Biotransformation of Phenolic Acids in Foods: Pathways, Key Enzymes, and Technological Applications
Abstract
1. Introduction
2. Methods, Pathways, and Significance of Phenolic Acid Biotransformation
2.1. Methods of Phenolic Acid Biotransformation
2.1.1. Chemical Methods
2.1.2. Microbial Methods
2.1.3. Enzymatic Methods
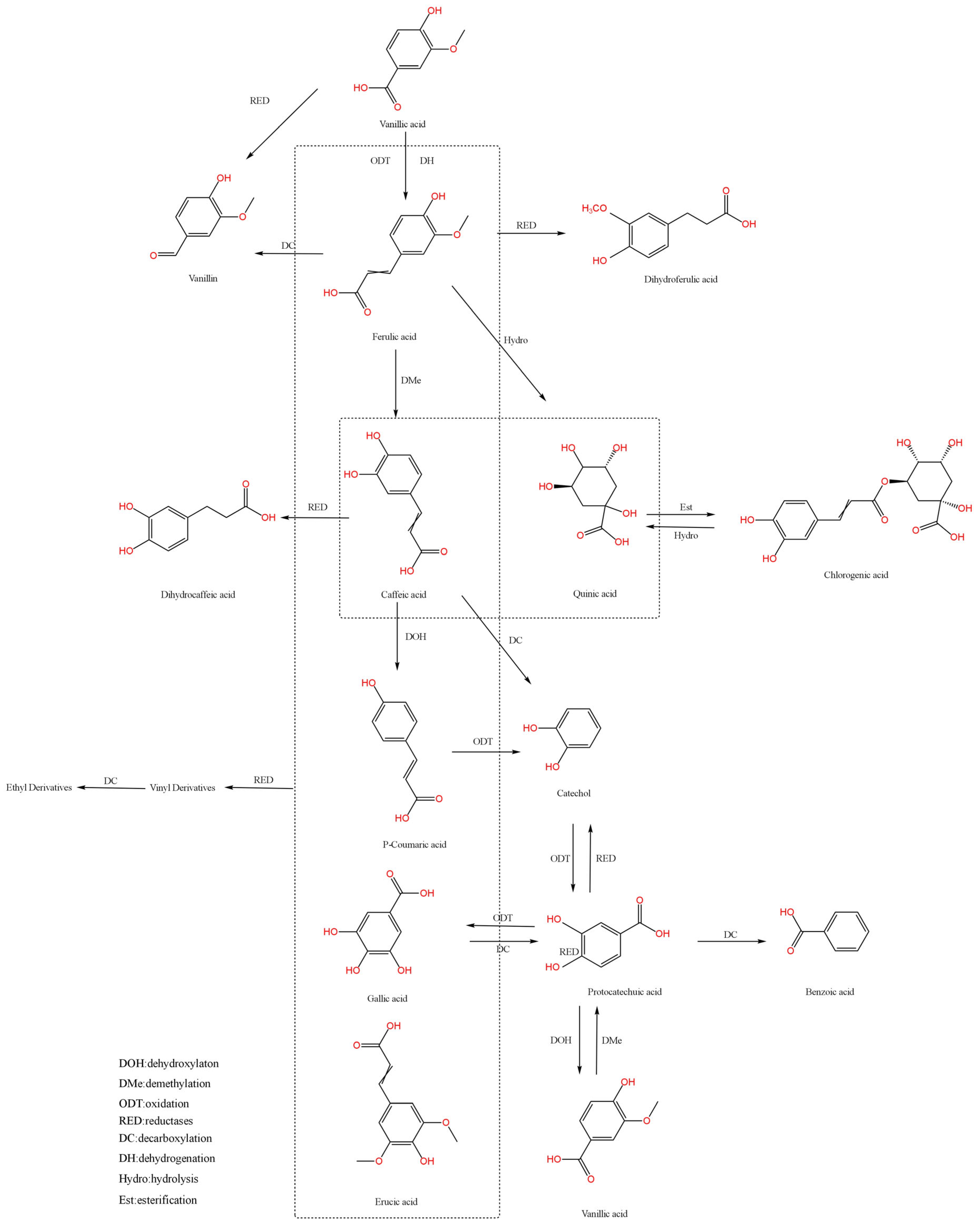
2.2. Pathways of Phenolic Acid Biotransformation
2.2.1. Decarboxylation
2.2.2. Reduction
2.2.3. Hydrolysis
2.3. Significance of Phenolic Acid Biotransformation
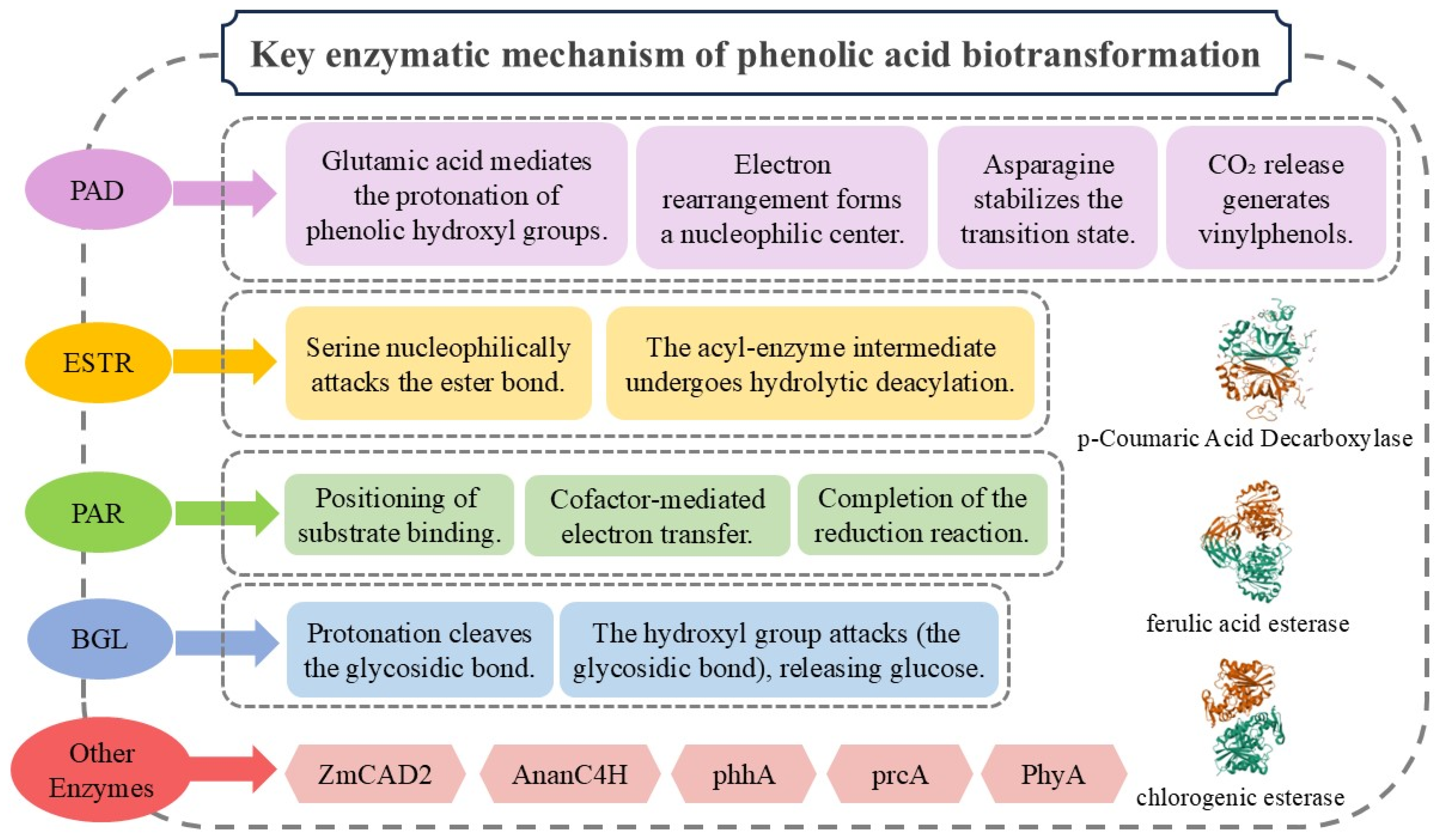
3. Structure and Mechanisms of Key Enzymes in Phenolic Acid Biotransformation
3.1. Phenolic Acid Decarboxylase
3.1.1. Structure of Phenolic Acid Decarboxylase
3.1.2. Mechanisms of Phenolic Acid Decarboxylase
3.2. Phenolic Acid Esterase
3.2.1. Structure of Phenolic Acid Esterase
3.2.2. Mechanisms of Phenolic Acid Esterase
3.3. Phenolic Acid Reductase
3.3.1. Structure of Phenolic Acid Reductase
3.3.2. Mechanisms of Phenolic Acid Reductase
3.4. β-Glucosidase
3.4.1. Structure of β-Glucosidase
3.4.2. Mechanisms of β-Glucosidase
3.5. Other Enzymes Involved in the Biotransformation of Phenolic Acids
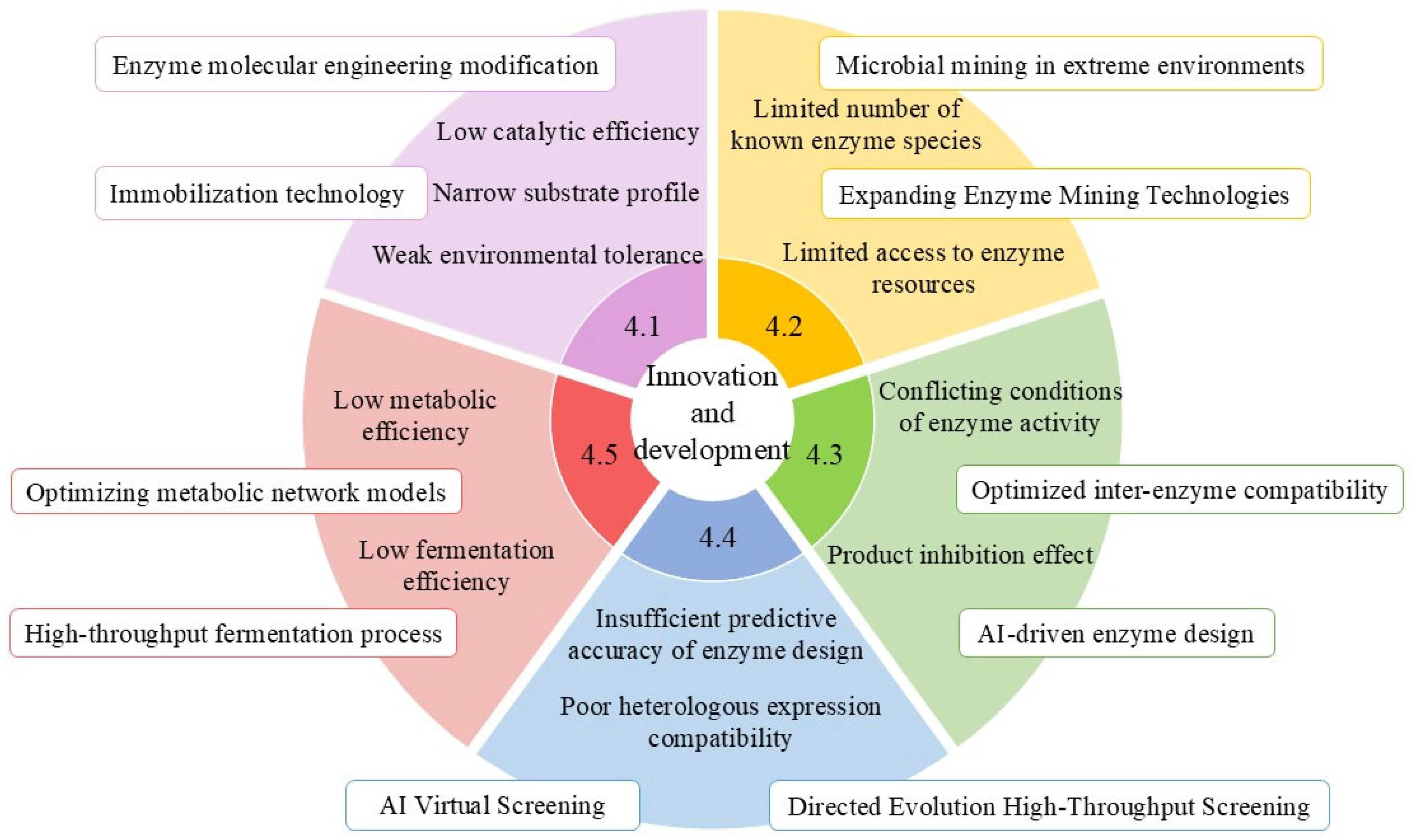
4. Multidimensional Innovation and Development in Key Enzyme Research
4.1. Systematic Enhancement of Key Enzyme Molecular Performance
4.2. In-Depth Exploration and Functional Development of Enzyme Resources
4.3. Construction of Multi-Enzyme Cascade Systems
4.4. Synthetic Biology-Driven Enzyme Function Enhancement
4.5. Precision Design of Microbial Cell Factories
5. Applications of Key Enzymes in Phenolic Acid Biotransformation in the Food Industry
5.1. Applications in Cereals
| Enzyme | Original Phenolics | Transformed Products | Microorganism | Food Matrix | Role | Reference |
|---|---|---|---|---|---|---|
| Cellulase | Ferulic acid and p-coumaric acid | Caffeic acid | Monascus anka GIM 3.592 | Oatmeal | Releases bound phenolic acids and improves antioxidant properties | [133] |
| AlPAD | Ferulic acid | 4-Vinylguaiacol | Aspergillus luchuensis | Steamed rice | Development of characteristic flavors | [134] |
| Lp_0796, Est_1092 | Ferulic acid | Dihydroferulic acid | Lactobacillus plantarum TMW1.460 | Whole wheat flour | Altering the sensory properties of food | [135] |
| par1, par2, estR, pad | Hydroxycinnamic acid | Vinyl Derivatives | Furfurilactobacillus milii FUA3583 | Sorghum | Improvement in antimicrobial activity | [136] |
| PCD | Ferulic acid | Vinyl guaiacol Ethyl guaiacol Dihydroferulic acid | Lactobacillus plantarum | Wheat sourdough | Affects the nutritional content and texture of bread | [137] |
| Cellulase, hydrolytic enzyme, and β-glucosidase | p-Coumaric acid and caffeic acid | Chlorogenic acid | Monascus anka GIM 3.592, Saccharomyces cerevisiae GIM 2.139, and Bacillus subtilis 784 | Avena sativa L. | Improvement in functional properties of cereal products | [138] |
| - | Soluble protocatechuic acid and soluble vanillic acid | Alcohol-soluble protocatechuic acid and alcohol-soluble vanillic acid | Aspergillus oryzae 6001, Aspergillus oryzae 6020, Aspergillus sojae 700, and Aspergillus luchuensis 8035 | Rice | Increased antioxidant activity | [139] |
| Hydroxycinnamic acid reductase | Caffeic acid and ferulic acid | Dihydrocaffeic acid and dihydroferulic acid | Candida milleri, Lactobacillus brevis, and Lactobacillus plantarum | Whole wheat and rye | Able to regulate blood lipid and blood sugar levels | [140] |
| - | Ferulic acid | 4-Vinylguaiacol | Streptomyces tunisiensis DSM 42037 | Barley bran | Potential applications in the food, pharmaceutical, and cosmetic industries | [40] |
| - | Ferulic acid and caffeic acid | Dihydroferulic acid and dihydrocaffeic acid | Lactobacillus plantarum DSMZ 13890 | Rye | Regulates gut and host health | [141] |
5.2. Applications in Fruits
5.3. Applications in Other Foods
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, F. The Reciprocal Interaction between Polyphenols and Other Dietary Compounds: Impact on Bioavailability, Antioxidant Capacity and Other Physico-Chemical and Nutritional Parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Chen, W.; Liu, Q.; Wang, L. Encapsulation of Phenolic Acids within Food-Grade Carriers Systems: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2025, 65, 2765–2784. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of Phenolic Compounds by Lactobacillus spp. during Fermentation of Cherry Juice and Broccoli Puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest Developments in Polyphenol Recovery and Purification from Plant By-Products: A Review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Krygier, K.; Sosulski, F.; Hogge, L. Free, Esterified, and Insoluble-Bound Phenolic Acids. 1. Extraction and Purification Procedure. J. Agric. Food Chem. 1982, 30, 330–334. [Google Scholar] [CrossRef]
- Memariani, Z.; Abbas, S.Q.; Ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and Naringenin as Anticancer Agents and Adjuvants in Cancer Combination Therapy: Efficacy and Molecular Mechanisms of Action, a Comprehensive Narrative Review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef]
- Pang, F.; Ding, S.; Li, N.; Li, Z.; Tian, N.; Shi, C.; Zhang, F.; Mai, Y.; Zhang, J.; Wang, J. Gallic Acid Mediates Tumor-Suppressive Effects on Osteosarcoma through the H19-Wnt/β-Catenin Regulatory Axis. J. Orthop. Transl. 2023, 39, 34–42. [Google Scholar] [CrossRef]
- Roszkowski, S. Application of Polyphenols and Flavonoids in Oncological Therapy. Molecules 2023, 28, 4080. [Google Scholar] [CrossRef]
- Chen, K.; Peng, C.; Chi, F.; Yu, C.; Yang, Q.; Li, Z. Antibacterial and Antibiofilm Activities of Chlorogenic Acid Against Yersinia Enterocolitica. Front. Microbiol. 2022, 13, 885092. [Google Scholar] [CrossRef]
- Krzemińska, M.; Owczarek, A.; Gonciarz, W.; Chmiela, M.; Olszewska, M.A.; Grzegorczyk-Karolak, I. The Antioxidant, Cytotoxic and Antimicrobial Potential of Phenolic Acids-Enriched Extract of Elicited Hairy Roots of Salvia Bulleyana. Molecules 2022, 27, 992. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants-Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Ansary, J.; Gil, E.; Amici, A.; Bompadre, S.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Phenolic Compounds from Mediterranean Foods as Nutraceutical Tools for the Prevention of Cancer: The Effect of Honey Polyphenols on Colorectal Cancer Stem-like Cells from Spheroids. Food Chem. 2020, 325, 126881. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y.; Cheng, N.; Li, F.; Zhao, H.; Bai, N.; El-Seedi, H.R.; Cao, W. Mitigation of DSS-Induced Colitis Potentially via Th1/Th2 Cytokine and Immunological Function Balance Induced by Phenolic-Enriched Buckwheat (Fagopyrum Esculentum Moench) Bee Pollen Extract. Foods 2022, 11, 1293. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Zhang, W.; Liu, C.; Chen, S. Natural Polyphenols in Metabolic Syndrome: Protective Mechanisms and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 6110. [Google Scholar] [CrossRef]
