Fermentation of Fruits and Vegetables: Bridging Traditional Wisdom and Modern Science for Food Preservation and Nutritional Value Improvements
Abstract
1. Introduction
2. Methodology of the Review
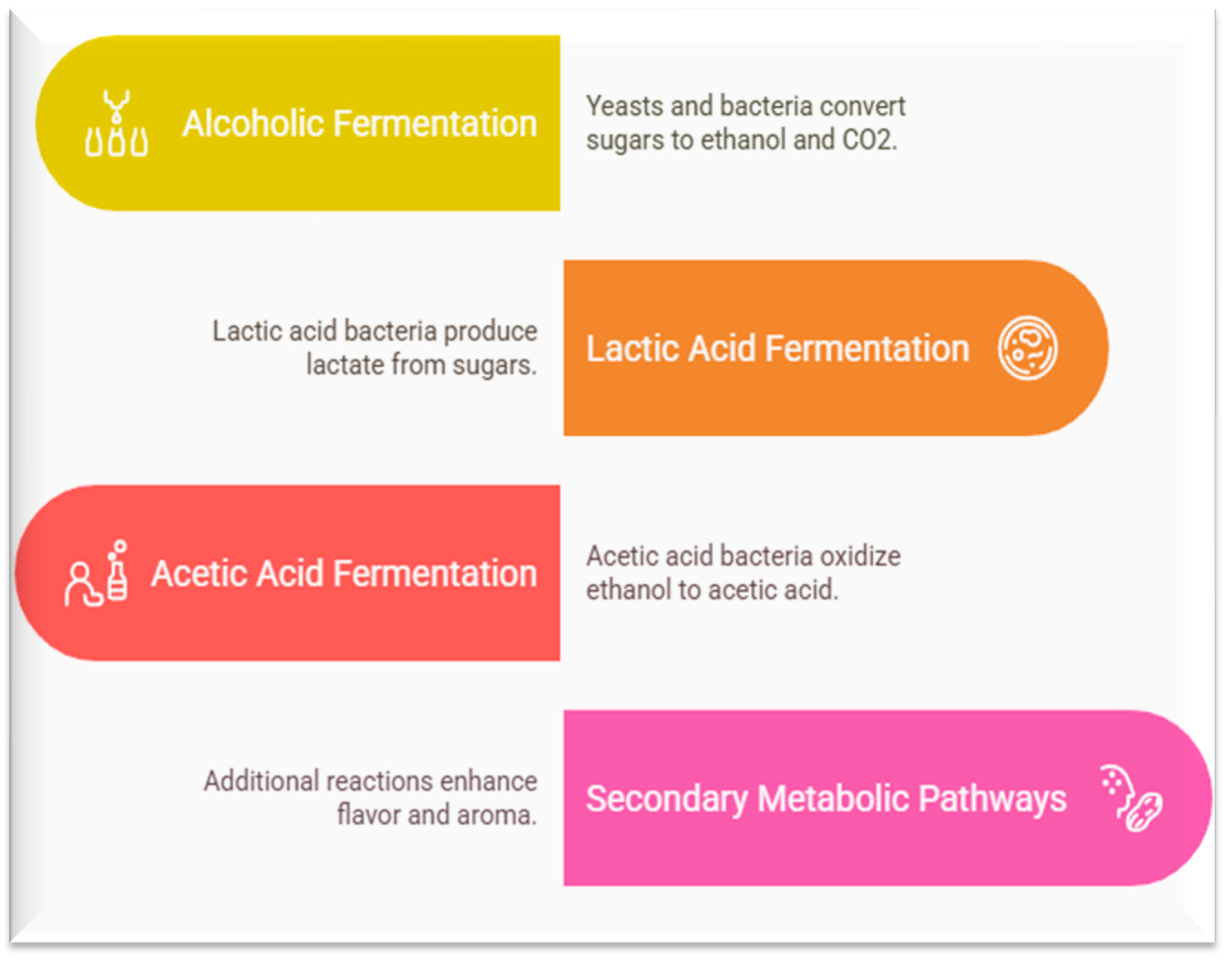
3. Biochemical Pathways in Fruit and Vegetable Fermentation
3.1. Alcoholic Fermentation
3.2. Lactic Acid Fermentation
3.2.1. Homolactic Fermentation
3.2.2. Heterolactic Fermentation
3.3. Acetic Acid Fermentation
- Ethanol→Acetaldehyde (catalyzed by membrane-bound alcohol dehydrogenase, ADH);
- Acetaldehyde→Acetic acid (via aldehyde dehydrogenase, ALDH).
- Periplasmic localization of ADH/ALDH;
- Proton extrusion mechanisms;
- Membrane lipid adaptations.
3.4. Secondary Metabolic Pathways
4. Current Market for Fermented Fruit and Vegetable Products
5. Fermentation Process and Role of Microorganisms
| Species | Fruit and Vegetable Sources | Optimal Temperature (°C) | Optimal pH Range | Refs. |
|---|---|---|---|---|
| Lactiplantibacillus pentosus | Eggplants, Cucumbers | 30–37 °C | 5.0–6.0 | [76] |
| Lactobacillus rossiae | Pineapple | 25–30 °C | 4.5–5.5 | [77] |
| Latilactobacillus curvatus | Peppers | 25–30 °C | 4.0–6.0 | [78] |
| Lactobacillus paraplantarum | Cabbages, Caper | 30–37 °C | 4.0–5.5 | [79] |
| Leuconostoc mesenteroides | White Cabbages, Carrots | 20–25 °C | 4.5–6.0 | [80] |
| Weissella confusa | Peppers, Blackberries, Papaya | 25–30 °C | 4.0–5.5 | [81] |
| Pediococcus pentosaceus | Cherries, Cucumbers, Cabbages | 25–30 °C | 4.0–5.5 | [82] |
| Limosilactobacillus fermentum | French Beans, Red Beets | 30–37 °C | 4.0–5.5 | [83] |
| Weissella soli | Carrots | 25–30 °C | 4.0–6.0 | [84] |
| Lactiplantibacillus plantarum | Tomatoes, Plums, Kiwi | 30–37 °C | 3.5–6.5 | [85] |
| Enterococcus faecium | French Beans, Tomatoes | 30–37 °C | 6.0–7.5 | [86] |
| Levilactobacillus brevis | Tomatoes, Melon pod | 25–30 °C | 4.5–6.5 | [87] |
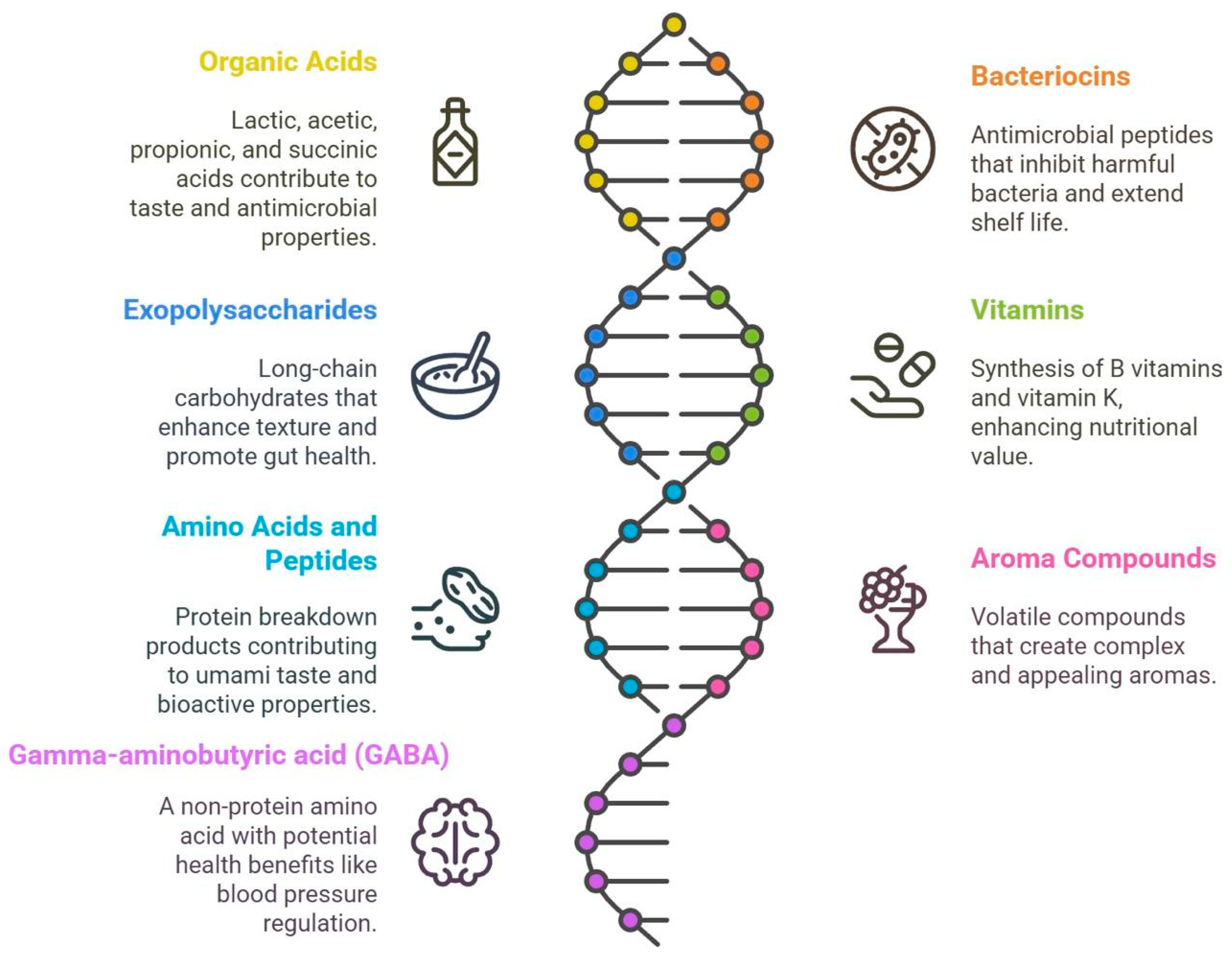
5.1. Metabolites Produced by Commonly Used Fermentation Microorganisms
5.2. Key Metabolites from LAB Fermentation
5.3. Specific Metabolites Involved in Fruit Fermentation
5.4. Specific Metabolites Involved in Vegetable Fermentation
6. Optimization of Fermentation Using the Newest Techniques and Equipment
6.1. Modern Fermentation Techniques
6.1.1. Categorization of Modern Fermentation Techniques
- (1)
- Spontaneous Fermentation
- (2)
- Starter Culture Fermentation
- (3)
- Solid-State Fermentation (SSF)
- (4)
- Submerged Fermentation (SMF)
- (5)
- Precision Fermentation
- (6)
- Continuous Fermentation
- (7)
- Artificial Intelligence (AI) and Machine Learning (ML) in Regard to Fermentation
6.1.2. Factors Influencing the Choice of Fermentation Method
6.2. Equipment for Optimized Fermentation
- (1)
- Fermentation chambers or bioreactors, featuring integrated controls for temperature, pH, and dissolved oxygen levels [130];
- (2)
- Airlocks and anaerobic lids are designed to regulate gas exchange and reduce oxygen infiltration [131];
- (3)
- Glass or stainless-steel weights are utilized to maintain solids submerged within brine [132];
- (4)
- Sensor-based monitoring systems, including pH meters, thermometers, and automated stirring mechanisms [133];
- (5)
- Cold chain apparatus for post-fermentation storage, which is especially vital for the stability of probiotic products [134].
