Comparison of Polygonatum sibiricum Polysaccharides from Different Extraction Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Main Instruments and Equipment
2.3. Experimental Process Chart
2.4. Different Extraction Methods of PsP
2.4.1. Pretreatment Process
2.4.2. Water–Ethanol Extraction (WEE) Method of PsP
2.4.3. Ultrasound-Assisted Extraction (UAE) Method of PsP
2.4.4. Deep Eutectic Solvent Extraction (DES) Method of PsP
2.4.5. Ultrasound-Assisted Extraction–Deep Eutectic Solvent Extraction (UAE–DES) Method of PsP
2.5. Separation and Purification of the PsP
2.6. Composition Analysis
2.6.1. Measurement for PsP
2.6.2. Measurement for Protein
2.7. Measurement for PsP’s Molecular Weight
2.8. Measurement for PsP’s Monosaccharide Component
2.9. Measurement for PsP’s Functional Groups
2.10. In Vitro Antioxidant Activity
2.10.1. DPPH and ABTS Radical Scavenging Rate
2.10.2. Total Antioxidant Capacity
Phosphorus Molybdenum Complexation (PMC) Method
Ferric Reducing Antioxidant Power (FRAP) Method
2.10.3. In Vitro Catalase (CAT) Activity Measurement
2.11. Statistical Analysis
3. Results
3.1. Comparisons of Extraction Yields of Different Extraction Methods for PsP
3.2. Separation and Purification of UDPsP
3.3. Comparison of Molecular Weight
3.4. Composition for Monosaccharide Compositions
3.5. Comparison for Functional Groups
3.6. Comparisons of Antioxidant Activity
3.6.1. Comparison of DPPH and ABTS Radical Scavenging Ability
3.6.2. Comparison of Total Antioxidant Ability
3.6.3. Comparison of In Vitro CAT Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| ABTS | 2,20–azino–bis(3–ethylbenzothiazoline–6–sulfoniacid) |
| BSA | Bovine serum albumin |
| DESs | Deep eutectic solvents |
| DPPH | 2,2–diphenyl–1–picrylhydrazyl |
| DPsP | Deep eutectic solvent extraction method–PsP |
| FRAP | Ferric reducing antioxidant power |
| P. sibiricum | Polygonatum sibiricum |
| PMC | Phosphorus molybdenum complexation |
| PsP | Polygonatum sibiricum Polysaccharide |
| TFA | Trifluoroacetic acid |
| UAE | Ultrasound-assisted extraction |
| UAE–DESs | Ultrasound-assisted extraction–deep eutectic solvents |
| UDPsP | Ultrasound-assisted extraction–deep eutectic solvent extraction method–PsP |
| UDPsP–1 | Separated and purified UDPsP |
| UPsP | Ultrasound-assisted extraction method–PsP |
