Bioactive Properties of Hazelnut-Derived Products in Colorectal Cancer Prevention: A Systematic Review of Preclinical and Epidemiological Studies
Abstract
1. Introduction
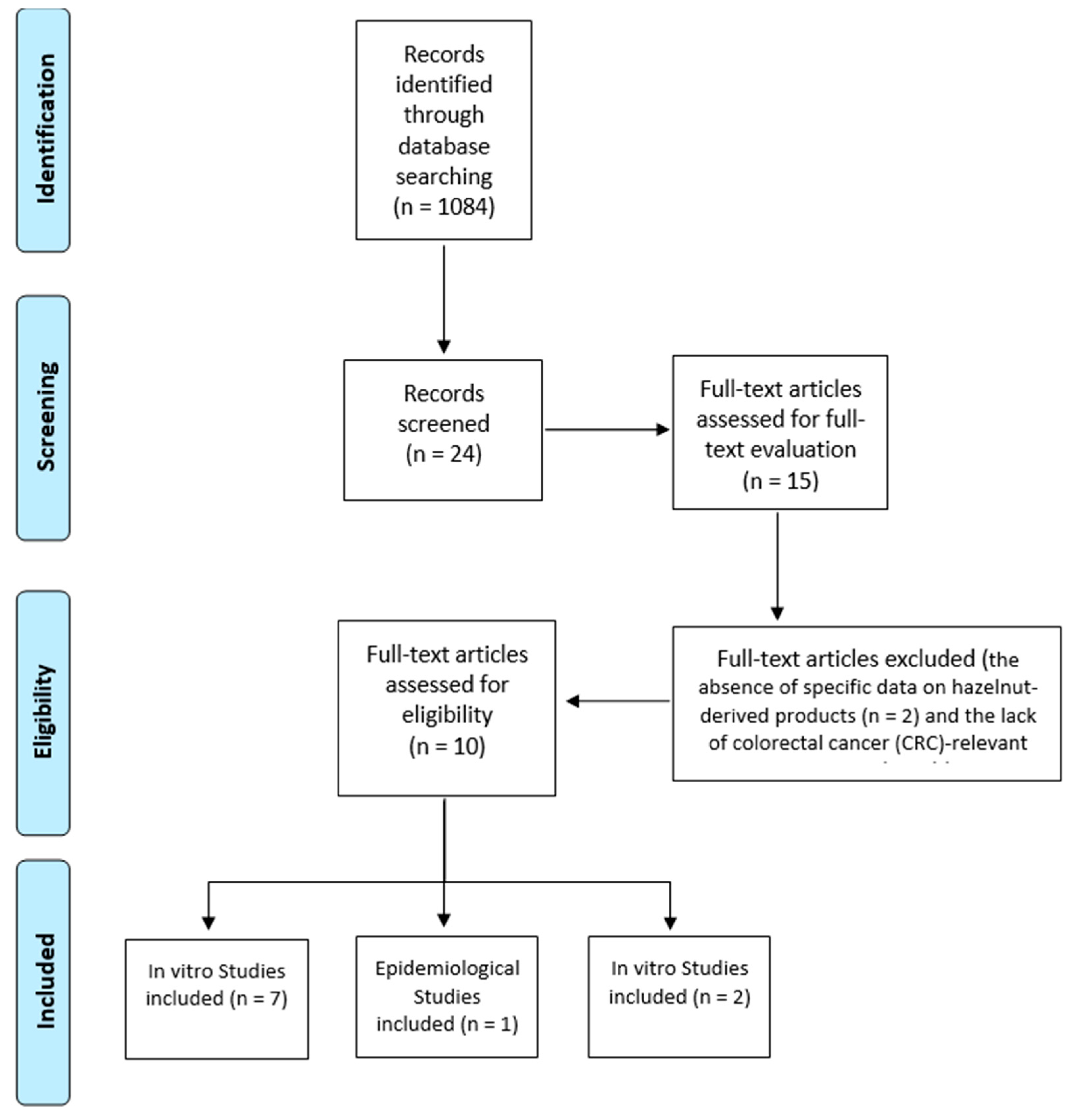
2. Materials and Methods
2.1. Design and Objectives
2.2. Literature Search Strategy
“Corylus avellana” OR “hazelnuts” OR “hazelnut oil” OR “hazelnut skin” AND “colorectal cancer” OR “colorectal neoplasms” OR “colon cancer” OR “rectal cancer” AND “apoptosis” OR “oxidative stress” OR “bile acids” OR “inflammation” AND “nutritional properties” OR “dietary fiber” OR “flavan-3-ols” OR “FIBEROX®”
2.3. Inclusion and Exclusion Criteria
- Involved in vitro, in vivo, or observational human models relevant to CRC;
- Assessed the effects of hazelnuts or hazelnut-derived compounds (including oils, skins, or fermented extracts);
- Reported at least one outcome related to CRC risk, tumorigenesis, or biological mechanisms involved in carcinogenesis (e.g., bile acid metabolism, oxidative stress, inflammatory pathways, apoptosis);
- Were original research articles published in full text in English.
