Au-Ag Bimetallic Nanoparticles for Surface-Enhanced Raman Scattering (SERS) Detection of Food Contaminants: A Review
Abstract
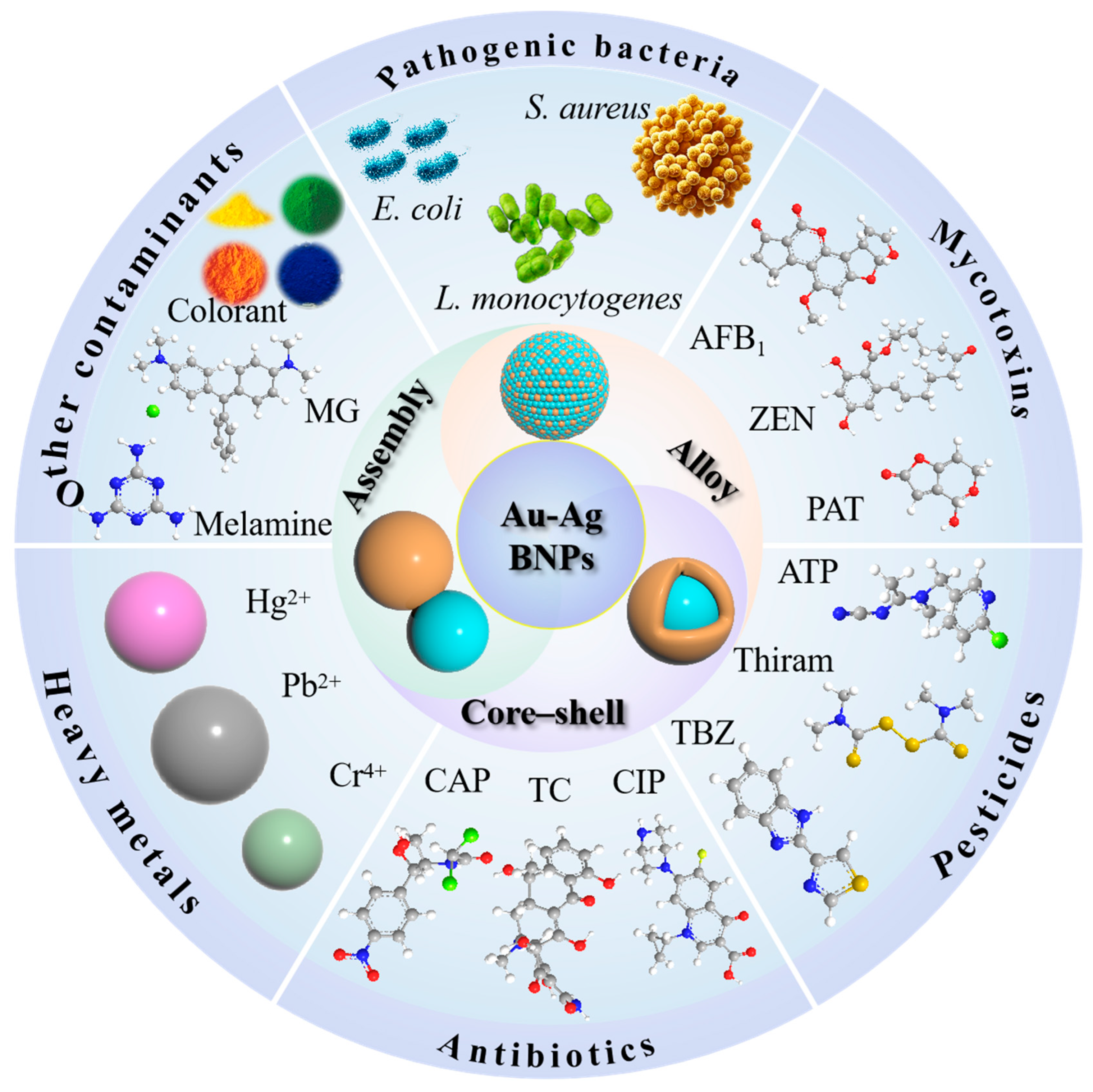
1. Introduction
2. Types and Synthesis of Au-Ag BNPs
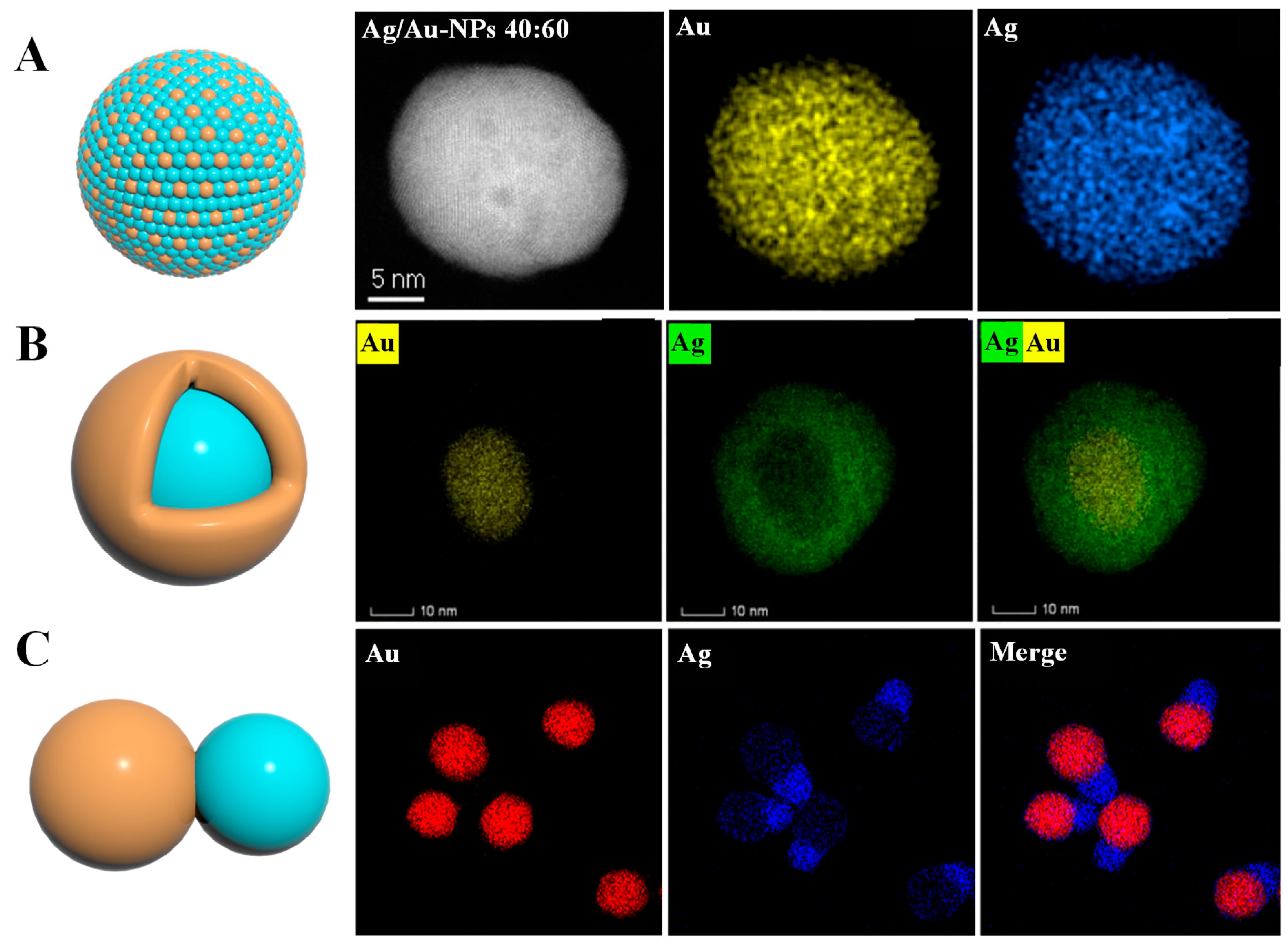
2.1. Types of Au-Ag BNPs
2.2. Synthesis of Au-Ag BNPs
2.2.1. Physical Synthesis Methods
2.2.2. Chemical Methods
2.2.3. Biological Green Synthesis Methods
3. Application of Au-Ag SERS Substrates with Bimetallic Synergistic Effects
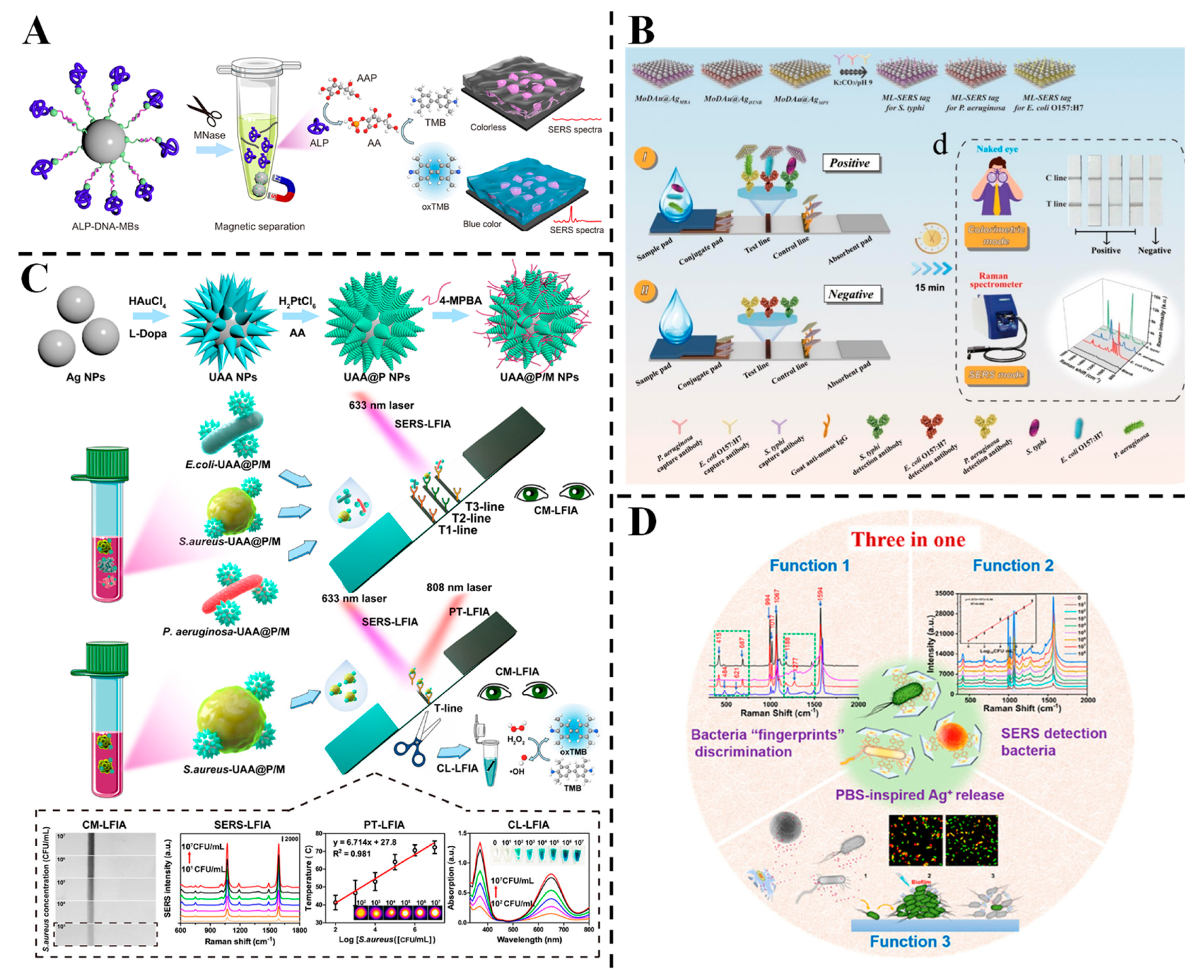
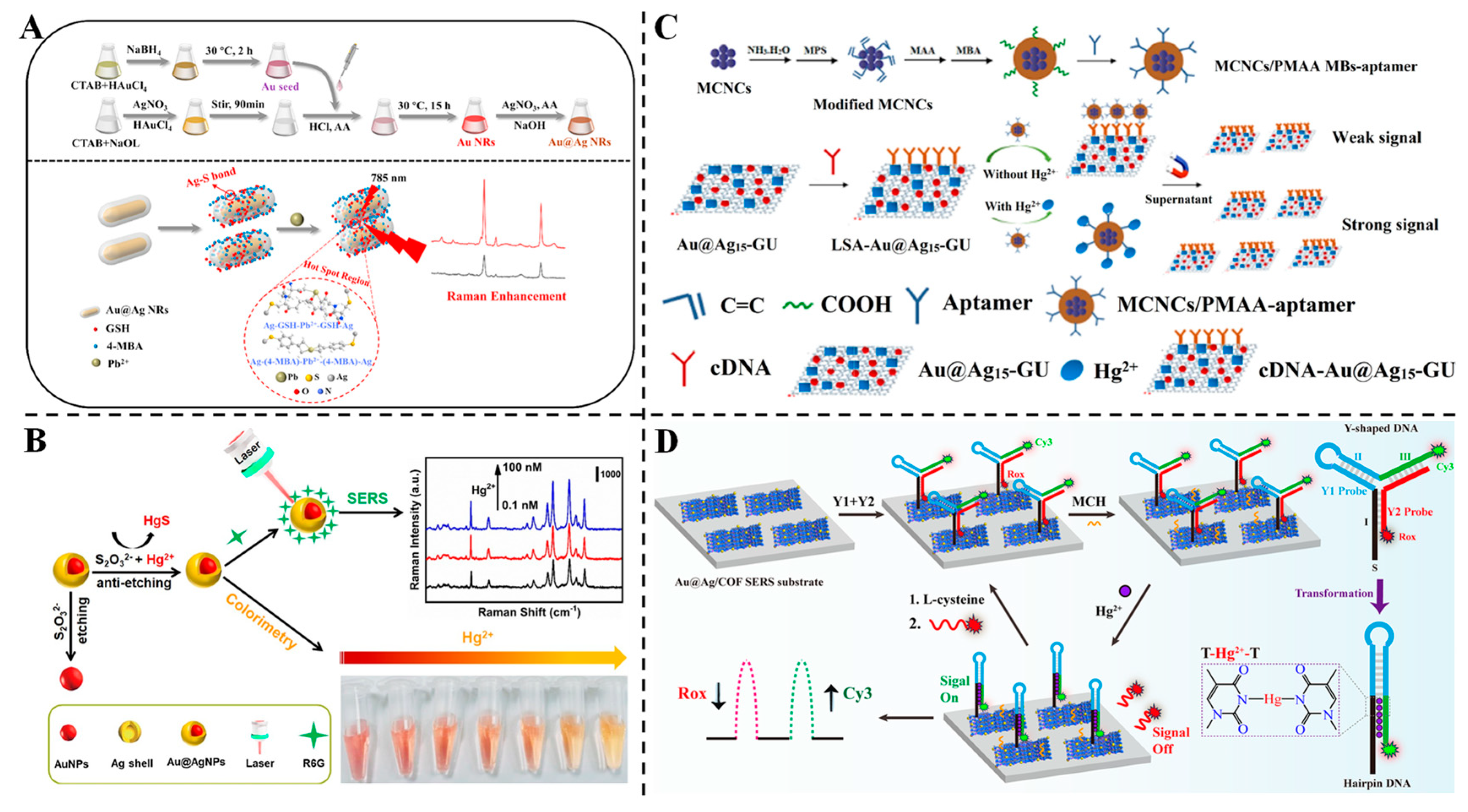
3.1. Detection of Harmful Microbes
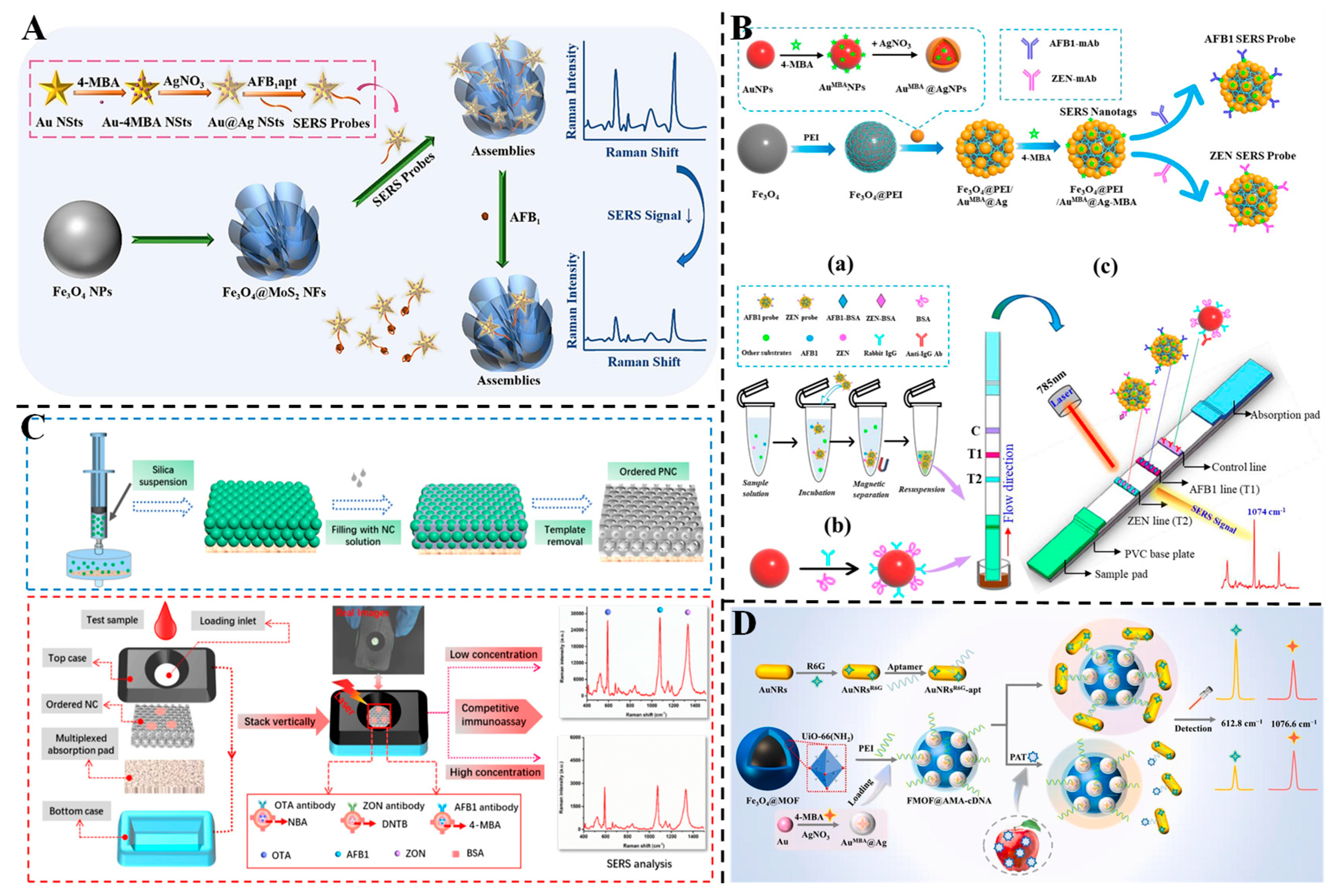
3.2. Detection of Mycotoxins
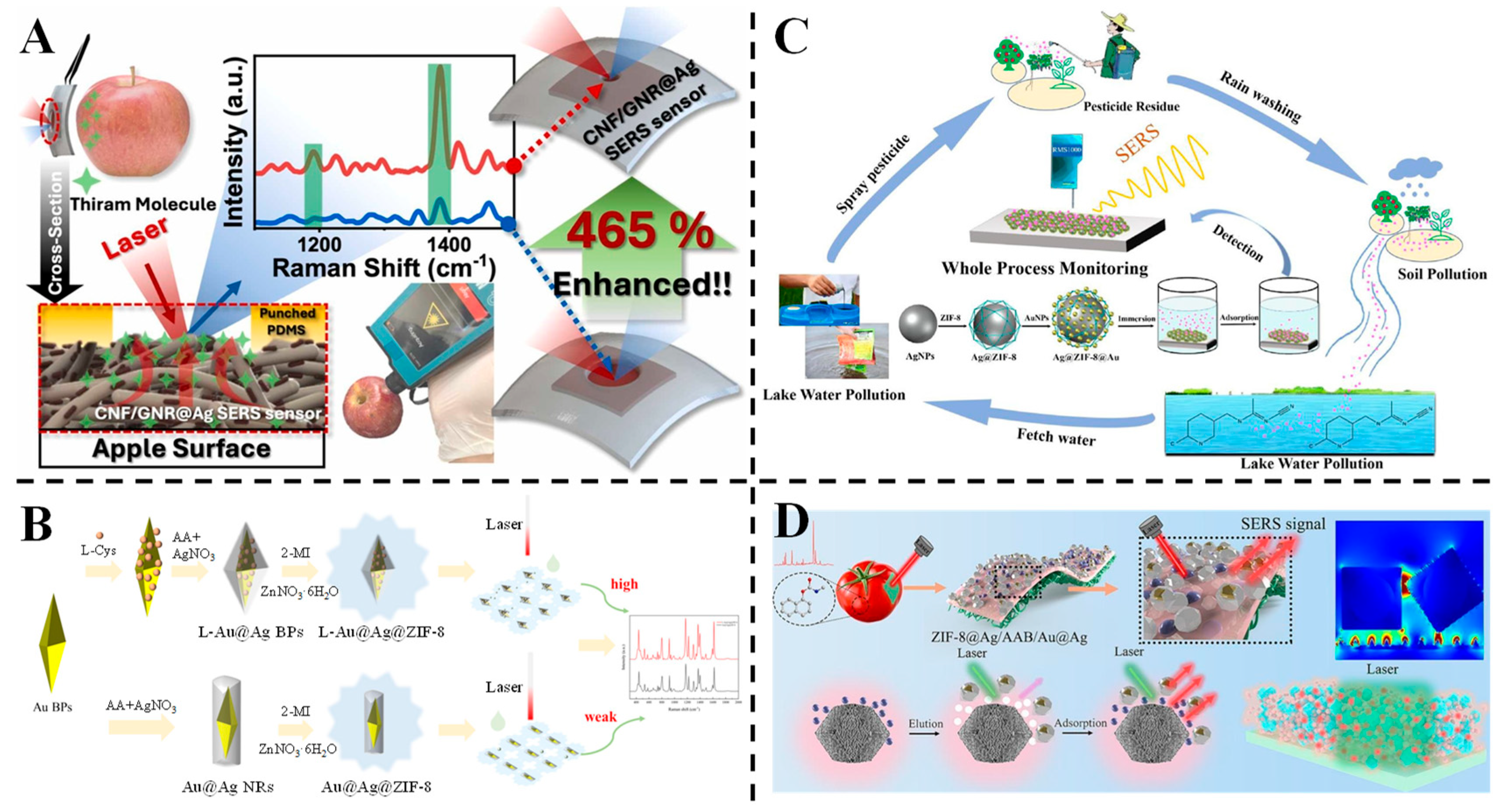
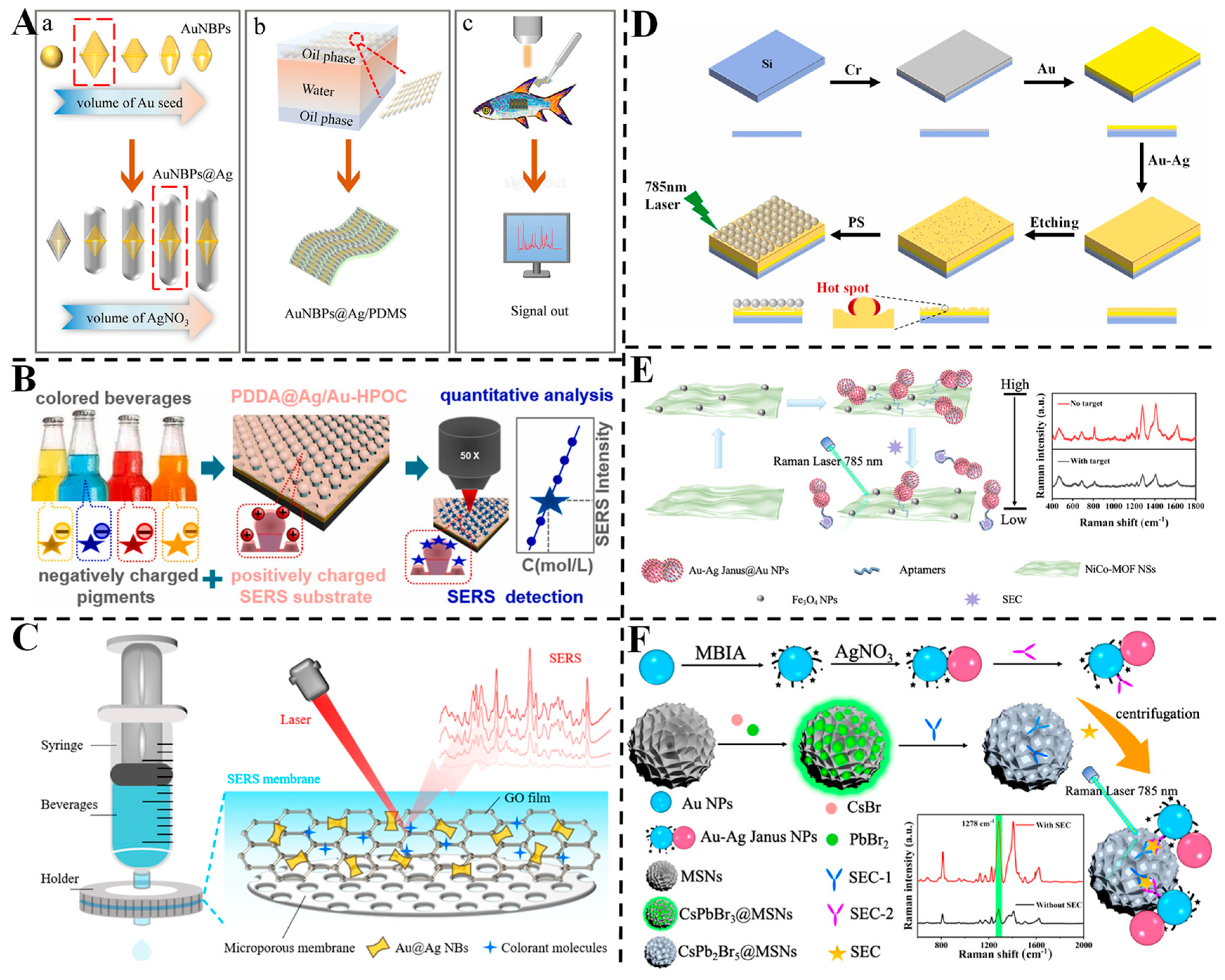
3.3. Detection of Pesticides
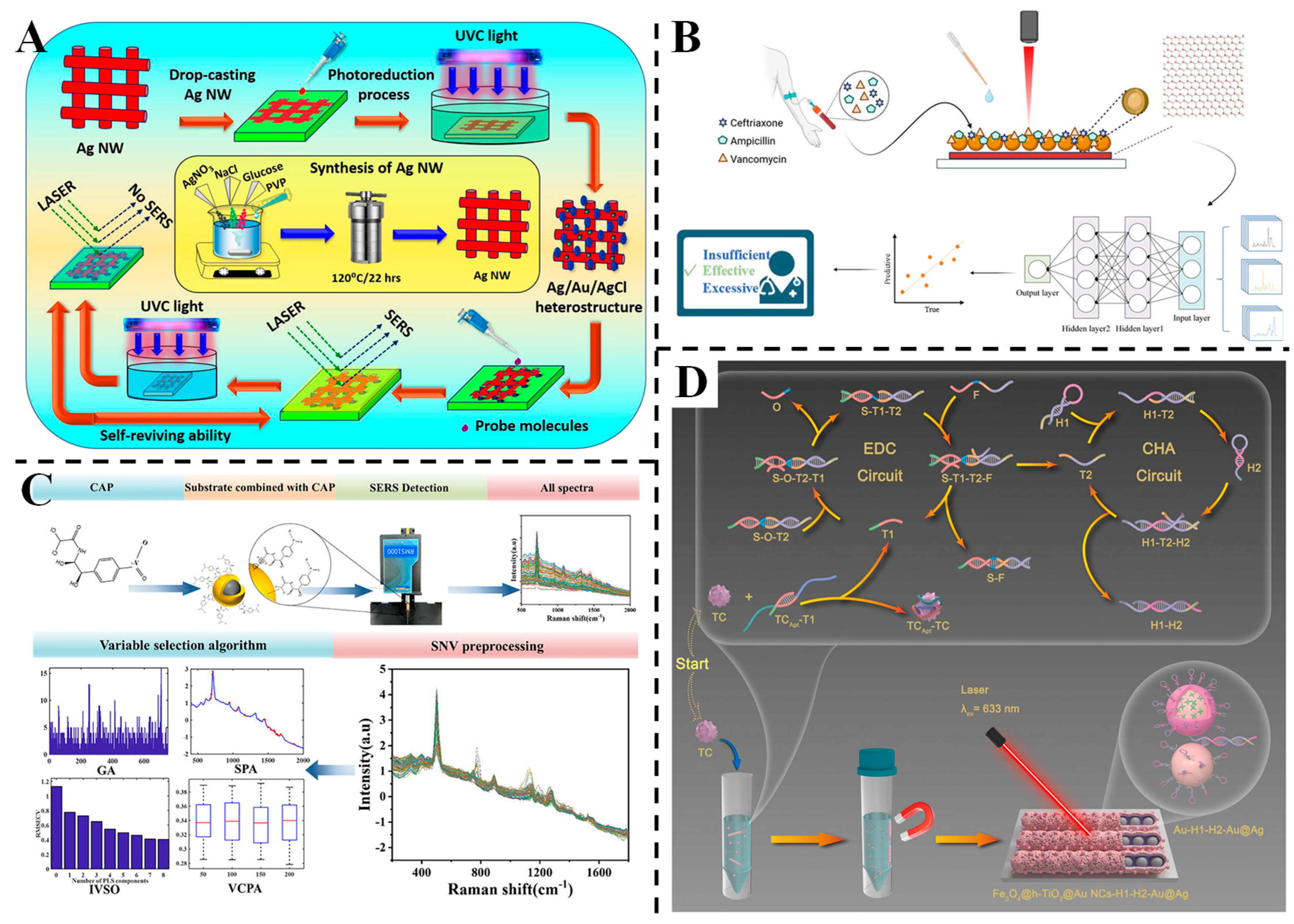
3.4. Detection of Antibiotics
3.5. Detection of Heavy Metals
3.6. Detection of Other Contaminants
4. Challenges and Outlook
4.1. Challenges
4.2. Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Huang, X.; Wang, C.; Tian, X.; Chang, X.; Ren, Y.; Yu, S. Applications of surface functionalized Fe3O4 NPs-based detection methods in food safety. Food Chem. 2021, 342, 128343. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Lin, H.; Jiang, H.; Yao-Say Solomon Adade, S.; Xue, Z.; Chen, Q. Advanced applications of chemo-responsive dyes based odor imaging technology for fast sensing food quality and safety: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5145–5172. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-H.; Gao, Z.-F.; Guo, X.; Chen, G.-H. Aptamer-Based Detection Methodology Studies in Food Safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, B.; Wang, J.; Li, B.; He, K.; Zhang, X. Rapid Detection of Escherichia coli O157:H7 by Loop-Mediated Isothermal Amplification Coupled with a Lateral Flow Assay Targeting the z3276 Genetic Marker. Food Anal. Methods 2022, 15, 908–916. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Cui, Y.; Hong, X.; Du, D. Using of Tyramine Signal Amplification to Improve the Sensitivity of ELISA for Aflatoxin B1 in Edible Oil Samples. Food Anal. Methods 2018, 11, 2553–2560. [Google Scholar] [CrossRef]
- Sharma, A.S.; Ali, S.; Sabarinathan, D.; Murugavelu, M.; Li, H.; Chen, Q. Recent progress on graphene quantum dots-based fluorescence sensors for food safety and quality assessment applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5765–5801. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Huang, X.; Hu, X.; Huang, X.; Yin, L.; Huang, Q.; Wen, Y.; Li, B.; Shi, J.; et al. Switchable aptamer-fueled colorimetric sensing toward agricultural fipronil exposure sensitized with affiliative metal-organic framework. Food Chem. 2023, 407, 135115. [Google Scholar] [CrossRef]
- Qin, C.; Guo, W.; Liu, Y.; Liu, Z.; Qiu, J.; Peng, J. A Novel Electrochemical Sensor Based on Graphene Oxide Decorated with Silver Nanoparticles–Molecular Imprinted Polymers for Determination of Sunset Yellow in Soft Drinks. Food Anal. Methods 2017, 10, 2293–2301. [Google Scholar] [CrossRef]
- Ma, L.; Yang, X.; Xue, S.; Zhou, R.; Wang, C.; Guo, Z.; Wang, Y.; Cai, J. “Raman plus X” dual-modal spectroscopy technology for food analysis: A review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70102. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Ma, L.; Han, E.; Yin, L.; Xu, Q.; Zou, C.; Bai, J.; Wu, W.; Cai, J. Simultaneous detection of mixed pesticide residues based on portable Raman spectrometer and Au@Ag nanoparticles SERS substrate. Food Control 2023, 153, 109951. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Z.; Wang, L.; Zhang, X.; Luo, C.; Hua, J.; Feng, M.; Chen, Z.; Wang, M.; Xu, C. Construction of a microcavity-based microfluidic chip with simultaneous SERS quantification of dual biomarkers for early diagnosis of Alzheimer’s disease. Talanta 2023, 261, 124677. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Chen, G.; Deng, J.; Gu, Y.; Mao, Y.; Zhou, X.; Li, G. Multiplex signal amplification strategy-based early-stage diagnosis of Parkinson’s disease on a SERS-enabled LoC system. Anal. Chim. Acta 2023, 1247, 340890. [Google Scholar] [CrossRef]