| WEE | Water–ethanol extraction |
| WPsP | Water–ethanol extraction method–PsP |
References
- Wan, P.; Liu, H.; Zhu, Y.; Xin, H.; Ma, Y.; Chen, Z. Effects of Polygonatum sibiricum on Physicochemical Properties, Biological Compounds, and Functionality of Fermented Soymilk. Foods 2023, 12, 2715. [Google Scholar] [CrossRef]
- Kakar, M.U.; Karim, H.; Shabir, G.; Iqbal, I.; Akram, M.; Ahmad, S.; Shafi, M.; Gul, P.; Riaz, S.; Rehman, R.U.; et al. A review on extraction, composition, structure, and biological activities of polysaccharides from different parts of Nelumbo nucifera. Food Sci. Nutr. 2023, 11, 3655–3674. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Gan, X.; Li, Y.; Chen, J.; Xu, Y.; Shi, S.; Li, T.; Li, B.; Wang, H.; Wang, S. Review on the genus Polygonatum polysaccharides: Extraction, purification, structural characteristics and bioactivities. Int. J. Biol. Macromol. 2023, 229, 909–930. [Google Scholar] [CrossRef]
- Zhao, X.; Patil, S.; Qian, A.; Zhao, C. Bioactive Compounds of Polygonatum sibiricum—Therapeutic Effect and Biological Activity. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, W.; Han, C.; Nie, S. Advances in Polygonatum sibiricum polysaccharides: Extraction, purification, structure, biosynthesis, and bioactivity. Front. Nutr. 2022, 9, 1074671. [Google Scholar] [CrossRef] [PubMed]
- Deka, H.; Sarmah, P.P.; Devi, A.; Tamuly, P.; Karak, T. Changes in major catechins, caffeine, and antioxidant activity during CTC processing of black tea from North East India. RSC Adv. 2021, 11, 11457–11467. [Google Scholar] [CrossRef]
- Ma, W.; Wei, S.; Peng, W.; Sun, T.; Huang, J.; Yu, R.; Zhang, B.; Li, W. Antioxidant Effect of Polygonatum sibiricum Polysaccharides in D-Galactose-Induced Heart Aging Mice. Biomed. Res. Int. 2021, 2021, 6688855. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Song, Y.X.; Zhang, W.X.; Chen, M.J.; Man, S.L. Research on anti-tumor natural product diosgenin. Zhongguo Zhong Yao Za Zhi 2021, 46, 4360–4366. [Google Scholar] [CrossRef]