- Narrative reviews, conference proceedings, editorials, or letters;
- Studies focusing on general “tree nut” consumption without hazelnut-specific data;
- Articles lacking CRC-relevant outcomes or mechanistic endpoints.
2.4. Study Selection and Data Synthesis
3. Results
3.1. In Vitro Studies
3.1.1. Cancer Biology Context: Hazelnut Extracts and Colorectal Cancer (CRC)
3.1.2. Fermented Hazelnut Skins and Antioxidant Defense Mechanisms
3.1.3. Hazelnut Oil and Mitochondrial Apoptotic Pathways
3.1.4. Comparative Effects of Non-Titrated vs. Titrated Extracts
3.1.5. Use of Human-Derived CRC Cell Lines
3.2. In Vivo and Epidemiological Studies
4. Discussion
4.1. Study Limitations
4.2. Clinical Implications and Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Buccafusca, G.; Proserpio, I.; Tralongo, A.C.; Giuliano, S.R.; Tralongo, P. Early colorectal cancer: Diagnosis, treatment and survivorship care. Crit. Rev. Oncol. Hematol. 2019, 136, 20–30. [Google Scholar] [CrossRef]
- Mangone, L.; Marinelli, F.; Bisceglia, I.; Braghiroli, M.B.; Banzi, M.; Damato, A.; Giorgi Rossi, P. Characteristics and Outcomes of Colorectal Cancer Patients Cared for by the Multidisciplinary Team in the Reggio Emilia Province, Italy. Cancers 2024, 16, 2390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Lin, H.; Lin, Z. Hazelnut and its by-products: A comprehensive review of nutrition, phytochemical profile, extraction, bioactivities and applications. Food Chem. 2023, 413, 135576. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Tonkaz, T.; Yarılgaç, T. Chemical composition, antioxidant capacity and total phenolic content of hazelnuts grown in different countries. Tekirdağ Ziraat Fakültesi Derg. 2022, 19, 262–270. [Google Scholar] [CrossRef]
- Ivanović, S.; Avramović, N.; Dojčinović, B.; Trifunović, S.; Novaković, M.; Tešević, V.; Mandić, B. Chemical composition, total phenols and flavonoids contents and antioxidant activity as nutritive potential of roasted hazelnut skins (Corylus avellana L.). Foods 2020, 9, 430. [Google Scholar] [CrossRef]
- Rondanelli, M.; Nichetti, M.; Martin, V.; Barrile, G.C.; Riva, A.; Petrangolini, G.; Giacosa, A. Phytoextracts for human health from raw and roasted hazelnuts and from hazelnut skin and oil: A narrative review. Nutrients 2023, 15, 2421. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; Watanabe, T. Colorectal cancer. Nature reviews. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Glei, M.; Fischer, S.; Lamberty, J.; Ludwig, D.; Lorkowski, S.; Schloermann, W. Chemopreventive potential of in vitro fermented raw and roasted hazelnuts in LT97 colon adenoma cells. Anticancer Res. 2018, 38, 83–93. [Google Scholar]
- Ramezan, M.; Hosseini, H.M.; Salimi, A.; Ramezan, Y. Study of the Apoptotic Impacts of Hazelnut Oil on the Colorectal Cancer Cell Line. Adv. Biomed. Res. 2023, 12, 76. [Google Scholar] [CrossRef]
- Schlörmann, W.; Lamberty, J.; Lorkowski, S.; Ludwig, D.; Mothes, H.; Saupe, C.; Glei, M. Chemopreventive potential of in vitro fermented nuts in LT97 colon adenoma and primary epithelial colon cells. Mol. Carcinog. 2017, 56, 1461–1471. [Google Scholar] [CrossRef]
- Caimari, A.; Puiggròs, F.; Suárez, M.; Crescenti, A.; Laos, S.; Ruiz, J.A.; Arola, L. The intake of a hazelnut skin extract improves the plasma lipid profile and reduces the lithocholic/deoxycholic bile acid faecal ratio, a risk factor for colon cancer, in hamsters fed a high-fat diet. Food Chem. 2015, 167, 138–144. [Google Scholar] [CrossRef]
- Li, H.; Parry, J. Phytochemical compositions, antioxidant properties, and colon cancer antiproliferation effects of turkish and oregon hazelnut. Food Nutr. Sci. 2011, 2, 1142–1149. [Google Scholar] [CrossRef]