6.3. Microbial Interactions During Fermentation
- (1)
- Synergism, exemplified by co-fermentation involving LAB and yeasts that augments food flavor and shelf life [136];
- (2)
- Competitive exclusion, where acid-producing microorganisms inhibit the proliferation of spoilage organisms [137];
- (3)
- Successional colonization, according to which the microbial dominance shifts in response to changing environmental conditions [138].

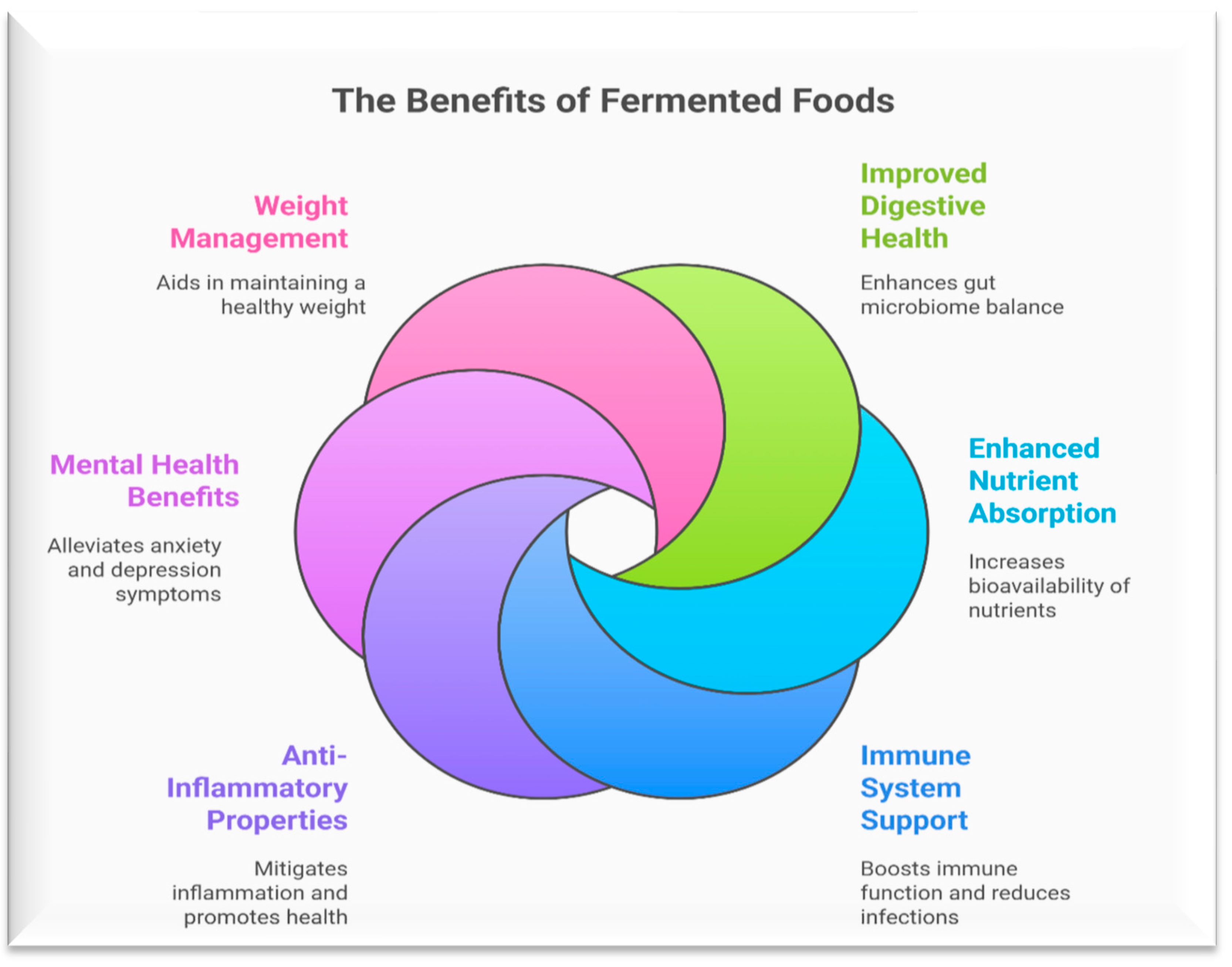
7. Health Benefits of Fermented Fruits and Vegetable Products
7.1. Probiotics
7.2. Antimicrobial Activity
7.3. Enhancing Intestinal Health
7.4. Anticancer
7.5. Diabetes Inhibition
7.6. Comparison of Bioactivities Before and After Fermentation
8. Food Security of Fermented Food
9. Safety Problems in Regard to Fermented Food and Current Solutions
9.1. Biogenic Amines
9.2. Nitrite
9.3. Microbial Safety
| Critical Control Points | Safety Concerns | Traditional Control Methods | Emerging Non-Thermal Technologies | Refs. |
|---|---|---|---|---|
| Pre-treatment Procedure | Biofilm-forming bacteria may affect flavor, texture, and safety of fermented products | Heat treatment to reduce initial microbial load | High-pressure processing (HPP), ozone treatment, pulsed electric fields (PEFs) | [174] |
| Salting Process | High salt levels control microbes, but may affect health and increase microbial deterioration risk | High sodium concentration and long salting duration | PEFs, ultrasound to accelerate salt penetration and reduce salt usage | [187] |
| Packaging and Storage | Pathogens in ready-to-eat fermented foods can pose health risks without final sterilization | Pasteurization before packaging | Pulsed light treatment, ozone treatment, HPP | [188] |
| Termination of Fermentation | Over-fermentation can negatively impact flavor and texture; pathogens can reduce shelf life | Heat treatment to stop fermentation and extend shelf life | Cold plasma, UV-C irradiation, ultrasound treatment | [189] |
10. Thoughts on the Future of Fruit and Vegetable Fermentation
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAB | Acetic acid bacteria |
| ADH | Alcohol dehydrogenase |
| ALDH | Aldehyde dehydrogenase |
| ATP | Adenosine triphosphate |
| CAD | Cadaverine |
| Caco-2 | Human epithelial colorectal adenocarcinoma cell line |
| CCD-18Co | Normal human colon myofibroblast cell line |
| CFU/g | Colony-forming units per gram |
| CI | Confidence interval |
| DHAP | 2,6-Dihydroxyacetophenone |
| DPP-IV | Dipeptidyl peptidase-IV |
| ED pathway | Entner–Doudoroff pathway |
| EFeLM | Extract of fermented leaf mustard |
| EFrLM | Extract of fresh leaf mustard |
| EMP pathway | Embden–Meyerhof–Parnas pathway |
| FDA | U.S. Food and Drug Administration |
| G2/M | Gap 2/Mitosis phase (cell cycle) |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GC-MS | Gas chromatography–mass spectrometry |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GLP-1 | Glucagon-like peptide-1 |
| GRAS | Generally Recognized as Safe |
| HBA | 4-Hydroxybenzaldehyde |
| HCT116 | Human colon cancer cell line |
| HIS | Histamine |
| HR | Hazard ratio |
| 4-HPEA | 4-Hydroxyphenylethanol |
| IBD | Inflammatory Bowel Disease |
| IBS | Irritable bowel syndrome |
| IC50 | Half-maximal inhibitory concentration |
| LAB | Lactic acid bacteria |
| LC-MS | Liquid chromatography–mass spectrometry |
| LDH | Lactate dehydrogenase |
| NAD+ | Nicotinamide adenine dinucleotide (oxidized form) |
| NADH | Nicotinamide adenine dinucleotide (reduced form) |
| n | Sample size |
| OR | Odds ratio |
| p | Probability value (statistical significance) |
| PDC | Pyruvate decarboxylase |
| pH | Potential of Hydrogen (measure of acidity/alkalinity) |
| PUT | Putrescine |
| SMF | Submerged fermentation |
| SSF | Solid-state fermentation |
| TCA cycle | Tricarboxylic acid cycle (Krebs cycle) |
| TYR | Tyramine |
| UV | Ultraviolet (light) |
| VOCs | Volatile organic compounds |
References
- Saud, S.; Xiaojuan, T.; Fahad, S. The consequences of fermentation metabolism on the qualitative qualities and biological activity of fermented fruit and vegetable juices. Food Chem. X 2024, 21, 101209. [Google Scholar] [CrossRef] [PubMed]
- Mounika, K.; Reddy, P.P.; Singh, R.P.; Bharty, S.K.; Kumar, A.; Jain, S. Post-harvest technologies and cold chain. Plant Arch. 2025, 25, 3275–3283. [Google Scholar]
- Srivastava, P.K.; Sit, N. A review on fruit and vegetable processing using traditional and novel methods. Future Foods Food Process. Preserv. 2025, 1, 4–26. [Google Scholar] [CrossRef]
- Melo, J.; Quintas, C. Minimally processed fruits as vehicles for foodborne pathogens. AIMS Microbiol. 2023, 9, 1–19. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Pasquali, M.B.; dos Anjos, A.I.; Sarinho, A.M.; de Melo, E.D.; Andrade, R.; Batista, L.; Lima, J.; Diniz, Y.; Barros, A. Innovative and Sustainable Food Preservation Techniques: Enhancing Food Quality, Safety, and Environmental Sustainability. Sustainability 2024, 16, 8223. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, R. Impact of chemical food preservatives on human health. Palarch’s J. Archaeol. Egypt/Egyptol. 2021, 15, 811–818. [Google Scholar]
- Ampemohotti, T.; Golneshin, A.; Pillidge, C.; Brennan, C.; Hao Van, T.T. Fermented Vegetables: Their Microbiology and Impact on Gut Microbiota and Overall Health Benefits. Food Rev. Int. 2025, 28, 102551. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, T.; Sun, L.; Qiao, Z.; Pan, H.; Zhong, Y.; Zhuang, Y. Recent advances of fermented fruits: A review on strains, fermentation strategies, and functional activities. Food Chem. X 2024, 22, 101482. [Google Scholar] [CrossRef]
- Lee, S.H.; Whon, T.W.; Roh, S.W.; Jeon, C.O. Unraveling microbial fermentation features in kimchi: From classical to meta-omics approaches. Appl. Microbiol. Biotechnol. 2020, 104, 7731–7744. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, Y.; Li, T.; Yang, Y.; Zeng, F.; Wang, H.; Suo, H.; Song, J.; Zhang, Y. Microbial Composition and Correlation between Microbiota and Quality-Related Physiochemical Characteristics in Chongqing Radish Paocai. Food Chem. 2022, 369, 130897. [Google Scholar] [CrossRef]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical composition and sensory quality of sauerkraut produced from different cabbage varieties. LWT—Food Sci. Technol. 2021, 136, 110325. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Y.; Sun, X.; Zhang, M.; Zhao, Y.; Zhao, X. Effect of dense phase carbon dioxide treatment on physicochemical and textural properties of pickled carrot. CYTA—J. Food 2019, 17, 988–996. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Liu, L.; Bi, X.; Liu, X. Characteristics of microbial communities in fermentation of pickled ginger and their correlation with its volatile flavors. Food Biosci. 2023, 53, 102736. [Google Scholar] [CrossRef]