- Krishnan, B.; Ramu Ganesan, A.; Balasubramani, R.; Nguyen, D.D.; Chang, S.W.; Wang, S.; Xiao, J.; Balasubramanian, B. Chrysoeriol Ameliorates Hyperglycemia by Regulating the Carbohydrate Metabolic Enzymes in Streptozotocin-Induced Diabetic Rats. Food Sci. Hum. Wellness 2020, 9, 346–354. [Google Scholar] [CrossRef]
- Suo, H.; Shishir, M.R.I.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K.-W. Red Wine High-Molecular-Weight Polyphenolic Complex: An Emerging Modulator of Human Metabolic Disease Risk and Gut Microbiota. J. Agric. Food Chem. 2021, 69, 10907–10919. [Google Scholar] [CrossRef] [PubMed]
- Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Giampieri, F.; Battino, M.; Dekanski, D. The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders. Nutrients 2022, 14, 5246. [Google Scholar] [CrossRef]
- Zabaleta, M.E.; Forbes-Hernández, T.Y.; Simal-Gandara, J.; Quiles, J.L.; Cianciosi, D.; Bullon, B.; Giampieri, F.; Battino, M. Effect of Polyphenols on HER2-Positive Breast Cancer and Related miRNAs: Epigenomic Regulation. Food Res. Int. 2020, 137, 109623. [Google Scholar] [CrossRef]
- Das, M.; Devi, K.P.; Belwal, T.; Devkota, H.P.; Tewari, D.; Sahebnasagh, A.; Nabavi, S.F.; Khayat Kashani, H.R.; Rasekhian, M.; Xu, S.; et al. Harnessing Polyphenol Power by Targeting eNOS for Vascular Diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 2093–2118. [Google Scholar] [CrossRef]
- Canas, S.; Rebollo-Hernanz, M.; Braojos, C.; Benitez, V.; Ferreras-Charro, R.; Duenas, M.; Aguilera, Y.; Martin-Cabrejas, M.A. Gastrointestinal Fate of Phenolic Compounds and Amino Derivatives from the Cocoa Shell: An in Vitro and in Silico Approach. Food Res. Int. 2022, 162, 112117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Zhang, W.; Ding, Y.; Zhao, T.; Zhang, M.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Bioaccessibility and Biotransformation of Anthocyanin Monomers Following in Vitro Simulated Gastric-Intestinal Digestion and in Vivo Metabolism in Rats. Food Funct. 2019, 10, 6052–6061. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and Metabolism of Three Mulberry Anthocyanin Monomers by Rat Gut Microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Li, M.; Ming, D.; Liu, W.; Xu, X.; Jiang, L. Phase Separation-Mediated Multienzyme Assembly In Vivo. J. Agric. Food Chem. 2025, 73, 7867–7876. [Google Scholar] [CrossRef]
- Tembeni, B.; Idowu, O.E.; Benrkia, R.; Boutahiri, S.; Olatunji, O.J. Biotransformation of Selected Secondary Metabolites by Alternaria Species and the Pharmaceutical, Food and Agricultural Application of Biotransformation Products. Nat. Prod. Bioprospect. 2024, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Yixuan, L.; Qaria, M.A.; Sivasamy, S.; Jianzhong, S.; Daochen, Z. Curcumin Production and Bioavailability: A Comprehensive Review of Curcumin Extraction, Synthesis, Biotransformation and Delivery Systems. Ind. Crops Prod. 2021, 172, 114050. [Google Scholar] [CrossRef]
- Cao, L.; Garcia, S.L.; Wurzbacher, C. Profiling Trace Organic Chemical Biotransformation Genes, Enzymes and Associated Bacteria in Microbial Model Communities. J. Hazard. Mater. 2025, 485, 136811. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, J.; Zhao, X.; Li, X.; Zhang, Y.; Wang, F. Attapulgite-Supported Magnetic Dual Acid-Base Catalyst for the Catalytic Conversion of Lignin to Phenolic Monomers. J. Chem. Technol. Biotechnol. 2019, 94, 1269–1281. [Google Scholar] [CrossRef]
- Feng, J.; Tong, L.; Ma, C.; Xu, Y.; Jiang, J.; Yang, Z.; Pan, H. Directional and Integrated Conversion of Whole Components in Biomass for Levulinates and Phenolics with Biphasic System. Bioresour. Technol. 2020, 315, 123776. [Google Scholar] [CrossRef]
- Mori, T.; Sakata, K.; Shirakawa, S. Direct and Chemoselective Transformation of Cysteine to Dehydroalanine on Peptides. Chem. Commun. 2025, 61, 4800–4803. [Google Scholar] [CrossRef]
- Hu, X.; Ming, C.; Li, Q.; Zhang, L.; Li, C.-Z. Polymerization of Sugar Furan Model Compounds and Bio-Oil during the Acid-Catalyzed Conversion-a Review. Fuel Process. Technol. 2021, 222, 106958. [Google Scholar] [CrossRef]
- Akpabli-Tsigbe, N.D.K.; Osabutey, J.; Mintah, B.K.; Tano-Debrah, K.; Ma, Y. Cleavage of Macromolecule (Protein Polysaccharide)-Phenolic Bond in Soybean Cell Wall through Lactobacillus casei and Lactobacillus helviticus Mixed Culture Solid-State Fermentation for Chlorogenic Acid Extraction. Food Biosci. 2023, 55, 102903. [Google Scholar] [CrossRef]
- Yao, M.; Yang, Y.; Fan, J.; Ma, C.; Liu, X.; Wang, Y.; Wang, B.; Sun, Z.; McClements, D.J.; Zhang, J.; et al. Production, Purification, and Functional Properties of Microbial Fibrinolytic Enzymes Produced by Microorganism Obtained from Soy-Based Fermented Foods: Developments and Challenges. Crit. Rev. Food Sci. Nutr. 2024, 64, 3725–3750. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xu, H.; Pan, J.; Dai, C.; Mintah, B.K.; Dabbour, M.; Zhou, R.; He, R.; Ma, H. Mixed-Strain Fermentation Conditions Screening of Polypeptides from Rapeseed Meal and the Microbial Diversity Analysis by High-Throughput Sequencing. Foods 2022, 11, 3285. [Google Scholar] [CrossRef]
- Mamy, D.; Boateng, I.D.; Chen, X. Two-Pot Optimization of Nutrient Sources to Enhance Antioxidants in Citrus reticulata Peel Powder through Solid-State Fermentation with Aspergillus niger CGMCC 3.6189. Food Biosci. 2024, 59, 104145. [Google Scholar] [CrossRef]
- Yang, S.; Tao, Y.; Maimaiti, X.; Su, W.; Liu, X.; Zhou, J.; Fan, L. Investigation on the Exopolysaccharide Production from Blueberry Juice Fermented with Lactic Acid Bacteria: Optimization, Fermentation Characteristics and Vis-NIR Spectral Model. Food Chem. 2024, 452, 139589. [Google Scholar] [CrossRef]
- Mamy, D.; Boateng, I.D.; Chen, X. Metabolomic Changes in Citrus reticulata Peel after Conventional and Ultrasound-Assisted Solid-State Fermentation with Aspergillus niger: A Focus on Flavonoid Metabolism. Food Chem. 2025, 467, 142224. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, B.; Sirhindi, G.; Guleria, N.; Kaur, J. Phenolic Biotransformations in Wheatgrass Juice after Primary and Secondary Fermentation. Foods 2023, 12, 1624. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, L.; Wang, H.; Zhang, Z.; Zhou, J.; Jiao, N. Development of a CRISPR/Cas9n-Based Tool for Metabolic Engineering of Pseudomonas putida for Ferulic Acid-to-Polyhydroxyalkanoate Bioconversion. Commun. Biol. 2020, 3, 98. [Google Scholar] [CrossRef]