- Wang, L.; Gao, X.; Han, J.; Yuan, J.; Wang, Z.; Ma, X. Core-shell SERS nanoprobes for ROS detection and imaging during apoptosis. Food Biosci. 2025, 67, 106339. [Google Scholar] [CrossRef]
- Zhang, A.; Ding, Z.; Shen, Z.; Yan, Z.; Han, K.; Li, J.; Zhang, M.; Zhang, W. Nano-arrayed Cu2S@MoS2 heterojunction SERS sensor for highly sensitive and visual detection of polystyrene in environmental matrices. Talanta 2025, 292, 127934. [Google Scholar] [CrossRef]
- Jiang, L.; Hassan, M.M.; Ali, S.; Li, H.; Sheng, R.; Chen, Q. Evolving trends in SERS-based techniques for food quality and safety: A review. Trends Food Sci. Technol. 2021, 112, 225–240. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Ouyang, Q.; Zhao, J. Fabricating a Novel Raman Spectroscopy-Based Aptasensor for Rapidly Sensing Salmonella typhimurium. Food Anal. Methods 2017, 10, 3032–3041. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Kneipp, J. Surface-Enhanced Raman Scattering in Local Optical Fields of Silver and Gold Nanoaggregates from Single-Molecule Raman Spectroscopy to Ultrasensitive Probing in Live Cells. Acc. Chem. Res. 2006, 39, 443–450. [Google Scholar] [CrossRef]
- Long, Y.; Li, H.; Du, Z.; Geng, M.; Liu, Z. Confined Gaussian-distributed electromagnetic field of tin(II) chloride-sensitized surface-enhanced Raman scattering (SERS) optical fiber probe: From localized surface plasmon resonance (LSPR) to waveguide propagation. J. Colloid Interface Sci. 2021, 581, 698–708. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Hassan, M.M.; Zhu, J.; Wang, A.; Ouyang, Q.; Zareef, M.; Chen, Q. Amplification of Raman spectra by gold nanorods combined with chemometrics for rapid classification of four Pseudomonas. Int. J. Food Microbiol. 2019, 304, 58–67. [Google Scholar] [CrossRef]
- Wang, J.; Ahmad, W.; Mehedi Hassan, M.; Zareef, M.; Viswadevarayalu, A.; Arslan, M.; Li, H.; Chen, Q. Landing microextraction sediment phase onto surface enhanced Raman scattering to enhance sensitivity and selectivity for chromium speciation in food and environmental samples. Food Chem. 2020, 323, 126812. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Zheng, Z.; Haruna, S.A.; Chen, Q. Flexible SERS sensor using AuNTs-assembled PDMS film coupled chemometric algorithms for rapid detection of chloramphenicol in food. Food Chem. 2023, 418, 135998. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; You, T.; Arslan, M.; El-Seedi, H.R.; Guo, Z.; Zou, X.; Cai, J. Dual-layers Raman reporter-tagged Au@Ag combined with core-satellite assemblies for SERS detection of Zearalenone. Food Chem. 2023, 429, 136834. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, Z.; Xiang, Q.; Xu, Z.; Yue, W.; Li, C.; Xu, Y.; Wang, L.; Cao, X.; Zhang, J. Boosting electromagnetic enhancement for detection of non-adsorbing analytes on semiconductor SERS substrates. Chem 2023, 9, 1464–1476. [Google Scholar] [CrossRef]
- Lv, Q.; Tan, J.; Wang, Z.; Gu, P.; Liu, H.; Yu, L.; Wei, Y.; Gan, L.; Liu, B.; Li, J.; et al. Ultrafast charge transfer in mixed-dimensional WO3−x nanowire/WSe2 heterostructures for attomolar-level molecular sensing. Nat. Commun. 2023, 14, 2717. [Google Scholar] [CrossRef]
- Qiu, B.; Xing, M.; Yi, Q.; Zhang, J. Chiral Carbonaceous Nanotubes Modified with Titania Nanocrystals: Plasmon-Free and Recyclable SERS Sensitivity. Angew. Chem. Int. Ed. 2015, 54, 10643–10647. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lin, Z.; Yang, J.; Wang, J.; Yue, J.; Liu, B.; Jiang, L. Fabrication of porous noble metal nanoparticles based on laser ablation toward water and dealloying for biosensing. Appl. Surf. Sci. 2022, 579, 152130. [Google Scholar] [CrossRef]
- Yin, L.; You, T.; El-Seedi, H.R.; El-Garawani, I.M.; Guo, Z.; Zou, X.; Cai, J. Rapid and sensitive detection of zearalenone in corn using SERS-based lateral flow immunosensor. Food Chem. 2022, 396, 133707. [Google Scholar] [CrossRef]
- Jiao, S.; Wu, L.; Jiang, H.; Zhang, S.; Han, Y.; Huang, H. A review on SERS-based techniques for mycotoxin detection: From construction to application. TrAC Trends Anal. Chem. 2025, 184, 118120. [Google Scholar] [CrossRef]