- Moore, J.F.; DuVivier, R.; Johanningsmeier, S.D. Changes in the free amino acid profile of pickling cucumber during lactic acid fermentation. J. Food Sci. 2022, 87, 599–611. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Tracz, K.; Bielińska, P.; Rybak, K.; Pobiega, K.; Gniewosz, M.; Woźniak, Ł.; Gramza-Michałowska, A. The Impact of the Fermentation Method on the Pigment Content in Pickled Beetroot and Red Bell Pepper Juices and Freeze-Dried Powders. Appl. Sci. 2022, 12, 5766. [Google Scholar] [CrossRef]
- Montaño, A.; Casado, F.J.; De Castro, A.; Sánchez, A.H.; Rejano, L. Vitamin content and amino acid composition of pickled garlic processed with and without fermentation. J. Agric. Food Chem. 2004, 52, 7324–7330. [Google Scholar] [CrossRef]
- Fayek, N.M.; Farag, M.A.; Saber, F.R. Metabolome classification via GC/MS and UHPLC/MS of olive fruit varieties grown in Egypt reveal pickling process impact on their composition. Food Chem. 2021, 339, 127861. [Google Scholar] [CrossRef]
- Shang, Z.; Li, M.; Zhang, W.W.; Cai, S.; Hu, X.; Yi, J. Analysis of phenolic compounds in pickled chayote and their effects on antioxidant activities and cell protection. Food Res. Int. 2022, 157, 111325. [Google Scholar] [CrossRef]
- Fabela-Mor, M.F. Bioactive compounds, sensory attributes, and flavor perceptions involved in taste-active molecules in fruits and vegetables. Front. Nutr. 2024, 11, 1427857. [Google Scholar] [CrossRef]
- Liu, Z.; Le Bourvellec, C.; Yu, J.; Zhao, L.; Wang, K.; Tao, Y.; Renard, C.M.G.C.; Hu, X. Trends and challenges on fruit and vegetable processing: Insights into sustainable, traceable, precise, healthy, intelligent, personalized and local innovative food products. Trends Food Sci. Technol. 2022, 126, 12–25. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Reale, A. A holistic review on Euro-Asian lactic acid bacteria fermented cereals and vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [CrossRef]
- Sucheta, P.; Singla, G.; Chaturvedi, K.; Sandhu, P.P. Status and recent trends in fresh-cut fruits and vegetables. In Fresh-Cut Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–49. [Google Scholar] [CrossRef]
- Gomathy, M.; Sabarinathan, K.G.; Rajakumar, D.; Manoj, M.; Bhimani, H.D.; John, P.; Ramya, R. Fermented foods of South Asia. In Microbial Fermentation: Nature as Design and Process; Elsevier: Amsterdam, The Netherlands, 2023; pp. 323–351. [Google Scholar]
- Owusu-Kwarteng, J.; Agyei, D.; Akabanda, F.; Atuna, R.A.; Amagloh, F.K. Plant-based alkaline fermented foods as sustainable sources of nutrients and health-promoting bioactive compounds. Front. Sustain. Food Syst. 2022, 6, 885328. [Google Scholar] [CrossRef]
- Yang, X.; Hong, J.; Wang, L.; Cai, C.; Mo, H.; Wang, J.; Fang, X.; Liao, Z. Effect of lactic acid bacteria fermentation on plant-based products. Fermentation 2024, 10, 48. [Google Scholar] [CrossRef]
- Marafon, K.; Prestes, A.A.; Carvalho, A.C.F.; De Souza, C.K.; Prudencio, E.S. Bioactive compounds’ importance in plant-based beverages: A review. Curr. Opin. Food Sci. 2025, 63, 101304. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Wang, N.; Liu, X.; Wang, L.; Ning, K. Synergy of traditional practices and modern technology: Advancing the understanding and applications of microbial resources and processes in fermented foods. Trends Food Sci. Technol. 2025, 157, 104891. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 2018, 60, 181–200. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, L. Encapsulated natural antimicrobials: A promising way to reduce microbial growth in different food systems. Food Control 2021, 123, 107678. [Google Scholar] [CrossRef]
- Suganthi, K.K.A.; Thangaraj, Y.S.J.; Anandham, E.R.; Murugan, P.R.M. Role of lactic acid bacteria in insecticide residue degradation. Probiotics Antimicrob. Proteins 2024, 17, 81–102. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Cui, L.; Cai, R.; Yuan, Y.; Gao, Z.; Yue, T.; Wang, W. The health benefits of fermented fruits and vegetables and their underlying mechanisms. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70072. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H. Antimicrobial activity of probiotic bacteria isolated from plants: A review. Foods 2025, 14, 495. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds from food and by-products. Curr. Opin. Food Sci. 2022, 43, 189–198. [Google Scholar] [CrossRef]
- Jaika, S. Africa’s contribution to global sustainable and healthy diets: A scoping review. Front. Nutr. 2025, 12, 1519248. [Google Scholar] [CrossRef]
- Jimoh, A.A. Evaluation of antiobesogenic properties of fermented foods: In silico insights. J. Food Sci. 2025, 90, e70074. [Google Scholar] [CrossRef] [PubMed]
- Şanlıbaba, P. Fermented nondairy functional foods based on probiotics. Ital. J. Food Sci. 2023, 35, 91–105. [Google Scholar] [CrossRef]
- Hassid, A.; Salla, M.; Krayem, M.; Khaled, S.; Hassan, H.F.; El Khatib, S. A review on the versatile applications of plant-based essential oils in food flavoring, culinary uses and health benefits. Discov. Food 2025, 5, 130. [Google Scholar] [CrossRef]
- Mikołajczuk-Szczyrba, A.; Wojtczak, A.; Kieliszek, M.; Sokołowska, B. Characteristics and in vitro properties of potential probiotic strain Fructobacillus tropaeoli KKP 3032 isolated from orange juice. Folia Microbiol. 2025, 70, 177–194. [Google Scholar] [CrossRef]
- Mojikon, F.D.; Kasimin, M.E.; Molujin, A.M.; Gansau, J.A.; Jawan, R. Probiotication of nutritious fruit and vegetable juices: An alternative to dairy-based probiotic functional products. Nutrients 2022, 14, 3457. [Google Scholar] [CrossRef]
- Lys, I.M. The role of lactic fermentation in ensuring the safety and extending the shelf life of African indigenous vegetables and its economic potential. Appl. Sci. 2025, 4, e202400131. [Google Scholar] [CrossRef]
- Rosa, L.M.R.d.; Borges, B.P.; Santos, S. Estudo de bebidas fermentadas de tomate italiano e tomate sweet grape. Periódico Tchê Química 2022, 19, 35–42. [Google Scholar] [CrossRef]
- Rathod, N.; Soni, A.; Meena, P.; Sharma, H. Non-dairy dietary approach for lactose intolerant. In Lactose Hydrolysis in Dairy Products; Rao, P.S., Sharma, H., Arora, S., Naik, N.L., Eds.; Springer: Cham, Switzerland, 2025; pp. 119–137. [Google Scholar] [CrossRef]
- Sharma, N.; Patial, S.; Sadana, K.; Shukla, G. Probiotication of beverages: The future of functional nutrition and gut health. Preprints 2025, 1–18. [Google Scholar] [CrossRef]
- Gaudioso, G.; Weil, T.; Marzorati, G.; Solovyev, P.; Bontempo, L.; Franciosi, E.; Bertoldi, L. Microbial and metabolic characterization of organic artisanal sauerkraut fermentation and study of gut health-promoting properties of sauerkraut brine. Front. Microbiol. 2022, 13, 929738. [Google Scholar] [CrossRef] [PubMed]
