Corn Steep Liquor as an Efficient Bioresource for Functional Components Production by Biotransformation Technology
Abstract
:1. Introduction
2. Organic Acids Production by CSL Bioconversion
2.1. Malic Acid Production by CSL
2.2. Citric Acid Production by CSL
2.3. Lactic Acid Production by CSL
3. Polysaccharide Production by CSL Bioconversion
4. Lipids Production by CSL Bioconversion
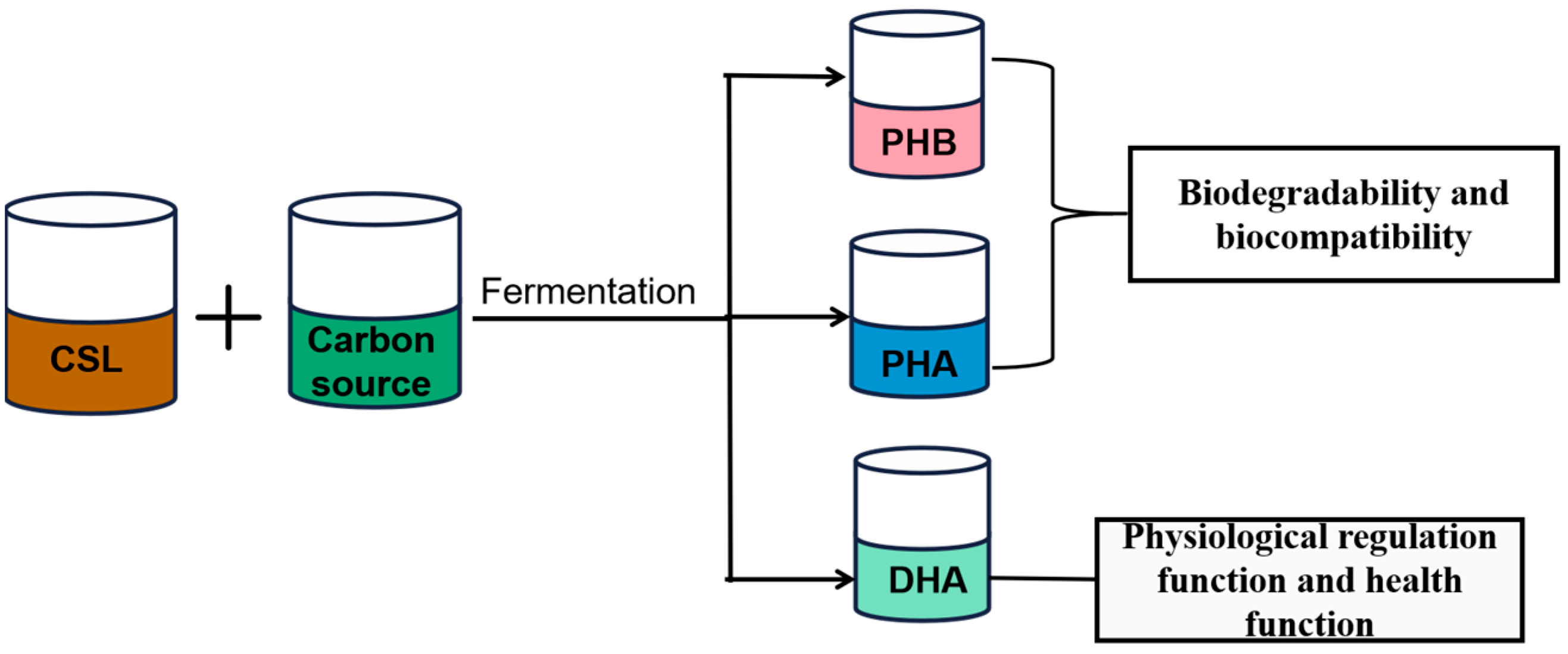
4.1. Polyhydroxybutyrate Production by CSL
4.2. PHA Production by CSL
4.3. DHA Production by CSL
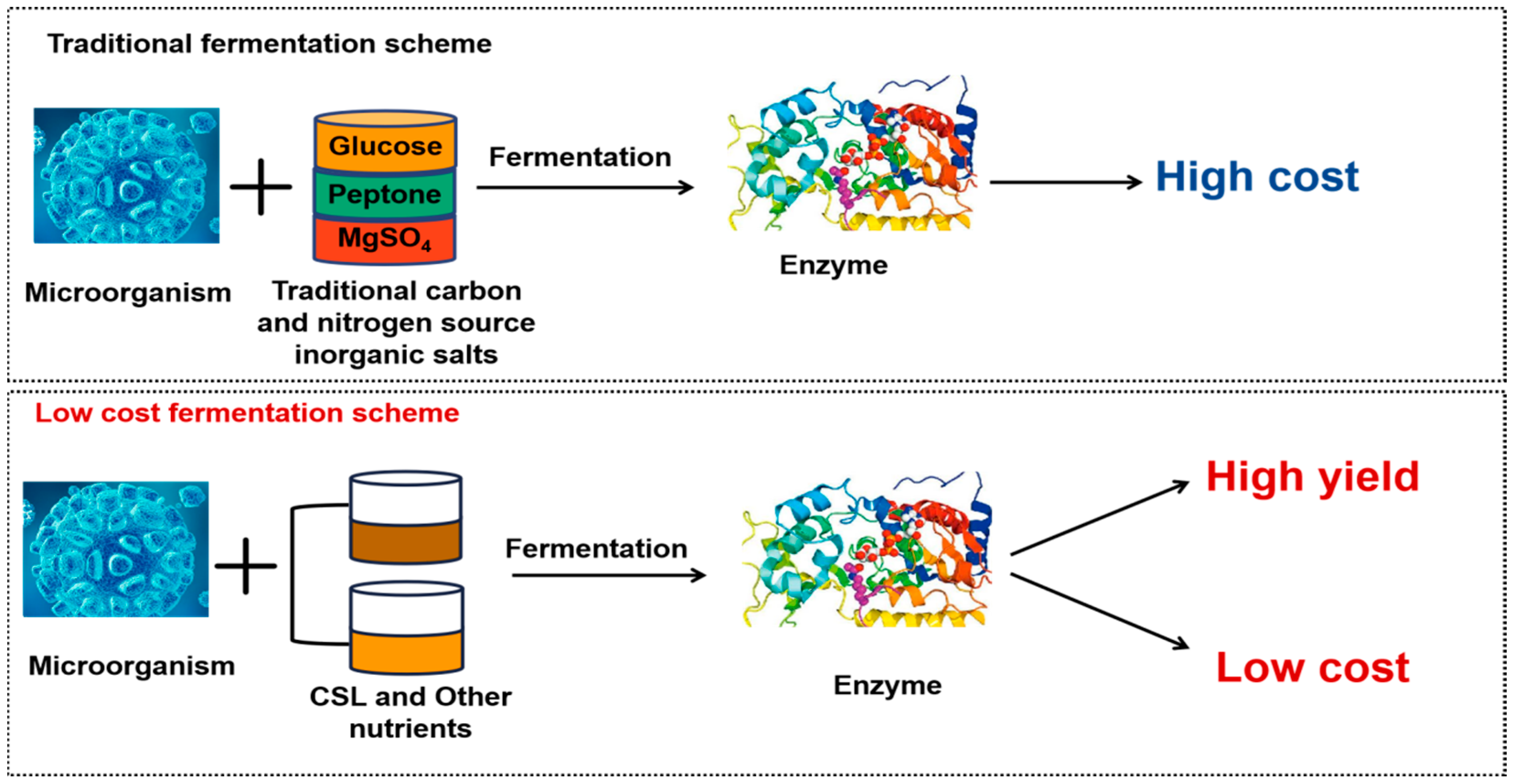
5. Enzyme Production by CSL Bioconversion
5.1. Corn Steep Liquor Fermentation Produces Cellulase
5.2. Lipase Production by CSL
5.3. Protease Production by CSL
5.4. Other Enzymes Production by CSL
6. Bioconversion of Corn Steep Liquor to Produce Natural Pigment
6.1. Lycopene Production by CSL
6.2. Astaxanthin Production by CSL
6.3. Carotenoid Production by CSL
7. Bioconversion of Corn Steep Liquor to Produce Other Novel Functional Components
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riccetto, S.; Davis, A.S.; Guan, K.; Pittelkow, C.M. Integrated assessment of crop production and resource use efficiency indicators for the U.S. Corn Belt. Glob. Food Secur.-Agric. 2020, 24, 100339. [Google Scholar] [CrossRef]
- Cornejo, M.W.d.l.Á.; Rincón, N.; Del Real, A.; Rodríguez, M.E. The effect of Ca2+ ions on the pasting, morphological, structural, vibrational, and mechanical properties of corn starch–water system. J. Cereal Sci. 2018, 79, 174–182. [Google Scholar] [CrossRef]
- Liu, H.; Su, B.B.; Guo, M.; Wang, J.B. Exploring R&D network resilience under risk propagation: An organizational learning perspective. Int. J. Prod. Econ. 2024, 273, 109266. [Google Scholar]
- Zhou, K.; Yu, J.; Ma, Y.; Cai, L.; Zheng, L.; Gong, W.; Liu, Q. Corn Steep Liquor: Green Biological Resources for Bioindustry. Appl. Biochem. Biotechnol. 2022, 194, 3280–3295. [Google Scholar] [CrossRef]
- Kona, R.; Qureshi, N.; Pai, J. Production of glucose oxidase using Aspergillus niger and corn steep liquor. Bioresour. Technol. 2001, 78, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Sekoai, P.T.; Ayeni, A.O.; Daramola, M.O. Parametric optimization of citric acid production from apple pomace and corn steep liquor by a wild type strain of Aspergillus niger: A Response surface methodology approach. Int. J. Eng. Res. Afr. 2018, 36, 98–113. [Google Scholar] [CrossRef]
- Saalia, F.K.; Amponsah, A.K.; Asante, N.D.; Owusu-Brafi, N.K.; Amoa, B.B.; Affrifah, N.S.; Sefa-Dedeh, S. Effects of corn steep water pretreatment on the rheological and microstructural properties of Ga-kenkey. J. Food Process Eng. 2017, 40, e12521. [Google Scholar] [CrossRef]
- Awaad, M.; Abdel-Alim, G.; Sayed, K.; Ahmed, A.; Nada, A.; Metwalli, A.; Alkhalaf, A.J.P.V.J. Immunostimulant effects of essential oils of peppermint and eucalyptus in chickens. Pak. Vet. J. 2010, 30, 61–66. [Google Scholar]
- Lawford, H.; Rousseau, J. Corn Steep Liquor as a Cost-Effective Nutrition Adjunct in High-Performance Zymomonas Ethanol Fermentations. Appl. Biochem. Biotechnol. 1997, 63, 287–304. [Google Scholar] [CrossRef]
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef]
- Mahr-un-Nisa; Sarwar, M.; Khan, M. Influence of ad libitum feeding of urea-treated wheat straw with or without corn steep liquor on intake, in situ digestion kinetics, nitrogen metabolism, and nutrient digestion in Nili-Ravi buffalo bulls. Aust. J. Agric. Res. 2004, 55, 229–236. [Google Scholar] [CrossRef]
- Obayori, O.S.; Adebusoye, S.A.; Ilori, M.O.; Oyetibo, G.O.; Omotayo, A.E.; Amund, O.O. Effects of Corn Steep Liquor on Growth Rate and Pyrene Degradation by Pseudomonas strains. Curr. Microbiol. 2009, 60, 407–411. [Google Scholar] [CrossRef]