- Slama, N.; Mankai, H.; Limam, F. Streptomyces tunisiensis DSM 42037 Mediated Bioconversion of Ferulic Acid Released from Barley Bran. World J. Microbiol. Biotechnol. 2021, 37, 70. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation Transforms the Phenolic Profiles and Bioactivities of Plant-Based Foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Wang, J.; Wei, B.-C.; Wei, B.; Yu, H.-Y.; Thakur, K.; Wang, C.-Y.; Wei, Z.-J. Evaluation of Phenolics Biotransformation and Health Promoting Properties of Blueberry Juice Following Lactic Acid Bacteria Fermentation. Food Sci. Technol. 2023, 43, e104522. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, C.; Cai, J.; Zhang, C.; Li, Y.; Xu, B.; Farooq, M.A. The Synergistic Inhibition and Mechanism of Epicatechin Gallate and Chitosan against Methicillin-Resistant Staphylococcus aureus and the Application in Pork Preservation. LWT 2022, 163, 113575. [Google Scholar] [CrossRef]
- Liu, J.; Zhuang, Y.; Hu, Y.; Xue, S.; Li, H.; Chen, L.; Fei, P. Improving the Color Stability and Antioxidation Activity of Blueberry Anthocyanins by Enzymatic Acylation with p-Coumaric Acid and Caffeic Acid. LWT 2020, 130, 109673. [Google Scholar] [CrossRef]
- Ji, T.; Liaqat, F.; Khazi, M.I.; Liaqat, N.; Nawaz, M.Z.; Zhu, D. Lignin Biotransformation: Advances in Enzymatic Valorization and Bioproduction Strategies. Ind. Crops Prod. 2024, 216, 118759. [Google Scholar] [CrossRef]
- Ai, J.; Yang, Z.; Liu, J.; Schols, H.A.; Battino, M.; Bao, B.; Tian, L.; Bai, W. Structural Characterization and In Vitro Fermentation Characteristics of Enzymatically Extracted Black Mulberry Polysaccharides. J. Agric. Food Chem. 2022, 70, 3654–3665. [Google Scholar] [CrossRef]
- Ni, W.; Zhang, P.; Long, L.; Ding, S. Engineering and Linker-Mediated Co-Immobilization of Carotenoid Cleavage Oxygenase with Phenolic Acid Decarboxylase for Efficiently Converting Ferulic Acid into Vanillin. Process Biochem. 2022, 122, 67–77. [Google Scholar] [CrossRef]
- Han, J.; Feng, H.; Wu, J.; Li, Y.; Zhou, Y.; Wang, L.; Luo, P.; Wang, Y. Construction of Multienzyme Co-Immobilized Hybrid Nanoflowers for an Efficient Conversion of Cellulose into Glucose in a Cascade Reaction. J. Agric. Food Chem. 2021, 69, 7910–7921. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; Wang, M.; Yu, X.; Cui, Y.; Xu, L.; Ma, A.; Ding, Z.; Huo, S.; Zou, B.; et al. Application of Immobilized Enzymes in Juice Clarification. Foods 2023, 12, 4258. [Google Scholar] [CrossRef]
- Wang, H.; Qi, X.; Gao, S.; Kan, G.; Damdindorj, L.; An, Y.; Lu, F. Characterization of a Novel Multifunctional β-Glucosidase/Xylanase/Feruloyl Esterase and Its Effects on Improving the Quality of Longjing Tea. Food Chem. 2024, 453, 139637. [Google Scholar] [CrossRef] [PubMed]
- Heng, X.; Chen, H.; Lu, C.; Feng, T.; Li, K.; Gao, E. Study on Synergistic Fermentation of Bean Dregs and Soybean Meal by Multiple Strains and Proteases. LWT 2022, 154, 112626. [Google Scholar] [CrossRef]
- Ai, J.; Tang, X.; Mao, B.; Zhang, Q.; Zhao, J.; Chen, W.; Cui, S. Gut Microbiota: A Superior Operator for Dietary Phytochemicals to Improve Atherosclerosis. Crit. Rev. Food Sci. Nutr. 2024, 1–23. [Google Scholar] [CrossRef]
- Spencer, G.W.K.; Li, X.; Lam, K.W.L.; Mutch, G.; Fry, F.H.; Gras, S.L. Codeine 3-O-Demethylase Catalyzed Biotransformation of Morphinan Alkaloids in Escherichia Coli: Site Directed Mutagenesis of Terminal Residues Improves Enzyme Expression, Stability and Biotransformation Yield. J. Biol. Eng. 2025, 19, 9. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Forstater, J.H.; McIntosh, J.A. Decarboxylation in Natural Products Biosynthesis. JACS Au 2024, 4, 2715–2745. [Google Scholar] [CrossRef]
- Lomascolo, A.; Odinot, E.; Villeneuve, P.; Lecomte, J. Challenges and Advances in Biotechnological Approaches for the Synthesis of Canolol and Other Vinylphenols from Biobased p-Hydroxycinnamic Acids: A Review. Biotechnol. Biofuels Bioprod. 2023, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, R.J.M.; de Vries, R.P. Production of Protocatechuic Acid from p-Hydroxyphenyl (H) Units and Related Aromatic Compounds Using an Aspergillus niger Cell Factory. mBio 2021, 12, e00391-21. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Q.; Wang, T.; Kan, Z.; Li, X.; Hu, L.; Peng, C.; Qian, F.; Wang, Y.; Granato, D. Green Tea Polyphenols and Epigallocatechin-3-Gallate Protect against Perfluorodecanoic Acid Induced Liver Damage and Inflammation in Mice by Inhibiting NLRP3 Inflammasome Activation. Food Res. Int. 2020, 127, 108628. [Google Scholar] [CrossRef]
- Chen, J.; Jia, X.; Hu, Y.; Zhao, X.; Cheng, Y.; Lu, L.; Zhong, S.; You, J.; Zou, T. Benzoic Acid as a Dietary Supplement Mitigates Inflammation and Intestinal Injury in Acute Enterotoxigenic Escherichia Coli-Infected Mice without Adverse Effects in Healthy Mice. Food Funct. 2025, 16, 3195–3210. [Google Scholar] [CrossRef]
- Gaur, G.; Oh, J.-H.; Filannino, P.; Gobbetti, M.; van Pijkeren, J.-P.; Gänzle, M.G. Genetic Determinants of Hydroxycinnamic Acid Metabolism in Heterofermentative Lactobacilli. Appl. Environ. Microbiol. 2020, 86, e02461. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Zeng, Y.; Yang, N.; Jiang, Y.; Suo, Y. Enhanced Tolerance of Clostridium Tyrobutyricum to Lignin-Derived Phenolic Acids by Overexpressing Native Reductases. J. Biotechnol. 2025, 404, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Marc, G.; Stana, A.; Tertis, M.; Cristea, C.; Ciorita, A.; Dragan, S.-M.; Toma, V.-A.; Borlan, R.; Focsan, M.; Pirnau, A.; et al. Discovery of New Hydrazone-Thiazole Polyphenolic Antioxidants through Computer-Aided Design and In Vitro Experimental Validation. Int. J. Mol. Sci. 2023, 24, 13277. [Google Scholar] [CrossRef]
- Zieniuk, B. Dihydrocaffeic Acid-Is It the Less Known but Equally Valuable Phenolic Acid? Biomolecules 2023, 13, 859. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of Polyphenols in Apple Pomace: A Comparative Study of Different Extraction and Hydrolysis Procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Bel-Rhlid, R.; Thapa, D.; Kraehenbuehl, K.; Hansen, C.E.; Fischer, L. Biotransformation of Caffeoyl Quinic Acids from Green Coffee Extracts by Lactobacillus johnsonii NCC 533. AMB Express 2013, 3, 28. [Google Scholar] [CrossRef]
