Synergistic Effects of Pressure, Temperature, CO2 Flow Rate and Co-Solvent on Bioactive Contents of Thai Fingerroot (Boesenbergia rotunda (L.) Mansf.) Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Maceration Extraction
2.3. Supercritical CO2 Fluid Extraction
2.4. Experimental Design
2.5. Total Yield Analysis
2.6. Total Phenolic Content (TPC) Analysis
2.7. Total Flavonoid Content (TFC) Analysis
2.8. Extract Composition Analysis
3. Results and Discussion
3.1. Extraction Yield, Phenolic and Flavonoid Contents from Maceration Extraction
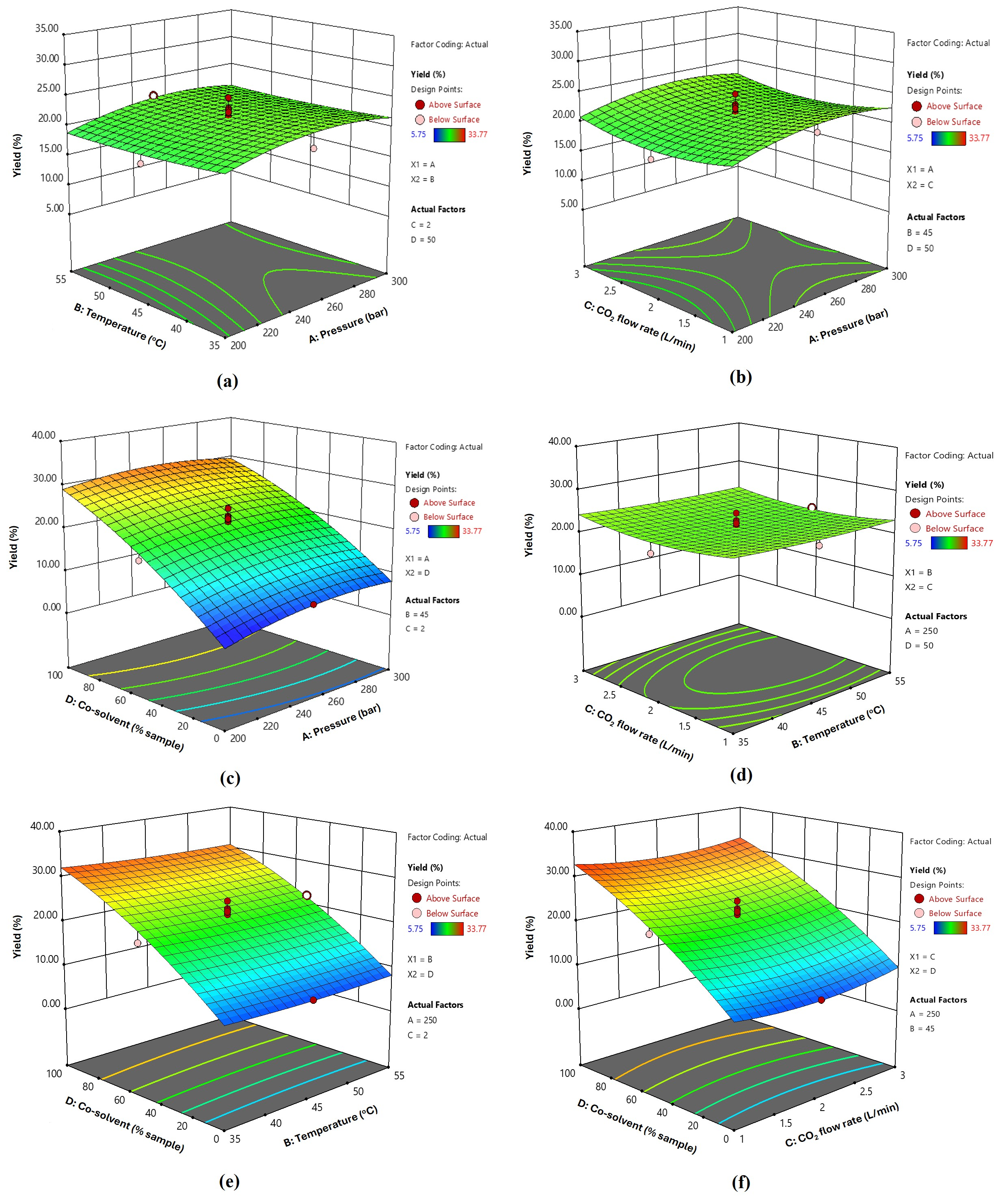
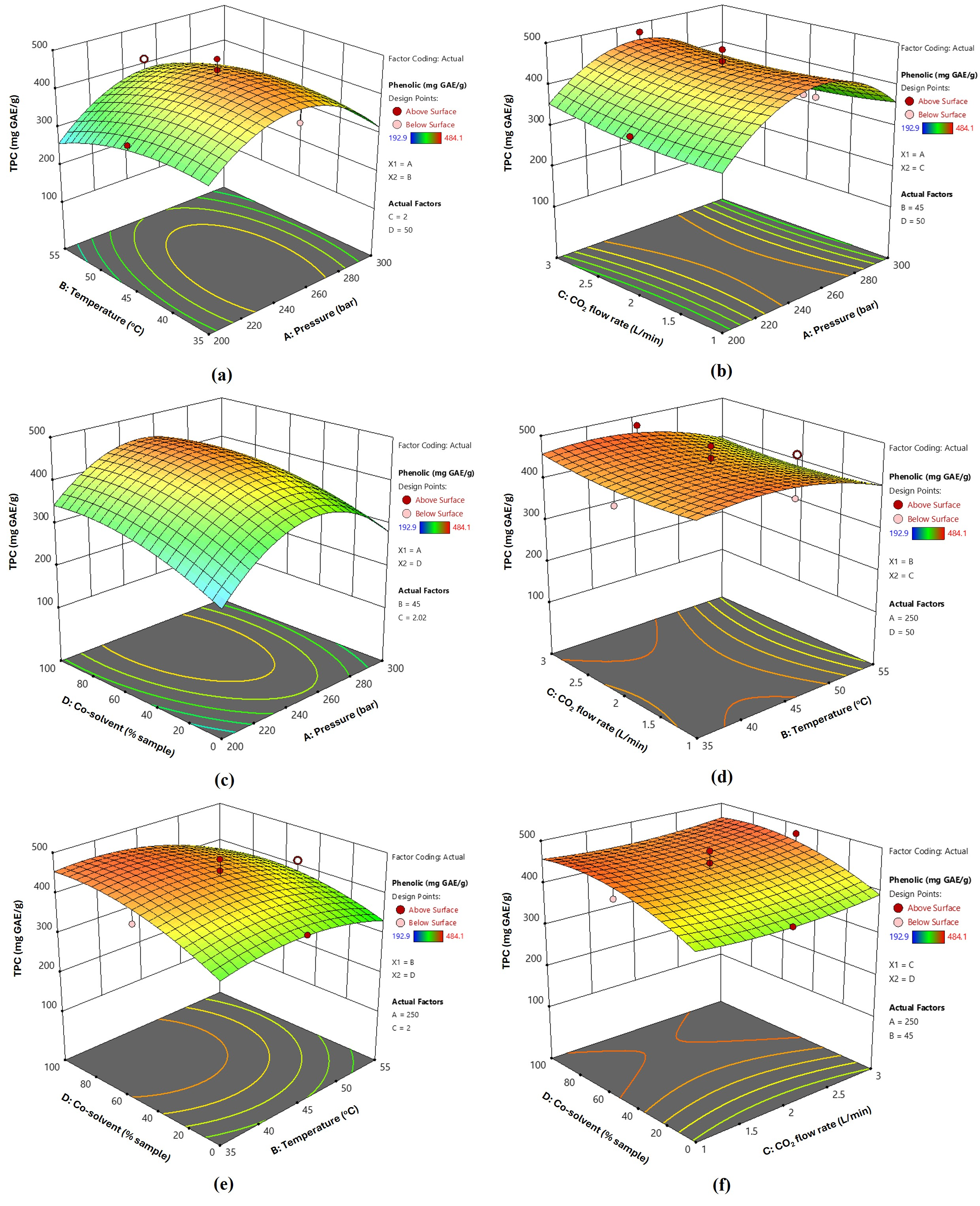
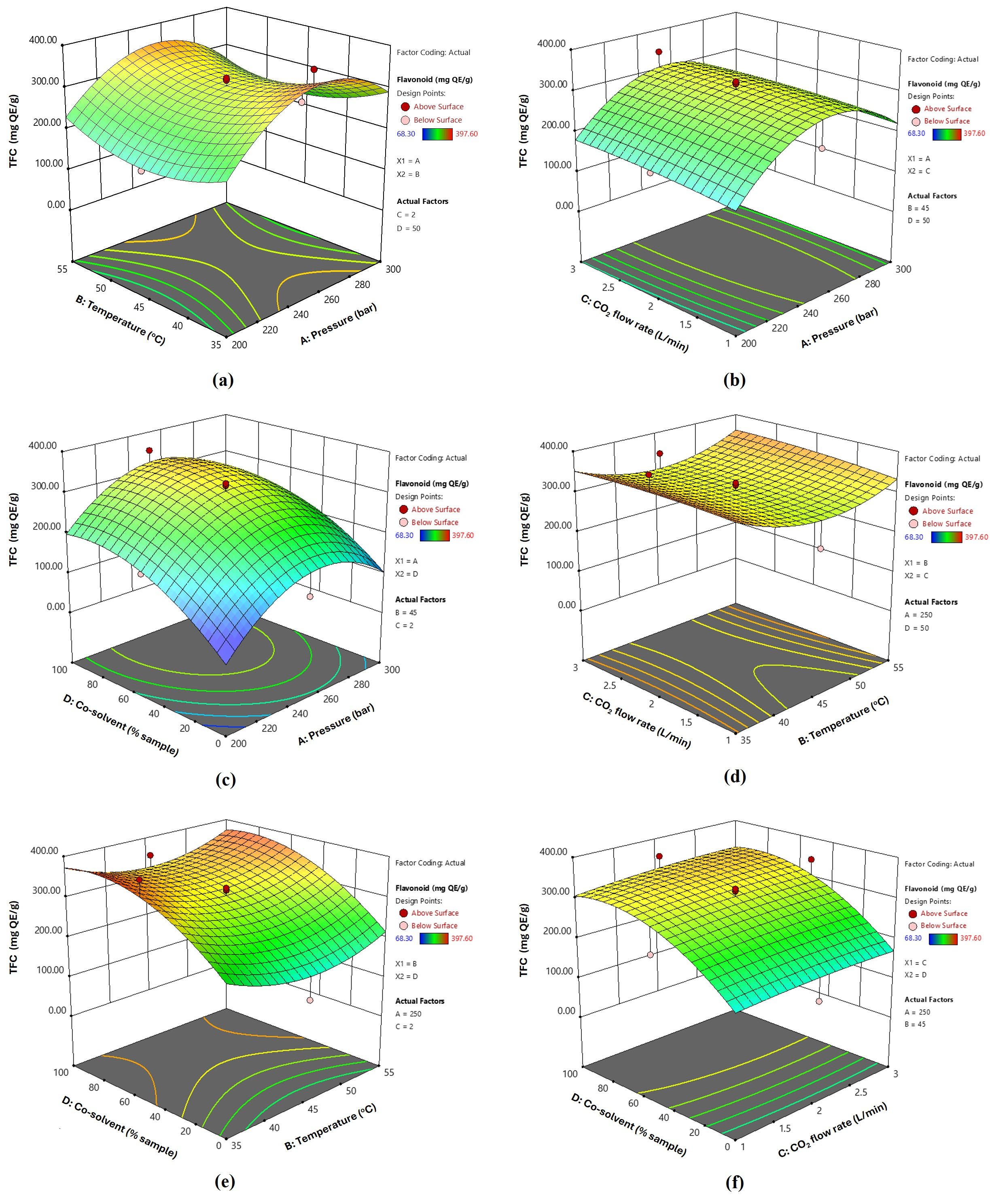
3.2. Extraction Yield and Bioactive Contents of Fingerroot Extracts from SFE
3.3. Optimization and Validation of SFE Conditions for Fingerroot Extract
3.4. Comparison of Conventional Extraction and Supercritical Fluid Extraction (SFE)
3.5. Main Compounds in Boesenbergia Rotunda Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alolga, R.N.; Wang, F.; Zhang, X.; Li, J.; Tran, L.-S.P.; Yin, X. Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses. Antioxidants 2022, 11, 1281. [Google Scholar] [CrossRef]
- Ng, T.L.M.; Karim, R.; Tan, Y.S.; Teh, H.F.; Danial, A.D.; Ho, L.S.; Khalid, N.; Appleton, D.R.; Harikrishna, J.A. Amino Acid and Secondary Metabolite Production in Embryogenic and Non-Embryogenic Callus of Fingerroot Ginger (Boesenbergia rotunda). PLoS ONE 2016, 11, e0156714. [Google Scholar] [CrossRef] [PubMed]
- Ongwisespaiboon, O.; Jiraungkoorskul, W. Fingerroot, Boesenbergia rotunda and Its Aphrodisiac Activity. Pharmacogn. Rev. 2017, 11, 27. [Google Scholar] [CrossRef]
- Kanjanasirirat, P.; Suksatu, A.; Manopwisedjaroen, S.; Munyoo, B.; Tuchinda, P.; Jearawuttanakul, K.; Seemakhan, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Rangkasenee, N.; et al. High-Content Screening of Thai Medicinal Plants Reveals Boesenbergia rotunda Extract and Its Component Panduratin A as Anti-SARS-CoV-2 Agents. Sci. Rep. 2020, 10, 19963. [Google Scholar] [CrossRef]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Phahom, T.; Mano, J. Integration of Multiple Linear Regression, Principal Component Analysis, and Hierarchical Cluster Analysis for Optimizing Dried Fingerroot (Boesenbergia rotunda) Extraction Process. J. Appl. Res. Med. Aromat. Plants 2023, 36, 100511. [Google Scholar] [CrossRef]
- Saah, S.; Siriwan, D.; Trisonthi, P. Biological Activities of Boesenbergia rotunda Parts and Extracting Solvents in Promoting Osteogenic Differentiation of Pre-Osteoblasts. Food Biosci. 2021, 41, 101011. [Google Scholar] [CrossRef]
