Chemical Traits and Microbial Population Characterization of ‘Asprinio’ Grape Must, a Local Vine Cultivated in Campania Region (Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sampling of ‘Asprinio’ Grape Must
2.3. Free Amino Acid Determination
2.4. Total Phenol Content and Antioxidant Evaluation
2.5. High-Throughput Sequencing
2.6. NMR and Partial Purification
2.7. Statistical Analysis
3. Results and Discussion
3.1. Free Amino Acid Content in ‘Asprinio’ Grape Must
3.2. Total Phenol Content and Antioxidant Evaluation of ‘Asprinio’ Grape Must
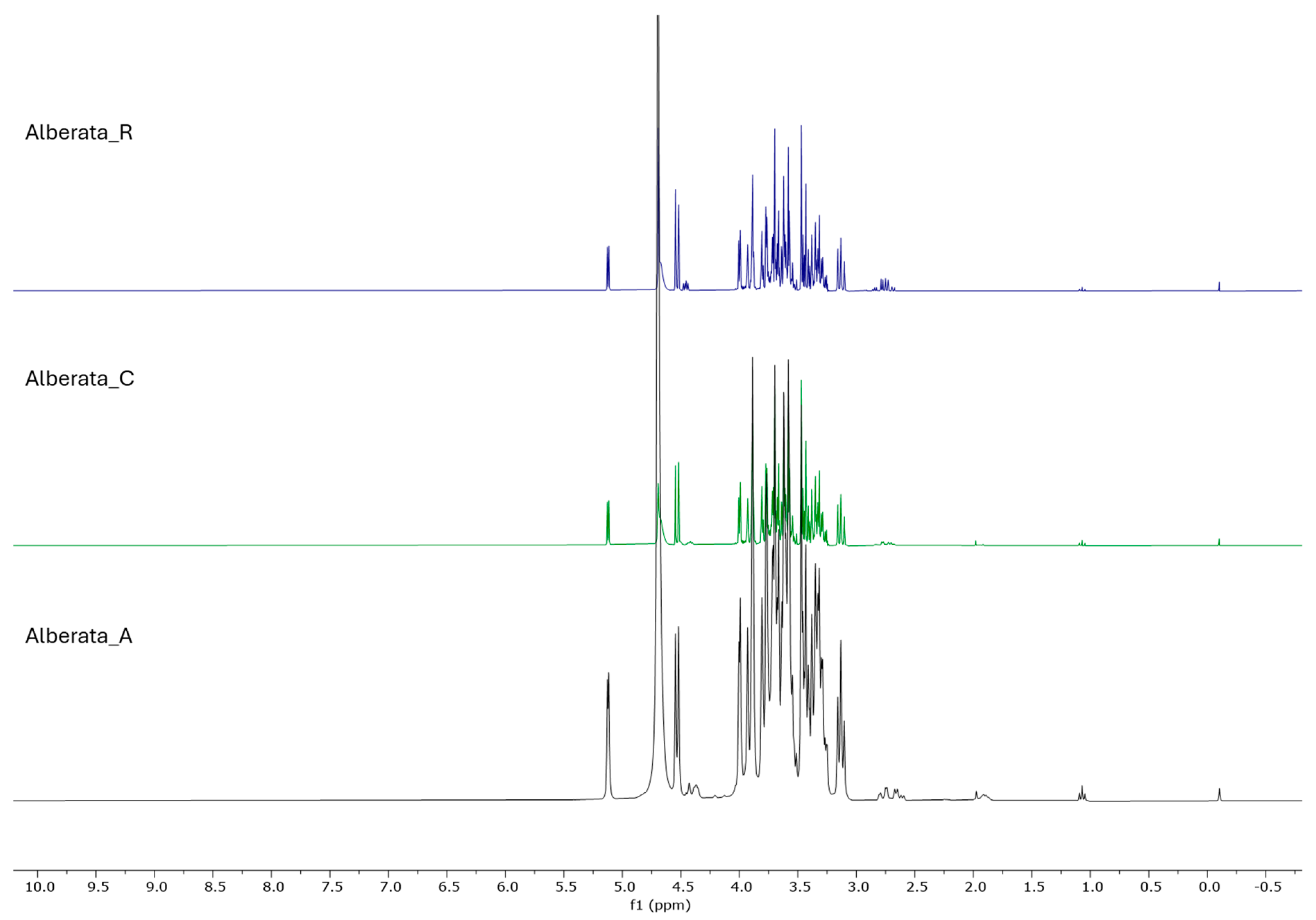
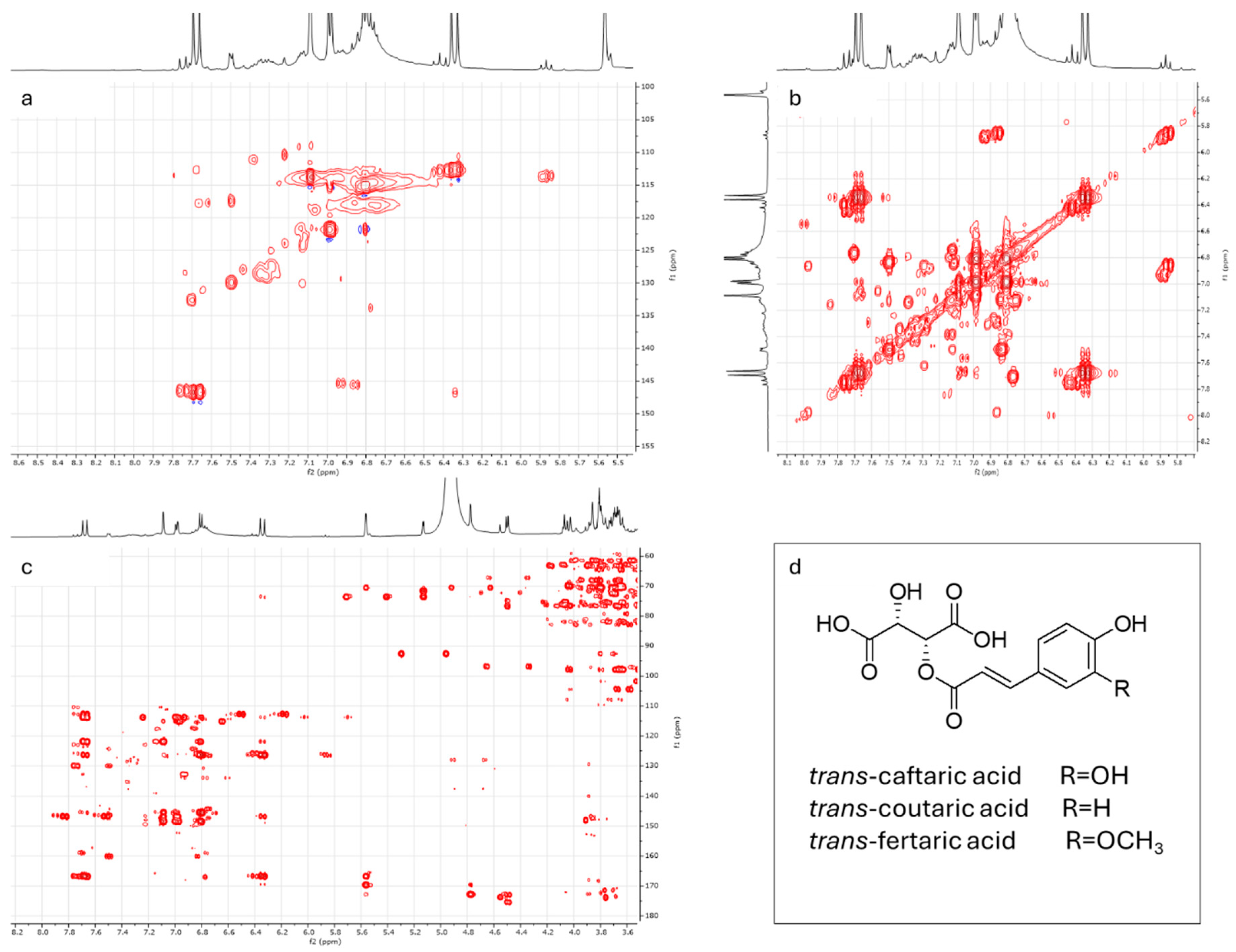
3.3. Metabolite Profiling of ‘Asprinio’ Grape Must
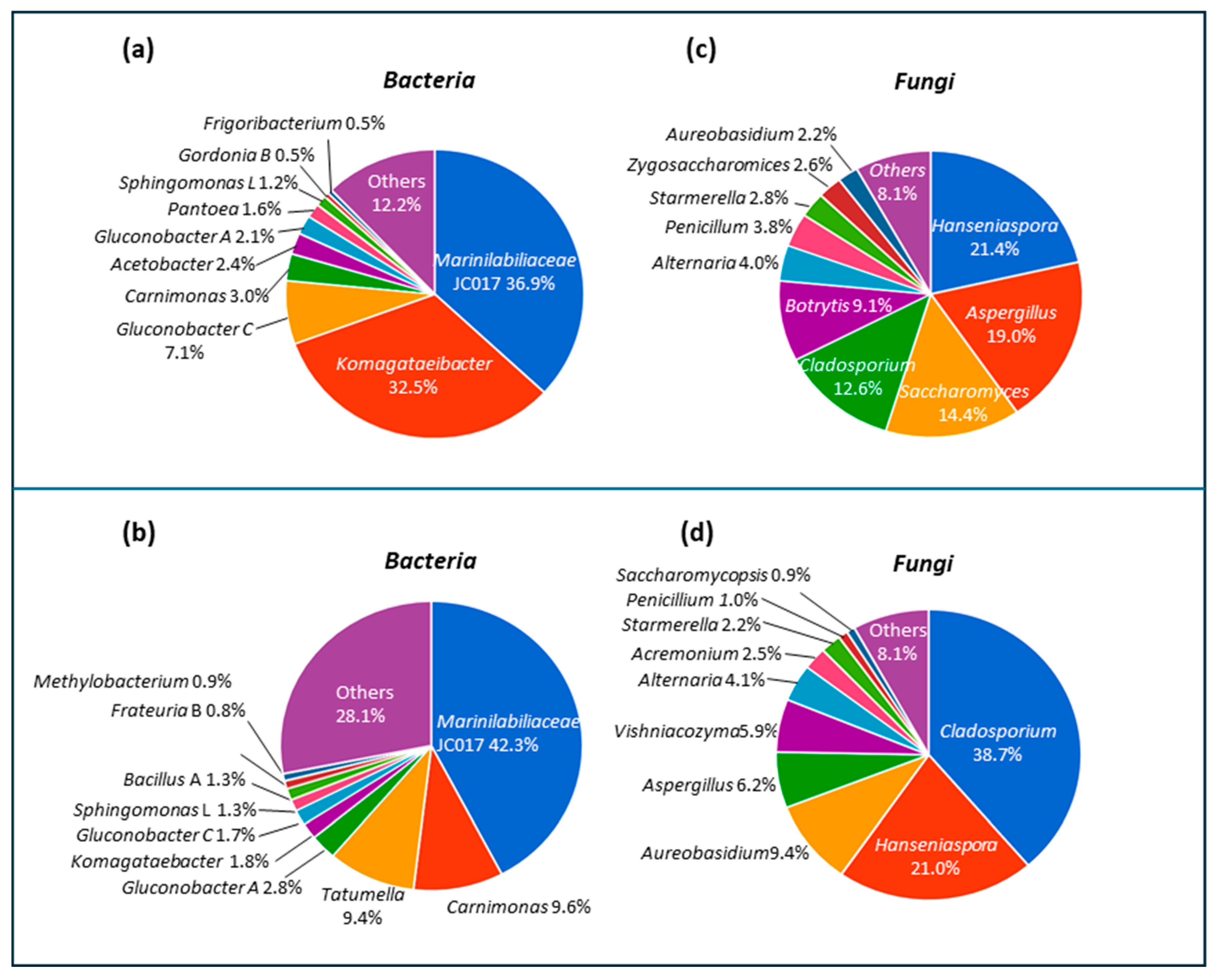
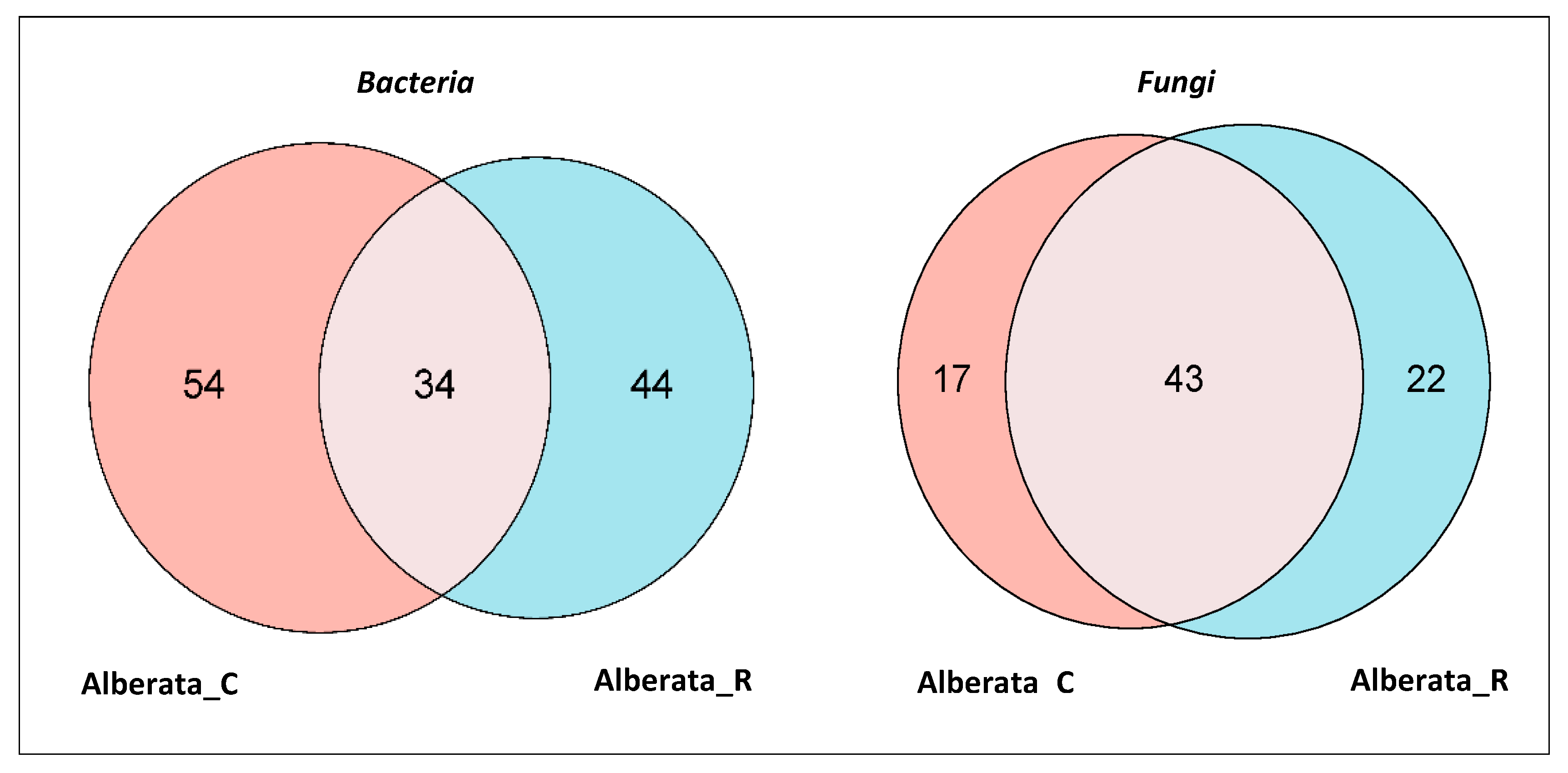
3.4. Composition of Microbial Community in ‘Asprinio’ Must from Different Alberata
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministero_Agricoltura_Foreste Asprinio di Aversa Denominazione di Origine Controllata (DOC), Decreto Ministeriale (DM) 31 July 1993. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=1993-08-12&atto.codiceRedazionale=093A4423&elenco30giorni=false (accessed on 7 April 2025).
- Ministero_Agricoltura_Foreste Asprinio di Aversa Denominazione di Origine Controllata (DOC), Decreto Ministeriale (DM) 30 November 2011. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2011-12-20&atto.codiceRedazionale=11A16146&elenco30giorni=false (accessed on 7 April 2025).
- D’Agata, I. Asprinio Bianco. In Native Wine Grapes of Italy, 1st ed.; University of California Press: Berkeley, CA, USA, 2014; pp. 184–186. [Google Scholar]
- Ncube, A.; Fiorentino, G.; Colella, M.; Ulgiati, S. Upgrading wineries to biorefineries within a Circular Economy perspective: An Italian case study. Sci. Total Environ. 2021, 775, 145809. [Google Scholar] [CrossRef] [PubMed]
- Buono, R.; Vallariello, G. La vite maritata in Campania. Delpinoa 2002, 44, 53–63. [Google Scholar]
- Scatozza, C. L’Alberata Aversana dell’Asprinio è Ufficialmente Patrimonio Nazionale. Available online: https://www.campaniaslow.it/2023/02/06/l-alberata-aversana-dell-asprinio-e-ufficialmente-patrimonio-nazionale/ (accessed on 7 April 2025).
