Metabolomic Profiling and Antioxidant Capacity Changes in Longzi Black Barley During Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Germination Process
2.3. Determination of Total Flavonoid Content
2.4. Determination of Total Phenol Content
2.5. Antioxidant Capacity Assays
2.5.1. DPPH Radical Scavenging Activity
2.5.2. ABTS Radical Cation Scavenging Activity
2.5.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.6. Metabolomic Analysis
2.6.1. Sample Preparation
2.6.2. Metabolite Extraction Procedure
2.6.3. Chromatography and Mass Spectrometry Data Acquisition
2.6.4. Differential Metabolite Analysis
2.7. Statistical Analysis
3. Results
3.1. Determination of Bioactive Substances and Antioxidant Capacity
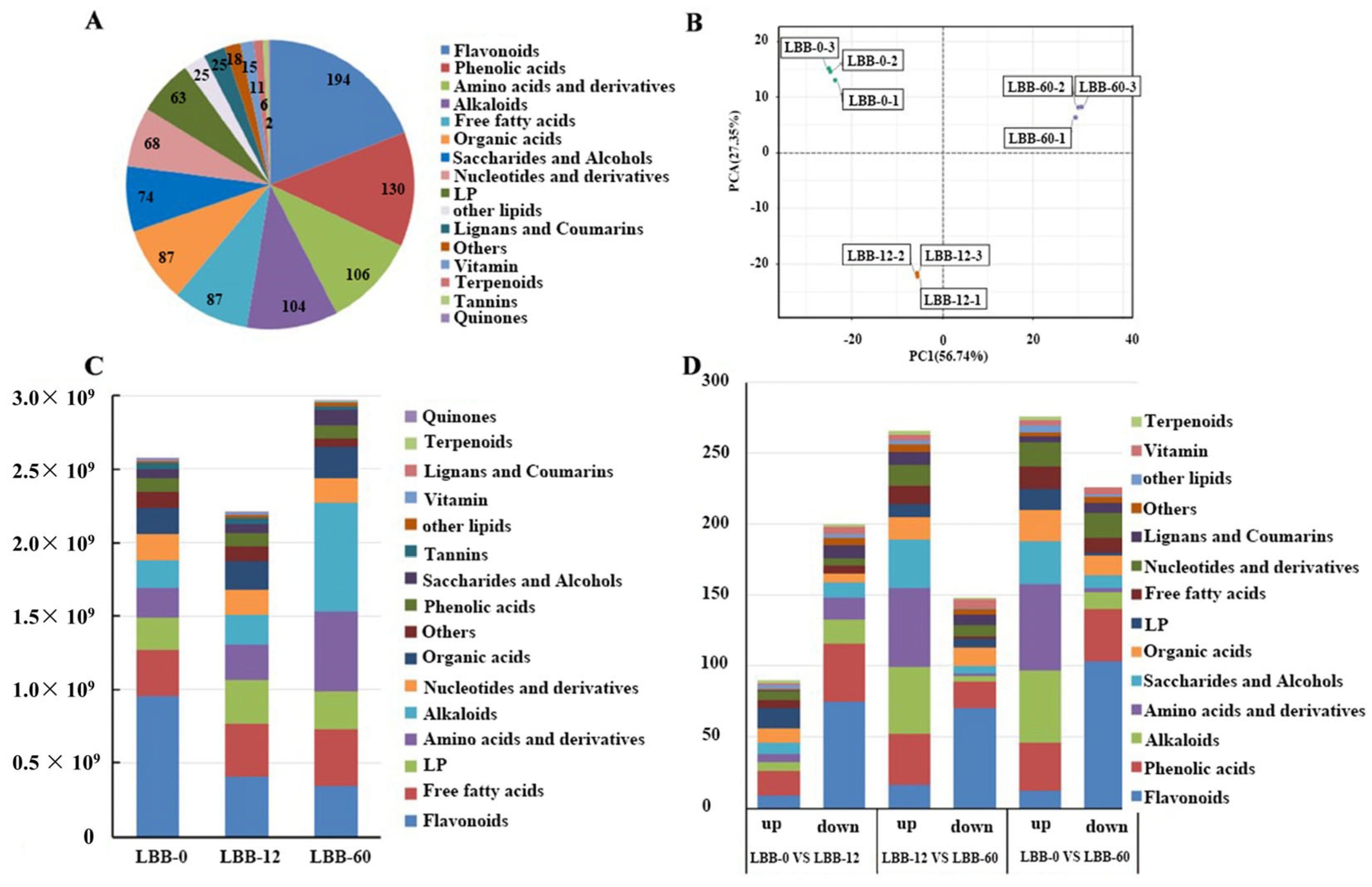
3.2. Metabolomic Analysis
3.2.1. Identification and Quantification of Metabolites
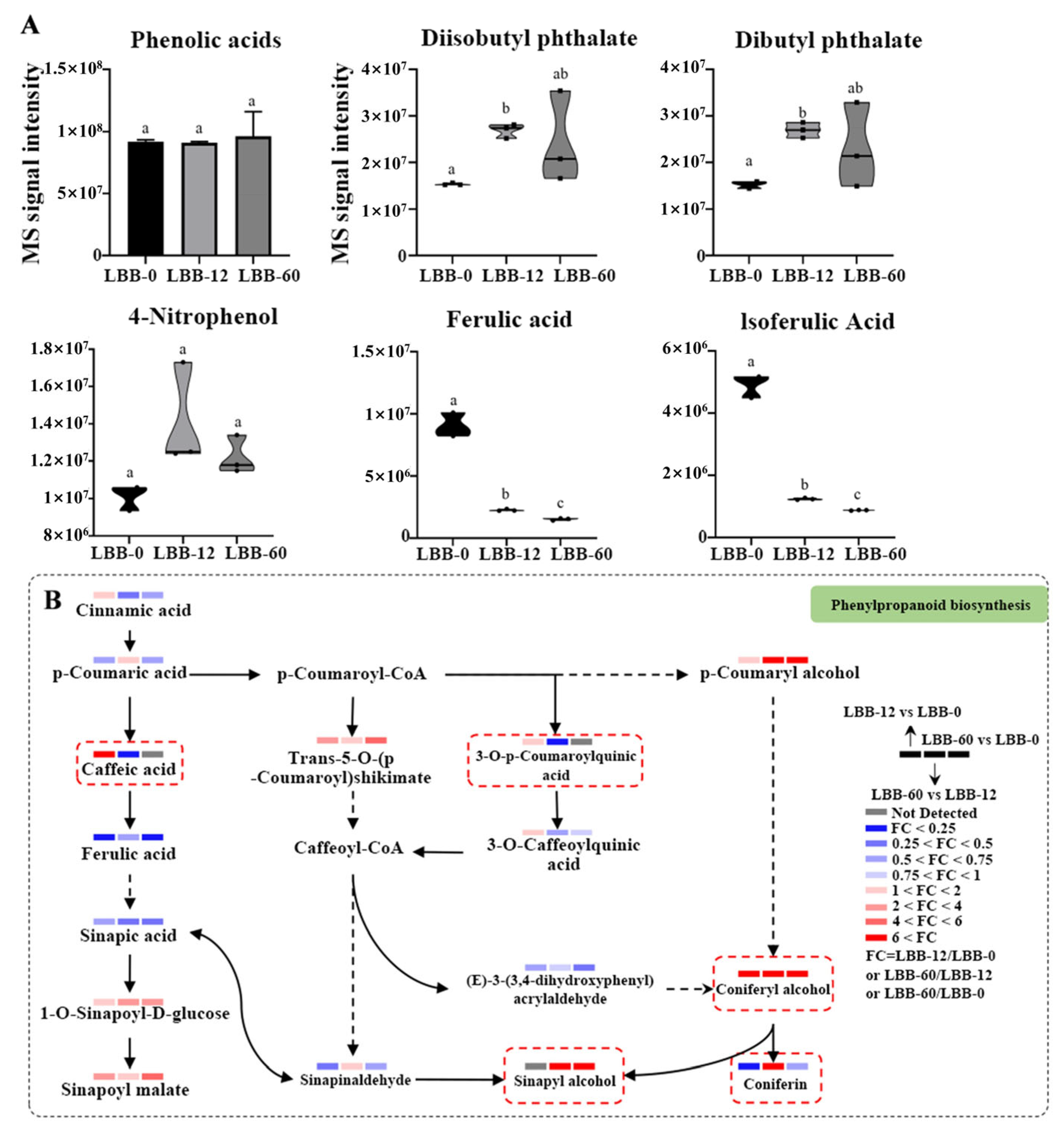
3.2.2. Phenolic Metabolites and Metabolic Pathways
- (1)
- Flavonoid metabolites and metabolic pathways
- (2)
- Phenolic Acid Metabolites and Metabolic Pathways
3.2.3. Amino Acids and Their Derivatives
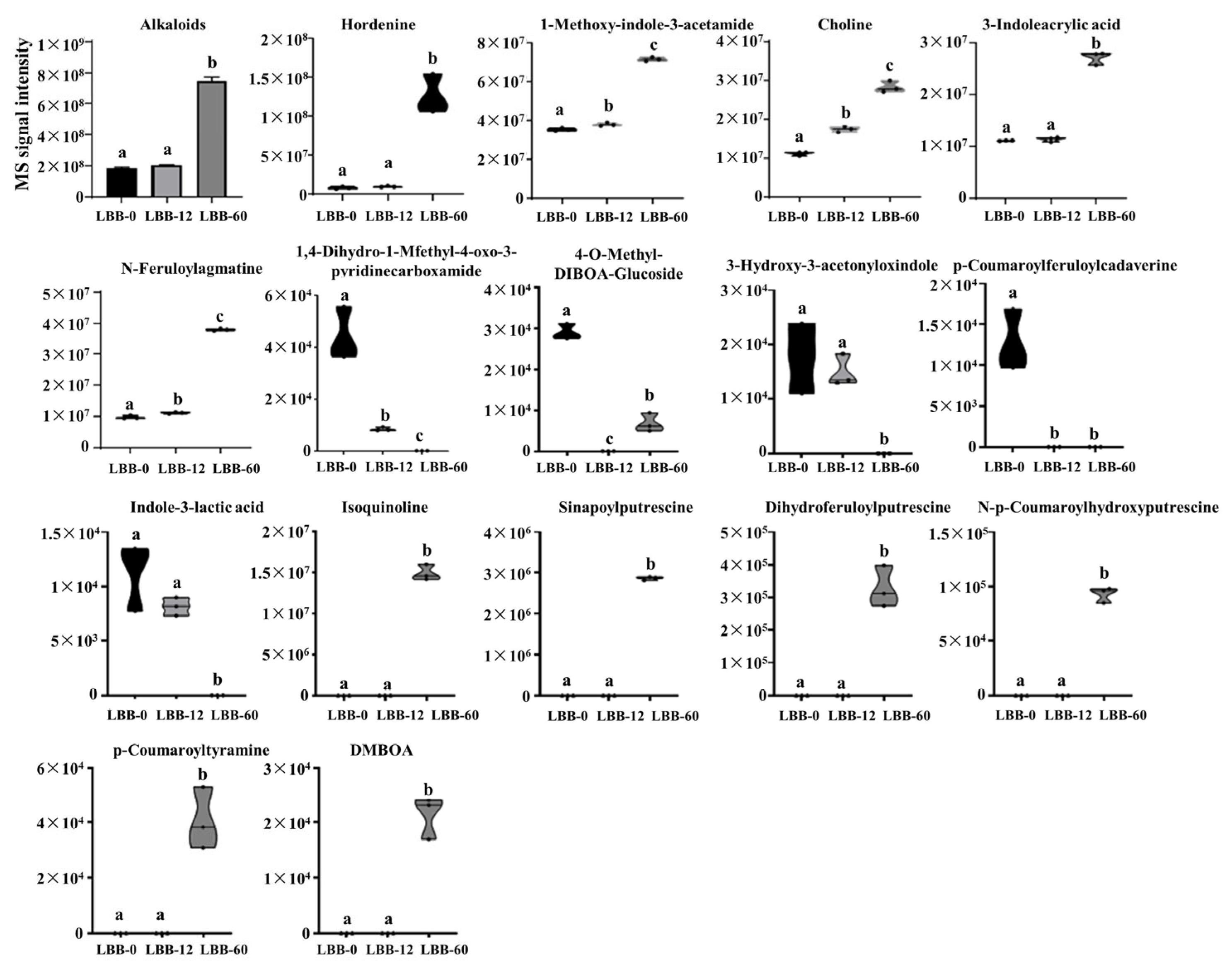
3.2.4. Alkaloid Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, D.; Tan, H.; Liu, G.; Xie, Y.; Zhang, H.; Biabba, Z. Analysis of Agronomic Characters and Nutritional Quality of Different Black Highland Barley Varieties in Tibet. J. Triticeae Crops 2018, 38, 142–147. [Google Scholar]
- Mohammed, O.; Jun, S.; Bin, X. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar]