- Sun, L.; Gong, M.; Lv, X.; Huang, Z.; Gu, Y.; Li, J.; Liu, L. Current advance in biological production of short-chain organic acid. Appl. Microbiol. Biotechnol. 2020, 104, 9109–9124. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, K.; Kabilan, S.C.B.; Sindhu, V.; Meenakshisundaram, S. A metabolic-engineering framework approach via fed-batch fermentation for enhancing glucaric acid production in Komagataella phaffii. Enzyme Microb. Technol. 2025, 187, 110627. [Google Scholar]
- Chae, T.U.; Ahn, J.H.; Ko, Y.S.; Kim, J.W.; Lee, J.A.; Lee, E.H.; Lee, S.Y. Metabolic engineering for the production of dicarboxylic acids and diamines. Metab. Eng. 2020, 58, 2–16. [Google Scholar] [CrossRef]
- Wang, J.; Lin, M.; Xu, M.; Yang, S.T. Anaerobic Fermentation for Production of Carboxylic Acids as Bulk Chemicals from Renewable Biomass. Adv. Biochem. Eng. Biotechnol. 2016, 156, 323–361. [Google Scholar] [PubMed]
- Kövilein, A.; Kubisch, C.; Cai, L.; Ochsenreither, K. Malic acid production from renewables: A review. Chem. Technol. Biotechnol. 2019, 95, 513–526. [Google Scholar] [CrossRef]
- Liu, P.P.; Shao, C.X.; Ren, H.; Yang, W. Transcription factor MdNAC18.1 regulates malic acid accumulation in apple fruits. Int. J. Biol. Macromol. 2025, 308, 14233. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, L.; Guo, J. Transcriptome Analysis Reveals Distinct Differences in Organic Acid Metabolism Between the Pericarp and the Pulp of Cerasus humilis During Fruit Maturation. Plants 2025, 14, 1105. [Google Scholar] [CrossRef]
- Da Silva, R.R.; Leal, G.F.; Da Costa Gomes, C.; De Oliveira, J.E.B.; Da Silva Soares, C.M. Chemical Characterization, Antioxidant Potential and Phenolic Profile of the Pulp and By-Products of Black puçá (Mouriri pusa), a Fruit from the Brazilian Cerrado region. Plant Foods Hum. Nutr. 2024, 80, 16. [Google Scholar] [CrossRef]
- Dai, Z.; Zhou, H.; Zhang, S.; Gu, H.; Yang, Q.; Zhang, W.; Xin, F. Current advance in biological production of malic acid using wild type and metabolic engineered strains. Bioresour. Technol. 2018, 258, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhou, Y.; Lin, M.; Wei, P.; Yang, S.T. Polymalic acid fermentation by Aureobasidium pullulans for malic acid production from soybean hull and soy molasses: Fermentation kinetics and economic analysis. Bioresour. Technol. 2017, 223, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Daunoraite, D.; de Souza, N.R.D.; Babinskas, J.; Cherubini, F.; Vares, L.; Matijosyte, I. Unlocking nature’s sweet secret for citric acid production from wood sugars: Evaluation of microbial strains and environmental impacts. J. Clean. Prod. 2024, 467, 142914. [Google Scholar] [CrossRef]
- Dementev, D.A.; Rybakov, Y.A.; Sineoky, S.P. Prospects of Development of Biotechnologies for Citric Acid Production. Appl. Biochem. Micro. 2024, 60, 1517–1525. [Google Scholar] [CrossRef]
- Díaz-Orozco, L.; Santillán, M.M.; Portales, R.E.D.; Rosales-Colunga, L.M.; Leyva-Porras, C.; Saavedra-Leos, Z. Advances in L-Lactic Acid Production from Lignocellulose Using Genetically Modified Microbial Systems. Polymers 2025, 17, 322. [Google Scholar] [CrossRef]
- Show, P.L.; Oladele, K.O.; Siew, Q.Y.; Aziz Zakry, F.A.; Lan, J.C.W.; Ling, T.C. Overview of citric acid production from Aspergillus niger. Front. Life Sci. 2015, 8, 271–283. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Xu, J.; Xia, J.; Lv, J.; Zhang, T.; He, J. Citric acid production by Yarrowia lipolytica SWJ-1b using corn steep liquor as a source of organic nitrogen and vitamins. Ind. Crops Prod. 2015, 78, 154–160. [Google Scholar] [CrossRef]
- Latif, A.; Hassan, N.; Ali, H.; Niazi, M.B.K.; Jahan, Z.; Ghuman, I.L.; Saqib, A. An overview of key industrial product citric acid production by Aspergillus niger and its application. J. Ind. Microbiol. Biot. 2024, 52, 1–16. [Google Scholar] [CrossRef]
- Wu, R.F.; Yang, J.H.; Jiang, Y.J.; Xin, F.X. Advances and prospects for lactic acid production from lignocellulose. Enzyme Microb. Technol. 2025, 182, 110542. [Google Scholar] [CrossRef]
- Zhang, Y.; Yoshida, M.; Vadlani, P.V. Biosynthesis of d-lactic acid from lignocell-ulosic biomass. Biotechnol. Lett. 2018, 40, 1167–1179. [Google Scholar] [CrossRef]