- Nieter, A.; Kelle, S.; Takenberg, M.; Linke, D.; Bunzel, M.; Popper, L.; Berger, R.G. Heterologous Production and Characterization of a Chlorogenic Acid Esterase from Ustilago maydis with a Potential Use in Baking. Food Chem. 2016, 209, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, Y.; Guan, T.; Zhang, Y.; Huang, K.; Zhang, Z.; Cao, W.; Guan, X. Quinic Acid Alleviates High-Fat Diet-Induced Neuroinflammation by Inhibiting DR3/IKK/NF-κB Signaling via Gut Microbial Tryptophan Metabolites. Gut Microbes. 2024, 16, 2374608. [Google Scholar] [CrossRef]
- Ahmad, A.; Javed, S. Biotransformation of Fruit Wastes into Bio-Vanillin: A Natural Aromatic Flavoring Compound. Biomass Convers. Biorefin. 2025, 15, 8267–8286. [Google Scholar] [CrossRef]
- Hu, H.; Li, L.; Ding, S. An Organic Solvent-Tolerant Phenolic Acid Decarboxylase from Bacillus licheniformis for the Efficient Bioconversion of Hydroxycinnamic Acids to Vinyl Phenol Derivatives. Appl. Microbiol. Biotechnol. 2015, 99, 5071–5081. [Google Scholar] [CrossRef]
- Hu, J.; Vinothkanna, A.; Wu, M.; Ekumah, J.; Akpabli-Tsigbe, N.D.K.; Ma, Y. Tracking the Dynamic Changes of a Flavor, Phenolic Profile, and Antioxidant Properties of Lactiplantibacillus plantarum- and Saccharomyces cerevisiae-fermented Mulberry Wine. Food Sci. Nutr. 2021, 9, 6294–6306. [Google Scholar] [CrossRef]
- Lentz, M. The Impact of Simple Phenolic Compounds on Beer Aroma and Flavor. Fermentation 2018, 4, 20. [Google Scholar] [CrossRef]
- Akpabli-Tsigbe, N.D.K.; Ma, Y.; Ekumah, J.-N.; Osabutey, J.; Hu, J.; Xu, M.; Johnson, N.A.N. Novel Solid-State Fermentation Extraction of 5-O-Caffeoylquinic Acid from Heilong48 Soybean Using Lactobacillus helviticus: Parametric Screening and Optimization. LWT 2021, 149, 111809. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Zhang, Z.-H.; Qiao, Z.-R.; Cai, W.-D.; Yan, J.-K. Construction and Characterization of Antioxidative Ferulic Acid-Grafted Carboxylic Curdlan Conjugates and Their Contributions on 8-Carotene Storage Stability. Food Chem. 2021, 349, 129166. [Google Scholar] [CrossRef]
- Skała, E.; Olszewska, M.A.; Makowczyńska, J.; Kicel, A. Effect of Sucrose Concentration on Rhaponticum Carthamoides (Willd.) Iljin Transformed Root Biomass, Caffeoylquinic Acid Derivative, and Flavonoid Production. Int. J. Mol. Sci. 2022, 23, 13848. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Eborall, W.; Hyde, R.; Hart, S.; Turkenburg, J.P.; Grogan, G. Mutational Analysis of Phenolic Acid Decarboxylase from Bacillus subtilis (BsPAD), Which Converts Bio-Derived Phenolic Acids to Styrene Derivatives. Catal. Sci. Technol. 2012, 2, 1568–1574. [Google Scholar] [CrossRef]
- Sheldon, R.A. Biocatalysis and Biomass Conversion: Enabling a Circular Economy. Philos. Trans. A Math. Phys. Eng. Sci. 2020, 378, 20190274. [Google Scholar] [CrossRef]
- Intasian, P.; Prakinee, K.; Phintha, A.; Trisrivirat, D.; Weeranoppanant, N.; Wongnate, T.; Chaiyen, P. Enzymes, In Vivo Biocatalysis, and Metabolic Engineering for Enabling a Circular Economy and Sustainability. Chem. Rev. 2021, 121, 10367–10451. [Google Scholar] [CrossRef]
- Degrassi, G.; Polverino De Laureto, P.; Bruschi, C.V. Purification and Characterization of Ferulate and p-Coumarate Decarboxylase from Bacillus pumilus. Appl. Environ. Microbiol. 1995, 61, 326–332. [Google Scholar] [CrossRef]
- Maeda, M.; Tokashiki, M.; Tokashiki, M.; Uechi, K.; Ito, S.; Taira, T. Characterization and Induction of Phenolic Acid Decarboxylase from Aspergillus luchuensis. J. Biosci. Bioeng. 2018, 126, 162–168. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, C.-Z.; Sawadogo, R.; Yuan, J.; Zeng, J.; Xu, M.; Tan, T.; Yuan, C.-S. 4-Vinylguaiacol, an Active Metabolite of Ferulic Acid by Enteric Microbiota and Probiotics, Possesses Significant Activities against Drug-Resistant Human Colorectal Cancer Cells. ACS Omega 2021, 6, 4551–4561. [Google Scholar] [CrossRef]
- Huang, H.-K.; Tokashiki, M.; Maeno, S.; Onaga, S.; Taira, T.; Ito, S. Purification and Properties of Phenolic Acid Decarboxylase from Candida guilliermondii. J. Ind. Microbiol. Biotechnol. 2012, 39, 55–62. [Google Scholar] [CrossRef]
- Gu, W.; Yang, J.; Lou, Z.; Liang, L.; Sun, Y.; Huang, J.; Li, X.; Cao, Y.; Meng, Z.; Zhang, K.-Q. Structural Basis of Enzymatic Activity for the Ferulic Acid Decarboxylase (FADase) from Enterobacter sp. Px6-4. PLoS ONE 2011, 6, e16262. [Google Scholar] [CrossRef]
- Sheng, X.; Lind, M.E.S.; Himo, F. Theoretical Study of the Reaction Mechanism of Phenolic Acid Decarboxylase. FEBS J. 2015, 282, 4703–4713. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Liu, Y. Mechanistic Insights into the Catalytic Reaction of Ferulic Acid Decarboxylase from Aspergillus niger: A QM/MM Study. Phys. Chem. Chem. Phys. 2017, 19, 7733–7742. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xia, Y.; Zhao, T.; Gong, Y.; Fang, S.; Chen, M. Improving the Catalytic Characteristics of Phenolic Acid Decarboxylase from Bacillus amyloliquefaciens by the Engineering of N-Terminus and C-Terminus. BMC Biotechnol. 2021, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-K.; Chen, L.-F.; Tokashiki, M.; Ozawa, T.; Taira, T.; Ito, S. An Endogenous Factor Enhances Ferulic Acid Decarboxylation Catalyzed by Phenolic Acid Decarboxylase from Candida guilliermondii. AMB Express 2012, 2, 4. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Dong, L.; Li, Y.; Liu, Y.; Liu, Y.; Liu, L.; Liu, L. Fermentation of Lactobacillus fermentum NB02 with Feruloyl Esterase Production Increases the Phenolic Compounds Content and Antioxidant Properties of Oat Bran. Food Chem. 2024, 437, 137834. [Google Scholar] [CrossRef]
- Rauwerdink, A.; Kazlauskas, R.J. How the Same Core Catalytic Machinery Catalyzes 17 Different Reactions: The Serine-Histidine-Aspartate Catalytic Triad of α/β-Hydrolase Fold Enzymes. ACS Catal. 2015, 5, 6153–6176. [Google Scholar] [CrossRef]
- Silveira, R.L.; Knott, B.C.; Pereira, C.S.; Crowley, M.F.; Skaf, M.S.; Beckham, G.T. Transition Path Sampling Study of the Feruloyl Esterase Mechanism. J. Phys. Chem. B. 2021, 125, 2018–2030. [Google Scholar] [CrossRef]
- Xu, Y.; Minhazul, K.A.H.M.; Wang, X.; Liu, X.; Li, X.; Meng, Q.; Li, H.; Zhang, C.; Sun, X.; Sun, B. Biodegradation of Phthalate Esters by Paracoccus kondratievae BJQ0001 Isolated from Jiuqu (Baijiu Fermentation Starter) and Identification of the Ester Bond Hydrolysis Enzyme. Environ. Pollut. 2020, 263, 114506. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; He, W.; Song, X.; Peng, Y.; Hu, X.; Bian, S.; Li, Y.; Nie, S.; Yin, J.; et al. Exploring the Biogenic Transformation Mechanism of Polyphenols by Lactobacillus plantarum NCU137 Fermentation and Its Enhancement of Antioxidant Properties in Wolfberry Juice. J. Agric. Food Chem. 2024, 72, 12752–12761. [Google Scholar] [CrossRef]