- Chang, K.; Zhao, Y.; Wang, M.; Xu, Z.; Zhu, L.; Xu, L.; Wang, Q. Advances in metal-organic framework-plasmonic metal composites based SERS platforms: Engineering strategies in chemical sensing, practical applications and future perspectives in food safety. Chem. Eng. J. 2023, 459, 141539. [Google Scholar] [CrossRef]
- Jiao, T.; Dong, C.; Zhu, A.; Ahmad, W.; Peng, L.; Wu, X.; Chen, Q.; Wei, J.; Chen, X.; Qin, O.; et al. AFB1-responsive mesoporous silica nanoparticles for AFB1 quantification based on aptamer-regulated release of SERS reporter. Food Chem. 2025, 463, 141417. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, A.N.; Triyono, D.; Herbani, Y.; Isnaeni; Saleh, R. The performance of Au-Ag nanoalloys as SERS substrate on silicon wafer in enhancement Raman signal of deltamethrin pesticides. AIP Conf. Proc. 2022, 2652, 020001. [Google Scholar] [CrossRef]
- Quyen, T.T.B.; Hwang, B.-J. Novel Ag/Au Nanocubes Modified the Negative/Positive Charge on the Surface and Their Application in Surface-Enhanced Raman Scattering. Procedia CIRP 2016, 40, 551–556. [Google Scholar] [CrossRef]
- Li, H.; Luo, X.; Haruna, S.A.; Zareef, M.; Chen, Q.; Ding, Z.; Yan, Y. Au-Ag OHCs-based SERS sensor coupled with deep learning CNN algorithm to quantify thiram and pymetrozine in tea. Food Chem. 2023, 428, 136798. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core–Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef] [PubMed]
- Awiaz, G.; Lin, J.; Wu, A. Recent advances of Au@Ag core–shell SERS-based biosensors. Exploration 2023, 3, 20220072. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yin, L.; Zhang, Y.; Zhou, R.; El-Seedi, H.R.; Zou, X.; Gong, Y.; Guo, Z. SERS aptasensor detection of aflatoxin B1 based on silicon-au-ag Janus nanocomposites. Food Chem. 2025, 467, 142325. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Argüello, A.; Medina-Cruz, D.; Pérez-Ramírez, Y.S.; Pérez-García, S.A.; Velasco-Soto, M.A.; Jafari, Z.; De Leon, I.; González, M.U.; Huttel, Y.; Martínez, L.; et al. Composition-Dependent Cytotoxic and Antibacterial Activity of Biopolymer-Capped Ag/Au Bimetallic Nanoparticles against Melanoma and Multidrug-Resistant Pathogens. Nanomaterials 2022, 12, 779. [Google Scholar] [CrossRef]
- Awiaz, G.; Wu, X.; Zhang, C.; Pan, T.; Xu, X.; Lin, J.; Wu, A. Au@Ag-Au core@double shell SERS bioprobes for high-resolution tumor cells imaging. Chin. J. Anal. Chem. 2023, 51, 100204. [Google Scholar] [CrossRef]
- Wang, J.; Luo, D.; Cai, Y.; Li, X.-L.; Chen, H.-Y.; Xu, J.-J. A plasmonic Au-Ag janus nanoprobe for monitoring endogenous hydrogen sulfide generation in living cells. Biosens. Bioelectron. 2022, 213, 114422. [Google Scholar] [CrossRef]
- Moram, S.S.B.; Byram, C.; Soma, V.R. Effect of wavelength and liquid on formation of Ag, Au, Ag/Au nanoparticles via picosecond laser ablation and SERS-based detection of DMMP. Beilstein J. Nanotechnol. 2024, 15, 1054–1069. [Google Scholar] [CrossRef] [PubMed]
- Alheshibri, M. Fabrication of Au–Ag Bimetallic Nanoparticles Using Pulsed Laser Ablation for Medical Applications: A Review. Nanomaterials 2023, 13, 2940. [Google Scholar] [CrossRef]
- Murugadoss, A.; Kai, N.; Sakurai, H. Synthesis of bimetallic gold–silver alloy nanoclusters by simple mortar grinding. Nanoscale 2012, 4, 1280–1282. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Kapoor, S.; Dave, D.P.; Mukherjee, T. Synthesis of Au, Au/Ag, Au/Pt and Au/Pd nanoparticles using the microwave-polyol method. Res. Chem. Intermed. 2006, 32, 103–113. [Google Scholar] [CrossRef]
- Lyu, X.; Liu, Q.; Yuan, Q.; Liang, X.; Chen, Q.; Luo, P.; Yang, Y.; Fang, Z.; Bao, H. Ultrafast synthesis of multi-branched Au/Ag bimetallic nanoparticles at room temperature for photothermal reduction of 4-nitrophenol. J. Catal. 2023, 428, 115174. [Google Scholar] [CrossRef]
- Hassan, M.M.; Li, H.; Ahmad, W.; Zareef, M.; Wang, J.; Xie, S.; Wang, P.; Ouyang, Q.; Wang, S.; Chen, Q. Au@Ag nanostructure based SERS substrate for simultaneous determination of pesticides residue in tea via solid phase extraction coupled multivariate calibration. LWT 2019, 105, 290–297. [Google Scholar] [CrossRef]