- Yamane, T.; Tanaka, R. Highly accumulative production of l(+)-lactate from glucose by crystallization fermentation with immobilized Rhizopus oryzae. J. Biosci. Bioeng. 2013, 115, 90–95. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V. Role of Microbial Fermentation in the Bio-Production of Food Aroma Compounds from Vegetable Waste. Fermentation 2024, 10, 132. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Q.; Wang, H. Microbial Characteristics and Functions in Coffee Fermentation: A Review. Fermentation 2024, 11, 5. [Google Scholar] [CrossRef]
- Alvarado, E.; Castro, R.; Rodríguez, C.A.J. Poly(lactic acid) Degradation by Recombinant Cutinases from Aspergillus nidulans. Polymers 2024, 16, 1994. [Google Scholar] [CrossRef] [PubMed]
- Bulut, S.; Elibol, M.; Ozer, D. Effect of different carbon sources on l(+) -lactic acid production by Rhizopus oryzae. Biochem. Eng. J. 2004, 21, 33–37. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Huang, F.; Wang, J.; Zhao, J.; Zhao, X.; Zhou, S. Engineering and adaptive evolution of Escherichia coli W for l-lactic acid fermentation from molasses and corn steep liquor without additional nutrients. Bioresour. Technol. 2013, 148, 394–400. [Google Scholar] [CrossRef]
- De Lima, C.J.; Coelho, L.F.; Contiero, J. The use of response surface methodology in optimization of lactic acid production: Focus on medium supplementation, temperature and pH control. Food Technol. Biotechnol. 2010, 48, 175–181. [Google Scholar]
- Tang, Q.; Huang, G. Preparation and antioxidant activities of cuaurbit polysaccharide. Int. J. Biol. Macromol. 2018, 117, 362–365. [Google Scholar] [CrossRef]
- Kealy, L.; Runting, J.; Thiele, D. An emerging maestro of immune regulation: How DOT1L orchestrates the harmonies of the immune system. Front. Immunol. 2024, 15, 1385319. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yao, Z.; Zhao, M.; Qi, H. Sulfation, anticoagulant and antioxidant activities of polysaccharide from green algae Enteromorpha linza. Int. J. Biol. Macromol. 2013, 58, 225–230. [Google Scholar] [CrossRef]
- Ren, L.; Perera, C.; Hemar, Y. Antitumor activity of mushroom polysaccharides: A review. Food Funct. 2012, 3, 1118–1130. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Xu, X.; Zhang, X.J.I.J.o.B.M. Purification, antitumor and anti-inflammation activities of an alkali-soluble and carboxymethyl polysaccharide CMP33 from Poria cocos. Int. J. Biol. Macromol. 2019, 127, 39–47. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 115, 77–82. [Google Scholar] [CrossRef]
- Eduardo, J.G.; Márcia, R.C.; Soraia, P.S.; Elisabete, C.; Manuel, A.C.; José, A.T.; Lígia, R.R. Sustainable Exopolysaccharide Production by Rhizobium viscosum CECT908 Using Corn Steep Liquor and Sugarcane Molasses as Sole Substrates. Polymers 2023, 15, 20. [Google Scholar]
- Jiao, Y.; Xu, F.; Chang, Y. Improvement of Cordyceps militaris Polysaccharides Production by a Sole Corn Steep Liquor Medium, and its Antioxidant Activity. Sugar Tech 2025. [Google Scholar] [CrossRef]
- Sharma, N.; Prasad, G.S.; Choudhury, A.R. Utilization of corn steep liquor for biosynthesis of pullulan, an important exopolysaccharide. Carbohyd. Polym. 2013, 93, 95–101. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, H.D.; Han, H.; Chang, Y. Development and Utilization of Corn Processing by-Products: A Review. Foods 2022, 11, 3709. [Google Scholar] [CrossRef]
- Utrilla, J.; Licona-Cassani, C.; Marcellin, E.; Gosset, G.; Nielsen, L.K.; Martinez, A. Engineering and adaptive evolution of Escherichia coli for d-lactate fermentation reveals GatC as a xylose transporter. Metab. Eng. 2012, 14, 469–476. [Google Scholar] [CrossRef]
- Wang, F.; Hu, J.H.; Guo, C.; Liu, C.Z.J.B.T. Enhanced laccase production by Trametes versicolor using corn steep liquor as both nitrogen source and inducer. Bioresour. Technol. 2014, 166, 602–605. [Google Scholar] [CrossRef]