- Vieitez, I.; Maceiras, L.; Jachmanián, I.; Alborés, S. Antioxidant and Antibacterial Activity of Different Extracts from Herbs Obtained by Maceration or Supercritical Technology. J. Supercrit. Fluids 2018, 133, 58–64. [Google Scholar] [CrossRef]
- Azwanida, N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Atun, S.; Sri, H.; Rakhmawati, A. Potential Bioactive Compounds Isolated from Boesenbergia rotunda as Antioxidant and Antimicrobial Agents. Pharmacogn. J. 2018, 10, 513–518. [Google Scholar] [CrossRef]
- Jirakiattikul, Y.; Rithichai, P.; Prachai, R.; Itharat, A. Elicitation Enhancement of Bioactive Compound Accumulation and Antioxidant Activity in Shoot Cultures of Boesenbergia rotunda L. Agric. Nat. Resour. 2021, 55, 456–463. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Smith, S.A.; Chen, M.; Liu, H.; Zhang, J.; Tang, L.; Li, J.; Liu, Q.; Wu, X. Optimization of Supercritical CO2 Extraction of Moringa Oleifera Seed Oil Using Response Surface Methodological Approach and Its Antioxidant Activity. Front. Nutr. 2022, 8, 829146. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical Fluid Extraction of Seed Oils—A Short Review of Current Trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Marzlan, A.A.; Muhialdin, B.J.; Zainal Abedin, N.H.; Mohammed, N.K.; Abadl, M.M.T.; Mohd Roby, B.H.; Meor Hussin, A.S. Optimized Supercritical CO2 Extraction Conditions on Yield and Quality of Torch Ginger (Etlingera Elatior (Jack) R.M. Smith) Inflorescence Essential Oil. Ind. Crops. Prod. 2020, 154, 112581. [Google Scholar] [CrossRef]
- Herzyk, F.; Piłakowska-Pietras, D.; Korzeniowska, M. Supercritical Extraction Techniques for Obtaining Biologically Active Substances from a Variety of Plant Byproducts. Foods 2024, 13, 1713. [Google Scholar] [CrossRef]
- de Aguiar, A.C.; Vardanega, R.; Viganó, J.; Silva, E.K. Supercritical Carbon Dioxide Technology for Recovering Valuable Phytochemicals from Cannabis Sativa L. and Valorization of Its Biomass for Food Applications. Molecules 2023, 28, 3849. [Google Scholar] [CrossRef]
- Pourmortazavi, S.; Rahimi-Nasrabadi, M.; Hajimirsadeghic, S. Supercritical Fluid Technology in Analytical Chemistry—Review. Curr. Anal. Chem. 2013, 10, 3–28. [Google Scholar] [CrossRef]
- Kuś, P.M.; Okińczyc, P.; Jakovljević, M.; Jokić, S.; Jerković, I. Development of Supercritical CO2 Extraction of Bioactive Phytochemicals from Black Poplar (Populus nigra L.) Buds Followed by GC–MS and UHPLC-DAD-QqTOF-MS. J. Pharm. Biomed. Anal. 2018, 158, 15–27. [Google Scholar] [CrossRef]
- Pao-la-or, P.; Posridee, K.; Buranakon, P.; Singthong, J.; Oonmetta-Aree, J.; Oonsivilai, R.; Oonsivilai, A. Beyond Traditional Methods: Deep-Learning Machines Empower Fingerroot (Boesenbergia rotunda)-Extract Production with Superior Antioxidant Activity. Foods 2024, 13, 2676. [Google Scholar] [CrossRef]
