192. https://doi.org/10.3390/foods14132192
APA StyleAihaiti, A., Li, Y., Huang, X., Yang, Y., Mulati, A., & Wang, J. (2025). The Effects of UV-LED Technology on the Quality of Ready-to-Eat Pomegranates: Epigenetic Indicators and Metabolomic Analysis. Foods, 14(13), 2192. https://doi.org/10.3390/foods14132192