- Page, C.A.; Pérez-Díaz, I.M. Whole-genome sequencing and annotation of selected Lactobacillales isolated from commercial cucumber fermentation. Microbiol. Resour. Announc. 2021, 10, e0062521. [Google Scholar] [CrossRef]
- Bavaro, A.R.; Tarantini, A.; Bruno, A.; Logrieco, A.F.; Gallo, A.; Mita, G.; Valerio, F.; Bleve, G.; Cardinali, A. Functional foods in Mediterranean diet: Exploring the functional features of vegetable case-studies obtained also by biotechnological approaches. Aging Clin. Exp. Res. 2024, 36, 208. [Google Scholar] [CrossRef]
- Song, J.; Peng, S.; Yang, J.; Zhou, F.; Suo, H. Isolation and identification of novel antibacterial peptides produced by Limosilactobacillus fermentum SHY10 in Chinese pickles. Food Chem. 2021, 348, 129097. [Google Scholar] [CrossRef]
- Ghimire, A.; Sah, A.K.; Poudel, R. Kinetics and modeling of growth and lactic acid production in Gundruk, a Himalayan fermented vegetable dish. Food Sci. Nutr. 2020, 8, 5591–5600. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhao, Y.; Guan, H.; Jin, C.; Gong, H.; Sun, X. Effect of LeviLeviLevilactobacillus brevis as a starter on the flavor quality of radish paocai. Food Res. Int. 2023, 168, 112780. [Google Scholar] [CrossRef]
- Ahmed, S.; Ashraf, F.; Tariq, M.; Zaidi, A. Aggrandizement of fermented cucumber through the action of autochthonous probiotic cum starter strains of Lactiplantibacillus plantarum and Pediococcus pentosaceus. Ann. Microbiol. 2021, 71, 33. [Google Scholar] [CrossRef]
- Kaur, P.; Zalpouri, R.; Modi, R.; Sahota, P.P.; Dhillon, T.S.; Kaur, A. Development and standardization of processing technique for ready-to-use lab fermented Kanji mix using refractance window dried black carrot powder. Sci. Rep. 2023, 13, 185. [Google Scholar] [CrossRef]
- Maicas, S. Advances in wine fermentation. Fermentation 2021, 7, 187. [Google Scholar] [CrossRef]
- Han, Y.; Du, J. A comparative study of the effect of bacteria and yeasts communities on inoculated and spontaneously fermented apple cider. Food Microbiol. 2023, 111, 104195. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, Q.; Zhang, Y.; Wu, D.; Li, G.; Liu, J.; Zhang, L.; Wang, H.-M.D. Three novel dietary phenolic compounds from pickled Raphanus sativus L. inhibit lipid accumulation in obese mice by modulating the gut microbiota composition. Mol. Nutr. Food Res. 2021, 65, 2000780. [Google Scholar] [CrossRef]
- Akpoghelie, P.O.; Edo, G.I.; Mafe, A.N.; Isoje, E.F.; Igbuku, U.A.; Ali, A.B.M.; Yousif, E.; Owheruo, J.O.; Oberhiri, S.O.; Essaghah, A.E.A.; et al. Food, health, and environmental impact of lactic acid bacteria: The superbacteria for posterity. Probiotics Antimicrob. Proteins 2025, 28, 1–37. [Google Scholar] [CrossRef]
- Adesemoye, E.T.; Sanni, A.I.; Spano, G.; Capozzi, V.; Fragasso, M. Lactic acid bacteria diversity in fermented foods as potential bio-resources contributing to alleviate malnutrition in developing countries: Nigeria as a case study. Fermentation 2025, 11, 103. [Google Scholar] [CrossRef]
- Ma, F.; Wang, J.; Zhao, M.; Tong, P.; Lv, L.; Gao, Z.; Long, Y. High hydrostatic pressure treatments improved properties of fermentation of apple juice accompanied by higher reserved Lactiplantibacillus plantarum. Foods 2023, 12, 678. [Google Scholar] [CrossRef]
- Hariprasath, K.; Dhanvarsha, M.; Mohankumar, S.; Sudha, M.; Saranya, N.; Saminathan, V.R. Characterization of gut microbiota in Apis cerana across different altitudes in the Peninsular India. BMC Ecol. Evol. 2025, 25, 39. [Google Scholar] [CrossRef]
- Seong, J.H.; Kang, C.N.; Lee, J.W. Taxonomic hierarchy of the phylum Firmicutes and novel Firmicutes species originated from various environments in Korea. J. Microbiol. 2018, 56, 1–10. [Google Scholar] [CrossRef]
- Sawant, S.S.; Park, H.; Sim, E.; Kim, H.; Choi, H. Microbial fermentation in food: Impact on functional properties and nutritional enhancement—A review of recent developments. Fermentation 2025, 11, 15. [Google Scholar] [CrossRef]
- Shi, C.; Wei, Y.; Lin, X.; Liang, H.; Zhang, S.; Chen, Y.; Dong, L.; Ji, L. Microbial metabolic transformation and antioxidant activity evaluation of polyphenols in kombucha. Food Biosci. 2022, 45, 102287. [Google Scholar] [CrossRef]
- Bilal, M.; Ibrahim, A.; Saleem, R.; Husnain, M.; Alam, H.M.; Mazhar, M.G.; Mushtaq, A. Microbes and their role in food sciences. Indus J. Biosci. Res. 2025, 3, 283–292. [Google Scholar] [CrossRef]
- Kapoor, J.; Nivethitha, M.J.; Krupa, S. Unlocking cold secrets; psychrophiles and their benefits in food. Int. J. Pharm. Sci. Res. 2025, 16, 315–324. [Google Scholar] [CrossRef]
- Widyastuti, Y.; Febrisiantosa, A.; Tidona, F. Health-promoting properties of lactobacilli in fermented dairy products. Front. Microbiol. 2021, 12, 673890. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Li, D.; Wen, G.; Zhou, J.; Manickam, S.; Han, Y.; Chai, W.S. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere 2021, 276, 130090. [Google Scholar] [CrossRef]
- Kouam, F.E.M.; Kaktcham, P.; Maffo, B.; Tchamani, P.; Fotso Techeu, U.D.; Zambou Ngoufack, F. Development of a non-dairy probiotic beverage based on sorrel and pineapple juices using Lacticaseibacillus paracasei 62L. J. Agric. Food Res. 2023, 14, 100688. [Google Scholar] [CrossRef]
- Petrović, T.Ž.; Ilić, P.; Grujović, M.; Mladenović, K. LatiLatiLatilactobacillus curvatus from fermented sausages as new probiotic functional foods. Food Sci. Technol. 2022, 13, 907393. [Google Scholar] [CrossRef]
- Le Marc, Y.; Bavaro, A.R.; Lonigro, S.L.; Lavermicocca, P.; Postollec, F. A predictive growth model for pro-technological and probiotic Lacticaseibacillus paracasei strains fermenting white cabbage. Front. Microbiol. 2022, 13, 907393. [Google Scholar] [CrossRef]
- Zubaidah, E.; Yuwono, S.S.; Srianta, I. Effect of Lactiplantibacillus plantarum and Leuconostoc mesenteroides starter cultures in lower salt concentration fermentation on the sauerkraut quality. Food Res. 2020, 4, 1038–1044. [Google Scholar] [CrossRef]
- Ahmed, S.; Singh, S.; Singh, V.; Roberts, K.D.; Zaidi, A.; Rodriguez-Palacios, A. The Weissella genus: Clinically treatable bacteria with antimicrobial/probiotic effects on inflammation and cancer. Microorganisms 2022, 10, 2427. [Google Scholar] [CrossRef]
- Li, C.C.; Li, B. Temperature and humidity regulate sporulation of Corynespora cassiicola that is associated with pathogenicity in cucumber (Cucumis sativus L.). Biology 2022, 11, 1675. [Google Scholar] [CrossRef]
- Jafar, N.B.; Ghaleb, Z.T.; Fadhil, Z.H. Production of fermented red beet juice using probiotic lactobacilli bacteria. Ann. Trop. Med. Public Health 2019, 22, S211. [Google Scholar] [CrossRef]
- Mun, S.Y.; Chang, H.C. Characterization of Weissella koreensis SK isolated from kimchi fermented at low temperature (~0 °C) based on complete genome sequence and corresponding phenotype. Microorganisms 2020, 8, 1147. [Google Scholar] [CrossRef]