- Eakwaropas, P.; Aye, N.M.M.; Ngawhirunpat, T.; Pamornpathomkul, B. Antioxidant Activity, Antimicrobial Activities, and Effect on the Viability of Fibroblast Cells of Boesenbergia rotunda—Loaded Hydrogel Patches. Key Eng. Mater. 2022, 914, 37–43. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Shin, E.-S.; Sim, E.-J.; Bae, Y.-J. Comparison of Antioxidant and Antimicrobial Activities of Fingerroot (Boesenbergia Pandura) and Ginger (Zingiber Officinale Roscoe). Korean J. Food Nutr. 2020, 33, 105–110. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.; Kwon, D.; Kim, M.-B.; Hwang, J.-K. Standardized Kaempferia parviflora Wall. Ex Baker (Zingiberaceae) Extract Inhibits Fat Accumulation and Muscle Atrophy in Ob/Ob Mice. Evid.-Based Complement. Altern. Med. 2018, 2018, 8161042. [Google Scholar] [CrossRef]
- Fahrudin, F.I.; Intipunya, P.; Phongthai, S.; Wirjantoro, T.I. Screening of Antioxidant Activity, Total Phenolics, and Flavonoid on Selected Northern Thailand Medicinal Rhizomes. In Proceedings of the 8th International Conference of Food, Agriculture and Natural Resource & the Second International Conference of Sustainable Industrial Agriculture (IC-FANRES-IC-SIA 2023); Atlantis Press: Dordrecht, The Netherlands, 2024; pp. 237–246. [Google Scholar]
- Kanchanapiboon, J.; Kongsa, U.; Pattamadilok, D.; Kamponchaidet, S.; Wachisunthon, D.; Poonsatha, S.; Tuntoaw, S. Boesenbergia rotunda Extract Inhibits Candida Albicans Biofilm Formation by Pinostrobin and Pinocembrin. J. Ethnopharmacol. 2020, 261, 113193. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of Solvent Polarity on Extraction Yield and Antioxidant Properties of Phytochemicals from Bean (Phaseolus vulgaris) Seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, L.; Xiao, P.; Lu, Y.; Bao, J. Total Phenolics, Flavonoids, Antioxidant Capacity in Rice Grain and Their Relations to Grain Color, Size and Weight. J. Cereal Sci. 2009, 49, 106–111. [Google Scholar] [CrossRef]
- Ameh, A.O.; Olakunle, M.S.; Shehu, H.U.; Oyegoke, T. Kinetics of the Extraction of Oleoresin from Ginger: Influence of Particle Size and Extraction Time Effects. NIPES J. Sci. Technol. Res. 2020, 2, 142. [Google Scholar] [CrossRef]
- Alencar, N.M.M.; Cazarin, C.B.B.; Corrêa, L.C.; Maróstica Junior, M.R.; Biasoto, A.C.T.; Behrens, J.H. Influence of Maceration Time on Phenolic Compounds and Antioxidant Activity of the Syrah Must and Wine. J. Food Biochem. 2018, 42, e12471. [Google Scholar] [CrossRef]