- Xi, Y.L.; Chen, K.Q.; Dai, W.Y.; Ma, J.F.; Zhang, M.; Jiang, M.; Ouyang, P.K.J.B.t. Succinic acid production by Actinobacillus succinogenes NJ113 using corn steep liquor powder as nitrogen source. Bioresour. Technol. 2013, 136, 775–779. [Google Scholar] [CrossRef]
- Sun, N.; Chen, J.; Wang, D.; Lin, S. Advance in food-derived phospholipids: Sources, molecular species and structure as well as their biological activities. Trends Food Sci. Technol. 2018, 80, 199–211. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Wang, L. Transcriptome Mechanism of Utilizing Corn Steep Liquor as the Sole Nitrogen Resource for Lipid and DHA Biosynthesis in Marine Oleaginous Protist Aurantiochytrium sp. Biomolecules 2019, 9, 695. [Google Scholar] [CrossRef]
- Pili, J.; Danielli, A.; Nyari, N.L.D.; Zeni, J.; Cansian, R.L.; Backes, G.T.; Valduga, E. Biotechnological potential of agro-industrial waste in the synthesis of pectin lyase from Aspergillus brasiliensis. Food Sci. Technol. Int. 2017, 24, 97–109. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Ghodake, G.S.; Shin, H.S.; Saratale, G.D.; Park, Y.; Kim, D.S. Utilization of Noxious Weed Water Hyacinth Biomass as a Potential Feedstock for Biopolymers Production: A Novel Approach. Polymers 2020, 12, 1704. [Google Scholar] [CrossRef]
- Purushothaman, M.; Anderson, R.; Narayana, S.; Jayaraman, V. Industrial byproducts as cheaper medium components influencing the production of polyhydroxyalkanoates (PHA)—Biodegradable plastics. Bioproc. Biosyst. Eng. 2001, 24, 131–136. [Google Scholar]
- Saratale, R.G.; Cho, S.K.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Shin, H.S.J.P. Developing microbial co-culture system for enhanced polyhydroxyalkanoates (PHA) production using acid pretreated lignocellulosic biomass. Polymers 2022, 14, 726. [Google Scholar] [CrossRef]
- Ye, J.; Huang, W.; Wang, D.; Chen, F.; Yin, J.; Li, T.; Chen, G.Q. Pilot Scale-up of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Production by Halomonas bluephagenesisvia Cell Growth Adapted Optimization Process. Biotechnol. J. 2018, 13–15. [Google Scholar]
- Moniz, P.; Silva, C.; Oliveira, A.C.; Reis, A.; Lopes da Silva, T.J.P. Raw glycerol based medium for DHA and lipids production, using the marine heterotrophic microalga Crypthecodinium cohnii. Processes 2021, 9, 2005. [Google Scholar] [CrossRef]
- Han, H.; Ling, Z.; Khan, A.; Virk, A.K.; Kulshrestha, S.; Li, X. Improvements of thermophilic enzymes: From genetic modifications to applications. Bioresour. Technol. 2019, 279, 350–361. [Google Scholar] [CrossRef]
- Zhou, P.; Gao, C.; Song, W.; Wei, W.; Wu, J.; Liu, L.; Chen, X. Engineering status of protein for improving microbial cell factories. Biotechnol. Adv. 2024, 70, 108282. [Google Scholar] [CrossRef] [PubMed]
- Suresh, G.; Ragunathan, R.; Johney, J. Assessing the impact of corn steep liquor as an inducer on enhancing laccase production and laccase gene (Lac1) transcription in Pleurotus pulmonarius during solid-state fermentation. Bioresour. Technol. 2024, 27, 101905. [Google Scholar] [CrossRef]
- Amany, H.; Elsayed, M.; Moataza, S.; Mohsen, S. Utilization of orange pulp and corn steep liquor for L-methioninase production by Wickerhamomyces subpelliculosus. Egypt Pharm. J. 2024, 20, 8–16. [Google Scholar]
- Bhatt, B.; Bhatt, K.; Lal, S.; Bhatt, V. Production of a novel cellulase by Bacillus amyloliquefaciens OKB3 isolated from soil: Purification and characterization. Int. J. Biol. Macromol. 2024, 282, 137454. [Google Scholar] [CrossRef]
- Eberini, I.; Chang, C.J.; Lee, C.C.; Chan, Y.T.; Trudeau, D.L.; Wu, M.H.; Chao, Y.C. Exploring the Mechanism Responsible for Cellulase Thermostability by Structure-Guided Recombination. PLoS ONE 2016, 11, e0147485. [Google Scholar]
- de Azevedo, A.R.; dos Santos, M.S.N.; Wancura, J.H.C.; Oro, C.E.D.; Pfeifenberg, R.; Zabot, G.L.; Tres, M.V. Semi-continuous subcritical hydrolysis of orange waste biomasses for integrated production of fermentable sugars and platform chemicals. Chem. Eng. Process. 2024, 197, 109719. [Google Scholar] [CrossRef]