- Lin, X.B.; Gänzle, M.G. Quantitative High-Resolution Melting PCR Analysis for Monitoring of Fermentation Microbiota in Sourdough. Int. J. Food Microbiol. 2014, 186, 42–48. [Google Scholar] [CrossRef]
- Gaur, G.; Gänzle, M.G. Conversion of (Poly)Phenolic Compounds in Food Fermentations by Lactic Acid Bacteria: Novel Insights into Metabolic Pathways and Functional Metabolites. Curr. Res. Food Sci. 2023, 6, 100448. [Google Scholar] [CrossRef]
- Gaur, G.; Gänzle, M. Biochemical Characterization of HcrF from Limosilactobacillus Fermentum, a NADH-Dependent 2-Ene Reductase with Activity on Hydroxycinnamic Acids. Lett. Appl. Microbiol. 2024, 77, ovae109. [Google Scholar] [CrossRef]
- Santamaría, L.; Reverón, I.; de Felipe, F.L.; de Las Rivas, B.; Muñoz, R. Ethylphenol Formation by Lactobacillus plantarum: Identification of the Enzyme Involved in the Reduction of Vinylphenols. Appl. Environ. Microbiol. 2018, 84, e01064-18. [Google Scholar] [CrossRef]
- Han, J.; Fang, S.; He, X.; Wang, L.; Li, C.; Wu, J.; Cai, Y.; Wang, Y. Combination of Aqueous Two-Phase Flotation and Inverse Transition Cycling: Strategies for Separation and Purification of Recombinant β-Glucosidase from Cell Lysis Solution. Food Chem. 2022, 373, 131543. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically Bound Aroma Precursors in Fruits: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, J.; Tang, N.; Chen, W.; Gu, Q.; Li, H. Screening, Cloning, Immobilization and Application Prospects of a Novel β-Glucosidase from the Soil Metagenome. Environ. Res. 2024, 244, 117676. [Google Scholar] [CrossRef]
- Zhong, S.; Yan, M.; Zou, H.; Zhao, P.; Ye, H.; Zhang, T.; Zhao, C. Spectroscopic and in Silico Investigation of the Interaction between GH1 β-Glucosidase and Ginsenoside Rb1. Food Sci. Nutr. 2021, 9, 1917–1928. [Google Scholar] [CrossRef]
- Qu, Y.; Luo, Y.; Yang, X.; Zhang, Y.; Yang, E.; Xu, H.; He, Y.; Chagan, I.; Yan, J. Highly Efficient Biotransformation of Phenolic Glycosides Using a Recombinant β-Glucosidase from White Rot Fungus Trametes Trogii. Front. Microbiol. 2022, 13, 762502. [Google Scholar] [CrossRef]
- Liu, X.; Van Acker, R.; Voorend, W.; Pallidis, A.; Goeminne, G.; Pollier, J.; Morreel, K.; Kim, H.; Muylle, H.; Bosio, M.; et al. Rewired Phenolic Metabolism and Improved Saccharification Efficiency of a Zea Mays Cinnamyl Alcohol Dehydrogenase 2 (Zmcad2) Mutant. Plant J. 2021, 105, 1240–1257. [Google Scholar] [CrossRef]
- Shan, B.; Mo, J.; Yang, J.; Qin, X.; Yu, H. Cloning and Functional Characterization of a Cinnamate 4-Hydroxylase Gene from the Hornwort Anthoceros angustus. Plant Sci. 2024, 341, 111989. [Google Scholar] [CrossRef]
- Rodríguez, H.; Angulo, I.; de las Rivas, B.; Campillo, N.; Páez, J.A.; Muñoz, R.; Mancheño, J.M. p-Coumaric Acid Decarboxylase from Lactobacillus plantarum: Structural Insights into the Active Site and Decarboxylation Catalytic Mechanism. Proteins Struct. Funct. Bioinf. 2010, 78, 1662–1676. [Google Scholar] [CrossRef]
- Holck, J.; Fredslund, F.; Møller, M.S.; Brask, J.; Krogh, K.B.; Lange, L.; Welner, D.H.; Svensson, B.; Meyer, A.S.; Wilkens, C. A carbohydrate-binding family 48 module enables feruloyl esterase action on polymeric arabinoxylan. J. Biol. Chem. 2019, 294, 17339–17353. [Google Scholar] [CrossRef]
- Finnigan, W.; Hepworth, L.J.; Flitsch, S.L.; Turner, N.J. RetroBioCat as a Computer-Aided Synthesis Planning Tool for Biocatalytic Reactions and Cascades. Nat. Catal. 2021, 4, 98–104. [Google Scholar] [CrossRef]
- Myrtollari, K.; Calderini, E.; Kracher, D.; Schöngaßner, T.; Galušić, S.; Slavica, A.; Taden, A.; Mokos, D.; Schrüfer, A.; Wirnsberger, G.; et al. Stability Increase of Phenolic Acid Decarboxylase by a Combination of Protein and Solvent Engineering Unlocks Applications at Elevated Temperatures. ACS Sustain. Chem. Eng. 2024, 12, 3575–3584. [Google Scholar] [CrossRef]
- Wang, K.; Jin, W.; Ding, Y.; Lyu, Y.; Liu, J.; Yu, X. Dual Enzyme Co-Immobilization on Reversibly Soluble Polymers for the One-Pot Conversion of Ferulic Acid from Wheat Bran. New J. Chem. 2022, 46, 7734–7740. [Google Scholar] [CrossRef]
- Lopez, I.L.; Sanchez-Costa, M.; Orrego, A.H.; Zeballos, N.; Padrosa, D.R.; Lopez-Gallego, F. Microtiter Plate Immobilization Screening for Prototyping Heterogeneous Enzyme Cascades. Angew. Chem.-Int. Edit. 2024, 63, e202407411. [Google Scholar] [CrossRef]
- Detering, T.; Mundry, K.; Berger, R.G. Generation of 4-Vinylguaiacol through a Novel High-Affinity Ferulic Acid Decarboxylase to Obtain Smoke Flavours without Carcinogenic Contaminants. PLoS ONE 2020, 15, e0244290. [Google Scholar] [CrossRef]
- Myrtollari, K.; Chánique, A.M.; Kracher, D.; Herrera, D.P.; Gutierrez Benavente, J.; Schüller, A.; Kourist, R. Engineering Substrate Acceptance of Resveratrol O-Methyltransferase from Vitis Vinifera for the Selective Synthesis of O-Methyl Protected Biobased Hydroxystyrenes. ChemCatChem 2025, 0, e202402027. [Google Scholar] [CrossRef]
- Petermeier, P.; Bittner, J.P.; Jonsson, T.; Domínguez de María, P.; Byström, E.; Kara, S. Integrated Preservation of Water Activity as Key to Intensified Chemoenzymatic Synthesis of Bio-Based Styrene Derivatives. Commun. Chem. 2024, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chao, H.; Guo, M.; Wang, R.; Gu, D.; Wang, Y.; He, D.; Yang, Y. Biomimetic Construction of Multi-Enzyme Reactor through Artificial Antibody-Antigen-Directed Immobilization for Cascade Catalytic Conversion of Cellulose to Gluconic Acid. Food Chem. 2025, 475, 143262. [Google Scholar] [CrossRef] [PubMed]
- Naaz, T.; Kim, B.S. Use of Nicotinamide Mononucleotide as Non-Natural Cofactor. Catalysts 2025, 15, 37. [Google Scholar] [CrossRef]
- Fokum, E.; Zabed, H.M.; Guo, Q.; Yun, J.; Yang, M.; Pang, H.; An, Y.; Li, W.; Qi, X. Metabolic Engineering of Bacterial Strains Using CRISPR/Cas9 Systems for Biosynthesis of Value-Added Products. Food Biosci. 2019, 28, 125–132. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, M.; Izquierdo-Canas, P.M.; Garcia-Romero, E.; Paniagua-Martinez, T.; Gomez-Alonso, S. The Effects of a Saccharomyces cerevisiae Strain Overexpressing the Endopolygalacturonase PGU1 Gene on the Aminoacidic, Volatile, and Phenolic Compositions of Cabernet Sauvignon Wines. Fermentation 2024, 10, 375. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, C.; Bian, X.; Lu, W. Metabolic Engineering of Saccharomyces Cerevisiae for Heterologous Carnosic Acid Production. Front. Bioeng. Biotechnol. 2022, 10, 916605. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, B.; Bai, J.; Fan, S.; Daglia, M.; Li, J.; Zhao, Y.; He, Y.; Zhu, L.; Xiao, X. Heterologous Expression and Enzymatic Characteristics of Sulfatase from Lactobacillus plantarum Dy-1. Food Funct. 2024, 15, 5439–5449. [Google Scholar] [CrossRef]