- Carrillo-Torres, R.C.; García-Soto, M.J.; Morales-Chávez, S.D.; Garibay-Escobar, A.; Hernández-Paredes, J.; Guzmán, R.; Barboza-Flores, M.; Álvarez-Ramos, M.E. Hollow Au–Ag bimetallic nanoparticles with high photothermal stability. RSC Adv. 2016, 6, 41304–41312. [Google Scholar] [CrossRef]
- Tsuji, M.; Nishio, M.; Jiang, P.; Miyamae, N.; Lim, S.; Matsumoto, K.; Ueyama, D.; Tang, X.-L. Role of chloride ions in the formation of Au@Ag core–shell nanocrystal structures by using a microwave–polyol method. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 247–255. [Google Scholar] [CrossRef]
- Larrañaga-Tapia, M.; Betancourt-Tovar, B.; Videa, M.; Antunes-Ricardo, M.; Cholula-Díaz, J.L. Green synthesis trends and potential applications of bimetallic nanoparticles towards the sustainable development goals 2030. Nanoscale Adv. 2023, 6, 51–71. [Google Scholar] [CrossRef]
- Al-Haddad, J.; Alzaabi, F.; Pal, P.; Rambabu, K.; Banat, F. Green synthesis of bimetallic copper–silver nanoparticles and their application in catalytic and antibacterial activities. Clean Technol. Environ. Policy 2020, 22, 269–277. [Google Scholar] [CrossRef]
- Lagashetty, A.; Ganiger, S.K.; Shashidhar. Synthesis, characterization and antibacterial study of Ag–Au Bi-metallic nanocomposite by bioreduction using piper betle leaf extract. Heliyon 2019, 5, e02794. [Google Scholar] [CrossRef]
- Newase, S.; Bankar, A.V. Synthesis of bio-inspired Ag–Au nanocomposite and its anti-biofilm efficacy. Bull. Mater. Sci. 2017, 40, 157–162. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Nundkumar, N.; Singh, M.; Iyekowa, O. Green synthesis of Ag, Au and Ag-Au bimetallic nanoparticles using Stigmaphyllon ovatum leaf extract and their in vitro anticancer potential. Mater. Lett. 2019, 243, 148–152. [Google Scholar] [CrossRef]
- Ganaie, S.U.; Tasneem, A.; Abbasi, S.A. Rapid and green synthesis of bimetallic Au–Ag nanoparticles using an otherwise worthless weed Antigonon leptopus. J. Exp. Nanosci. 2016, 11, 395–417. [Google Scholar] [CrossRef]
- Bi, L.; Wang, X.; Cao, X.; Liu, L.; Bai, C.; Zheng, Q.; Choo, J.; Chen, L. SERS-active Au@Ag core-shell nanorod (Au@AgNR) tags for ultrasensitive bacteria detection and antibiotic-susceptibility testing. Talanta 2020, 220, 121397. [Google Scholar] [CrossRef]
- Jia, N.; Xiong, Y.; Wang, Y.; Lu, S.; Zhang, R.; Kang, Y.; Du, Y. A novel surface-enhanced Raman scattering method for detecting fish pathogenic bacteria with Fe3O4@PEI nanocomposite and concentrated Au@Ag. J. Raman Spectrosc. 2022, 53, 211–221. [Google Scholar] [CrossRef]
- Xu, Y.; He, P.; Ahmad, W.; Hassan, M.M.; Ali, S.; Li, H.; Chen, Q. Catalytic hairpin activated gold-magnetic/gold-core-silver-shell rapid self-assembly for ultrasensitive Staphylococcus aureus sensing via PDMS-based SERS platform. Biosens. Bioelectron. 2022, 209, 114240. [Google Scholar] [CrossRef]
- Cai, J.; Lin, Y.; Yu, X.; Yang, Y.; Hu, Y.; Gao, L.; Xiao, H.; Du, J.; Wang, H.; Zhong, X.; et al. Multifunctional AuAg-doping Prussian Blue-based MOF: Enhanced colorimetric catalytic activities and amplified SERS signals for bacteria discrimination and detection. Sens. Actuators B Chem. 2023, 394, 134279. [Google Scholar] [CrossRef]
- Nazarovskaia, D.A.; Domnin, P.A.; Gyuppenen, O.D.; Tsiniaikin, I.I.; Ermolaeva, S.A.; Gonchar, K.A.; Osminkina, L.A. Advanced Bacterial Detection with SERS-Active Gold- and Silver-Coated Porous Silicon Nanowires. Bull. Russ. Acad. Sci. Phys. 2023, 87, S41–S46. [Google Scholar] [CrossRef]
- Jin, L.; Yang, J.; You, G.; Ge, C.; Cao, Y.; Shen, S.; Wang, D.; Hui, Q. A characteristic bacterial SERS marker for direct identification of Salmonella in real samples assisted by a high-performance SERS chip and a selective culture medium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 301, 122941. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Zhao, L.; Wang, N.; Li, M.; Liang, D.; Zhao, G.; Liu, W.; Sun, L.; Xu, L.; et al. Preparation of a multifunctional and ultrasensitive Au@AgNPs-Van/PDMS film SERS substrate for rapid detection of foodborne pathogens in beef. Microchem. J. 2023, 193, 109209. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Y.; Zhou, H.; Hu, L.; Yang, A.; Jin, H.; Zheng, B.; Pi, J.; Xu, J.; Sun, P.; et al. Coupling of an Au@AgPt nanozyme array with an micrococcal nuclease-specific responsiveness strategy for colorimetric/SERS sensing of Staphylococcus aureus in patients with sepsis. J. Pharm. Anal. 2025, 15, 101085. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Li, J.; Zheng, S.; Wang, S.; Wang, C.; Yin, J.; Wang, C. 3D membrane-like tag mediated SERS encoding-lateral flow immunoassay for ultrasensitive and multiplex diagnosis of pathogens. Chem. Eng. J. 2025, 514, 163223. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, X.; Huang, H.; Huang, X.; Zhou, X.; Zhang, Z.; Sun, G.; Cai, H.; Zhou, H.; Sun, P. Triple-Function Au–Ag-Stuffed Nanopancakes for SERS Detection, Discrimination, and Inactivation of Multiple Bacteria. Anal. Chem. 2022, 94, 5785–5796. [Google Scholar] [CrossRef]
- Wu, X.; Yin, L.; Gao, S.; Zhou, R.; Zhang, Y.; Xue, S.; Jayan, H.; El-Seedi, H.R.; Zou, X.; Guo, Z. Core-satellite nanoassembly system with aptamer-conjugated Au@Ag nanoparticles for SERS detection of patulin in apples. Food Control 2024, 159, 110293. [Google Scholar] [CrossRef]
- Chen, R.; Sun, Y.; Huo, B.; Mao, Z.; Wang, X.; Li, S.; Lu, R.; Li, S.; Liang, J.; Gao, Z. Development of Fe3O4@Au nanoparticles coupled to Au@Ag core-shell nanoparticles for the sensitive detection of zearalenone. Anal. Chim. Acta 2021, 1180, 338888. [Google Scholar] [CrossRef]
- Shao, B.; Ma, X.; Zhao, S.; Lv, Y.; Hun, X.; Wang, H.; Wang, Z. Nanogapped Au(core) @ Au-Ag(shell) structures coupled with Fe3O4 magnetic nanoparticles for the detection of Ochratoxin A. Anal. Chim. Acta 2018, 1033, 165–172. [Google Scholar] [CrossRef]
- Chen, P.; Li, C.; Ma, X.; Wang, Z.; Zhang, Y. A surface-enhanced Raman scattering aptasensor for ratiometric detection of aflatoxin B1 based on graphene oxide-Au@Ag core-shell nanoparticles complex. Food Control 2022, 134, 108748. [Google Scholar] [CrossRef]
- Chen, P.; Jiang, C.; Wang, Z.; Lian, H.-Z.; Ma, X. 3D plasmonic SERS aptasensor for rapid detection of aflatoxin B1 combined with Au@Ag bimetallic nanostars and Fe3O4@MoS2 magnetic nanoflowers. Microchem. J. 2024, 196, 109598. [Google Scholar] [CrossRef]
- Tan, X.; Kang, K.; Zhang, R.; Dong, J.; Wang, W.; Kang, W. An ultrasensitive dual-mode approach for AFB1 detection: Colorimetric/label-free SERS aptasensor based on a self-assembled core-shell structured Ag@Au IP6 bifunctional nanozyme. Sens. Actuators B Chem. 2024, 412, 135854. [Google Scholar] [CrossRef]
- Zhao, Z.; Ren, M.; Zhang, W.; Chen, Z.; Zhang, L.; Qu, X.; Shi, J.; Xia, W.; Xu, X.; Yang, Y. SERS-lateral flow immunoassay based on AuNR@Ag@SiO2-AuNP assembly for ultra-sensitive detection of deoxynivalenol in grain. LWT 2024, 212, 117015. [Google Scholar] [CrossRef]
- Yin, L.; Cai, J.; Ma, L.; You, T.; Arslan, M.; Jayan, H.; Zou, X.; Gong, Y. Dual function of magnetic nanocomposites-based SERS lateral flow strip for simultaneous detection of aflatoxin B1 and zearalenone. Food Chem. 2024, 446, 138817. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hu, J.; Wang, H.; Li, C.; Kang, H.; Chen, Y.; Yang, L.; Tang, X.; Xiong, B.; Zhao, X. Vertical flow immunoassay for multiplex mycotoxins based on photonic nitrocellulose and SERS nanotags. Food Chem. X 2025, 25, 102152. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, X.; Xue, S.; Yin, L.; Gao, S.; Zhang, Y.; Wang, C.; Wang, Y.; El-Seedi, H.R.; Zou, X.; et al. Magnetic metal-organic frameworks-based ratiometric SERS aptasensor for sensitive detection of patulin in apples. Food Chem. 2025, 466, 142200. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Shi, J.; Zhang, W.; Zhang, X.; Huang, X.; Zou, X.; Li, Z.; Wei, R. Facile synthesis of Au@Ag core–shell nanorod with bimetallic synergistic effect for SERS detection of thiabendazole in fruit juice. Food Chem. 2022, 370, 131276. [Google Scholar] [CrossRef]
- Zhu, A.; Xu, Y.; Ali, S.; Ouyang, Q.; Chen, Q. Au@Ag nanoflowers based SERS coupled chemometric algorithms for determination of organochlorine pesticides in milk. LWT 2021, 150, 111978. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, L.; Jayan, H.; Jiang, S.; El-Seedi, H.R.; Zou, X.; Guo, Z. In situ self-cleaning PAN/Cu2O@Ag/Au@Ag flexible SERS sensor coupled with chemometrics for quantitative detection of thiram residues on apples. Food Chem. 2025, 473, 143032. [Google Scholar] [CrossRef]