- Sithisarn, P.; Nantateerapong, P.; Rojsanga, P.; Sithisarn, P. Screening for Antibacterial and Antioxidant Activities and Phytochemical Analysis of Oroxylum Indicum Fruit Extracts. Molecules 2016, 21, 446. [Google Scholar] [CrossRef]
- Onyebuchi, C.; Kavaz, D. Effect of Extraction Temperature and Solvent Type on the Bioactive Potential of Ocimum Gratissimum L. Extracts. Sci. Rep. 2020, 10, 21760. [Google Scholar] [CrossRef] [PubMed]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Gharby, S.; Ainane, T.; Rharrabti, Y. A Multivariate Approach to Qualify “Moroccan Picholine” Virgin Olive Oil According to Extraction and Environmental Factors. J. Am. Oil Chem. Soc. 2024, 101, 173–185. [Google Scholar] [CrossRef]
- Segura, N.; Pinchak, Y.; Merlinski, N.; Amarillo, M.; Feller, C.; Irigaray, B.; Raggio, L.; Orono, M.; Grompone, M.A. Deterioration of Extra Virgin Olive Oil Caused by Different Processes. J. Food Res. 2017, 6, 59. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Moumneh, M.; Hamad, H.J.; Abughoush, M.; Abuawad, B.; Al-Nawasrah, B.A.; Al-Jaloudi, R.; Iqbal, S. Impact of Processing and Preservation Methods and Storage on Total Phenolics, Flavonoids, and Antioxidant Activities of Okra (Abelmoschus esculentus L.). Foods 2023, 12, 3711. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. Total Phenolic Content and Antioxidant Properties of Eclipse Black Beans ( Phaseolus Vulgaris L.) as Affected by Processing Methods. J. Food Sci. 2008, 73, H19–H27. [Google Scholar] [CrossRef]
- Buranachokpaisan, K.; Chalermchat, Y.; Muangrat, R. Economic Evaluation of the Production of Oil Extracted from Pressed Sesame Seed Cake Using Supercritical CO2 in Thailand. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100410. [Google Scholar] [CrossRef]
- He, H.-P.; Corke, H.; Cai, J.-G. Supercritical Carbon Dioxide Extraction of Oil and Squalene from Amaranthus Grain. J. Agric. Food Chem. 2003, 51, 7921–7925. [Google Scholar] [CrossRef]
- He, J.-Z.; Shao, P.; Liu, J.-H.; Ru, Q.-M. Supercritical Carbon Dioxide Extraction of Flavonoids from Pomelo (Citrus grandis (L.) Osbeck) Peel and Their Antioxidant Activity. Int. J. Mol. Sci. 2012, 13, 13065–13078. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and Emerging Extraction Processes of Flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Cid-Ortega, S.; Monroy-Rivera, J.A.; González-Ríos, Ó. Extraction of Kaempferitrin and Astragalin from Justicia Spicigera by Supercritical Fluid Extraction and Its Comparison with Conventional Extraction. J. Food Eng. Technol. 2021, 10, 35–44. [Google Scholar] [CrossRef]