- Aldalin, H.K.; Alharbi, N.K.; Hadi, A.M.; Sharaf, M.; Mekky, A.E.; Ragab, S.M.; Mahmoud, N.; Al-Hoshani, N.; Alwutayd, K.M.; Abdelnour, S.A. Bioactivity screening and molecular identification of Anchusa milleri L. sunflower crud extract for antioxidant, antiviral, antimicrobial, and anticancer properties. Nat. Prod. Res. 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Tan, M.; Zhong, X.; Xue, H.; Cao, Y.; Tan, G.; Li, K. Polysaccharides from pineapple peel: Structural characterization, film-forming properties and its effect on strawberry preservation. Int. J. Biol. Macromol. 2024, 279, 135192. [Google Scholar] [CrossRef]
- Wang, H.; Luan, F.; Shi, Y.; Yan, S.; Xin, B.; Zhang, X.; Guo, D.; Sun, J.; Zou, J. Extraction, structural features, and pharmacological effects of the polysaccharides from Porphyra yezoensis: A review. Int. J. Biol. Macromol. 2024, 279, 134745. [Google Scholar] [CrossRef]
- Lesgourgues, M.; Latire, T.; Terme, N.; Douzenel, P.; Leschiera, R.; Lebonvallet, N.; Bourgougnon, N.; Bedoux, G. Ultrasound Depolymerization and Characterization of Poly- and Oligosaccharides from the Red Alga Solieria chordalis (C. Agardh) J. Agardh 1842. Mar. Drugs 2024, 22, 367. [Google Scholar] [CrossRef]
- Sarkhel, S.; Mondal, M.; Datta, D.; Sahoo, B.; Kumari, A.; Saha, S.; Bera, S.; Jana, M.; Tiwari, A.; Roy, A. Ultrasonic high-yield extraction of non-toxic fucose-containing Abroma augusta polysaccharide bearing emulsifying properties. J. Sci. Food Agric. 2024, 104, 8858–8868. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, G.; Huang, H. Ultrasonic-assisted extraction, analysis and properties of purple mangosteen scarfskin polysaccharide and its acetylated derivative. Ultrason. Sonochem 2024, 109, 107010. [Google Scholar] [CrossRef]
- Lin, Z.L.; Jin, X.H.; Zhang, C.H. Study on ultrasound-assisted extraction of polysaccharides from Polygonatum cyrtonema and itsbiological activity. Jiangsu Agric. Sci. 2019, 47, 221–225. [Google Scholar] [CrossRef]
- Jing, Y.; Yan, M.; Zhang, H.; Liu, D.; Qiu, X.; Hu, B.; Zhang, D.; Zheng, Y.; Wu, L. Effects of Extraction Methods on the Physicochemical Properties and Biological Activities of Polysaccharides from Polygonatum sibiricum. Foods 2023, 12, 2088. [Google Scholar] [CrossRef]
- Morozova, O.V.; Vasil’eva, I.S.; Shumakovich, G.P.; Zaitseva, E.A.; Yaropolov, A.I. Deep Eutectic Solvents for Biotechnology Applications. Biochemistry 2023, 88, S150–S175. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, L.; Su, X.; Li, Q.; Guo, H.; Liu, Z.; Wei, Z.; Wang, F. Research on the Impact of Deep Eutectic Solvent and Hot-Water Extraction Methods on the Structure of Polygonatum sibiricum Polysaccharides. Molecules 2023, 28, 6981. [Google Scholar] [CrossRef]