- You, C.-B.; Lee, E.-S.; Lee, M.-K.; Lee, G.-Y. Antioxidant and anti-inflammatory activities of heat-killed Lactiplantibacillus plantarum isolated from kimchi. Curr. Top. Lact. Acid Bact. 2022, 8, 66–78. [Google Scholar] [CrossRef]
- Singh, G.; Gupta, S.K. Role of temperature, relative humidity and rainfall in the development of French bean rust (Uromyces appendiculatus). Indian Phytopathol. 2019, 72, 271–280. [Google Scholar] [CrossRef]
- Fevria, R.; Hartanto, I. Isolation and characterization of lactic acid bacteria (Lactobacillus sp.) from tomato (Solanum lycopersicum). Bioscience 2018, 2, 45. [Google Scholar] [CrossRef]
- Mujahid, K.; Wakeel, M.; Ali, A.M.; Saeed, S.; Nawaz, A.S.; Hafeez, K. Food fermentation: Traditional practices and modern applications in food industry. Int. J. Food Ferment. Technol. 2024, 14, 239–273. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Shiferaw Terefe, M.A.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef]
- Dwivedi, A.; Chaudhary, M.; Awasthi, A. Solid-state fermentation of plant-based food to enhance bioactive components. In Fermentation Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2025; pp. 29–61. [Google Scholar]
- Katu, J.K.; Tóth, T.; Varga, L. Enhancing the nutritional quality of low-grade poultry feed ingredients through fermentation: A review. Agriculture 2025, 15, 476. [Google Scholar] [CrossRef]
- Nkemnaso, C. Solid state fermentation: Substrates uses and applications in biomass and metabolites production—A review. South Asian Res. J. Biol. Appl. Biosci. 2019, 1, 20–29. [Google Scholar]
- Hu, Z.; Ye, A.; Cheng, L.; Lee, S.J.; Yang, Y. Recent progress in fabrication, characterization and application of functional protein aggregates derived from plant proteins. Crit. Rev. Food Sci. Nutr. 2025, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Han, G.; Seo, Y.S.; Park, I. Evolution of food fermentation processes and the use of multi-omics in deciphering the roles of the microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeon, H.S.; Yoo, J.Y.; Kim, J.H. Some important metabolites produced by lactic acid bacteria originated from Kimchi. Foods 2021, 10, 2148. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Rodríguez, L.G.; Mohamed, F.; Bleckwedel, J.; Medina, R.; De Vuyst, L.; Hebert, E.M.; Mozzi, F. Diversity and Functional Properties of Lactic Acid Bacteria Isolated from Wild Fruits and Flowers Present in Northern Argentina. Front. Microbiol. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Tang, H.; Huang, W.; Yao, Y.F.; Ruiz Rodríguez, L.G.; Mohamed, F.; Bleckwedel, J.; Medina, R.; De Vuyst, L.; Hebert, E.M.; Mozzi, F. The metabolites of lactic acid bacteria: Classification, biosynthesis and modulation of gut microbiota. Microb. Cell 2023, 10, 49–62. [Google Scholar] [CrossRef]
- Peters, A.; Krumbholz, P.; Jäger, E.; Heintz-Buschart, A.; Çakir, M.V.; Rothemund, S.; Gaudl, A.; Ceglarek, U.; Schöneberg, T.; Stäubert, C. Metabolites of lactic acid bacteria present in fermented foods are highly potent agonists of human hydroxycarboxylic acid receptor 3. PLoS Genet. 2019, 15, e1008145. [Google Scholar] [CrossRef]
- Elhalis, H.; See, X.Y.; Osen, R.; Chin, X.H.; Chow, Y. The potentials and challenges of using fermentation to improve the sensory quality of plant-based meat analogs. Front. Microbiol. 2023, 14, 1267227. [Google Scholar] [CrossRef]
- Joshi, T.J.; Salini , S.V.; Mohan, L.; Nandagopal, P.; Arakal, J.J. Functional metabolites of probiotic lactic acid bacteria in fermented foods. Curr. Res. Food Sci. 2024, 8, 100612. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Oliveira, K.A.R.; de Oliveira, M.E.G. Influence of lactic acid bacteria metabolites on physical and chemical food properties. Curr. Opin. Food Sci. 2023, 49, 100981. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, F.; Cai, W.; Zhao, X.; Shan, C. Evaluating the effect of lactic acid bacteria fermentation on quality, aroma, and metabolites of chickpea milk. Front. Nutr. 2022, 9, 1069714. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Cui, F.; Wang, D.; Lv, X.; Li, X.; Li, J. Fermented vegetables: Health benefits, defects, and current technological solutions. Foods 2023, 13, 38. [Google Scholar] [CrossRef]
- Moresi, M. Design and operation of a multifunctional pilot-scale bioreactor for enhanced aerobic fermentation. Fermentation 2025, 11, 101. [Google Scholar] [CrossRef]
- Groff, M.C.; Fernández Puchol, C.; Gil, R.; Pedrozo, L.P.; Albareti, S.; Manzanares, A.B.; Sánchez, E.; Scaglia, G. Integrated system of microalgae photobioreactor and wine fermenter: Growth kinetics for sustainable CO2 biocapture. Fermentation 2025, 11, 58. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Shen, H.; Shi, N.; Zhou, H.; Li, Y.; Guo, Y.; Luo, H.; Yu, L. Screening, identification, and fermentation characteristics of lactic acid bacteria from pickled potherb mustard and potential applications. Foods 2025, 14, 1431. [Google Scholar] [CrossRef]
- Keskin, E.; Yıldırım, İ.O. Cultivating precision: Comparative analysis of sensor-based yogurt fermentation monitoring techniques. arxiv 2025, arXiv:2501.08781. [Google Scholar]
- Avirvarei, T.E.; Salanță, A.-C.; Pop, R.; Mudura, C.; Pasqualone, A.; Anjos, O.; Barboza, N.; Usaga, J.; Pompei Darab, C.; Burja-Udrea, C.; et al. Fruit-based fermented beverages: Contamination sources and emerging technologies applied to assure their safety. Foods 2023, 12, 838. [Google Scholar] [CrossRef]
- Remize, F.; De Santis, E. Spore-Forming Bacteria. In The Microbiological Quality of Food; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2025; pp. 157–174. [Google Scholar]
- Sun, J.; AL-Ansi, W.; Lu, L.; Gu, Y.; Fan, M.; Li, Y.; Qian, H.; Fan, L.; Wang, L. Exploring the transformative effects of solid-state fermentation on the structure, texture, and metabolism of oat-based dough. Int. J. Food Sci. Technol. 2025, 60, vvae014. [Google Scholar] [CrossRef]
- Basharat, S.; Zhai, L.; Jiang, F.; Asjad, T.; Khan, A.; Liao, X. Screening and comparative genomics of probiotic lactic acid bacteria from bee bread of Apis cerana: Influence of stevia and stevioside on bacterial cell growth and the potential of fermented stevia as an antidiabetic, antioxidant, and antifungal agent. Microorganisms 2025, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Parveen Rani, R. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N. Fermented Fruits and Vegetables. In Food Processing: Principles and Applications; Academic Press: Cambridge, MA, USA, 2017; pp. 43–60. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An overview of fermentation in the food industry—Looking back from a new perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef]
- Pandey, A.; Selvakumar, P.; Soccol, C.R.; Singh, P.; Nigam, N. Solid-state fermentation for the production of industrial enzymes. Curr. Sci. 2000, 79, 1199–1206. Available online: https://www.jstor.org/stable/24102923 (accessed on 25 May 2025).