- Liu, K.; Zhang, B.; Chen, L.; Li, X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2019, 271, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (Qingke): A review. Trends Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, L.; Wu, X.; Li, W.; Wu, T.; Zhang, P. Impact of germination pretreatment on the polyphenol profile, antioxidant activities, and physicochemical properties of three color cultivars of highland barley. J. Cereal Sci. 2021, 97, 103152. [Google Scholar] [CrossRef]
- Pan, W.; Liu, W.; Li, J.; Chen, Y.; Yu, Q.; Xie, J. The role of guar gum in improving the gel and structural characteristics of germinated highland barley starch. Int. J. Biol. Macromol. 2023, 238, 124052. [Google Scholar] [CrossRef]
- Moza, J.; Gujral, H.S. Starch digestibility and bioactivity of high altitude hulless barley. Food Chem. 2016, 194, 561–568. [Google Scholar] [CrossRef]
- Obadi, M.; Qi, Y.; Xu, B. Highland barley starch (Qingke): Structures, properties, modifications, and applications. Int. J. Biol. Macromol. 2021, 185, 725–738. [Google Scholar] [CrossRef]
- Baik, B.-K.; Ullrich, S.E. Barley for food: Characteristics, improvement, and renewed interest. J. Cereal Sci. 2008, 48, 233–242. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, X.; Xu, N.; Tao, J.; Zhang, L.; Zhang, G. Effect of Illumination on Melatonin, Phenolic Content and Antioxidant Activity during Germination of Black Highland Barley. J. Triticeae Crops 2021, 41, 1503–1511. [Google Scholar]
- Li, T.; Du, Y.; Chen, Z.; Zhou, W.; Tu, Z.; Li, J. Hypoglycemic and Hypolipidemic Effect of Germinated Highland Barley. J. Chin. Inst. Food Sci. Technol. 2022, 22, 114–122. [Google Scholar]
- Diez-Gutierrez, L.; San Vicente, L.; Barron, L.J.R.; del Carmen Villaran, M.; Chavarri, M. Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 2020, 64, 103669. [Google Scholar] [CrossRef]