- Sun, C.; Wang, G.; Sun, J.; Yin, J.; Huang, J.; Li, Z.; Mu, D.; He, M.; Liu, T.; Cheng, J.; et al. A New Method of Extracting Polygonatum sibiricum Polysaccharide with Antioxidant Function: Ultrasound-Assisted Extraction-Deep Eutectic Solvents Method. Foods 2023, 12, 3438. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, Q.; Zhao, J.; Mao, G.; Feng, W.; Chen, Y.; Li, Q.; Wu, X.; Yang, L.; Zhao, T. Purification, structural elucidation and physicochemical properties of a polysaccharide from Abelmoschus esculentus L. (okra) flowers. Int. J. Biol. Macromol. 2020, 155, 740–750. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, N.; Xue, X.; Li, Q.; Sun, D.; Zhao, Z. Purification, structural characterization and in vivo immunoregulatory activity of a novel polysaccharide from Polygonatum sibiricum. Int. J. Biol. Macromol. 2020, 160, 688–694. [Google Scholar] [CrossRef]
- Bigard, A.; Cardinael, P.; Agasse, V. Anion Exchange Chromatography Coupled to Electrospray-Mass Spectrometry: An Efficient Tool for Food, Environment, and Biological Analysis. Crit. Rev. Anal. Chem. 2023, 53, 1591–1603. [Google Scholar] [CrossRef]
- Luo, C.; DeStefano, J.J.; Langlois, T.J.; Boyes, B.E.; Schuster, S.A.; Godinho, J.M. Fundamental to achieving fast separations with high efficiency: A review of chromatography with superficially porous particles. Biomed. Chromatogr. 2021, 35, e5087. [Google Scholar] [CrossRef]
- Cai, J.L.; Li, X.P.; Zhu, Y.L.; Yi, G.Q.; Wang, W.; Chen, X.Y.; Deng, G.M.; Yang, L.; Cai, H.Z.; Tong, Q.Z.; et al. Polygonatum sibiricum polysaccharides (PSP) improve the palmitic acid (PA)-induced inhibition of survival, inflammation, and glucose uptake in skeletal muscle cells. Bioengineered 2021, 12, 10147–10159. [Google Scholar] [CrossRef]
- Chen, P.; Ding, S.; Yan, Z.; Liu, H.; Tu, J.; Chen, Y.; Zhang, X. Structural Characteristic and In-Vitro Anticancer Activities of Dandelion Leaf Polysaccharides from Pressurized Hot Water Extraction. Nutrients 2022, 15, 80. [Google Scholar] [CrossRef]