- Wang, K.; Li, J. Reliable SERS detection of pesticides with a large-scale self-assembled Au@4-MBA@Ag nanoparticle array. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120218. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, X.; Yin, L.; Han, E.; Wang, C.; Zhou, R.; Bai, J.; Wang, Y.; Guo, Z.; Cai, J. Rapid dual-modal detection of two types of pesticides in fruits using SERS-based immunoassay. J. Food Compos. Anal. 2024, 136, 106781. [Google Scholar] [CrossRef]
- Cho, H.-S.; Yang, C.-H.; Kim, Y.-H.; Kim, Y.J.; Yoo, K.; Shin, M.; Bae, H.-J.; Jun, B.-H. Silica nanoparticles surface-decorated with Au-Ag alloy nanoparticles as highly sensitive, reproducible, and stable surface-enhanced Raman scattering (SERS) substrates. J. Alloys Compd. 2025, 1010, 177523. [Google Scholar] [CrossRef]
- Park, M.; Kim, Y.-S.; Kim, S.; Lim, J.Y. Ultra-sensitive, on-site pesticide detection for environmental and food safety monitoring using flexible cellulose nano fiber/Au nanorod@Ag SERS sensor. J. Hazard. Mater. 2025, 487, 137197. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Pu, H.; Sun, D.-W. Chiral spiny L-au@ag@ZIF-8 three-layer core-shell SERS substrate for sensitive detection of quinalphos in tangerines. Food Chem. 2025, 485, 144433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; He, M.; Qin, C.; Wu, Z.; Cao, M.; Ni, D.; Yu, Z.; Liang, P. A highly effective SERS platform formed by the fabrication of Ag@ZIF-8@Au nanoparticles for rapid detection of acetamiprid in environment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 308, 123754. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhao, P.; Cai, W.; Fang, H.; Wang, X.; Zhao, J.; Zhu, P.; Yang, H.; Ge, S.; Yu, J. MOF@Ag/AAB/Au@Ag composite matrix full-dimensional divergence effect-based SERS paper sensor for rapid carbaryl quantification. Food Chem. 2025, 472, 142885. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Wei, S.; Xu, Y.; Zareef, M.; Li, H.; Sayada, J.; Chen, Q. Ascorbate functionalized Au@AgNPs SERS sensor combined random frog-partial least squares for the prediction of chloramphenicol in milk. J. Food Compos. Anal. 2024, 129, 106106. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Liu, J.; Xiong, R.; He, Y.; Luo, X.; Yang, X. Well-ordered Au@Ag NBPs/SiO2 nanoarray for sensitive detection of chloramphenicol via DNAzyme-assisted SERS sensing. Food Chem. 2024, 454, 139806. [Google Scholar] [CrossRef]
- Nguyen, M.T.-T.; Le, T.A.; Nguyen, N.T.; Pham-Van, H.; Ngo, T.C. Controlled synthesis of Ag–Au alloy nanoparticles for optimizing surface enhanced Raman scattering-based detection of antibiotic and pesticide residues. Opt. Mater. 2025, 159, 116550. [Google Scholar] [CrossRef]
- Mao, S.; Pei, F.; Feng, S.; Hao, Q.; Zhang, P.; Tong, Z.; Mu, X.; Lei, W.; Liu, B. Detection of trace Rhodamine B using stable, uniformity, and reusable SERS substrate based on Ag@SiO2-Au nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130595. [Google Scholar] [CrossRef]
- Riswana Barveen, N.; Wang, T.-J.; Chang, Y.-H. Photochemical synthesis of Ag/Au/AgCl heterostructure from Ag nanowires as a reusable SERS substrate for ultrasensitive detection of analgesics and antibiotics. Chem. Eng. J. 2021, 423, 130191. [Google Scholar] [CrossRef]
- Jiao, A.; Cui, Q.; Li, S.; Li, H.; Xu, L.; Tian, Y.; Ma, H.; Zhang, M.; Liu, X.; Chen, M. Aligned TiO2 nanorod arrays decorated with closely interconnected Au/Ag nanoparticles: Near-infrared SERS active sensor for monitoring of antibiotic molecules in water. Sens. Actuators B Chem. 2022, 350, 130848. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, Y.; Zhao, W.; Liu, H.; Zhang, X.; Chen, H.; Sui, M.; Ma, P. SERS based determination of ceftriaxone, ampicillin, and vancomycin in serum using WS2/Au@Ag nanocomposites and a 2D-CNN regression model. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 333, 125850. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhu, A.; Adade, S.Y.-S.S.; Ali, S.; Chen, Q.; Wei, J.; Chen, X.; Jiao, T.; Chen, Q. Ag@Au core–shell nanoparticle-based surface-enhanced Raman scattering coupled with chemometrics for rapid determination of chloramphenicol residue in fish. Food Chem. 2024, 438, 138026. [Google Scholar] [CrossRef]