- Li, J.; Lu, X.; Zou, X.; Ye, B.-C. Recent Advances in Microbial Metabolic Engineering for Production of Natural Phenolic Acids. J. Agric. Food Chem. 2024, 72, 4538–4551. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Gao, J.; Yu, W.; Chen, X.; Zhai, X.; Chen, Y.; Zhang, L.; Zhou, Y.J. Engineering Cofactor Supply and Recycling to Drive Phenolic Acid Biosynthesis in Yeast. Nat. Chem. Biol. 2022, 18, 520–529. [Google Scholar] [CrossRef]
- Lemke, P.; Schneider, L.; Kunz, W.; Rieck, A.L.; Jäger, P.S.; Bruckmann, A.; Nestler, B.; Rabe, K.S.; Niemeyer, C.M. Flow-induced Microfluidic Assembly for Advanced Biocatalysis Materials. Adv. Funct. Mater. 2024, 34, 2313944. [Google Scholar] [CrossRef]
- Ma, H.; Huang, S.; Li, F.; Pang, Z.; Luo, J.; Sun, D.; Liu, J.; Chen, Z.; Qu, J.; Qu, Q. Development and Validation of an Automatic Machine Learning Model to Predict Abnormal Increase of Transaminase in Valproic Acid-Treated Epilepsy. Arch. Toxicol. 2024, 98, 3049–3061. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic Compounds in Fruits—An Overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Shamanin, V.P.; Tekin-Cakmak, Z.H.; Gordeeva, E.I.; Karasu, S.; Pototskaya, I.; Chursin, A.S.; Pozherukova, V.E.; Ozulku, G.; Morgounov, A.I.; Sagdic, O.; et al. Antioxidant Capacity and Profiles of Phenolic Acids in Various Genotypes of Purple Wheat. Foods 2022, 11, 2515. [Google Scholar] [CrossRef]
- Wang, X.; Tsang, Y.F.; Li, Y.; Ma, X.; Cui, S.; Zhang, T.-A.; Hu, J.; Gao, M.-T. Inhibitory Effects of Phenolic Compounds of Rice Straw Formed by Saccharification during Ethanol Fermentation by Pichia stipitis. Bioresour. Technol. 2017, 244, 1059–1067. [Google Scholar] [CrossRef]
- Aalim, H.; Hashim, S.B.H.; Zhou, C.; Zou, X.; Luo, Z. Matrix Characteristics Modulate Black Rice Phenolic Compounds Bioaccessibility and Antioxidant Activity during Simulated Gastrointestinal Digestion. Food Biosci. 2024, 58, 103628. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal Dietary Fibre: A Natural Functional Ingredient to Deliver Phenolic Compounds into the Gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, F.; Luo, H.; He, W.; Li, D.; Bao, Y.; Zhang, Z.; Zhou, C. Changes in Phytochemical Profiles, Relevant Enzyme Activity and Antioxidant Capacity of Different Germinated Maize Varieties. Food Biosci. 2023, 56, 103410. [Google Scholar] [CrossRef]
- Varga, M.; Jójárt, R.; Fónad, P.; Mihály, R.; Palágyi, A. Phenolic Composition and Antioxidant Activity of Colored Oats. Food Chem. 2018, 268, 153–161. [Google Scholar] [CrossRef]
- Rao, M.; Muralikrishna, G. Evaluation of the Antioxidant Properties of Free and Bound Phenolic Acids from Native and Malted Finger Millet (Ragi, Eleusine Coracana Indaf-15). J. Agric. Food Chem. 2002, 50, 889–892. [Google Scholar] [CrossRef]
- Boasiako, T.A.; Hua, F.; YuQing, X.; Boateng, I.D.; Ma, Y. Enzymatic Catalytic Dynamics of Lactic-Acetic Acid Co-Fermentation: Effect of Cellulase on the Physicochemical, Phytochemicals, Volatiles, and Antioxidant Activity of Jujube Puree Extracts. Ind. Crops Prod. 2024, 222, 119590. [Google Scholar] [CrossRef]
- Chen, C.; Yao, Y.; Wang, X.; Chen, W.; Wang, L. Interaction of Oat Avenanthramides with Starch and Effects on in Vitro Avenanthramide Bioaccessibility and Starch Digestibility. Food Chem. 2024, 437, 137770. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.; Yarley, O.P.N.; Wang, Y.; Wang, Y.; Zhang, A.; Fan, X.; Zhou, C.; Lv, W. Effects of Combined Drying Techniques and Cellulase Hydrolysis on the Nutritional Value and Sensory Properties of Shiitake Mushrooms (Lentinus edodes). Food Chem. 2024, 450, 139387. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, D.; Li, T.; Zhao, W.; Chen, G. Application of Cellulase for Contributing Phenolic Release and Conversion in Oats (Avena sativa L.) during Microbial Fermentation. Appl. Biochem. Biotechnol. 2023, 195, 4277–4291. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Motosoko, M.; Tokashiki, T.; Tokashiki, J.; Mizutani, O.; Uechi, K.; Goto, M.; Taira, T. Phenolic Acid Decarboxylase of Aspergillus luchuensis Plays a Crucial Role in 4-Vinylguaiacol Production during Awamori Brewing. J. Biosci. Bioeng. 2020, 130, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Gaur, G.; Chen, C.; Gänzle, M.G. Characterization of Isogenic Mutants with Single or Double Deletions of Four Phenolic Acid Esterases in Lactiplantibacillus plantarum TMW1.460. Int. J. Food Microbiol. 2023, 388, 110100. [Google Scholar] [CrossRef]
- Gaur, G.; Damm, S.; Passon, M.; Lo, H.K.; Schieber, A.; Gänzle, M.G. Conversion of Hydroxycinnamic Acids by Furfurilactobacillus milii in Sorghum Fermentations: Impact on Profile of Phenolic Compounds in Sorghum and on Ecological Fitness of Ff. milii. Food Microbiol. 2023, 111, 104206. [Google Scholar] [CrossRef]
- Ripari, V.; Bai, Y.; Gänzle, M.G. Metabolism of Phenolic Acids in Whole Wheat and Rye Malt Sourdoughs. Food Microbiol. 2019, 77, 43–51. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, D.; Xu, W.; Liu, H.; Pang, M.; Chen, G. Regulation of the Phenolic Release and Conversion in Oats (Avena sativa L.) by Co-Microbiological Fermentation with Monascus anka, Saccharomyces cerevisiae and Bacillus subtilis. Bioprocess. Biosyst. Eng. 2025, 48, 287–299. [Google Scholar] [CrossRef]
- Jitpakdee, J.; Yamashita, H.; Nakagawa, T.; Nitoda, T.; Kanzaki, H. Solid-State Cultivation of Multiple Industrial Strains of Koji Mold on Different Thai Unpolished Rice Cultivars: Biotransformation of Phenolic Compounds and Their Effects on Antioxidant Activity. Biosci. Biotechnol. Biochem. 2024, 88, 1117–1125. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Mattila, O.; Katina, K.; Poutanen, K.; Aura, A.-M.; Hanhineva, K. Metabolic Profiling of Sourdough Fermented Wheat and Rye Bread. Sci. Rep. 2018, 8, 5684. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Hedberg, M.; Shi, L.; Johansson, A.; Savolainen, O.; Lehtonen, M.; Aura, A.; Hanhineva, K.; Landberg, R. Metabolite Pattern Derived from Lactiplantibacillus plantarum—Fermented Rye Foods and In Vitro Gut Fermentation Synergistically Inhibits Bacterial Growth. Mol. Nutr. Food Res. 2022, 66, 2101096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, M.; Zhao, Y.; Zhu, Y.; Bai, J.; Fan, S.; Zhu, L.; Song, C.; Xiao, X. Recent Developments in Fermented Cereals on Nutritional Constituents and Potential Health Benefits. Foods 2022, 11, 2243. [Google Scholar] [CrossRef] [PubMed]