- Scienza, A.; Boselli, M. Vini e Vitigni Della Campania: Tremila Anni di Storia; Prismi: Napoli, Italy, 2003; p. 222. [Google Scholar]
- Onorati, N.C. Delle Cose Rustiche Ovvero Dell’agricoltura Teorica Trattata Secondo i Principj Della Chimica Moderna Opera; Stamperia Flautina: Naples, Italy, 1804. [Google Scholar]
- Monaco, A.; Nasi, A.; Paparelli, L.; Spada, E. Caratterizzazione aromatica ed enologica di uve e vini della varietà Asprinio: Un confronto analitico e storico per una identità lunga sei secoli. In Proceedings of the Vinitaly Conference, Verona, Italy, 8–12 April 2011. [Google Scholar]
- Cipriani, G.; Spadotto, A.; Jurman, I.; Di Gaspero, G.; Crespan, M.; Meneghetti, S.; Frare, E.; Vignani, R.; Cresti, M.; Morgante, M.; et al. The SSR-based molecular profile of 1005 grapevine (Vitis vinifera L.) accessions uncovers new synonymy and parentages, and reveals a large admixture amongst varieties of different geographic origin. Theor. Appl. Genet. 2010, 121, 1569–1585. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Monaco, A.; Vouillamoz, J.; Forlani, M.; Grando, M.S. Genetic relationship among local Vitis vinifera cultivars from Campania (Italy). Vitis 2005, 44, 25–34. [Google Scholar]
- Landi, N.; Scognamiglio, M.; Woodrow, P.; Ciarmiello, L.F.; Ragucci, S.; Clemente, A.; Hussain, H.Z.F.; Fiorentino, A.; Di Maro, A. Biochemical traits, (1)H NMR profile and residual DNA content of ‘Asprinio’, white wine from Campania region (Southern Italy). Foods 2022, 11, 2322. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Harding, J. The Oxford Companion to Wine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Camin, F.; Bontempo, L.; Larcher, R.; Grando, M.S.; Moreno Sanz, P.; Fauhl-Hassek, C.; Hajslova, J.; Hurkova, J.; Uttl, L.; Thomas, F. Wine and must. In Foodintegrity Handbook; Morin, J.-F., Lees, M., Eds.; Eurofins Analytics France: Nantes, France, 2018; pp. 205–228. [Google Scholar]
- Jackson, R.S. 6—Chemical constituents of grapes and wine. In Wine Science, 4th ed.; Jackson, R.S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 347–426. [Google Scholar]
- Moreno, J.; Peinado, R. Chapter 2—Composition of Grape Must. In Enological Chemistry; Academic Press: San Diego, CA, USA, 2012; pp. 13–22. [Google Scholar]
- Butnariu, M.; Butu, A. 10—The Evolution and the Development Phases of Wine. In Alcoholic Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 303–345. [Google Scholar]
- König, H.; Unden, G.; Fröhlich, J. Biology of Microorganisms on Grapes, in Must and in Wine, 2nd ed.; Springer Nature: Cham, Switzerland, 2009. [Google Scholar]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Vion, C.; Yeramian, N.; Hranilovic, A.; Masneuf-Pomarède, I.; Marullo, P. Influence of yeasts on wine acidity: New insights into Saccharomyces cerevisiae. OENO One 2024, 58, 7877. [Google Scholar] [CrossRef]
- Aranda, A.; Matallana, E.; Olmo, M.L.D. Chapter 1—Saccharomyces Yeasts I: Primary Fermentation. In Molecular Wine Microbiology; Carrascosa, A.V., Muñoz, R., González, R., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 1–31. [Google Scholar]
- Waterhouse, A.L. Wine phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Martínez, M.; Rubires, X.; Yuste-Rojas, M.; Torres, M. Chapter 14—Applied Wine Microbiology. In Molecular Wine Microbiology; Carrascosa, A.V., Muñoz, R., González, R., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 341–355. [Google Scholar]
- Landi, N.; Alberico, L.; Clemente, A.; Peddio, S.; Hussain, H.Z.F.; Ragucci, S.; Zucca, P.; Woodrow, P.; Di Maro, A. Nutritional, metabolic and genetic profiling of ‘Cerato’ and ‘Curniciello’ bean landraces from Caserta, Southern Italy. Food Biosci. 2023, 55, 102975. [Google Scholar] [CrossRef]
- Takahashi, M.; Masaki, K.; Mizuno, A.; Goto-Yamamoto, N. Modified COLD-PCR for detection of minor microorganisms in wine samples during the fermentation. Food Microbiol. 2014, 39, 74–80. [Google Scholar] [CrossRef][Green Version]