- Pan, J.; Wang, H.; Chen, C.; Chang, J. Extraction of Astaxanthin from Haematococcus pluvialis by Supercritical Carbon Dioxide Fluid with Ethanol Modifier. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar] [CrossRef]
- Yerena-Prieto, B.J.; Gonzalez-Gonzalez, M.; García-Alvarado, M.Á.; Casas, L.; Palma, M.; Rodríguez-Jimenes, G.D.C.; Barbero, G.F.; Cejudo-Bastante, C. Evaluation of the Effect of Different Co-Solvent Mixtures on the Supercritical CO2 Extraction of the Phenolic Compounds Present in Moringa Oleifera Lam. Leaves. Agronomy 2022, 12, 1450. [Google Scholar] [CrossRef]
- Reis, J.H.D.O.; Machado, B.A.S.; Barreto, G.D.A.; Anjos, J.P.D.; Fonseca, L.M.D.S.; Santos, A.A.B.; Pessoa, F.L.P.; Druzian, J.I. Supercritical Extraction of Red Propolis: Operational Conditions and Chemical Characterization. Molecules 2020, 25, 4816. [Google Scholar] [CrossRef] [PubMed]
- Putra, N.R.; Rizkiyah, D.N.; Machmudah, S.; Shalleh, L.M.; Che Yunus, M.A. Recovery and Solubility of Flavonoid and Phenolic Contents from Arachis Hypogea in Supercritical Carbon Dioxide Assisted by Ethanol as Cosolvent. J. Food Process. Preserv. 2020, 44, e14768. [Google Scholar] [CrossRef]
- Rizkiyah, D.N.; Jusoh, W.M.S.W.; Idham, Z.; Putra, N.R.; Che Yunus, M.A. Investigation of Phenolic, Flavonoid, and Antioxidant Recovery and Solubility from Roselle Using Supercritical Carbon Dioxide: Experiment and Modeling. J. Food Process. Preserv. 2022, 46, e16670. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Mamat, H.; Jusoh, W.M.S.W.; Idham, Z.; Yunus, M.A.C.; Irianto, I. Influence of Particle Size in Supercritical Carbon Dioxide Extraction of Roselle (Hibiscus sabdariffa) on Bioactive Compound Recovery, Extraction Rate, Diffusivity, and Solubility. Sci. Rep. 2023, 13, 10871. [Google Scholar] [CrossRef]
| Independent Variables | Symbol | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Pressure (Bar) | A | 200 | 250 | 300 |
| Temperature (°C) | B | 35 | 45 | 55 |
| Flow rate (L/min) | C | 1 | 2 | 3 |
| Co-solvent (% of sample mass) | D | 0 | 50 | 100 |
| Run | Pressure (Bar) | Temperature (°C) | CO2 Flow Rate (L/min) | Co-Solvent (% of Sample Mass) |
|---|---|---|---|---|
| 1 | (1) 300 | (1) 55 | (−1) 1 | (−1) 0 |
| 2 | (−1) 200 | (1) 55 | (1) 3 | (1) 100 |
| 3 | (1) 300 | (0) 45 | (0) 2 | (0) 50 |
| 4 | (1) 300 | (−1) 35 | (−1) 1 | (−1) 0 |
| 5 | (0) 250 | (0) 45 | (0) 2 | (0) 50 |
| 6 | (0) 250 | (0) 45 | (0) 2 | (0) 50 |
| 7 | (0) 250 | (0) 45 | (1) 3 | (0) 50 |
| 8 | (1) 300 | (1) 55 | (1) 3 | (−1) 0 |
| 9 | (1) 300 | (1) 55 | (−1) 1 | (1) 100 |
| 10 | (−1) 200 | (−1) 35 | (−1) 1 | (−1) 0 |
| 11 | (−1) 200 | (−1) 35 | (−1) 1 | (1) 100 |
| 12 | (0) 250 | (0) 45 | (0) 2 | (−1) 0 |
| 13 | (1) 300 | (1) 55 | (1) 3 | (1) 100 |