- Samal, R.R.; Kumari, K.; Sahoo, Y.; Mishra, S.K.; Subudhi, U. Interaction of artemisinin protects the activity of antioxidant enzyme catalase: A biophysical study. Int. J. Biol. Macromol. 2021, 172, 418–428. [Google Scholar] [CrossRef]
- Deore, U.V.; Mahajan, H.S. Isolation and structural characterization of mucilaginous polysaccharides obtained from the seeds of Cassia uniflora for industrial application. Food Chem. 2021, 351, 129262. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Xu, Y.; Sun, C.; Qu, W.; Du, H.; He, M.; Huo, J.; Sun, J.; Huang, J.; et al. Comparison of Polygonatum sibiricum Polysaccharides Found in Young and Mature Rhizomes. Foods 2024, 13, 2010. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Pan, J.; Shi, Y.; Zou, J.; Zhang, X.; Xin, B.; Zhai, B.; Guo, D.; Sun, J.; Luan, F. Preparation technologies, structural features, and biological activities of polysaccharides from Mesona chinensis Benth.: A review. J. Ethnopharmacol. 2024, 326, 117979. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, J.; Shi, Y.; Zhang, X.; Zhai, B.; Guo, D.; Sun, J.; Luan, F. Extraction techniques, structural features and biological functions of Hippophae rhamnoides polysaccharides: A review. Int. J. Biol. Macromol. 2024, 263, 130206. [Google Scholar] [CrossRef]
- Ji, R.; Wang, Z.; Kuang, H. Extraction, purification, structural characterization, and biological activity of polysaccharides from Schisandra chinensis: A review. Int. J. Biol. Macromol. 2024, 271, 132590. [Google Scholar] [CrossRef]
- Li, D.; Chen, M.; Meng, X.; Sun, Y.; Liu, R.; Sun, T. Extraction, purification, structural characteristics, bioactivity and potential applications of polysaccharides from Avena sativa L.: A review. Int. J. Biol. Macromol. 2024, 265, 130891. [Google Scholar] [CrossRef]
- Yang, M.-H.; Zhou, X.; Yang, Y.; Chen, H.-G. Effects of different extraction methods on the structural characterization and bioactivities of polysaccharides extracted from Polygonatum sibiricum. J. Food Meas. Charact. 2024, 18, 940–954. [Google Scholar] [CrossRef]
- Chemat, F.; Zill, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Liu, S.; Geng, J.; Chen, W.; Zong, Y.; Zhao, Y.; Du, R.; He, Z. Isolation, structure, biological activity and application progress of ginseng polysaccharides from the Araliaceae family. Int. J. Biol. Macromol. 2024, 276, 133925. [Google Scholar] [CrossRef]