- Ding, Y.; Wei, R.; Wang, L.; Wang, W.; Wang, H.; Li, H. Exploring the ecological characteristics of natural microbial communities along the continuum from grape berries to winemaking. Food Res. Int. 2023, 167, 112718. [Google Scholar] [CrossRef]
- Andrade, C. The P Value and Statistical Significance: Misunderstandings, Explanations, Challenges, and Alternatives. Indian J. Psychol. Med. 2019, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Ingledew, W.M.; Kunkee, R.E. Factors influencing sluggish fermentations of grape juice. Am. J. Enol. Vitic. 1985, 36, 65–76. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Cacho, J.F.; Ferreira, V. Relationship between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solutions and chemometric study. J. Agric. Food Chem. 2002, 50, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Etiévant, P.; Schlich, P.; Bouvier, J.-C.; Symonds, P.; Bertrand, A. Varietal and geographic classification of French red wines in terms of elements, amino acids and aromatic alcohols. J. Sci. Food Agric. 1988, 45, 25–41. [Google Scholar] [CrossRef]
- Procopio, S.; Krause, D.; Hofmann, T.; Becker, T. Significant amino acids in aroma compound profiling during yeast fermentation analyzed by PLS regression. LWT—Food Sci. Technol. 2013, 51, 423–432. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q. Aroma characteristics of volatile compounds brought by variations in microbes in winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef]
- Petrovic, G.; Aleixandre, J.; Buica, A. Grape must profiling and cultivar discrimination based on amino acid composition and general discriminant analysis with best subset. S. Afr. J. Enol. Vitic. 2019, 40, 266–278. [Google Scholar] [CrossRef]
- Stines, A.P.; Grubb, J.; Gockowiak, H.; Henschke, P.A.; HØJ, P.B.; van Heeswijck, R. Proline and arginine accumulation in developing berries of Vitis vinifera L. in Australian vineyards: Influence of vine cultivar, berry maturity and tissue type. Aust. J. Grape Wine Res. 2000, 6, 150–158. [Google Scholar] [CrossRef]
- Beltran, G.; Novo, M.; Rozès, N.; Mas, A.; Guillamón, J.M. Nitrogen catabolite repression in Saccharomyces cerevisiae during wine fermentations. FEMS Yeast Res. 2004, 4, 625–632. [Google Scholar] [CrossRef]
- Mekoue Nguela, J.; Vernhet, A.; Julien-Ortiz, A.; Sieczkowski, N.; Mouret, J.R. Effect of grape must polyphenols on yeast metabolism during alcoholic fermentation. Food Res. Int. 2019, 121, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Soleas, G.J.; Tomlinson, G.; Goldberg, D.M. Kinetics of polyphenol release into wine must during fermentation of different cultivars. J. Wine Res. 1998, 9, 27–41. [Google Scholar] [CrossRef]
- Onache, P.A.; Florea, A.; Geana, E.-I.; Ciucure, C.T.; Ionete, R.E.; Sumedrea, D.I.; Tița, O. Assessment of bioactive phenolic compounds in musts and the corresponding wines of white and red grape varieties. Appl. Sci. 2023, 13, 5722. [Google Scholar] [CrossRef]
- Somers, T.C.; Vérette, E.; Pocock, K.F. Hydroxycinnamate esters of Vitis vinifera: Changes during white vinification, and effects of exogenous enzymic hydrolysis. J. Sci. Food Agric. 1987, 40, 67–78. [Google Scholar] [CrossRef]
- Singleton, V.L.; Zaya, J.; Trousdale, E.K. Caftaric and coutaric acids in fruit of Vitis. Phytochemistry 1986, 25, 2127–2133. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. A Comprehensive review on polyphenols of white wine: Impact on wine quality and potential health benefits. Molecules 2024, 29, 5074. [Google Scholar] [CrossRef]
- Singleton, V.L.; Salgues, M.; Zaya, J.; Trousdale, E. Caftaric acid disappearance and conversion to products of enzymic oxidation in grape must and wine. Am. J. Enol. Vitic. 1985, 36, 50. [Google Scholar] [CrossRef]