- Anica, D.; Shilpa, S.; Tulasi, S. Thermostable Recombinant Cellulases of the Thermophilic Mold Myceliophthora thermophila in the Bioconversion of Paddy Straw and Sugarcane Bagasse to Ethanol. BioEnergy Res. 2023, 17, 1029–1040. [Google Scholar] [CrossRef]
- Nascimento, R.P.; Junior, N.A.; Pereira, N., Jr.; Bon, E.P.S.; Coelho, R.R.R. Brewer’s spent grain and corn steep liquor as substrates for cellulolytic enzymes production by Streptomyces malaysiensis. Lett. Appl. Microbiol. 2009, 48, 529–535. [Google Scholar] [CrossRef]
- Da Vinha, F.N.M.; Gravina-Oliveira, M.P.; Franco, M.N.; Macrae, A.; da Silva Bon, E.P.; Nascimento, R.P.; Coelho, R.R.R. Cellulase Production by Streptomyces viridobrunneus SCPE-09 Using Lignocellulosic Biomass as Inducer Substrate. Appl. Biochem. Biotechnol. 2010, 164, 256–267. [Google Scholar] [CrossRef]
- Ladeira, S.A.; Cruz, E.; Delatorre, A.B.; Barbosa, J.B.; Martins, M.L.L. Cellulase production by thermophilic Bacillus sp. SMIA-2 and its detergent compatibility. Electron. J. Biotechn. 2015, 18, 110–115. [Google Scholar] [CrossRef]
- Franco-Cirigliano, M.N.; Rezende, R.d.C.; Gravina-Oliveira, M.P.; Pereira, P.H.F.; do Nascimento, R.P.; Bon, E.P.d.S.; Coelho, R.R.R. Streptomyces misionensis PESB-25 Produces a Thermoacidophilic Endoglucanase Using Sugarcane Bagasse and Corn Steep Liquor as the Sole Organic Substrates. Biomed. Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Xue, J.; Wang, Y. Development and characterization of zein/gum Arabic nanocomposites incorporated edible films for improving strawberry preservation. Adv. Compos. Hybrid. Mater. 2024, 7, 249. [Google Scholar] [CrossRef]
- Srivastav, K.A.; Jaiswal, J.; Kumar, U. Unraveling the physiochemical characteristics and molecular insights of Zein protein through structural modeling and conformational dynamics: A synergistic approach between machine learning and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2024, 2, 1–20. [Google Scholar] [CrossRef]
- Nascimento, D.P.D.S.; Souza, D.M.R.R.; Sobral, V.M. Gelatin-Oxidized Alginate and Chitosan-Coated Zein Nanoparticle Hydrogel Composite to Enhance Breast Cancer Cytotoxicity in Dual-Drug Delivery. ACS Omega 2024, 9, 45190–45202. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, D.; Lin, P. Preparation, characterization, and binding mechanism of quercetin-loaded composite nanoparticles based on zein-soybean protein isolate. Food Chem. 2024, 463, 141359. [Google Scholar] [CrossRef] [PubMed]
- Edwinoliver, N.G.; Thirunavukarasu, K.; Purushothaman, S.; Rose, C.; Gowthaman, M.K.; Kamini, N.R. Corn Steep Liquor as a Nutrition Adjunct for the Production of Aspergillus niger Lipase and Hydrolysis of Oils Thereof. J. Agric. Food Chem. 2009, 57, 10658–10663. [Google Scholar] [CrossRef]
- Maldonadoa, R.; Pancieraa, L.; Macedob, A.; Mazuttic, M.; Maugeria, F.; Rodriguesa, I. Improvement of lipase production from Geotrichum sp. in shaken flasks. Chem. Ind. Chem. Eng. Q. 2012, 18, 459–464. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Li, X. Tea saponin-Zein binary complex as a quercetin delivery vehicle: Preparation, characterization, and functional evaluation. Int. J. Biol. Macromol. 2024, 279, 135485. [Google Scholar] [CrossRef]
- He, J.; Tang, H.; Liao, R. Gemini surfactant stabilized zein nanoparticles: Preparation, characterization, interaction mechanism, and antibacterial activity. Int. J. Biol. Macromol. 2025, 305, 141264. [Google Scholar] [CrossRef]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial Proteases Applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef]
- De Azeredo, L.A.I.; De Lima, M.B.; Coelho, R.R.R.; Freire, D.M.G. A Low-Cost Fermentation Medium for Thermophilic Protease Production by Streptomyces sp. 594 Using Feather Meal and Corn Steep Liquor. Curr. Microbiol. 2006, 53, 335–339. [Google Scholar] [CrossRef]