- Song, Z.; Huang, G.; Huang, H. The ultrasonic-assisted enzymatic extraction, characteristics and antioxidant activities of lychee nuclear polysaccharide. Ultrason. Sonochem 2024, 110, 107038. [Google Scholar] [CrossRef]
- Yahaya, N.; Mohamed, A.H.; Sajid, M.; Zain, N.N.M.; Liao, P.C.; Chew, K.W. Deep eutectic solvents as sustainable extraction media for extraction of polysaccharides from natural sources: Status, challenges and prospects. Carbohydr. Polym. 2024, 338, 122199. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef]
- Liu, S.; Li, M.; Liu, W.; Zhang, Z.; Wang, X.; Dong, H. Structure and properties of acidic polysaccharides isolated from Massa Medicata Fermentata: Neuroprotective and antioxidant activity. Int. J. Biol. Macromol. 2024, 259, 129128. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Huang, R.; Li, S.; Jiang, L.; Shao, L.; Zhang, Q.; Shan, C. Polysaccharides from sea buckthorn—Ultrasound-assisted enzymatic extraction, purification, structural characterization, and antioxidant activity analysis. Food Chem. X 2025, 26, 102265. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; He, Z.; Hong, C.; Xie, S.; Zha, X. Extraction, purification, structural characterization and pharmacological activities of polysaccharides from sea buckthorn (Hippophae rhamnoides L.): A review. J. Ethnopharmacol. 2024, 324, 117809. [Google Scholar] [CrossRef] [PubMed]
- Abbasirad, S.; Ghotbi-Ravandi, A.A. Toxicity of copper oxide nanoparticles in barley: Induction of oxidative stress, hormonal imbalance, and systemic resistances. BMC Plant Biol. 2025, 25, 187. [Google Scholar] [CrossRef]



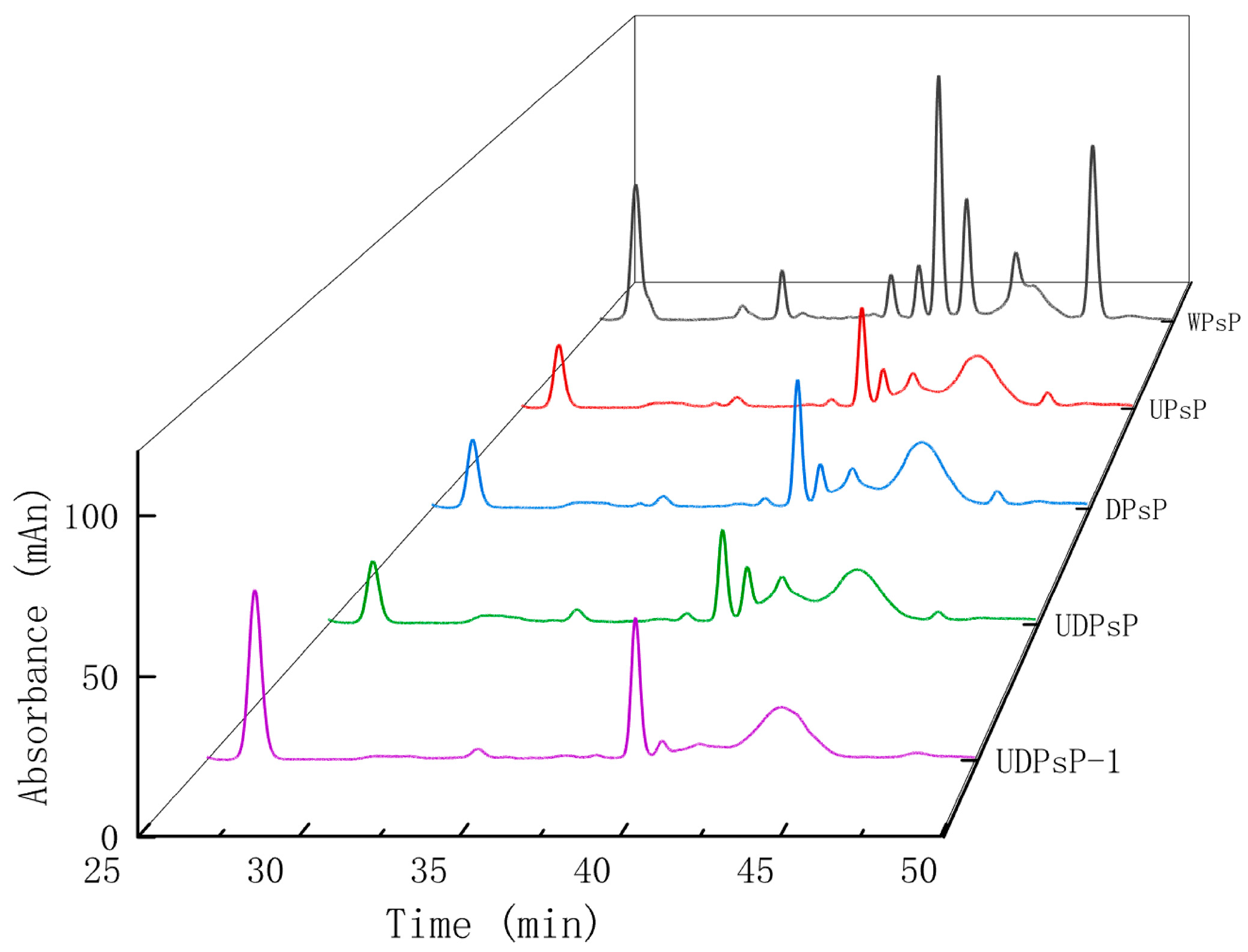


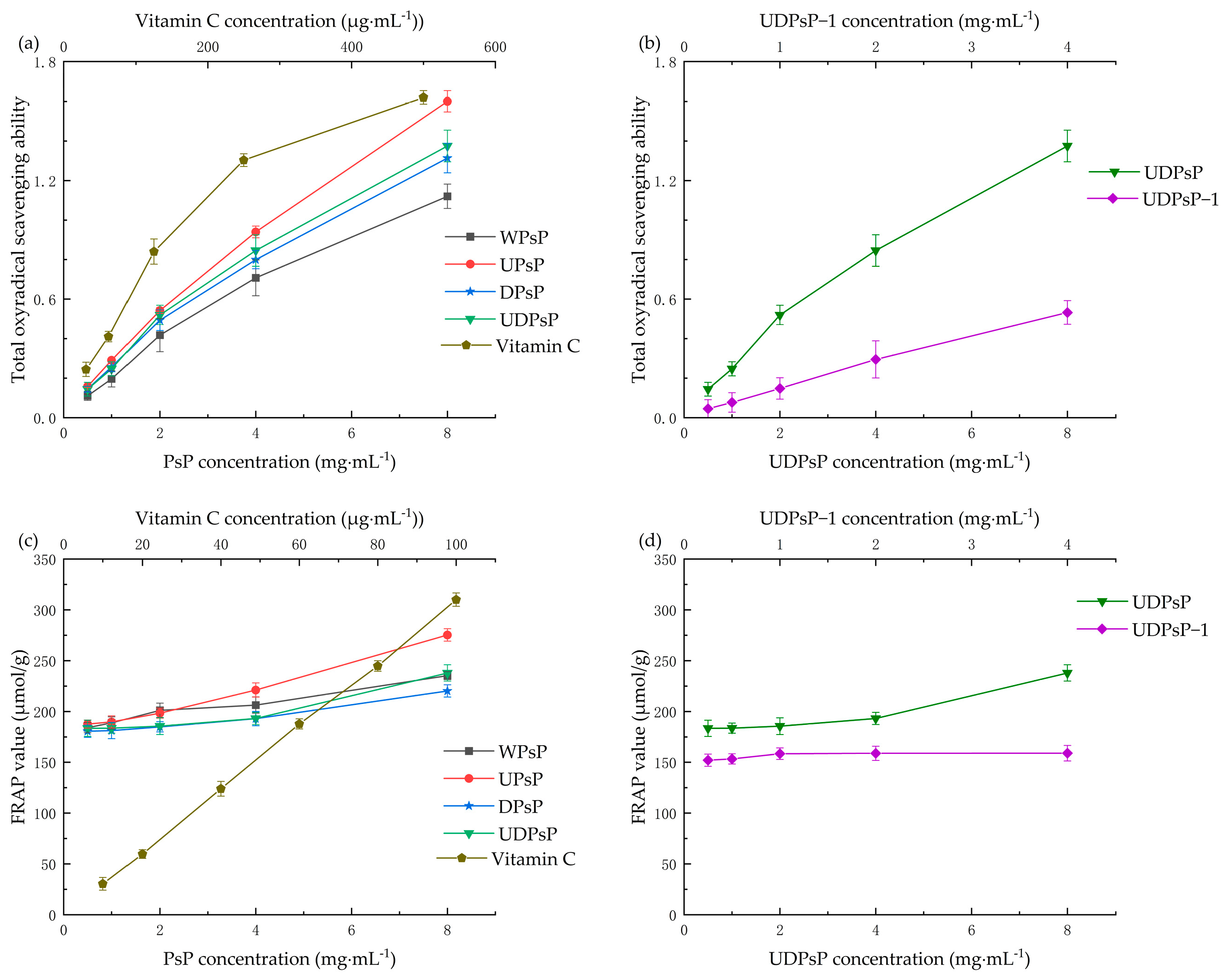

| Sample (mg) | Extraction Yield mg (%) | After Deproteinization mg (%) | Dialysis mg (%) | End Product mg (%) | Protein Residue % | Purity % |
|---|---|---|---|---|---|---|
| WPsP (700) | 70.91 ± 5.18 (10.13 ± 0.74)% | 45.43 ± 1.05 (6.49 ± 0.15)% | 39.19 ± 2.66 (5.60 ± 0.38)% | 23.80 ± 1.25 (3.40 ± 0.18)% | (0.63 ± 0.35)% * | (98.24 ± 0.47)% * |
| UPsP (700) | 143.71 ± 5.18 (20.53 ± 1.43)% | 95.44 ± 0.84 (13.63 ± 0.12)% | 76.73 ± 1.26 (10.96 ± 0.18)% | 57.24 ± 1.07 (8.18 ± 0.15)% | (0.57 ± 0.53)% * | (98.28 ± 0.30)% * |