- Liu, W.; Li, S.; Han, N.; Bian, H.; Song, D. Effects of germinated and ungerminated grains on the production of non-dairy probiotic-fermented beverages. Qual. Assur. Saf. Crops Foods 2022, 14, 32–39. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Zeng, Q.; Da, R.; Yan, W.; Ruan, M. Effect of Germinated Highland Barley Powder on Properties of Highland Barley-Wheat Dough and Quality of Non-Fried Twist. J. Chin. Cereals Oils Assoc. 2021, 36, 45–51, 65. [Google Scholar]
- Zeinivand, F.; Ghanbari, F. Improvement of germination, sprout weight and nutraceutical potential of mung bean (Vigna radiata) sprouts by melatonin, as an elicitor. Acta Agric. Slov. 2024, 120, 1–9. [Google Scholar] [CrossRef]
- Ge, X.; Saleh, A.S.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Li, W. Germination and drying induced changes in the composition and content of phenolic compounds in naked barley. J. Food Compos. Anal. 2021, 95, 103594. [Google Scholar] [CrossRef]
- Bangar, S.P.; Sandhu, K.S.; Trif, M.; Manjunatha, V.; Lorenzo, J.M. Germinated barley cultivars: Effect on physicochemical and bioactive properties. Food Anal. Methods 2022, 15, 2505–2512. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.; Huang, Y.; Liang, Q.; Cai, S.; Zhang, G. Time-course comparative metabolome analysis of different barley varieties during malting. J. Agric. Food Chem. 2022, 70, 2051–2059. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, B.; Mu, Y.; Li, Y.; Tong, L.; Wang, L.; Liu, L.; Li, M.; Sun, P.; Sun, J. A comparative metabolomics study of polyphenols in highland barley (Hordeum vulgare L.) grains with different colors. Food Res. Int. 2023, 174, 113672. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Carbon 2011, 100, 12–35. [Google Scholar]