- Obadi, M.; Xu, B. Effect of Processing Methods and Storage on the Bioactive Compounds of Black Rice (Oryza sativa L.): A Review. Food Funct. 2023, 14, 9100–9122. [Google Scholar] [CrossRef]
- Hellström, J.; Karhu, S.; Karhu, J.; Järvenpää, E.; Välimaa, A.-L. Phenolic Profiles Differentiate Wild Bilberry and Cultivated Blueberry Fruit. LWT 2024, 199, 116080. [Google Scholar] [CrossRef]
- Simat, V.; Cagalj, M.; Mekinic, I.G.; Mozina, S.S.; Malin, V.; Tabanelli, G.; Ozogul, F.; Skroza, D. Antioxidant and Antimicrobial Activity of Extracts from Selected Mediterranean Agro-Food by-Products, Their Mutual Interaction and Interaction with Phenolic Compounds. Food Biosci. 2024, 61, 104599. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q. Aroma Characteristics of Volatile Compounds Brought by Variations in Microbes in Winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef]
- Smit, A.; Cordero Otero, R.R.; Lambrechts, M.G.; Pretorius, I.S.; van Rensburg, P. Enhancing Volatile Phenol Concentrations in Wine by Expressing Various Phenolic Acid Decarboxylase Genes in Saccharomyces cerevisiae. J. Agric. Food Chem. 2003, 51, 4909–4915. [Google Scholar] [CrossRef]
- Deng, H.; Gu, Q.; Yu, X.; Zhou, J.; Liu, X. Surface-Displayed Phenolic Acid Decarboxylase for Increased Vinylphenolic Pyranoanthocyanins in Blueberry Wine. Curr. Res. Food Sci. 2024, 8, 100730. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of Lactobacillus Strains on Phenolic Profile, Color Attributes and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Maoloni, A.; Del Rio, D.; Calani, L.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation. Nutrients 2019, 11, 213. [Google Scholar] [CrossRef]
- Li, X.; Xia, X.; Wang, Z.; Wang, Y.; Dai, Y.; Yin, L.; Xu, Z.; Zhou, J. Cloning and Expression of Lactobacillus brevis Β-glucosidase and Its Effect on the Aroma of Strawberry Win. J. Food Process. Preserv. 2022, 46, e16368. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Xiao, L.; Li, X.; Hu, M. Effect of Fermentation Parameters and Their Optimization on the Phytochemical Properties of Lactic-Acid-Fermented Mulberry Juice. Food Meas. 2017, 11, 1462–1473. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of Lactic Acid Fermentation-Based Biotransformation on Phenolic Profiles, Antioxidant Capacity and Flavor Volatiles of Apple Juice. LWT 2020, 122, 109064. [Google Scholar] [CrossRef]
- Kucukgoz, K.; Echave, J.; Garcia-Oliveira, P.; Seyyedi-Mansour, S.; Donn, P.; Xiao, J.; Trzaskowska, M.; Prieto, M.A. Polyphenolic Profile, Processing Impact, and Bioaccessibility of Apple Fermented Products. Crit. Rev. Food Sci. Nutr. 2025, 65, 507–526. [Google Scholar] [CrossRef]
- Sarpong, F.; Oteng-Darko, P.; Golly, M.K.; Amenorfe, L.P.; Rashid, M.T.; Zhou, C. Comparative Study of Enzymes Inactivation and Browning Pigmentation of Apple (Malus Domestica) Slices by Selected Gums during Low Temperature Storage. J. Food Biochem. 2018, 42, e12681. [Google Scholar] [CrossRef]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in Color Expression and Antioxidant Activity of Strawberry Juice Fermented with Lactic Acid Bacteria: A Phenolic-Based Research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef]
- Godoy, L.; García, V.; Peña, R.; Martínez, C.; Ganga, M.A. Identification of the Dekkera bruxellensis Phenolic Acid Decarboxylase (PAD) Gene Responsible for Wine Spoilage. Food Control 2014, 45, 81–86. [Google Scholar] [CrossRef]
- Duan, Y.; Meng, F.; Manickam, S.; Zhu, X.; Yang, J.; Han, Y.; Tao, Y. Four Distinct Pathways Involved in a “Tug-of-War” Lead to the Non-Linear Nature of Phenolic Chemistry during Lactic Acid Fermentation of Fruits and Vegetables. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Q.; Huang, K.; Huang, Z.; Ding, W.; Sun, J.; Bai, W. Accelerating the Conversion of Black Chokeberry Anthocyanins toward Vinylphenolic Pyranoanthocyanins by Displaying Phenolic Acid Decarboxylase from Lactiplantibacillus plantarum on the Surface of Pichia pastoris. Food Chem. 2025, 476, 143408. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, M.; Zhang, S.; Wang, J.; Zhang, R.; Dong, L.; Huang, F.; Su, D.; Deng, M. Unveiling Biotransformation of Free Flavonoids into Phenolic Acids and Chromones alongside Dynamic Migration of Bound Phenolics in Lactobacillus-Fermented Lychee Pulp. Food Chem. 2024, 457, 140115. [Google Scholar] [CrossRef]
- Lv, M.; Liu, X.; Liu, R.; Aihaiti, A.; Hong, J.; Zheng, L.; Xing, J.; Cui, Y.; Wang, L. Analysis of the Antioxidant Efficacy Substances in Fermented Black Mulberry Juice and Their Preventive Effects on Oxidative Stress in C2C12 Cells. Food Chem. 2025, 473, 142988. [Google Scholar] [CrossRef]
- Suwannachot, J.; Ogawa, Y. Changes in Polyphenolic Compounds and Antioxidant Activity of Japanese Pickled Apricot with Salted Red Perilla Leaf during Pickling and Digestion Process. Food Res. Int. 2024, 192, 114752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nie, M.; Xiao, Y.; Zhu, L.; Gao, R.; Zhou, C.; Li, D.; Xu, Y.; Dai, Z. Positive Effects of Ultrasound Pretreatment on the Bioaccessibility and Cellular Uptake of Bioactive Compounds from Broccoli: Effect on Cell Wall, Cellular Matrix and Digesta. LWT 2021, 149, 112052. [Google Scholar] [CrossRef]
- Chae, S.-H.; Lee, S.-H.; Kim, S.-H.; Song, S.-H.; Moon, J.-H.; Kim, H.-W.; Cho, J.-Y. Changes in Quality and Metabolites of Pickled Purple Radish during Storage. Foods 2025, 14, 1259. [Google Scholar] [CrossRef]
- Gao, Q.; Li, Y.; Li, Y.; Zhang, Z.; Liang, Y. Antioxidant and Prooxidant Activities of Phenolic Acids Commonly Existed in Vegetables and Their Relationship with Structures. Food Sci. Technol. 2022, 42, e07622. [Google Scholar] [CrossRef]
- Pham, V.D.; Korver, D.R.; Gänzle, M.G. Conversion of Phenolic Acids in Canola Fermentation: Impact on Antimicrobial Activity against Salmonella enterica and Campylobacter jejuni. J. Agric. Food Chem. 2023, 71, 2059–2069. [Google Scholar] [CrossRef]
- Ding, Q.; Wu, R.A.; Yin, L.; Zhang, W.; He, R.; Zhang, T.; Jiang, H.; Luo, L.; Ma, H.; Dai, C. Antioxidation and Memory Protection Effects of Solid-State-Fermented Rapeseed Meal Peptides on D-Galactose-Induced Memory Impairment in Aging-Mice. J. Food Process Eng. 2019, 42, e13145. [Google Scholar] [CrossRef]
- Narnoliya, L.K.; Sangwan, N.; Jadaun, J.S.; Bansal, S.; Sangwan, R.S. Defining the Role of a Caffeic Acid 3-O-Methyltransferase from Azadirachta Indica Fruits in the Biosynthesis of Ferulic Acid through Heterologous over-Expression in Ocimum Species and Withania Somnifera. Planta 2021, 253, 20. [Google Scholar] [CrossRef]