- Escaramboni, B.; Garnica, B.C.; Abe, M.M.; Palmieri, D.A.; Fernández Núñez, E.G.; de Oliva Neto, P. Food Waste as a Feedstock for Fungal Biosynthesis of Amylases and Proteases. Waste Biomass Valoriz. 2021, 13, 213–226. [Google Scholar] [CrossRef]
- Nascimento, R.P.d.; Reis, A.D.; Gírio, F.; Pereira Jr, N.; Bon, E.P.d.S.; Coelho, R.R.R. A thermotolerant xylan-degrading enzyme is produced by Streptomyces malaysiensis AMT-3 using by-products from the food industry. Braz. Arch. Biol. Technol. 2020, 63, e20190243. [Google Scholar] [CrossRef]
- Han, M.; He, Q.; Zhang, W.G. Carotenoids production in different culture conditions by sporidiobolus pararoseus. Prep. Biochem. Biotechnol. 2012, 42, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Pranta, A.D.; Rahaman, M.T. Extraction of eco-friendly natural dyes and biomordants for textile coloration: A critical review. Nano-Struct. Nano-Objects 2024, 39, 101243. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Renuka Devi, P. Bacterial Pigments: Sustainable Compounds with Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Silva, T.R.; Tavares, R.S.N.; Canela-Garayoa, R.; Eras, J.; Rodrigues, M.V.N.; Neri-Numa, I.A.; Oliveira, V.M. Chemical Characterization and Biotechnological Applicability of Pigments Isolated from Antarctic Bacteria. Mar. Biotechnol. 2019, 21, 416–429. [Google Scholar] [CrossRef]
- Bhandari, M.; Sharma, R.; Sharma, S.; Bobade, H.; Singh, B.J.P.; Technology, R. Recent advances in nanoencapsulation of natural pigments: Emerging technologies, stability, therapeutic properties and potential food applications. Pigm. Resin. Technol. 2022, 53, 53–61. [Google Scholar] [CrossRef]
- Shafe, M.O.; Gumede, N.M.; Nyakudya, T.T.; Chivandi, E. Lycopene: A Potent Antioxidant with Multiple Health Benefits. J. Nutr. Metab. 2024, 2024, 6252426. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef]
- Chaudhary, K.; Khalid, S.; Zahid, M.; Ansar, S.; Zaffar, M.; Hassan, S.A.; Aadil, R.M. Emerging ways to extract lycopene from waste of tomato and other fruits, a comprehensive review. J. Food Process Eng. 2024, 47, e14720. [Google Scholar] [CrossRef]
- Marchena, A.M.; Franco, L.; Romero, A.M.; Barriga, C.; Rodríguez, A.B. Lycopene and melatonin: Antioxidant compounds in cosmetic formulations. Skin. Pharmacol. Phys. 2020, 33, 237–243. [Google Scholar] [CrossRef]
- Hernández-Almanza, A.; Montañez, J.; Martínez, G.; Aguilar-Jiménez, A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Lycopene: Progress in microbial production. Trends Food Sci. Technol. 2016, 56, 142–148. [Google Scholar] [CrossRef]
- Kang, C.K.; Jeong, S.; Yang, J.E.; Choi, Y.J. High-Yield Production of Lycopene from Corn Steep Liquor and Glycerol Using the Metabolically Engineered Deinococcus radiodurans R1 Strain. J. Agric. Food Chem. 2020, 68, 5147–5153. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Favara, C.; Tomasello, M.F.; Sica, C.; Grasso, A.; Dominguez, H.G.; Ferrante, M. Astaxanthin in cancer therapy and prevention. Biomed. Rep. 2025, 22, 66. [Google Scholar] [CrossRef]
- Chen, Y.T.; Kao, C.J.; Huang, H.Y.; Huang, S.Y.; Chen, C.Y.; Lin, Y.S.; Wang, H.M.D. Astaxanthin reduces MMP expressions, suppresses cancer cell migrations, and triggers apoptotic caspases of in vitro and in vivo models in melanoma. J. Funct. Foods 2017, 31, 20–31. [Google Scholar] [CrossRef]
- Babalola, J.A.; Stracke, A.; Loeffler, T.; Schilcher, I.; Sideromenos, S.; Flunkert, S.; Hoefler, G. Corrigendum to “Effect of astaxanthin in type-2 diabetes-induced APPxhQC transgenic and NTG mice”. Mol. Metab. 2024, 86, 101972. [Google Scholar] [CrossRef]