- Xu, M.; Rao, J.; Chen, B. Phenolic compounds in germinated cereal and pulse seeds: Classification, transformation, and metabolic process. Crit. Rev. Food Sci. Nutr. 2020, 60, 740–759. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, Z.; Meenu, M.; Xu, B. Impact of Germination Time on Resveratrol, Phenolic Acids, and Antioxidant Capacities of Different Varieties of Peanut (Arachis hypogaea Linn.) from China. Antioxidants 2021, 10, 1714. [Google Scholar] [CrossRef]
- Mejias, N.; Vega-Galvez, A.; Pasten, A.; Uribe, E.; Andrés, A.; Muñoz-Pina, S.; Khvostenko, K.; García-Segovia, P. Evaluating the Microstructure and Bioaccessibility of Bioactive Compounds and Antioxidant Activity After the Dehydration of Red Cabbage. Foods 2025, 14, 1932. [Google Scholar] [CrossRef]
- Ha, K.S.; Jo, S.H.; Mannam, V.; Kwon, Y.I.; Apostolidis, E. Stimulation of Phenolics, Antioxidant and α-Glucosidase Inhibitory Activities During Barley (Hordeum vulgare L.) Seed Germination. Plant Foods Hum. Nutr. 2016, 71, 211–217. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y.; Su, W.; Sun, P.; Yang, H.; Li, Q.; Du, S.; Yu, X. Differential metabolic networks in three energy substances of flaxseed (Linum usitatissimum L.) during germination. Food Chem. 2024, 443, 138463. [Google Scholar] [CrossRef]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Amir, R.; Galili, G.; Cohen, H. The metabolic roles of free amino acids during seed development. Plant Sci. 2018, 275, 11–18. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Qin, W.; Wang, Y.; Mouhamed, F.; Hamaker, B.; Zhang, G. Impact of the solubility of phenolic compounds from Highland Barley (Hordeum vulgare L.) on their antioxidant property and protein binding affinity. Lwt-Food Sci. Technol. 2023, 186, 115251. [Google Scholar] [CrossRef]
- Avezum, L.; Rondet, E.; Mestres, C.; Achir, N.; Madode, Y.; Gibert, O.; Lefevre, C.; Hemery, Y.; Verdeil, J.-L.; Rajjou, L. Improving the nutritional quality of pulses via germination. Food Rev. Int. 2023, 39, 6011–6044. [Google Scholar] [CrossRef]
- Zhao, P.; Chu, L.; Wang, K.; Zhao, B.; Li, Y.; Yang, K.; Wan, P. Analyses on the pigment composition of different seed coat colors in adzuki bean. Food Sci. Nutr. 2022, 10, 2611–2619. [Google Scholar] [CrossRef]
- Ali, A.S.; Elozeiri, A.A. Metabolic processes during seed germination. Adv. Seed Biol. 2017, 2017, 141–166. [Google Scholar]
- Mnich, E.; Bjarnholt, N.; Eudes, A.; Harholt, J.; Holland, C.; Jorgensen, B.; Larsen, F.H.; Liu, M.; Manat, R.; Meyer, A.S.; et al. Phenolic cross-links: Building and de-constructing the plant cell wall. Nat. Prod. Rep. 2020, 37, 919–961. [Google Scholar] [CrossRef]
- Monson, R.K.; Trowbridge, A.M.; Lindroth, R.L.; Lerdau, M.T. Coordinated resource allocation to plant growth–defense tradeoffs. New Phytol. 2022, 233, 1051–1066. [Google Scholar] [CrossRef]
- Bonnot, T.; Bachelet, F.; Boudet, J.; Le Signor, C.; Bancel, E.; Vernoud, V.; Ravel, C.; Gallardo, K. Sulfur in determining seed protein composition: Present understanding of its interaction with abiotic stresses and future directions. J. Exp. Bot. 2023, 74, 3276–3285. [Google Scholar] [CrossRef]