- Odinot, E.; Bisotto-Mignot, A.; Frezouls, T.; Bissaro, B.; Navarro, D.; Record, E.; Cadoret, F.; Doan, A.; Chevret, D.; Fine, F.; et al. A New Phenolic Acid Decarboxylase from the Brown-Rot Fungus Neolentinus Lepideus Natively Decarboxylates Biosourced Sinapic Acid into Canolol, a Bioactive Phenolic Compound. Bioengineering 2024, 11, 181. [Google Scholar] [CrossRef]
- Ramoza, S.A.; Aminin, A.L.N.; Cahyono, B. Biotransformation of Bitter Gourd (Momordica charantia) by Lactobacillus plantarum and Its Bioactivities. Int. Food Res. J. 2024, 31, 253–265. [Google Scholar] [CrossRef]
- Mazlan, F.A.; Annuar, M.S.M.; Sharifuddin, Y. Biotransformation of Momordica Charantia Fresh Juice by Lactobacillus plantarum BET003 and Its Putative Anti-Diabetic Potential. PeerJ. 2015, 3, e1376. [Google Scholar] [CrossRef]
- Li, M.; Xiao, Y.; Zhong, K.; Wu, Y.; Gao, H. Delving into the Biotransformation Characteristics and Mechanism of Steamed Green Tea Fermented by Aspergillus niger PW-2 Based on Metabolomic and Proteomic Approaches. Foods 2022, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wang, X.; Zhang, L.; Jiao, S.; Song, H.; Sun, J.; Wang, D. Changes and Biotransformation Mechanism of Main Functional Compounds during Kombucha Fermentation by the Pure Cultured Tea Fungus. Food Chem. 2024, 458, 140242. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, Y.; Sun, Y.; Lu, T.; Cao, Q.; Chen, X. Characterization of Kombucha Prepared from Black Tea and Coffee Leaves: A Comparative Analysis of Physiochemical Properties, Bioactive Components, and Bioactivities. J. Food Sci. 2024, 89, 3430–3444. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Lee, J.H.; Yun, H.D.; Ahn, B.Y.; Kim, H.; Seo, W.T. Changes of Phytochemical Constituents (Isoflavones, Flavanols, and Phenolic Acids) during Cheonggukjang Soybeans Fermentation Using Potential Probiotics Bacillus subtilis CS90. J. Food Compos. Anal. 2011, 24, 402–410. [Google Scholar] [CrossRef]
- Cárdenas-Castro, A.P.; Rochín-Medina, J.J.; Ramírez, K.; Tovar, J.; Sáyago-Ayerdi, S.G. In Vitro Intestinal Bioaccessibility and Colonic Biotransformation of Polyphenols from Mini Bell Peppers (Capsicum annuum L.). Plant Foods Hum. Nutr. 2022, 77, 77–82. [Google Scholar] [CrossRef]
| Enzyme | Original Phenolics | Transformed Products | Microorganism | Food Matrix | Role | Reference |
|---|---|---|---|---|---|---|
| - | Caffeic acid | Chlorogenic acid | Lactobacillus plantarum NCU137 | Wolfberry juice | Enhances antioxidant activity | [91] |
| β-Glucosidase | Caffeic acid | Vanillic acid and p-Coumaric acid | Lactobacillus plantarum and Lactobacillus acidophilus | Strawberry juice | Enhances antioxidant activity | [156] |
| - | Caffeic acid and p-coumaric acid | Dihydrocaffeic acid and 4-ethylphenol | Lactobacillus plantarum | Prunus avium L. | Enhance flavors | [150] |
| DbPAD | Ferulic acid and p-coumaric acid | Vinyl and derivatives | Saccharomyces cerevisiae BY4722 | Wine | Produces a unique aroma | [157] |
| SDPAD | hydroxycinnamic acid | 4-vinyl derivatives | Saccharomyces cerevisiae | Blueberry wine | Enhances color stability of blueberry wines | [148] |
| PADC, PDC | p-coumaric acid | Vinyl derivatives | Saccharomyces cerevisiae and Lactobacillus plantarum | Wine | Influence on wine aroma | [147] |
| padC and bglB | - | Gallic acid | Lactobacillus plantarum T7 | Mango | Gives new nutrients and flavors | [158] |
| reLPPAD and dLPPAD | Hydroxycinnamic acids | 4-vinyl derivatives | Pichia pastoris GS115 | Aronia melanocarpa | Maintains color stability and enhances the sensory evaluation of the product | [159] |
| - | Protocatechuic acid | Catechin | Lactiplantibacillus plantarum ATCC 14917 and Limosilactobacillus fermentum YL-11 | Lychee | Enhanced nutritional and flavor properties | [160] |
| - | 3,5-di-O-caffeoylquinic acid | Shikimic acid | Lactobaccilus paracei, Lactobacillus casei, Lactobacillus delbrueckii subsp. Bifidobacterium animalis subsp., and Lactobacillus fermentum | Black mulberries | Enhances antioxidant activity | [161] |
| Enzyme | Original Phenolics | Transformed Products | Microorganism | Food Matrix | Role | References |
|---|---|---|---|---|---|---|
| Lp_0796, Est_1092 | Ferulic acid and caffeic acid | Dihydroferulic acid and dihydrocaffeic acid | Lactobacillus plantarum TMW1.460 | Broccoli | Altering the sensory properties of food | [135] |
| - | Hydroxycinnamic acids | Dihydro, 4-vinyl, and 4-ethyl derivatives | Lactobacillus plantarum TMW1.460 and Furfurilactobacillus milii FUA3583 | Canola meal (CM) | Increases antimicrobial activity | [166] |
| NCOMT | Caffeic acid | Ferulic acid | - | Azadirachta indica | Enhanced pharmacological activity | [168] |
| NlePAD | Sinapic acid | Canolol | Neolentinus lepideus BRFM15 | Rapeseed meal (RSM) | Possesses antioxidant and anti-inflammatory activity | [169] |
| padC and bglB | - | Protocatechuic acid | Lactobacillus plantarum T7 | Cress | Gives new nutrients and flavors | [158] |
| - | Tannic acid | Syringic acid | Lactobacillus plantarum InaCC B1002 | Bitter gourd | Possesses antidiabetic activity | [170,171] |
| Tannase | Catechin gallate | Gallic acid | Aspergillus niger PW-2 | Steamed green tea | Developing a unique sensory profile | [172] |
| β-Glucosidase, cellulase, and esterase | Epicatechin gallate and epigallocatechin gallate | Epicatechin and epigallocatechin | Saccharomyces cerevisiae Y-01, Wickerhamomyces anomalus ZX-1, Lacticaseibacillus paracasei SJ-2 and Komagataeibacter oboediens CGMCC 22548 | Kombucha | Enhances antioxidant activity | [173,174] |
| β-glucosidase and esterase | - | Gallic acid | Bacillus subtilis CS90 | Cheonggukjang | Improvement of antioxidant capacity | [175] |
| - | Hydroxycinnamates | 3-(3-hydroxyphenyl)propionic acid | - | Bell pepper | Potential for cardiovascular disease protection | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Zhang, J.; Zhao, X.; Zi, Y.; Xiao, X. Biotransformation of Phenolic Acids in Foods: Pathways, Key Enzymes, and Technological Applications. Foods 2025, 14, 2187. https://doi.org/10.3390/foods14132187
Lu C, Zhang J, Zhao X, Zi Y, Xiao X. Biotransformation of Phenolic Acids in Foods: Pathways, Key Enzymes, and Technological Applications. Foods. 2025; 14(13):2187. https://doi.org/10.3390/foods14132187
Chicago/Turabian StyleLu, Chenxi, Jiayan Zhang, Xiangcheng Zhao, Yuancui Zi, and Xiang Xiao. 2025. "Biotransformation of Phenolic Acids in Foods: Pathways, Key Enzymes, and Technological Applications" Foods 14, no. 13: 2187. https://doi.org/10.3390/foods14132187
APA StyleLu, C., Zhang, J., Zhao, X., Zi, Y., & Xiao, X. (2025). Biotransformation of Phenolic Acids in Foods: Pathways, Key Enzymes, and Technological Applications. Foods, 14(13), 2187. https://doi.org/10.3390/foods14132187