- Lenucci, M.S.; Durante, M.; Anna, M.; Dalessandro, G.; Piro, G. Possible Use of the Carbohydrates Present in Tomato Pomace and in Byproducts of the Supercritical Carbon Dioxide Lycopene Extraction Process as Biomass for Bioethanol Production. J. Agric. Food Chem. 2013, 61, 3683–3692. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P.J.M. Astaxanthin for the food industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, J.; Xu, N.; Cai, C.; Lu, Y.; Liu, Y.; Wang, Y. Optimization of culture medium and scale-up production of astaxanthin using corn steep liquor as substrate by response surface methodology. Prep. Biochem. Biotechnol. 2023, 53, 443–453. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2019, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, P.; Huang, H.; Zeng, Y.; Zhang, M.; Chen, Z.; Ge, H. The associations between serum carotenoids and hyperuricemia among US National Health and Nutrition Examination Survey. BMC Public. Health 2025, 25, 1278. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G.J.M. Carotenoids and their biosynthesis in fungi. Molecules 2022, 27, 1431. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.P.; Remedi, R.D.; dos Santos Sá, C.; Rodrigues, A.B.; Ramos, J.M.G.; Burkert, C.A.V.; Biotechnology, A. Use of agroindustrial byproducts as substrate for production of carotenoids with antioxidant potential by wild yeasts. Biocatal. Agric. Biotechnol. 2019, 20, 101208. [Google Scholar] [CrossRef]
- Fomich, M.; Dia, V.; Wang, T. Characterization of soy protein hydrolysate and lecithin complex for its novel antifreeze activity. Food Hydrocoll. 2025, 167, 111389. [Google Scholar] [CrossRef]
- Bibek, B.; Swastik, S.; Thomas, M.; Buddhi, P.L. Evaluation of corn steep liquor as fermentation media for recombinant Lactococcus lactis producing antifreeze proteins. J. Sci. Food Agric. 2023, 103, 2512–2521. [Google Scholar]
- Wei, L.; Zhao, J.; Wang, Y.; Gao, J.; Du, M.; Zhang, Y.; Xu, N.; Du, H.; Ju, J.; Liu, Q.; et al. Engineering of Corynebacterium glutamicum for high-level γ-aminobutyric acid production from glycerol by dynamic metabolic control. Metab. Eng. 2021, 69, 134–146. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Zhang, B.; Bao, J. Production of γ-aminobutyric acid using corncob residue as carbohydrate feedstock by engineered Corynebacterium glutamicum. Biochem. Eng. J. 2025, 215, 109629. [Google Scholar] [CrossRef]
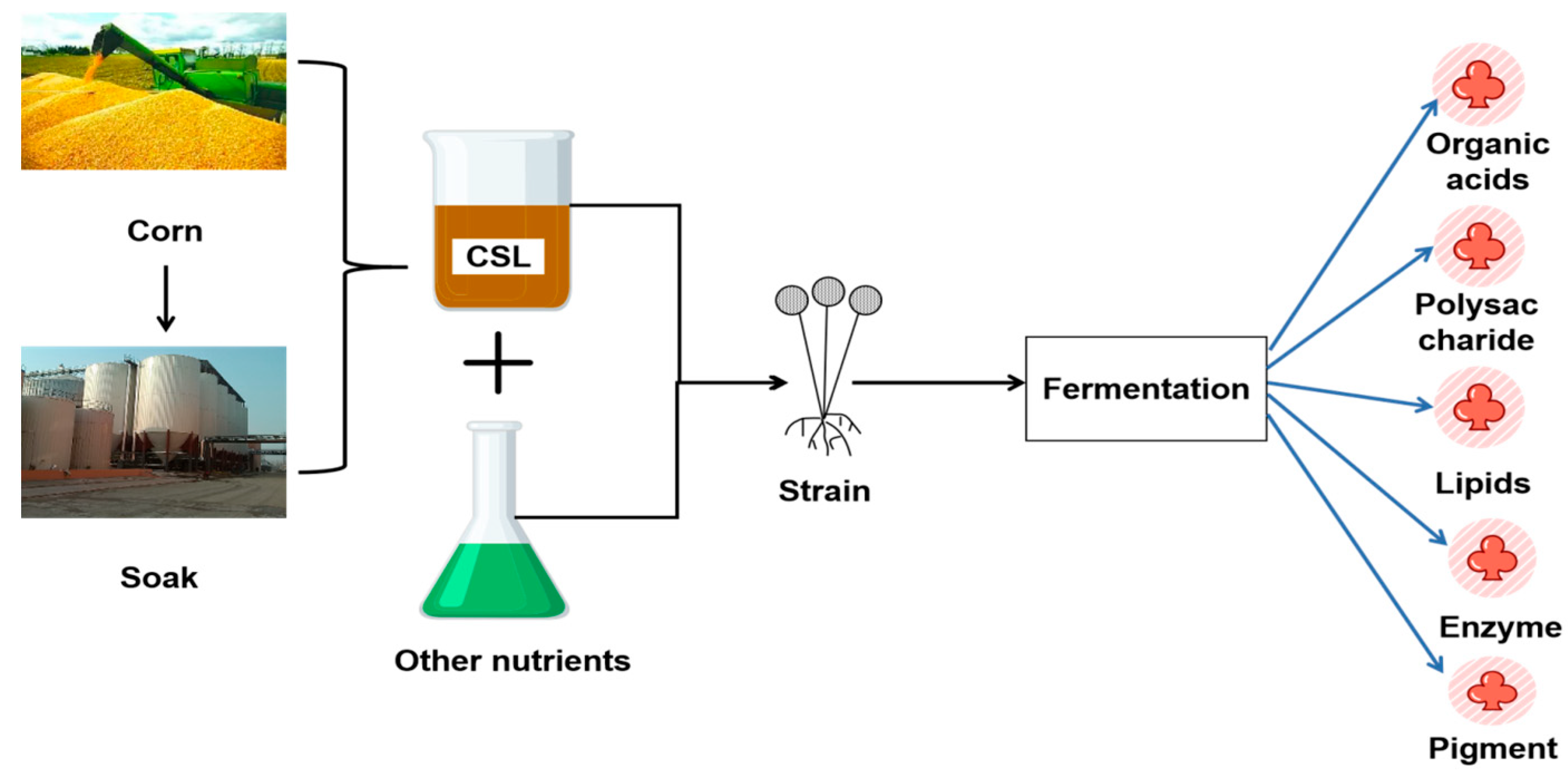
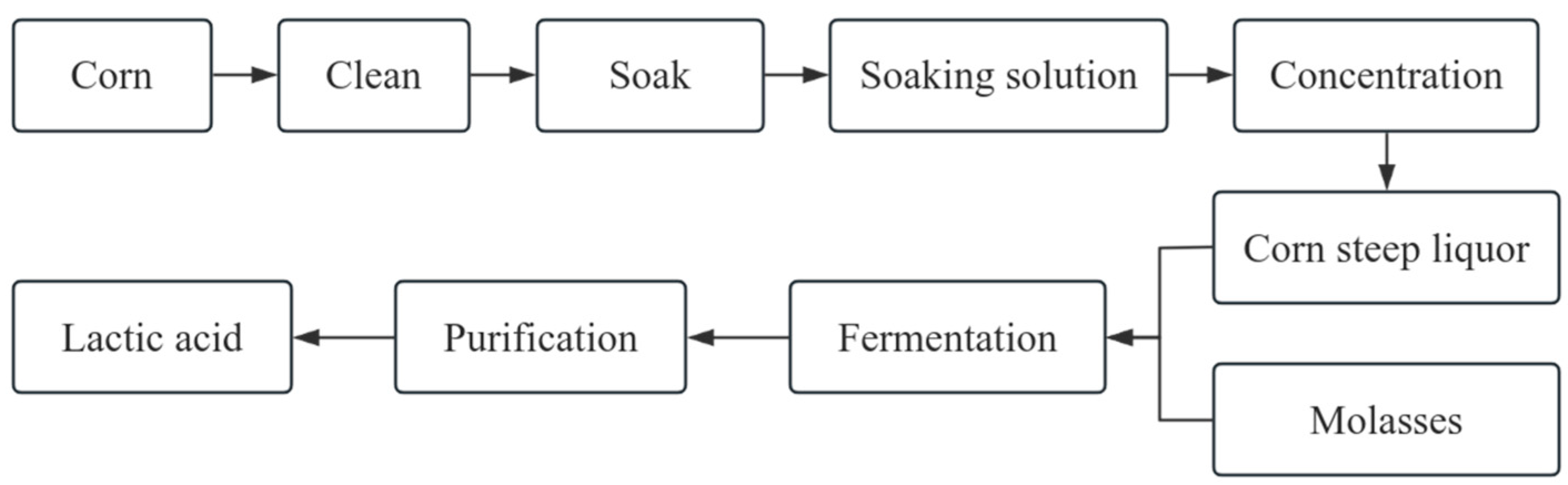
| Composition | As a Percentage of CSL | As a Percentage of the Dry Base |
|---|---|---|
| Protein | 3.19 | 51.04 |
| Total sugar | 0.82 | 13.12 |
| Starch | — | — |
| Total acid | 1.07 | 17.12 |
| Ash content | 1.17 | 18.72 |
| Solid | 6.25 | 100 |
| By-Product Type | Corn Gluten Powder | Corn Germ Meal | Corn Steep Liquor |
|---|---|---|---|
| Yield T/(100 T of corn) | 6 | 3.5 | 8–9.5 |
| Protein content % (w/w) | 60–70 | 25 | 45 |
| Current utilization | fodder | fodder | fodder, medium |
| Name | Organic Acids Type | Amount of CSL Addition | Addition of Other Substances | Result | Reference |
|---|---|---|---|---|---|
| Organic acid | Malic acid | 5–15 g/L | 300 g/L soybean molasses | The PMA yield of soybean shell hydrolysate supplemented with 5 to 15 g/L CSL was significantly higher than that of control supplemented with 2 g/L NH4NO3. | [22] |
| Citric acid | 1.0 g/L | 60.0 g/L of glucose, 7.5 g/L Na2HPO4 | The citric acid (CA) produced by the strain in the medium containing 1.0 g/L CSL was 27.5 g/L, which was 1.24 times higher than that in the control medium containing yeast extract. | [27] | |
| Lactic acid | 15 g/L | 55 g/L lactose, 5.625 g/L ammonium sulfate. | Adding corn steer liquor to ammonium sulfate can increase the yield of lactic acid. | [37] |
| Name | Species | Amount of CSL Addition | Addition of Other Substances | Result | Reference |
|---|---|---|---|---|---|
| Enzyme | Cellulase | 1.2% (w/v) | Brewer’s spent grain 0.5% | The maximum yield of cellulase reached 720 U/L after 4 days of fermentation in the medium containing 0.5% BSG and 1.2% CSL | [68] |
| 0.19% (w/v) | Wheat bran 2.0% | When the wheat bran was added with 2.0% and CSL with 0.19%, the cellulase yield reached 2.0 U/mL on the 5th day of fermentation. | [69] | ||