| DPsP (700) | 244.16 ± 4.76 (34.88 ± 0.68)% | 158.38 ± 1.19 (22.63 ± 0.17)% | 107.79 ± 0.42 (15.40 ± 0.06)% | 67.42 ± 0.89 (9.63 ± 0.13)% | (0.33 ± 0.08)% * | (98.76 ± 0.17)% * |
| UDPsP (700) | 315.56 ± 9.73 (45.08 ± 1.39)% | 235.66 ± 1.02 (33.67 ± 0.86)% | 154.64 ± 1.96 (22.09 ± 0.28)% | 102.12 ± 0.94 (14.59 ± 0.13)% | (0.42 ± 0.31)% | (98.15 ± 0.32)% |
| Sample | Retention Times (min) | Mw (Da) |
|---|---|---|
| WPsP | 16.83 | 2069 |
| UPsP | 16.61 | 2118 |
| DPsP | 16.40 | 2173 |
| UDPsP | 16.27 | 2512 |
| UDPsP–1 | 16.35 | 2339 |
| Sample | Man | Rha | GlcA | GalA | Glc | Gal | Ara | Fuc |
|---|---|---|---|---|---|---|---|---|
| WPsP | 1.97 | 0.34 | 0.00 | 2.21 | 1.00 | 1.96 | 1.29 | 2.58 |
| UPsP | 0.47 | 0.15 | 0.00 | 0.00 | 1.00 | 0.16 | 0.18 | 0.1 |
| DPsP | 0.39 | 0.12 | 0.00 | 0.17 | 1.00 | 0.16 | 0.29 | 0.08 |
| UDPsP | 0.49 | 0.08 | 0.00 | 0.00 | 1.00 | 0.28 | 0.48 | 0.07 |
| UDPsP–1 | 0.86 | 0.00 | 0.00 | 0.00 | 1.00 | 0.05 | 0.09 | 0.00 |
| Sample | IC50 of DPPH Radical Scavenging Rate (mg·mL−1) | IC50 of ABTS Radical Scavenging Rate (mg·mL−1) |
|---|---|---|
| WPsP | 60.83 | 45.51 |
| UPsP | 61.92 | 45.68 |
| DPsP | 57.15 | 62.36 |
| UDPsP | 54.77 | 41.64 |
| UDPsP–1 | 28.46 | 38.29 |
| Vitamin C | 16.49 | 9.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Du, H.; Qu, W.; Sun, C.; Chen, Q.; Du, Y.; Zhang, Z.; Guo, Y.; Wang, C.; Huang, J.; et al. Comparison of Polygonatum sibiricum Polysaccharides from Different Extraction Methods. Foods 2025, 14, 2188. https://doi.org/10.3390/foods14132188
Chen Y, Du H, Qu W, Sun C, Chen Q, Du Y, Zhang Z, Guo Y, Wang C, Huang J, et al. Comparison of Polygonatum sibiricum Polysaccharides from Different Extraction Methods. Foods. 2025; 14(13):2188. https://doi.org/10.3390/foods14132188
Chicago/Turabian StyleChen, Yan, Hanchen Du, Wenjie Qu, Chaoqun Sun, Qu Chen, Yuping Du, Zhuoyuan Zhang, Yiran Guo, Chonglin Wang, Jian Huang, and et al. 2025. "Comparison of Polygonatum sibiricum Polysaccharides from Different Extraction Methods" Foods 14, no. 13: 2188. https://doi.org/10.3390/foods14132188
APA StyleChen, Y., Du, H., Qu, W., Sun, C., Chen, Q., Du, Y., Zhang, Z., Guo, Y., Wang, C., Huang, J., & Yin, J. (2025). Comparison of Polygonatum sibiricum Polysaccharides from Different Extraction Methods. Foods, 14(13), 2188. https://doi.org/10.3390/foods14132188