- Abdel-Hamid, H.A.; Zenhom, N.M.; Toni, N.D. Melatonin reduced endometrial hyperplasia induced by estradiol in female albino rats. Gen. Physiol. Biophys. 2019, 38, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Saima; Anjum, I.; Najm, S.; Barkat, K.; Nafidi, H.-A.; Bin Jardan, Y.A.; Bourhia, M. Caftaric acid ameliorates oxidative stress, inflammation, and bladder overactivity in rats having interstitial cystitis: An in silico study. ACS Omega 2023, 8, 28196–28206. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Campos, V.R. Unlocking the potential of hydroxycinnamic acid bioconjugates: Tailored derivatives for biomedical, cosmetic, and food applications. Compounds 2024, 4, 604–625. [Google Scholar] [CrossRef]
- Barka Essaid, A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel Gilles, P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of Grapevine Flowers, Berries, and Seeds: Identification of Cultivable Bacteria, Comparison with Other Plant Parts, and Visualization of Niches of Colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Godálová, Z.; Kraková, L.; Puškárová, A.; Bučková, M.; Kuchta, T.; Piknová, Ľ.; Pangallo, D. Bacterial consortia at different wine fermentation phases of two typical Central European grape varieties: Blaufränkisch (Frankovka modrá) and Grüner Veltliner (Veltlínske zelené). Int. J. Food Microbiol. 2016, 217, 110–116. [Google Scholar] [CrossRef]
- Jumbam, B.; Toro, M.; Hu, M. Comparative analysis of grape berry microbiota uncovers sour rot associates from a Maryland vineyard. PLoS ONE 2025, 20, e0314397. [Google Scholar] [CrossRef]
- Africa, A.J.; Setati, M.E.; Hitzeroth, A.C.; Blancquaert, E.H. Exploring the evolution of microbial communities from the phyllosphere and carposphere to the grape must of Vitis vinifera L. cv’s Chardonnay and Pinot noir. Food Microbiol. 2025, 130, 104780. [Google Scholar] [CrossRef]
- Ohwofasa, A.; Dhami, M.; Zhang, J.; Tian, B.; Winefield, C.; On, S.L.W. Influence of climatic variation on microbial communities during organic Pinot noir wine production. PLoS ONE 2024, 19, e0296859. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Jiang, T.; Liang, Z.; Zhang, N.; Dong, B.; Wu, Q.; Gu, B. Carbohydrate-active enzyme profiles of Lactiplantibacillus plantarum strain 84-3 contribute to flavor formation in fermented dairy and vegetable products. Food Chem. X 2023, 20, 101036. [Google Scholar] [CrossRef]
- Tang, Q.-H.; Miao, C.-H.; Chen, Y.-F.; Dong, Z.-X.; Cao, Z.; Liao, S.-Q.; Wang, J.-X.; Wang, Z.-W.; Guo, J. The composition of bacteria in gut and beebread of stingless bees (Apidae: Meliponini) from tropics Yunnan, China. Antonie Van Leeuwenhoek 2021, 114, 1293–1305. [Google Scholar] [CrossRef]
- Kordowska-Wiater, M.; Pytka, M.; Stój, A.; Kubik-Komar, A.; Wyrostek, J.; Waśko, A. A Metagenetic Insight into Microbial Diversity of Spontaneously Fermented Polish Red Wines and an Analysis of Selected Physicochemical Properties. Appl. Sci. 2022, 12, 4373. [Google Scholar] [CrossRef]
- Wei, R.; Wang, L.; Ding, Y.; Zhang, L.; Gao, F.; Chen, N.; Song, Y.; Li, H.; Wang, H. Natural and sustainable wine: A review. Crit. Rev. Food. Sci. Nutr. 2023, 63, 8249–8260. [Google Scholar] [CrossRef]
- Gallego-Martínez, J.J.; Cañete-Carmona, E.; Gersnoviez, A.; Brox, M.; Sánchez-Gil, J.J.; Martín-Fernández, C.; Moreno, J. Devices for monitoring oenological processes: A review. Measurement 2024, 235, 114922. [Google Scholar] [CrossRef]
- Rossouw, D.; Bauer, F.F. Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol. 2016, 55, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Nardi, T. Microbial resources as a tool for enhancing sustainability in winemaking. Microorganisms 2020, 8, 507. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.; Mane, S.; Kulkarni, G.; Shaikh, S.; Ghormade, V.; Nerkar, D.P.; Shouche, Y.; Deshpande, M.V. Natural yeast flora of different varieties of grapes used for wine making in India. Food Microbiol. 2009, 26, 801–808. [Google Scholar] [CrossRef]