| 14 | (0) 250 | (0) 45 | (0) 2 | (0) 50 |
| 15 | (0) 250 | (0) 45 | (0) 2 | (0) 50 |
| 16 | (0) 250 | (0) 45 | (0) 2 | (1) 100 |
| 17 | (0) 250 | (0) 45 | (−1) 1 | (0) 50 |
| 18 | (0) 250 | (1) 55 | (0) 2 | (0) 50 |
| 19 | (1) 300 | (−1) 35 | (1) 3 | (1) 100 |
| 20 | (−1) 200 | (−1) 35 | (1) 3 | (−1) 0 |
| 21 | (−1) 200 | (0) 45 | (0) 2 | (0) 50 |
| 22 | (−1) 200 | (1) 55 | (1) 3 | (−1) 0 |
| 23 | (−1) 200 | (1) 55 | (−1) 1 | (−1) 0 |
| 24 | (−1) 200 | (−1) 35 | (1) 3 | (1) 100 |
| 25 | (0) 250 | (0) 45 | (0) 2 | (0) 50 |
| 26 | (1) 300 | (−1) 35 | (1) 3 | (−1) 0 |
| 27 | (−1) 200 | (1) 55 | (−1) 1 | (1) 100 |
| 28 | (0) 250 | (−1) 35 | (0) 2 | (0) 50 |
| 29 | (0) 250 | (0) 45 | (0) 2 | (0) 50 |
| 30 | (1) 300 | (−1) 35 | (−1) 1 | (1) 100 |
| Extraction Time (min) | Total Yield (%) | TPC (mg GAE/g) | TFC (mg QE/g) |
|---|---|---|---|
| 40 | 9.00 ± 0.10 b | 276.16 ± 7.36 a | 109.39 ± 3.67 a |
| 60 | 9.91 ± 0.08 a | 332.86 ± 8.42 a | 77.57 ± 0.97 b |
| 80 | 9.12 ± 0.16 b | 273.35 ± 10.51 a | 81.29 ± 0.38 b |
| Run | A/Pressure (Bar) | B/Temperature (°C) | C/CO2 Flow Rate | D/Co-Solvent (% Sample) | Yield (%) | Phenolic mg GAE/g | Flavonoid (mg QE/g) |
|---|---|---|---|---|---|---|---|
| 1 | (1) 300 | (1) 55 | (−1) 1 | (−1) 0 | 10.50 | 249.10 | 168.80 |
| 2 | (−1) 200 | (1) 55 | (1) 3 | (1) 100 | 31.15 | 262.30 | 254.70 |
| 3 | (1) 300 | (0) 45 | (0) 2 | (0) 50 | 20.25 | 318.80 | 206.80 |
| 4 | (1) 300 | (−1) 35 | (−1) 1 | (−1) 0 | 8.75 | 322.90 | 179.30 |
| 5 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 21.75 | 434.20 | 317.70 |
| 6 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 22.40 | 484.10 | 323.60 |
| 7 | (0) 250 | (0) 45 | (1) 3 | (0) 50 | 22.60 | 476.70 | 344.70 |
| 8 | (1) 300 | (1) 55 | (1) 3 | (−1) 0 | 8.25 | 274.20 | 114.00 |
| 9 | (1) 300 | (1) 55 | (−1) 1 | (1) 100 | 32.00 | 305.70 | 263.30 |
| 10 | (−1) 200 | (−1) 35 | (−1) 1 | (−1) 0 | 6.25 | 212.90 | 72.80 |
| 11 | (−1) 200 | (−1) 35 | (−1) 1 | (1) 100 | 33.78 | 368.90 | 232.70 |
| 12 | (0) 250 | (0) 45 | (0) 2 | (−1) 0 | 8.00 | 378.60 | 115.70 |
| 13 | (1) 300 | (1) 55 | (1) 3 | (1) 100 | 31.08 | 272.90 | 306.90 |
| 14 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 22.75 | 438.80 | 319.70 |
| 15 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 22.25 | 436.40 | 318.40 |
| 16 | (0) 250 | (0) 45 | (0) 2 | (1) 100 | 28.75 | 440.00 | 352.80 |
| 17 | (0) 250 | (0) 45 | (−1) 1 | (0) 50 | 21.90 | 429.80 | 226.80 |
| 18 | (0) 250 | (1) 55 | (0) 2 | (0) 50 | 22.05 | 413.60 | 283.40 |
| 19 | (1) 300 | (−1) 35 | (1) 3 | (1) 100 | 31.85 | 350.10 | 274.80 |
| 20 | (−1) 200 | (−1) 35 | (1) 3 | (−1) 0 | 6.75 | 305.50 | 68.30 |