- Bhargav, S.; Panda, B.P.; Ali, M.; Javed, S. Solid-state fermentation: An overview. Chem. Biochem. Eng. Q. 2008, 22, 49–70. [Google Scholar]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar]
- López-Gómez, J.P.; Venus, J. Potential Role of Sequential Solid-State and Submerged-Liquid Fermentations in a Circular Bio-economy. Fermentation 2021, 7, 76. [Google Scholar]
- Ismail, A.; Wentzel, M.; Bux, F. Solid-state fermentation bioreactors: A review. Biotechnol. Bioeng. 2006, 95, 779–788. [Google Scholar] [CrossRef]
- Verduzco-Oliva, R.; Gutierrez-Uribe, J.A. Beyond Enzyme Production: Solid State Fermentation (SSF) as an Alternative Approach to Produce Antioxidant Polysaccharides. Sustainability 2020, 12, 495. [Google Scholar]
- Krishna, C. Solid-state fermentation systems—A review. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Singh, A.K.; Upadhyay, S.K.; Singh, S.P. Microbial Bioreactors: An Introduction. Microb. Bioreact. Ind. Mol. 2023, 1–16. [Google Scholar]
- Glick, B.R.; Patten, C.L. Molecular Biotechnology: Principles and Applications of Recombinant DNA; ASM Press: Washington, DC, USA, 2010. [Google Scholar]
- Papagianni, M. Advances in citric acid fermentation by Aspergillus niger: Biochemical aspects, strain improvement and fermentation technology. Biotechnol. Adv. 2004, 22, 244–263. [Google Scholar] [CrossRef]
- Crueger, W.; Crueger, A. Biotechnology: A Textbook of Industrial Microbiology; Sinauer Associates: Sunderland, MA, USA, 1990. [Google Scholar]
- Yan, K.; Li, M.; Ma, X.; Chen, S.; Ding, B.; Huo, J.; Zhai, R.; Sha, Y.; Xu, Z.; Jin, M. Harnessing native nitrogen in lignocellulosic biomass for cellulosic ethanol production by ancestral xylose isomerase-engineered Saccharomyces cerevisiae. Bioresour. Technol. 2025, 432, 132662. [Google Scholar] [CrossRef]
- Lee, J.M. Biochemical Engineering; Prentice Hall: Upper Saddle River, NJ, USA, 2013. [Google Scholar]
- Karimian, P.; Johnston, E.; Kasprzak, A.; Liu, J.W.; Stockwell, S.; Vanhercke, T.; Hartley, C. Use of a dual biosensor for identification of novel secretion signal peptides and efficient screening of precision fermentation production strains. Future Foods 2025, 11, 100640. [Google Scholar] [CrossRef]
- Pereira, A.A.; Yaverino-Gutierrez, M.A.; Monteiro, M.C.; Souza, B.A.; Bachheti, R.K.; Chandel, A.K. Precision fermentation in the realm of microbial protein production: State-of-the-art and future insights. Food Res. Int. 2025, 200, 115527. [Google Scholar] [CrossRef]
- Niyigaba, T.; Küçükgöz, K.; Kołożyn-Krajewska, D.; Królikowski, T.; Trząskowska, M. Advances in fermentation technology: A focus on health and safety. Appl. Sci. 2025, 15, 3001. [Google Scholar] [CrossRef]
- Han, X.; Liu, Q.; Li, Y.; Zhang, M.; Liu, K.; Kwok, L.; Zhang, H.; Zhang, W. Synergizing artificial intelligence and probiotics: A comprehensive review of emerging applications in health promotion and industrial innovation. Trends Food Sci. Technol. 2025, 159, 104938. [Google Scholar] [CrossRef]
- Tun, K.J.G.; León-Becerril, E.; García-Depraect, O. Optimal control strategy based on artificial intelligence applied to a continuous dark fermentation reactor for energy recovery from organic wastes. Green Energy Resour. 2025, 3, 100112. [Google Scholar] [CrossRef]
- Mengesha, Y.; Terefe, Z.; Sintayehu, F.; Derbew, M. A Review on Factors Influencing the Fermentation Process of Teff (Eragrostis teff) and Other Cereal-Based Ethiopian Injera. Int. J. Food Sci. 2022, 2022, 8970856. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Zheng, Y.; Sun, X. Unveiling microbial dynamics: How forest aging shapes the microbial communities of Pinus massoniana. Ecol. Evol. 2025, 15, e71132. [Google Scholar] [CrossRef]
- Han, R.; Shi, S.; Yao, B.; Shinali, T.S.; Shang, N.; Wang, N. Recent insights in Lactobacillus-fermented fruit and vegetable juice: Compositional analysis, quality evaluation, and functional properties. Food Rev. Int. 2025, 1–35. [Google Scholar] [CrossRef]
- Galena, A.E.; Chai, J.; Zhang, J.; Bednarzyk, M.; Perez, D.; Ochrietor, J.D.; Jahan-Mihan, A. The effects of fermented vegetable consumption on the composition of the intestinal microbiota and levels of inflammatory markers in women: A pilot and feasibility study. PLoS ONE 2022, 17, e0265681. [Google Scholar] [CrossRef]
- Steinert, R.E.; Rehman, A.; Sadabad, M.S.; Milanese, A.; Wittwer-Schegg, J.; Burton, J.P.; Spooren, A. Microbial micronutrient sharing, gut redox balance and keystone taxa as a basis for a new perspective to solutions targeting health from the gut. Gut Microbes 2025, 17, 2477816. [Google Scholar] [CrossRef]
- Monciozo Domingos, M.; Tanure Werneck, H.; Sant’Ana, M.R.; de São José, J.F.B. Saccharomyces boulardii as a promising microorganism for the development of probiotic beverages: An overview. Food Rev. Int. 2025, 41, 1592–1607. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, N.; Ma, S.X.; Cheng, X.; Yang, X.; Wang, G. Gut microbiota dysbiosis: Pathogenesis, diseases, prevention, and therapy. MedComm 2025, 6, e70168. [Google Scholar] [CrossRef]
- Savitri; Lata, P. Probiotics for human health. In Advances in Probiotics for Sustainable Food and Medicine; Goel, G., Kumar, A., Eds.; Microorganisms for Sustainability Series; Springer: Cham, Switzerland, 2021; pp. 181–212. [Google Scholar] [CrossRef]
- Azeem, K.; Fatima, S.; Ali, A.; Ubaid, A.; Husain, F.M.; Abid, M. Biochemistry of bacterial biofilm: Insights into antibiotic resistance mechanisms and therapeutic intervention. Life 2025, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.R.; Mumtaz, M.; Sharif, S.; Mustafa, I.; Nayila, I. Helicobacter pylori and gastric cancer: Current insights and nanoparticle-based interventions. RSC Adv. 2025, 15, 5558–5570. [Google Scholar] [CrossRef]
- Hu, J.; Tian, X.; Wei, T.; Wu, H.; Lu, J.; Lyu, M.; Wang, S. Anti-Helicobacter pylori activity of a Lactobacillus sp. PW-7 exopolysaccharide. Foods 2021, 10, 2453. [Google Scholar] [CrossRef]
- Gong, X.; Han, D.; Zhang, L.; Yin, G.; Yang, J.; Jia, H.; Cao, Z. Comprehensive analysis of the LysM protein family and functional characterization of the key LysM effector StLysM1, which modulates plant immunity in Setosphaeria turcica. J. Integr. Agric. 2024, 24, 1860–1874. [Google Scholar] [CrossRef]
- Seon, H.S.; Hee, S.; Woo, S.; Yong, J.; Rhee, J.; Roh, W. Effects of the main ingredients of the fermented food, kimchi, on bacterial composition and metabolite profile. Food Res. Int. 2021, 149, 110668. [Google Scholar] [CrossRef]
- Sa’aid, N.; Tan, T. From probiotic fermentation to functional drinks: A review on fruit juices with lactic acid bacteria and prebiotics. Prep. Biochem. Biotechnol. 2025, 1–20. [Google Scholar] [CrossRef]
- Memel, Z.N.; Shah, N.D.; Beck, K.R. Diet, nutraceuticals, and lifestyle interventions for the treatment and management of irritable bowel syndrome. Nutr. Clin. Pract. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Šola, K.F.; Vladimir-Knežević, S.; Hrabač, P.; Mucalo, I.; Saso, L.; Verbanac, D. The effect of multistrain probiotics on functional constipation in the elderly: A randomized controlled trial. Eur. J. Clin. Nutr. 2022, 76, 1675–1681. [Google Scholar] [CrossRef]
- Prasetyawan, F.; Saristiana, Y.; Megasari, E.; Indrayanti, D. Halal studies and prediction of sulforaphane from cabbage (Brassica oleracea var. capitata L.) as glutathione S-transferase (GST) substrate. J. Studi Ilmu Keagamaan Islam 2025, 1, 64–77. [Google Scholar]