- Watanabe, D.; Hashimoto, W. Adaptation of yeast Saccharomyces cerevisiae to grape-skin environment. Sci. Rep. 2023, 13, 9279. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Q.; Tian, B.; Zhu, S.; Du, F.; Mao, R.; Li, S.; Liu, L.; Zhu, Y. Evaluation of Four Indigenous Non-Saccharomyces Yeasts Isolated from the Shangri-La Wine Region (China) for Their Fermentation Performances and Aroma Compositions in Synthetic Grape Juice Fermentation. J. Fungi 2022, 8, 146. [Google Scholar] [CrossRef]
- Gołębiewski, M.; Tretyn, A. Generating amplicon reads for microbial community assessment with next-generation sequencing. J. Appl. Microbiol. 2020, 128, 330–354. [Google Scholar] [CrossRef]
- Tatangelo, V.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Ambrosini, R. Effect of preservation method on the assessment of bacterial community structure in soil and water samples. FEMS Microbiol. Lett. 2014, 356, 32–38. [Google Scholar] [CrossRef]



| Amino Acid a | Alberta_A | Alberata_C | Alberata_R |
|---|---|---|---|
| Ala | 14.13 ± 1.63 a | 18.10 ± 4.97 a | 15.72 ± 2.24 a |
| Arg | 57.42 ± 4.10 a | 76.57 ± 7.36 a | 62.78 ± 7.59 a |
| Asn | 7.09 ± 1.39 a | 5.54 ± 2.48 a | 6.90 ± 0.27 a |
| Asp | 13.21 ± 1.12 a | 12.68 ± 3.33 a | 13.46 ± 0.36 a |
| β-ala | 5.65 ± 0.84 a | 4.61 ± 1.33 a | 5.41 ± 0.21 a |
| Ethan | 7.87 ± 1.04 a | 4.76 ± 1.40 a | 7.16 ± 1.01 a |
| GABA | 24.81 ± 2.19 a | 22.91 ± 4.44 a | 24.73 ± 0.11 a |
| Gln | 30.04 ± 6.56 a | 16.82 ± 18.06 a | 28.76 ± 1.80 a |
| Glu | 24.34 ± 2.68 a | 21.21 ± 6.46 a | 24.23 ± 0.16 a |
| Gly | 2.72 ± 0.40 a | 2.46 ± 0.96 a | 2.75 ± 0.05 a |
| His | 10.02 ± 0.59 a | 8.07 ± 2.53 a | 9.87 ± 0.20 a |
| Ile | 7.90 ± 0.94 a | 4.43 ± 1.39 a | 7.11 ± 1.12 a |
| Leu | 10.48 ± 1.39 a | 6.66 ± 2.18 a | 9.67 ± 1.15 a |
| Lys | 2.50 ± 0.22 a | 2.50 ± 0.75 a | 2.60 ± 0.13 a |
| Orn | 0.79 ± 0.01 a | 2.06 ± 0.56 a | 1.21 ± 0.59 a |
| Pea | 2.49 ± 0.16 a | 2.21 ± 0.70 a | 2.52 ± 0.04 a |
| Phe | 8.04 ± 1.33 a | 6.47 ± 2.48 a | 7.58 ± 0.27 a |
| Phser | 22.46 ± 1.63 a | 20.73 ± 2.68 a | 22.21 ± 0.35 a |
| Pro | 334.55 ± 29.90 a | 203.49 ± 58.65 a | 306.87 ± 39.15 a |
| Sarc | 3.14 ± 0.02 a | 3.21 ± 0.93 a | 3.32 ± 0.25 a |
| Ser | 13.45 ± 1.28 a | 11.69 ± 3.52 a | 13.41 ± 0.06 a |
| Taur | 0.50 ± 0.04 a | 0.40 ± 0.41 a | 0.56 ± 0.08 a |
| Thr | 13.53 ± 1.40 a | 14.39 ± 4.5 a | 14.30 ± 1.09 a |
| Trp | 9.32 ± 1.27 a | 3.62 ± 2.86 a | 8.17 ± 1.62 a |
| Tyr | 6.74 ± 0.89 a | 3.82 ± 1.34 a | 6.09 ± 0.92 a |
| Val | 13.33 ± 1.19 a | 9.79 ± 3.84 a | 12.91 ± 0.59 a |
| Total | 646.4 | 489.2 | 620.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landi, N.; Scognamiglio, M.; Muscariello, L.; Marasco, R.; Palazzo, A.; De Chiara, I.; Ragucci, S.; Pedone, P.V.; Fiorentino, A.; Di Maro, A. Chemical Traits and Microbial Population Characterization of ‘Asprinio’ Grape Must, a Local Vine Cultivated in Campania Region (Italy). Foods 2025, 14, 2110. https://doi.org/10.3390/foods14122110
Landi N, Scognamiglio M, Muscariello L, Marasco R, Palazzo A, De Chiara I, Ragucci S, Pedone PV, Fiorentino A, Di Maro A. Chemical Traits and Microbial Population Characterization of ‘Asprinio’ Grape Must, a Local Vine Cultivated in Campania Region (Italy). Foods. 2025; 14(12):2110. https://doi.org/10.3390/foods14122110
Chicago/Turabian StyleLandi, Nicola, Monica Scognamiglio, Lidia Muscariello, Rosangela Marasco, Alessia Palazzo, Ida De Chiara, Sara Ragucci, Paolo V. Pedone, Antonio Fiorentino, and Antimo Di Maro. 2025. "Chemical Traits and Microbial Population Characterization of ‘Asprinio’ Grape Must, a Local Vine Cultivated in Campania Region (Italy)" Foods 14, no. 12: 2110. https://doi.org/10.3390/foods14122110
APA StyleLandi, N., Scognamiglio, M., Muscariello, L., Marasco, R., Palazzo, A., De Chiara, I., Ragucci, S., Pedone, P. V., Fiorentino, A., & Di Maro, A. (2025). Chemical Traits and Microbial Population Characterization of ‘Asprinio’ Grape Must, a Local Vine Cultivated in Campania Region (Italy). Foods, 14(12), 2110. https://doi.org/10.3390/foods14122110