| 5.0 g/L | Bagasse 1.0% | The highest enzyme production rate of 1.01 U/mL was achieved at 1.0% bagasse addition and 1.2% corn steep liquor addition. | [70] | ||
| Lipase | 8.0% (w/v) | Soybean oil 0.6% | Extracellular lipase was produced by fermentation with CSL as the main nitrogen source. Under optimal conditions, the maximum production rate of lipase was 0.438 U/mL/h | [75] | |
| 2.0% (w/v) | Sesame oil 2.0%, ammonium dihydrogen phosphate 0.05%, and disodium hydrogen phosphate 0.75% | Aspergillus niger was fermented by CSL to produce lipase. The output of lipase was 26.7 U/mL and the activity of lipase was increased by 2.16 times after optimized medium conditions. | [76] | ||
| 15% (w/v) | Soybean oil concentration | The maximum yield of lipase was 35.20 ± 0.8 U/mL when CSL was used as the nitrogen source. | [77] | ||
| Protease | 0.32% (w/v) | 1% Feather meal | Using FM and CSL as carbon and nitrogen sources, the protease production of solid and liquid fermentation was 21.5 U/g and 13.4 U/g, respectively, which were 39% and 86% higher than that of traditional yeast meal fermentation. | [81] | |
| 20% (w/v) | Food waste 50%, Sugarcane bagasse 10%, Wheat bran 40%, | The highest protease level (665.5 U/g) was obtained when FW 50%, SCB 10%, WB 40% were added to the salt solution and 20% CSL was added to the same mix. | [82] | ||
| Others | 1.2% (w/v) | Wheat bran 2.5% | The highest xylanase activity was 45.8 U·mL−1 when wheat bran and corn steep liquor were added to the medium. The results show that endoxylanase can also be produced by by-products of food industry. | [83] | |
| 160 g/L | 46 g/L orange peel | Maximum pectin lyase activity was 300 U/mL in agricultural medium (46 g/Lorange peel, 160 g/L corn steep liquor, and 150 g/L steamed rice water) | [84] |
| Name | Species | Amount of CSL Addition | Addition of Other Substances | Result | Reference |
|---|---|---|---|---|---|
| Natural pigment | lycopene | 20 g/L | 50 g/L glycerol and minerals | Compared with glucose fermentation, the titer and content of lycopene obtained by glycerol supplemented with CSL were significantly increased by 26.0% (470.6 mg/L) and 28% (138.2 mg/g DCW), respectively. | [94] |
| Astaxanthin | 12 g/Lsolids content | 32 g/L glucose | CSL has been found to be a valuable and cost-effective supplement to culture P. rhodozyma D3, which could both reduce the production cost of astaxanthin and increased the to 1.41 mg/g. | [100] | |
| Carotenoids | 3.4 g/L | 70 g/L sugar cane molasses | Using agricultural medium (6.5 g/L corn soaking solution and 30 g/L sugarcane molasses) for feeding batch fermentation, 830.3 μg/L ± 27.0 μg/L carotenoid was obtained, CSL can replace traditional medium to obtain carotenoids. | [104] | |
| 20 g/L | 40 g/L Glucose, 1 g/L K2HPO4 and KH2PO4, 0.05 g/L FeSO4. | Cell biomass of CSL solution at 0–5% dissolved oxygen saturation, β-carotene content increased by 271% compared to the level before addition.The biomass was 56.32 ± 0.5 g/L, and the β-carotene content was 18.92 ± 0.3 mg/L. | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Zhao, X.-Q.; Zhang, X.; Jiao, Y. Corn Steep Liquor as an Efficient Bioresource for Functional Components Production by Biotransformation Technology. Foods 2025, 14, 2158. https://doi.org/10.3390/foods14132158
Chang Y, Zhao X-Q, Zhang X, Jiao Y. Corn Steep Liquor as an Efficient Bioresource for Functional Components Production by Biotransformation Technology. Foods. 2025; 14(13):2158. https://doi.org/10.3390/foods14132158
Chicago/Turabian StyleChang, Ying, Xin-Qi Zhao, Xin Zhang, and Yan Jiao. 2025. "Corn Steep Liquor as an Efficient Bioresource for Functional Components Production by Biotransformation Technology" Foods 14, no. 13: 2158. https://doi.org/10.3390/foods14132158
APA StyleChang, Y., Zhao, X.-Q., Zhang, X., & Jiao, Y. (2025). Corn Steep Liquor as an Efficient Bioresource for Functional Components Production by Biotransformation Technology. Foods, 14(13), 2158. https://doi.org/10.3390/foods14132158