- Lv, Y.; Qi, S.; Khan, I.M.; Dong, X.; Qin, M.; Yue, L.; Zhang, Y.; Wang, Z. Concatenated dynamic DNA network modulated SERS aptasensor based on gold-magnetic nanochains and Au@Ag nanoparticles for enzyme-free amplification analysis of tetracycline. Anal. Chim. Acta 2023, 1270, 341238. [Google Scholar] [CrossRef]
- Xu, R.; Tan, L.; Xu, W.; Xiao, L.; Zheng, Y.; Li, Y.; Lou, Y. Ultrasensitive surface-enhanced Raman scattering sensing of Cr(vi) with a Au@Ag nano-sea urchin paper-tip substrate. Chem. Commun. 2024, 60, 12872–12875. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zareef, M.; Zhu, A.; Wei, W.; Li, H.; Chen, Q. SERS-based Au@Ag core-shell nanoprobe aggregates for rapid and facile detection of lead ions. Food Control 2024, 155, 110078. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Zhang, Y.; Zhou, X.; Hu, Z.; Liao, X.; Sheng, B.; Yuan, K.; Wu, X.; Cai, H.; et al. Colorimetric and SERS dual-mode sensing of mercury (II) based on controllable etching of Au@Ag core/shell nanoparticles. Sens. Actuators B Chem. 2021, 330, 129364. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Mehedi Hassan, M.; Zuo, M.; Wu, X.; Chen, Y.; Chen, Q. Dual-channel biosensor for Hg2+ sensing in food using Au@Ag/graphene-upconversion nanohybrids as metal-enhanced fluorescence and SERS indicators. Microchem. J. 2020, 154, 104563. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, N.; Yan, J.; Cui, K.; Chu, Q.; Chen, X.; Luo, X.; Deng, X. A dual-signaling surface-enhanced Raman spectroscopy ratiometric strategy for ultrasensitive Hg2+ detection based on Au@Ag/COF composites. Food Chem. 2024, 456, 139998. [Google Scholar] [CrossRef]
- Li, H.; Zhong, D.; Yan, S.; Tian, Y.; Lei, H.; Zhao, W.; Wei, F.; Dou, S. Multilayer Au@SiO2@Ag@SiO2 composites enhanced surface Raman scattering for non-destructive trace detection. J. Photochem. Photobiol. A Chem. 2025, 467, 116439. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Zhao, L.; Li, M.; Liang, D.; Li, M.; Zhao, G.; Ma, Y.; Tu, Q. Characteristic substance analysis and rapid detection of bacteria spores in cooked meat products by surface enhanced Raman scattering based on Ag@AuNP array substrate. Anal. Chim. Acta 2024, 1308, 342616. [Google Scholar] [CrossRef]
- Niu, F.; Jiang, P.; Wang, L.; Gao, Y.; Zhu, H.; Gao, N.; Cai, Z.; He, H.; He, Y.; Chang, G. Controllable growth of Au NBPs@Ag nanorods modified PDMS as SERS substrate for rapid detection of malachite green in aquatic products. Microchem. J. 2024, 201, 110569. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Jin, J.; Zhou, M.; Zhong, J.-H.; Liu, B. Quantitative SERS detection of weakly adsorbed dye molecules by PDDA@Ag/Au-HPOC substrates. Sens. Actuators B Chem. 2025, 422, 136683. [Google Scholar] [CrossRef]
- Kong, L.; Chen, J.; Huang, M. GO/Au@Ag nanobones decorated membrane for simultaneous enrichment and on-site SERS detection of colorants in beverages. Sens. Actuators B Chem. 2021, 344, 130163. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Jia, X.; Zhou, J.; Li, H.; Wang, X.; Wang, G. Direct detection of polystyrene microspheres by using easily fabricated Ag core embedded Au film SERS substrates with high sensitivity. J. Environ. Chem. Eng. 2024, 12, 113311. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, Z.; Zhao, Y. Tunable Preparation of SERS-Active Au–Ag Janus@Au NPs for Label-Free Staphylococcal Enterotoxin C Detection. J. Agric. Food Chem. 2023, 71, 1224–1233. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, L.; Jing, X.; Miao, H.; Zhao, Y. SERS-Active Composites with Au–Ag Janus Nanoparticles/Perovskite in Immunoassays for Staphylococcus aureus Enterotoxins. ACS Appl. Mater. Interfaces 2022, 14, 3293–3301. [Google Scholar] [CrossRef]
- Duan, N.; Yao, T.; Li, C.; Wang, Z.; Wu, S. Surface-enhanced Raman spectroscopy relying on bimetallic Au–Ag nanourchins for the detection of the food allergen β-lactoglobulin. Talanta 2022, 245, 123445. [Google Scholar] [CrossRef]