- Madhu; Sharma, A.; Kaur, A.; Tyagi, S.; Upadhyay, S.K. Glutathione Peroxidases in Plants: Innumerable Role in Abiotic Stress Tolerance and Plant Development. J. Plant Growth Regul. 2023, 42, 598–613. [Google Scholar] [CrossRef]
- Hassan Mekky, R.; Thabet, M.M.; Rodriguez-Perez, C.; Elnaggar, D.M.Y.; Mahrous, E.A.; Segura-Carretero, A.; Abdel-Sattar, E. Comparative metabolite profiling and antioxidant potentials of seeds and sprouts of three Egyptian cultivars of Vicia faba L. Food Res. Int. 2020, 136, 109537. [Google Scholar]
- De-la-Cruz Chacón, I.; Riley-Saldaña, C.A.; González-Esquinca, A.R. Secondary metabolites during early development in plants. Phytochem. Rev. 2013, 12, 47–64. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Wei, Y.; Ren, Y.; Wang, X.; Xiang, D.; Li, B.; Wang, J.; Geng, F. Metabolomic Profiling and Antioxidant Capacity Changes in Longzi Black Barley During Germination. Foods 2025, 14, 2113. https://doi.org/10.3390/foods14122113
Hu G, Wei Y, Ren Y, Wang X, Xiang D, Li B, Wang J, Geng F. Metabolomic Profiling and Antioxidant Capacity Changes in Longzi Black Barley During Germination. Foods. 2025; 14(12):2113. https://doi.org/10.3390/foods14122113
Chicago/Turabian StyleHu, Gan, Yan Wei, Yuanhang Ren, Xinhui Wang, Dabing Xiang, Bin Li, Jinqiu Wang, and Fang Geng. 2025. "Metabolomic Profiling and Antioxidant Capacity Changes in Longzi Black Barley During Germination" Foods 14, no. 12: 2113. https://doi.org/10.3390/foods14122113
APA StyleHu, G., Wei, Y., Ren, Y., Wang, X., Xiang, D., Li, B., Wang, J., & Geng, F. (2025). Metabolomic Profiling and Antioxidant Capacity Changes in Longzi Black Barley During Germination. Foods, 14(12), 2113. https://doi.org/10.3390/foods14122113