- Nieuwenhuis, L.; van den Brandt, P.A. Nut and peanut butter intake are not directly associated with the risk of endometrial or ovarian cancer: Results from a Dutch prospective cohort study. Clin. Nutr. 2020, 39, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olofinnade, A.T.; Onaolapo, A.Y.; Onaolapo, O.J.; Olowe, O.A. Hazelnut Modulates Neurobehaviour and Ameliorates Ageing-induced Oxidative Stress, and Caspase-3-Mediated Apoptosis in Mice. Curr. Aging Sci. 2021, 14, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lux, S.; Scharlau, D.; Schlörmann, W.; Birringer, M.; Glei, M. In vitro fermented nuts exhibit chemopreventive effects in HT29 colon cancer cells. Br. J. Nutr. 2012, 108, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Güner, A.; Güner, Ö.; Karabay, Ü. Igdir University, Antiangiogenic and cytotoxic activity of giresun tombul hazelnut (Corylus avellana L.) oil on cervix, breast, and colon cancer cells. J. Inst. Sci. Technol. 2021, 11, 916–926. [Google Scholar] [CrossRef]
- Hong, M.Y.; Moore, J.; Nakagawa, A.; Nungaray, V. Effects of Mixed Nuts on Colonic Cell Proliferation and Ptgs2 and Rela Gene Expression. Anticancer Res. 2022, 42, 4285–4292. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Singh, A.; O’Keefe, J.H. Nuts: Natural Pleiotropic Nutraceuticals. Nutrients 2021, 13, 3269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naghshi, S.; Sadeghian, M.; Nasiri, M.; Mobarak, S.; Asadi, M.; Sadeghi, O. Association of Total Nut, Tree Nut, Peanut, and Peanut Butter Consumption with Cancer Incidence and Mortality: A Comprehensive Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 793–808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Author/Year | Sample | Posology/Dosage | Findings |
|---|---|---|---|
| Glei et al., 2018 [9] | Fermented skins of raw and roasted hazelnuts (RC1 = 140.6 °C/25 min, RC2 = 155.1 °C/20 min, RC3 = 180.4 °C/21 min) | 2.5% and 5% concentrations | Treatment of HT29 cells with 5% fermented hazelnut skin extracts significantly upregulated SOD2 (3.0-fold increase) and GSTP1 (2.1-fold increase) gene expression, while GPx1 mRNA levels were decreased (0.6-fold reduction). Caspase-3 activity was markedly increased (6.4-fold vs. control, p ≤ 0.05). DNA damage induced by H2O2 exposure was significantly reduced (p ≤ 0.05). These results were consistent across both raw and moderately roasted hazelnut skins, suggesting roasting at moderate temperatures does not impair bioactivity. Two-way ANOVA with Bonferroni post hoc analysis confirmed the statistical significance of all main outcomes. |
| Ramezan et al., 2023 [10] | Hazelnut oil (cold-pressed Iranian cultivar) | Serial dilutions: 1/2, 1/4, 1/8, 1/16 | Exposure of HT29 cells to hazelnut oil resulted in significant, dose-dependent reductions in cell viability, with the most pronounced effect observed at 1/2 and 1/4 dilutions (p < 0.05). Quantitative PCR analysis revealed downregulation of pro-apoptotic Bax and anti-apoptotic Bcl-2 mRNA levels. Apoptosis was significantly increased based on Annexin V/PI staining (p < 0.05). These findings suggest hazelnut oil induces apoptosis through mitochondrial pathways. |
| Li et al., 2011 [13] | Hazelnut extracts from Oregon and Turkey | 6 mg/mL (Oregon), 5 mg/mL (Turkish) | Turkish hazelnut extract inhibited 89% of HT29 cell proliferation after 4 days of treatment, compared to untreated controls. The Oregon extract showed a similar but slightly less potent effect. While MTT assays confirmed inhibition, specific statistical values (e.g., p-values) were not reported. Discussion highlighted differences in polyphenolic profiles between cultivars as a potential factor influencing antiproliferative efficacy. |