| 21 | (−1) 200 | (0) 45 | (0) 2 | (0) 50 | 17.50 | 341.40 | 169.70 |
| 22 | (−1) 200 | (1) 55 | (1) 3 | (−1) 0 | 7.00 | 192.90 | 124.90 |
| 23 | (−1) 200 | (1) 55 | (−1) 1 | (−1) 0 | 5.75 | 216.90 | 76.20 |
| 24 | (−1) 200 | (−1) 35 | (1) 3 | (1) 100 | 31.65 | 396.40 | 220.80 |
| 25 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 23.05 | 434.50 | 322.40 |
| 26 | (1) 300 | (−1) 35 | (1) 3 | (−1) 0 | 12.25 | 220.70 | 157.60 |
| 27 | (−1) 200 | (1) 55 | (−1) 1 | (1) 100 | 29.05 | 271.40 | 242.20 |
| 28 | (0) 250 | (−1) 35 | (0) 2 | (0) 50 | 20.00 | 404.40 | 397.60 |
| 29 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 24.80 | 456.60 | 318.30 |
| 30 | (1) 300 | (−1) 35 | (−1) 1 | (1) 100 | 32.35 | 332.60 | 282.90 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Extraction Yield | |||||
| Model | 2482.91 | 14 | 177.35 | 57.54 | <0.0001 * |
| Residual | 46.23 | 15 | 3.08 | ||
| Lack of Fit | 40.61 | 10 | 4.06 | 3.61 | 0.0846 ** |
| Pure error | 5.62 | 5 | 1.12 | ||
| Cor Total | 2529.14 | 29 | |||
| R2 | 0.9817 | ||||
| Total Phenolic (TPC) | |||||
| Model | 2.047 × 105 | 14 | 14,618.70 | 14.93 | <0.0001 * |
| Residual | 14,689.42 | 15 | 979.29 | ||
| Lack of Fit | 12,722.29 | 10 | 1272.23 | 3.23 | 0.1036 ** |
| Pure error | 1967.13 | 5 | 393.43 | ||
| Cor Total | 2.194 × 105 | 29 | |||
| R2 | 0.9330 | ||||
| Total Flavonoid (TFC) | |||||
| Model | 2.24 × 108 | 14 | 16,020.03 | 10.35 | <0.0001 * |
| Residual | 23,215.1 | 15 | 1547.67 | ||
| Lack of Fit | 23,185.55 | 10 | 2318.56 | 392.33 | <0.0001 * |
| Pure error | 29.55 | 5 | 5.91 | ||
| Cor Total | 2.48 × 108 | 29 | |||
| R2 | 0.9062 |
| Values | Optimized Process Parameters | Yield (%) | TPC (mg GAE/g) | TFC (mg QE/g) | |||
|---|---|---|---|---|---|---|---|
| Pressure (Bar) | Temperature (°C) | CO2 Flow Rate (L/min) | Co-Solvent (% Sample) | ||||
| Predicted | 250.103 | 45 | 3 | 99.99 | 32.51 | 463.600 | 328.905 |
| Actual | 250 | 45 | 3 | 100 | 28.67 | 354.578 | 273.479 |
| % error | 0.04 | 0 | 0 | 0.01 | 11.81 | 23.516 | 16.852 |
| Run | Pressure (Bar) | Temperature (°C) | CO2 Flow Rate (L/min) | Co-Solvent (% Sample) | Pinocembrin mg/g | Pinostrobin mg/g |
|---|---|---|---|---|---|---|
| 1 | (1) 300 | (1) 55 | (−1) 1 | (−1) 0 | 33.97 | 73.82 |
| 2 | (−1) 200 | (1) 55 | (1) 3 | (1) 100 | 32.79 | 75.29 |
| 3 | (1) 300 | (0) 45 | (0) 2 | (0) 50 | 37.43 | 92.47 |
| 4 | (1) 300 | (−1) 35 | (−1) 1 | (−1) 0 | 20.93 | 65.14 |
| 5 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 51.06 | 108.62 |
| 6 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 51.66 | 107.83 |
| 7 | (0) 250 | (0) 45 | (1) 3 | (0) 50 | 58.97 | 112.59 |
| 8 | (1) 300 | (1) 55 | (1) 3 | (−1) 0 | 40.58 | 85.36 |