- John, O.D.; Surugau, N.; Kansedo, J.; Panchal, S.K.; Brown, L. Plant-based functional foods from Borneo. Nutrients 2025, 17, 200. [Google Scholar] [CrossRef]
- Lee, Y.J.; Pan, Y.; Kwack, K.B.; Chung, J.H.; Park, K.Y. Increased anticancer activity of organic kimchi with starters demonstrated in HT-29 cancer cells. Appl. Sci. 2023, 13, 6654. [Google Scholar] [CrossRef]
- Hershock, M.J. “Seems to me you have plenty of nerve”: Polish American women, Detroit’s Federal Screw Works strike of 1938, and the fate of the UAW. Pol. Am. Stud. 2025, 82, 63–87. [Google Scholar] [CrossRef]
- Pathak, D.R.; Stein, A.D.; He, J.-P.; Noel, M.M.; Hembroff, L.; Nelson, D.A.; Vigneau, F.; Shen, T.; Scott, L.J.; Charzewska, J.; et al. Cabbage and sauerkraut consumption in adolescence and adulthood and breast cancer risk among US-resident Polish migrant women. Int. J. Environ. Res. Public Health 2021, 18, 10795. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhao, J.; Cui, X. Hypoglycemic potential of sugarcane molasses-derived polyphenols: Exploring the hypoglycemic mechanisms through in vitro α-glucosidase inhibition and intracellular glucose transport inhibition. Food Funct. 2025, 16, 4562–4574. [Google Scholar] [CrossRef]
- Coassolo, L.; Wiggenhorn, A.; Svensson, K.J. Understanding peptide hormones: From precursor proteins to bioactive molecules. Trends Biochem. Sci. 2025, 50, 481–494. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, X.; Chen, S.; Tian, K.; Xu, S.; Deng, R.; Chen, M.; Yang, Y.; Liu, T. Regular consumption of pickled vegetables and fermented bean curd reduces the risk of diabetes: A prospective cohort study. Front. Public Health 2023, 11, 1155989. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Aamarpali, T. Review of biochemical processes in food preservation techniques: Impact of fermentation, canning, and freezing on food safety and shelf life. Genes 2024, 11, 84–92. [Google Scholar]
- Haard, N.F. Fermented Cereals—A Global Perspective. In FAO Agricultural Services Bulletin No. 138; Food and Agriculture Organization: Rome, Italy, 1999. [Google Scholar]
- Tian, Y.; Deng, F.; Zhao, L.; Du, H.; Li, T.; Lai, D.; Zhou, T.; Qing, Z. Characterization of Extractable Components of Fresh and Fermented Huarong Large-Leaf Mustard and Their Inhibitory Effects on Human Colon Cancer Cells. Food Biosci. 2021, 43, 101280. [Google Scholar] [CrossRef]
- Jin, J.; den Besten, H.M.W.; Rietjens, I.M.C.M.; Van den Ende, F.W. Chemical and microbiological hazards arising from new plant-based foods, including precision fermentation–produced food ingredients. Annu. Rev. Food Sci. Technol. 2025, 16, 171–194. [Google Scholar] [CrossRef]
- Almpounioti, K.; Papagianni, O.I.; Koutelidakis, A.E. Traditional foods, the Mediterranean diet, and health promotion. In Handbook of Public Health Nutrition: International, National, and Regional Perspectives; Springer: Cham, Switzerland, 2025; pp. 1–23. [Google Scholar]
- Das, S.; Polley, S.; Bhattacharyya, S. The vital role of food safety in mitigating microbial hazards. Int. J. Curr. Microbiol. Appl. Sci. 2025, 14, 11. [Google Scholar] [CrossRef]
- Singh, A.; Vellapandian, C. High risk of metabolic complications due to high consumption of processed foods. Curr. Nutr. Food Sci. 2023, 19, 198–208. [Google Scholar] [CrossRef]
- Mirza Alizadeh, M.; Mohammadi, F.; Hashempour-Baltork, F.; Hosseini, H.; Shahidi, F. Process-induced toxicants in food: An overview on structures, formation pathways, sensory properties, safety and health implications. Food Prod. Process. Nutr. 2025, 7, 7. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Ye, L.; Hou, X.; Li, Z.; Tang, L.; Deng, N.; Li, H. Correlation between the physicochemical and bacteriological qualities of pickled chili pepper (Paojiao) and its nitrite degradation study. Food Biosci. 2025, 66, 106246. [Google Scholar] [CrossRef]
- Turna, N.S.; Chung, R.; McIntyre, L. A review of biogenic amines in fermented foods: Occurrence and health effects. Heliyon 2024, 10, e24501. [Google Scholar] [CrossRef]
- Semjon, B.; Bartkovský, M.; Očenáš, P.; Regecová, I.; Eftimová, Z.M.; Výrostková, J.; Mesarčová, L.; Kováčová, M.; Várady, M.; Šuľáková, L.; et al. The impact of grape maceration on quality and biogenic amine formation in Slovak Tokaj wines: Examination of microbial, chemical and sensory properties. Fermentation 2025, 11, 27. [Google Scholar] [CrossRef]
- Ekici, K.; Omer, A.K. Biogenic amines formation and their importance in fermented foods. BIO Web Conf. 2020, 17, 00232. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.; Lu, D.; Zhao, J.; Duan, G.; Zhu, T.; Hu, Y. The Fermentation Law of Biogenic Amines in the Pre-Fermentation Process Is Revealed by Correlation Analysis. Foods 2025, 14, 583. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Lang, X.; Ji, Y.; Li, S.; Xin, N.; Meng, X.; Zhang, T.; Shen, X.; Zhao, Z. The interaction between Lactiplantibacillus plantarum SC-5 and its biogenic amine formation with different salt concentrations in Chinese Dongbei Suancai. Food Res. Int. 2021, 150, 110813. [Google Scholar] [CrossRef]
- Tahmouzi, S.; Alizadeh Salmani, B.; Eskandari, S.; Arab, M. Effects of plant substitutes for nitrite on the technological characteristics of fermented sausages: A comprehensive review. Food Sci. Nutr. 2025, 13, 70186. [Google Scholar] [CrossRef]
- Paramithiotis, S. Lactiplantibacillus plantarum, the integral member of vegetable fermentations. Appl. Biosci. 2025, 4, 7. [Google Scholar] [CrossRef]
- Yu, Z.X.; Yangyang, Y.; Yuanshan, Y. Evaluation of nitrite, ethyl carbamate, and biogenic amines in four types of fermented vegetables. Foods 2021, 10, 1150. [Google Scholar] [CrossRef]
- Ahmad, K.A.; Abdo, A.A.; Khan, S.; Aleryani, H.; Mi, S.; Wang, Y. Advancing pickling techniques to enhance bioactive compounds and probiotic content in pickled vegetables. Food Rev. Int. 2025, 1–27. [Google Scholar] [CrossRef]
- Hang, S.; Zeng, L. Lactiplantibacillus plantarum ZJ316 improves the quality of Stachys sieboldii Miq. pickle by inhibiting harmful bacteria growth, degrading nitrite and promoting the gut microbiota health in vitro. Food Funct. 2022, 13, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Song, G.; Zhao, D.; Sun, J.; Ping, W.; Ge, J. Lacticaseibacillus casei starter culture improves vitamin content, increases acidity and decreases nitrite concentration during sauerkraut fermentation. Int. J. Food Sci. Technol. 2018, 53, 1925–1931. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Gong, C.; Tan, X.; Ren, Y.; Yao, K.; Zhang, Q.; Chi, Y. Physicochemical, microbial, and aroma characteristics of Chinese pickled red peppers (Capsicum annuum) with and without biofilm. RSC Adv. 2020, 10, 6609–6617. [Google Scholar] [CrossRef]
- Xing, Z.; Fu, X.; Huang, H.; Xu, Y.; Wei, L.; Shan, C.; Du, Y. Recent advances in Lactiplantibacillus plantarum fermentation in modifying fruit-based products: Flavor property, bioactivity, and practical production applications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70160. [Google Scholar] [CrossRef]
- Standard’s GB 2762-2017; National Food Safety Standard-Maximum Levels of Contaminants in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2017.