| Olofinnade et al., 2021 [16] | Hazelnut extract | IC50 values between 459 and 584 μg/mL | Hazelnut extracts induced a significant, dose-dependent inhibition of proliferation in Hela and SK-Mel-28 cancer cells. Apoptosis induction was confirmed by PARP-1 cleavage and Caspase-3 activation after 24–48 h exposure. Although IC50 values were provided, detailed p-values or statistical analyses were not explicitly reported. The discussion proposed oxidative stress modulation and mitochondrial apoptosis as key mechanisms underlying these effects. |
| Lux et al., 2012 [17] | Fermented nut samples (hazelnuts) | 2.5%, 5%, 10%, and 20% (v/v) | Treatment of HT29 cells with 20% fermented hazelnut extracts led to a statistically significant reduction in cell proliferation compared to medium control (p < 0.05). The reduction was time- and concentration-dependent, with higher doses producing more pronounced effects. The study emphasized the potential role of fermentation in enhancing the anticancer properties of nut-derived bioactives. |
| Schlörmann et al., 2017 [11] | LT97 colon adenoma cells | Fermented skin (FS) of hazelnuts at 2.5% and 5% | Significant induction of CAT (up to 4.0-fold), SOD2 (up to 2.5-fold), GSTP1 (up to 2.3-fold) mRNA; significant reduction in GPx1 (down to 0.8-fold) (all p < 0.05); upregulation of p21 (up to 2.6-fold), downregulation of cyclin D2 (0.4-fold); significant inhibition of LT97 cell growth (down to 46–1.6% depending on dose/time; p < 0.05); increased early apoptotic cells (mean +8.4%; p < 0.05); increased caspase-3 activity (+4.6-fold; p < 0.05). |
| Güner et al., 2021 [18] | Colon cancer cell line (CaCo-2) | Hazelnut oil at 0.5, 5, and 50 mg/L | Significant antiproliferative activity (IC50 = 26.26 ± 3.15 mg/L, p < 0.05); significant increase in total antioxidant capacity (TAC) (+49% in CaCo-2 cells at highest dose, p < 0.05); significant reduction in total oxidative stress (TOS) (−25% in CaCo-2 cells, p < 0.05); strong correlation between MTT viability inhibition and LDH release inhibition (R2 = 0.999); inhibition of LDH release by 40% in CaCo-2 cells (p < 0.05). |
| Author/Year | Randomization | Blinding | Sample Size Justification | Selective Reporting | Other Bias | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Glei et al., 2018 [9] | Low | Unclear | Low | Low | Low | Low |
| Ramezan et al., 2023 [10] | Low | Unclear | Low | Low | Low | Low |
| Li et al., 2011 [13] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Olofinnade et al., 2021 [16] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Lux et al., 2011 [17] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Schlörmann et al., 2017 [11] | Low | Unclear | Low | Low | Low | Low |
| Güner et al., 2021 [18] | Low | Unclear | Low | Low | Low | Low |
| Author/Year | Sample | Posology/Dosage | Findings |
|---|---|---|---|
| Nieuwenhuis et al., 2020 [14] | A population-based prospective cohort study in the Netherlands (NLCS) with 120,852 men and women aged 55–69 years. | Measured by a 150-item food frequency questionnaire (FFQ), assessing intake of “other mixed nuts” (“tree nuts”) in the previous year | No association between “tree nut” consumption and CRC molecular subtypes was observed in either sex in categorical analyses. In continuous analyses, the HR (95% CI) per 5 g/d increment of tree nut was 0.52 (0.28–0.95) for MSI tumors in women. This inconsistent finding between categorical and continuous analyses is likely due to chance. |
| Author/Year | Selection | Comparability | Outcome Assessment | Follow-Up Adequacy | Overall Risk of Bias |
|---|---|---|---|---|---|