| 9 | (1) 300 | (1) 55 | (−1) 1 | (1) 100 | 38.08 | 82.14 |
| 10 | (−1) 200 | (−1) 35 | (−1) 1 | (−1) 0 | 14.56 | 87.25 |
| 11 | (−1) 200 | (−1) 35 | (−1) 1 | (1) 100 | 37.44 | 94.18 |
| 12 | (0) 250 | (0) 45 | (0) 2 | (−1) 0 | 42.41 | 88.47 |
| 13 | (1) 300 | (1) 55 | (1) 3 | (1) 100 | 30.93 | 76.91 |
| 14 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 50.37 | 108.19 |
| 15 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 48.87 | 107.95 |
| 16 | (0) 250 | (0) 45 | (0) 2 | (1) 100 | 62.10 | 115.28 |
| 17 | (0) 250 | (0) 45 | (−1) 1 | (0) 50 | 44.71 | 95.63 |
| 18 | (0) 250 | (1) 55 | (0) 2 | (0) 50 | 41.51 | 89.42 |
| 19 | (1) 300 | (−1) 35 | (1) 3 | (1) 100 | 13.93 | 82.75 |
| 20 | (−1) 200 | (−1) 35 | (1) 3 | (−1) 0 | 17.47 | 92.31 |
| 21 | (−1) 200 | (0) 45 | (0) 2 | (0) 50 | 24.66 | 96.84 |
| 22 | (−1) 200 | (1) 55 | (1) 3 | (−1) 0 | 61.91 | 88.12 |
| 23 | (−1) 200 | (1) 55 | (−1) 1 | (−1) 0 | 36.45 | 91.27 |
| 24 | (−1) 200 | (−1) 35 | (1) 3 | (1) 100 | 8.27 | 84.69 |
| 25 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 56.96 | 109.52 |
| 26 | (1) 300 | (−1) 35 | (1) 3 | (−1) 0 | 10.93 | 79.46 |
| 27 | (−1) 200 | (1) 55 | (−1) 1 | (1) 100 | 9.59 | 99.37 |
| 28 | (0) 250 | (−1) 35 | (0) 2 | (0) 50 | 42.93 | 89.58 |
| 29 | (0) 250 | (0) 45 | (0) 2 | (0) 50 | 58.33 | 108.71 |
| 30 | (1) 300 | (−1) 35 | (−1) 1 | (1) 100 | 61.91 | 93.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahrudin, F.I.; Phongthai, S.; Wirjantoro, T.I.; Intipunya, P. Synergistic Effects of Pressure, Temperature, CO2 Flow Rate and Co-Solvent on Bioactive Contents of Thai Fingerroot (Boesenbergia rotunda (L.) Mansf.) Extracts. Foods 2025, 14, 2189. https://doi.org/10.3390/foods14132189
Fahrudin FI, Phongthai S, Wirjantoro TI, Intipunya P. Synergistic Effects of Pressure, Temperature, CO2 Flow Rate and Co-Solvent on Bioactive Contents of Thai Fingerroot (Boesenbergia rotunda (L.) Mansf.) Extracts. Foods. 2025; 14(13):2189. https://doi.org/10.3390/foods14132189
Chicago/Turabian StyleFahrudin, Fahmi Ilman, Suphat Phongthai, Tri Indrarini Wirjantoro, and Pilairuk Intipunya. 2025. "Synergistic Effects of Pressure, Temperature, CO2 Flow Rate and Co-Solvent on Bioactive Contents of Thai Fingerroot (Boesenbergia rotunda (L.) Mansf.) Extracts" Foods 14, no. 13: 2189. https://doi.org/10.3390/foods14132189
APA StyleFahrudin, F. I., Phongthai, S., Wirjantoro, T. I., & Intipunya, P. (2025). Synergistic Effects of Pressure, Temperature, CO2 Flow Rate and Co-Solvent on Bioactive Contents of Thai Fingerroot (Boesenbergia rotunda (L.) Mansf.) Extracts. Foods, 14(13), 2189. https://doi.org/10.3390/foods14132189