- Ye, J.; Shang, Z.; Li, M.; Qu, Y.; Long, H.; Yi, J. Evaluation of the physiochemical and aromatic qualities of pickled Chinese pepper (Paojiao) and their influence on consumer acceptability by using targeted and untargeted multivariate approaches. Food Res. Int. 2020, 13, 109535. [Google Scholar] [CrossRef]
- Kenesei, G.; Boylu-Kovács, M.; Gashi, A.; Mednyánszky, Z.; Takács, K.; Simon-Sarkadi, L. Effect of thermal and non-thermal pretreatments and fermentation on the amino acid and biogenic amine content of oyster mushroom. Appl. Sci. 2025, 15, 3509. [Google Scholar] [CrossRef]
- Yan, X.A.; Li, B.; Liang, J.; Huang, Q.C.; Cao, S.L.; Wang, L.H.; Zeng, Y. From laboratory to industry: The evolution and impact of pulsed electric field technology in food processing. Food Rev. Int. 2025, 41, 373–398. [Google Scholar] [CrossRef]
- Zhang, N.; Guo, C.; Luo, N.; Wang, X.; Yin, X.; Qian, L.; Cao, J.; Wang, X. Microwave processing effect on salt reduction and saltiness enhancement in muscle foods: A review. Food Res. Int. 2025, 203, 115872. [Google Scholar] [CrossRef]
- Molina-Hernandez, J.B.; Grande-Tovar, C.D.; Neri, L.; Delgado-Ospina, J.; Rinaldi, M.; Cordero-Bueso, G.A.; Chaves-López, C. Enhancing postharvest food safety: The essential role of non-thermal technologies in combating fungal contamination and mycotoxins. Front. Microbiol. 2025, 16, 1543716. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S. Cold plasma technology for sustainable food production: Meeting the United Nations sustainable development goals. Sustain. Food Technol. 2024, 3, 32–53. [Google Scholar] [CrossRef]
- Casalta, E.; Sabatier, C.; Girardi-Piva, G.; Dournes, G.; Roland, A.; Mouret, J. Impact of phytosterol addition on fermentation progress and volatile compounds synthesis during alcoholic fermentation in synthetic and natural grape musts. OENO One 2023, 57, 41–52. [Google Scholar] [CrossRef]
- Stanzer, D.; Hanousek Čiča, K.; Blesić, M.; Smajić Murtić, M.; Mrvčić, J.; Spaho, N. Alcoholic fermentation as a source of congeners in fruit spirits. Foods 2023, 12, 1951. [Google Scholar] [CrossRef] [PubMed]
- Mitsi, J.; Echeverría, C.; Cubillos, F.A.; Monardes-Carvajal, C.; Forero-Doria, O.; Echeverría, C. Alcoholic fermentation enhances in vitro and cellular antioxidant capacity and in vitro antihypertensive potential of native Chilean fruits algarrobo (Neltuma chilensis) and chañar (Geoffroea decorticans). Food Biosci. 2025, 69, 106852. [Google Scholar] [CrossRef]
- Chiarini, E.; Alessandria, V.; Buzzanca, D.; Giordano, M.; Mancuso, F.; Zeppa, G. Valorization of fruit by-products through lactic acid fermentation for innovative beverage formulation: Microbiological and physiochemical effects. Foods 2024, 13, 3715. [Google Scholar] [CrossRef]
- Wang, R.; He, H.; Li, J.; Wu, J.; Jiang, S.; Xue, H.; Wang, X. Lactic acid bacteria fermentation improves physicochemical properties, bioactivity, and metabolic profiles of Opuntia ficus-indica fruit juice. Food Chem. 2024, 453, 139646. [Google Scholar] [CrossRef]
- Luzón-Quintana, L.M.; Castro, R.; Durán-Guerrero, E. Biotechnological processes in fruit vinegar production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Liu, Y.; Zhen, H.; Wu, D.; Wang, F.; Kong, Z.; Li, X.; Sun, Y. Co-production of lactate and volatile fatty acids through repeated-batch fermentation of fruit and vegetable waste: Effect of cycle time and replacement ratio. Bioresour. Technol. 2023, 387, 129678. [Google Scholar] [CrossRef]
- Nwulu, N.; Adebo, O.A. Internet of Things (IoT) in the food fermentation process: A bibliometric review. J. Food Process Eng. 2023, 46, e14321. [Google Scholar] [CrossRef]
- Guan, Q.; Xiong, T.; Xie, M. Influence of probiotic fermented fruit and vegetables on human health and the related industrial development trend. Engineering 2020, 7, 212–218. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F. Fermentation for future food systems. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef] [PubMed]

| Fermented Product | Fruits and Vegetables Used | Lactic Acid Bacteria | Type of Fermentation | Refs. |
|---|---|---|---|---|
| Sauerkraut | Cabbage | Leuconostoc mesenteroides, Levilactobacillus brevis | Lactic Acid Fermentation | [54] |
| Fermented Cucumbers | Cucumbers | Pediococcus pentosaceus, Lactiplantibacillus plantarum | Lactic Acid Fermentation | [55] |
| Fermented Caper Berries | Capers | Lactiplantibacillus pentosus, Limosilactobacillus fermentum, Pediococcus pentosaceus, Lactobacillus paraplantarum, Enterococcus faecium | Lactic Acid Fermentation | [56] |
| Kimchi | Cabbage, Radish | Lactobacillus kimchiensis, Leuconostoc citreum, Lacticaseibacillus gasicomitatum, Lactobacillus bavaricus, Weissella confusa, Weissella kimchii, Latilactobacillus curvatus, Lactobacillus sakei, Loigolactobacillus maltaromicus | Mixed Fermentation | [57] |
| Gundruk | Cabbage, Mustard Leaves, Cauliflower Leaves | Lacticaseibacillus casei, Leuconostoc pseudoplantarum, Limosilactobacillus fermentum, Pediococcus pentosaceus | Lactic Acid Fermentation | [58] |
| Sinki | Radish Roots | Limosilactobacillus fermentum, Lactiplantibacillus plantarum, Levilactobacillus brevis, Levilactobacillus fallax | Lactic Acid Fermentation | [59] |
| Khalpi | Cucumber | Lactiplantibacillus plantarum, Pediococcus sp., Levilactobacillus brevis, Levilactobacillus fallax | Lactic Acid Fermentation | [60] |
| Kanji | Carrot and Beetroot | Levilactobacillus brevis | Lactic Acid Fermentation | [61] |
| Wine | Grape | Saccharomyces cerevisiae | Alcoholic Fermentation | [62] |
| Apple Cider | Apple | Enterococcus spp. | Alcoholic and Acetic Fermentation | [63] |
| Fermented Strawberry Juice | Strawberry | Lactiplantibacillus plantarum, Lactobacillus acidophilus | Lactic Acid Fermentation | [64] |
| Fermented Blueberry Juice | Blueberry | Lacticaseibacillus rhamnosus, Lactiplantibacillus plantarum | Lactic Acid Fermentation | [65] |
| Comparison Item | Before Fermentation | After Fermentation | Hypothetical Mechanisms |
|---|---|---|---|
| Enhanced bioavailability and bioactivity of phenolic compounds | Phenolic compounds are present, but their absorption and utilization by the human body can be limited due to their bound forms and complex matrix interactions | The hydrolysis of glycosides and esters increases the concentration of free, more readily absorbable phenolic aglycones. This often leads to a significant increase in antioxidant capacity, as demonstrated by higher DPPH radical scavenging activity and ferric-reducing antioxidant power (FRAP) in fermented products compared to the raw materials | The enhanced antioxidant activity is attributed to the increased concentration of free phenolic compounds, which can directly scavenge free radicals, chelate metal ions, and upregulate endogenous antioxidant enzymes. The smaller molecular size of aglycones facilitates their absorption across the intestinal barrier, allowing them to exert systemic antioxidant and anti-inflammatory effects |
| Production of bioactive peptides and free amino acids | Proteins are present, but their bioactive potential is largely latent within the larger protein structure | Fermented products often exhibit enhanced antihypertensive (ACE inhibitory), antioxidant, and immunomodulatory activities, due to the release of specific bioactive peptides. For example, peptides with ACE-inhibitory activity have been identified in fermented vegetable products, contributing to blood pressure regulation | Bioactive peptides can exert their effects by binding to specific receptors, modulating enzyme activities, or acting as signaling molecules. For instance, ACE-inhibitory peptides compete with angiotensin I in regard to binding to the angiotensin-converting enzyme, thereby preventing the formation of angiotensin II, a potent vasoconstrictor |
| Synthesis of vitamins | Vitamin content is dependent on the raw material and can vary | Fermented products often show increased levels of folate, riboflavin, and vitamin B12, which are essential for various metabolic processes, including DNA synthesis, energy production, and nerve function. This enhancement directly contributes to the improved nutritional status of such foods | The increased vitamin content directly contributes to their respective physiological roles. For example, increased folate levels support cell division and growth, while higher vitamin B12 contributes to red blood cell formation and neurological function |
| Production of short-chain fatty acids (SCFAs) | Raw fruits and vegetables contain dietary fibers that are precursors to SCFAs upon gut microbial fermentation | Fermented products can contain pre-formed SCFAs or promote their production in the gut. Butyrate, in particular, is a crucial energy source for colonocytes and plays a significant role in maintaining gut barrier integrity and modulating immune responses | SCFAs, particularly butyrate, exert their beneficial effects by acting as signaling molecules, modulating gene expression, and influencing immune cell differentiation. They contribute to improved gut health by strengthening the intestinal barrier, reducing inflammation, and promoting the growth of beneficial gut bacteria |
| Reduction of anti-nutritional factors | Anti-nutritional factors can chelate essential minerals (e.g., iron, zinc, calcium), reducing their absorption | The reduction in anti-nutritional factors leads to the increased bioavailability of minerals, enhancing the nutritional value of the fermented product | By degrading anti-nutritional factors, fermentation liberates bound minerals, making them more accessible for absorption in the gastrointestinal tract. This directly improves the nutritional impact of consuming these foods |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangakhedkar, P.S.; Deshpande, H.W.; Törős, G.; El-Ramady, H.; Elsakhawy, T.; Abdalla, N.; Shaikh, A.; Kovács, B.; Mane, R.; Prokisch, J. Fermentation of Fruits and Vegetables: Bridging Traditional Wisdom and Modern Science for Food Preservation and Nutritional Value Improvements. Foods 2025, 14, 2155. https://doi.org/10.3390/foods14132155
Gangakhedkar PS, Deshpande HW, Törős G, El-Ramady H, Elsakhawy T, Abdalla N, Shaikh A, Kovács B, Mane R, Prokisch J. Fermentation of Fruits and Vegetables: Bridging Traditional Wisdom and Modern Science for Food Preservation and Nutritional Value Improvements. Foods. 2025; 14(13):2155. https://doi.org/10.3390/foods14132155
Chicago/Turabian StyleGangakhedkar, Prasad S., Hemant W. Deshpande, Gréta Törős, Hassan El-Ramady, Tamer Elsakhawy, Neama Abdalla, Ayaz Shaikh, Béla Kovács, Rushikesh Mane, and József Prokisch. 2025. "Fermentation of Fruits and Vegetables: Bridging Traditional Wisdom and Modern Science for Food Preservation and Nutritional Value Improvements" Foods 14, no. 13: 2155. https://doi.org/10.3390/foods14132155
APA StyleGangakhedkar, P. S., Deshpande, H. W., Törős, G., El-Ramady, H., Elsakhawy, T., Abdalla, N., Shaikh, A., Kovács, B., Mane, R., & Prokisch, J. (2025). Fermentation of Fruits and Vegetables: Bridging Traditional Wisdom and Modern Science for Food Preservation and Nutritional Value Improvements. Foods, 14(13), 2155. https://doi.org/10.3390/foods14132155