| Nieuwenhuis et al., 2020 [14] | Low Risk | Low Risk | Low Risk | Low Risk | Low |
| Author/Year | Sample | Posology/Dosage | Findings |
|---|---|---|---|
| Caimari et al., 2015 [12] | n = 48 male Golden Syrian hamsters randomly assigned to four groups: standard chow, high-fat diet (HFD), HFD supplemented with FIBEROX® for 8 weeks, or HFD supplemented with FIBEROX® during the final 4 weeks | FIBEROX® (hazelnut skin extract) incorporated into the HFD at 10% w/w for either 8 weeks or 4 weeks | FIBEROX® supplementation reversed the HFD-induced increases in total plasma cholesterol and LDL-cholesterol, significantly reduced circulating free fatty acids and triglycerides, and decreased the lithocholic/deoxycholic bile acid fecal ratio—a recognized biomarker of colon carcinogenesis risk (p < 0.05 for all main outcomes). An increased total bile acid excretion was observed, suggesting an enhanced elimination mechanism. Additionally, a significant improvement in the plasma antioxidant capacity and a reduction in plasma malondialdehyde levels were reported in the group supplemented during the final 4 weeks. |
| Hong et al., 2022 [19] | n = 30 21-day-old male Sprague Dawley rats divided into three groups (n = 10 per group): control diet without nuts, pistachio-enriched diet (8.1% pistachio), or mixed nuts diet (7.5% mixed nuts, including hazelnuts) | Nut supplementation provided approximately 10% of daily caloric intake over 8 weeks | The mixed nut diet group showed a significant reduction in serum 8-oxo-deoxyguanosine (8-oxo-dG) levels, indicating lower oxidative DNA damage (p < 0.05 vs. control). A significant downregulation of the inflammatory gene Rela (NF-κB p65 subunit) in colonic tissue was also observed (p < 0.05). Although a decrease in Ptgs2 expression (COX-2) was noted mainly with pistachio intake, no significant differences in colonic cell proliferation (Ki-67 immunostaining) or apoptosis (TUNEL assay) were found between groups. These findings suggest a potential anti-inflammatory and antioxidant protective effect of nut consumption against early events in colorectal carcinogenesis. |
| Author/Year | Sequence Generation (Randomization) | Allocation Concealment | Blinding of Investigators | Incomplete Outcome Data | Selective Outcome Reporting | Other Bias | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| Caimari et al., 2015 [12] | Low | Unclear | Unclear | Low | Low | Low | Low |
| Hong et al., 2022 [19] | Low | Unclear | Unclear | Low | Low | Low | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzola, G.; Rondanelli, M.; Buga, F.; Riso, P.; Perna, S. Bioactive Properties of Hazelnut-Derived Products in Colorectal Cancer Prevention: A Systematic Review of Preclinical and Epidemiological Studies. Foods 2025, 14, 2154. https://doi.org/10.3390/foods14132154
Mazzola G, Rondanelli M, Buga F, Riso P, Perna S. Bioactive Properties of Hazelnut-Derived Products in Colorectal Cancer Prevention: A Systematic Review of Preclinical and Epidemiological Studies. Foods. 2025; 14(13):2154. https://doi.org/10.3390/foods14132154
Chicago/Turabian StyleMazzola, Giuseppe, Mariangela Rondanelli, Federico Buga, Patrizia Riso, and Simone Perna. 2025. "Bioactive Properties of Hazelnut-Derived Products in Colorectal Cancer Prevention: A Systematic Review of Preclinical and Epidemiological Studies" Foods 14, no. 13: 2154. https://doi.org/10.3390/foods14132154
APA StyleMazzola, G., Rondanelli, M., Buga, F., Riso, P., & Perna, S. (2025). Bioactive Properties of Hazelnut-Derived Products in Colorectal Cancer Prevention: A Systematic Review of Preclinical and Epidemiological Studies. Foods, 14(13), 2154. https://doi.org/10.3390/foods14132154