- Xu, Y.; Mehedi, H.M.; Selva, S.A.; Huanhuan, L.; Chen, Q. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 486–504. [Google Scholar] [CrossRef]
- Zhu, A.; Ali, S.; Jiao, T.; Wang, Z.; Ouyang, Q.; Chen, Q. Advances in surface-enhanced Raman spectroscopy technology for detection of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1466–1494. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Shi, Y.; Hu, X.; Wang, X.; Liang, N.; Shen, T.; Zou, X.; Shi, J. A dual-modal biosensor coupling cooperative catalysis strategy for sensitive detection of AFB1 in agri-products. Food Chem. 2023, 426, 136553. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Zhi, W.; Zeng, R.; Ji, G.; Cai, H.; Xu, J.; Wang, J.; Chen, S.; Tang, Y.; et al. Urchin-Shaped Au–Ag@Pt Sensor Integrated Lateral Flow Immunoassay for Multimodal Detection and Specific Discrimination of Clinical Multiple Bacterial Infections. Anal. Chem. 2023, 95, 13101–13112. [Google Scholar] [CrossRef]
- Ma, S.; Wang, M.; You, T.; Wang, K. Using Magnetic Multiwalled Carbon Nanotubes as Modified QuEChERS Adsorbent for Simultaneous Determination of Multiple Mycotoxins in Grains by UPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 8035–8044. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Y.; Li, M.; Garba, B.; Zhang, Q.; Wang, Y.; Zhang, H.; Li, P. Isolation and characterization of a Bacillus subtilis strain with aflatoxin B1 biodegradation capability. Food Control 2017, 75, 92–98. [Google Scholar] [CrossRef]
- Duan, N.; Chang, B.; Zhang, H.; Wang, Z.; Wu, S. Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Zareef, M.; Xu, Y.; Li, H.; Chen, Q. SERS based sensor for mycotoxins detection: Challenges and improvements. Food Chem. 2021, 344, 128652. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Mehedi Hassan, M.; Yang, W.; Shi, Z.; Zhou, X.; Xu, Y.; Ouyang, Q.; Chen, Q. Rapid and stable detection of three main mycotoxins in rice using SERS optimized AgNPs@K30 coupled multivariate calibration. Food Chem. 2023, 398, 133883. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Mahoney, N.E.; Pan, Z.; Khir, R.; Wu, B.; Ma, H.; Zhao, L. Effectiveness of pulsed light treatment for degradation and detoxification of aflatoxin B1 and B2 in rough rice and rice bran. Food Control 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Shen, G.; Cao, Y.; Yin, X.; Dong, F.; Xu, J.; Shi, J.; Lee, Y.-W. Rapid and nondestructive quantification of deoxynivalenol in individual wheat kernels using near-infrared hyperspectral imaging and chemometrics. Food Control 2022, 131, 108420. [Google Scholar] [CrossRef]
- Wu, S.; Liu, L.; Duan, N.; Li, Q.; Zhou, Y.; Wang, Z. Aptamer-Based Lateral Flow Test Strip for Rapid Detection of Zearalenone in Corn Samples. J. Agric. Food Chem. 2018, 66, 1949–1954. [Google Scholar] [CrossRef]
- Luo, L.; Ma, S.; Li, L.; Liu, X.; Zhang, J.; Li, X.; Liu, D.; You, T. Monitoring zearalenone in corn flour utilizing novel self-enhanced electrochemiluminescence aptasensor based on NGQDs-NH2-Ru@SiO2 luminophore. Food Chem. 2019, 292, 98–105. [Google Scholar] [CrossRef]
- Gao, S.; Wei, Z.; Zheng, X.; Wang, T.; Huang, X.; Shen, T.; Zhang, D.; Guo, Z.; Zhang, Y.; Zou, X. Multiplexed lateral-flow immunoassays for the simultaneous detection of several mycotoxins in foodstuffs. Trends Food Sci. Technol. 2025, 156, 104858. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Wang, M.; Zuo, M.; El-Seedi, H.R.; Chen, Q.; Shi, J.; Zou, X. Rapid enrichment detection of patulin and alternariol in apple using surface enhanced Raman spectroscopy with coffee-ring effect. LWT 2021, 152, 112333. [Google Scholar] [CrossRef]
- Xue, S.; Yin, L.; Gao, S.; Zhou, R.; Zhang, Y.; Jayan, H.; El-Seedi, H.R.; Zou, X.; Guo, Z. A film-like SERS aptasensor for sensitive detection of patulin based on GO@Au nanosheets. Food Chem. 2024, 441, 138364. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, M.; Xu, K.; Song, W.; Chen, Q.; Wen, H. Safeguarding food safety: Nanomaterials-based fluorescent sensors for pesticide tracing. Food Chem. 2025, 463, 141288. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Zou, X.-M.; Song, S.-H.; Chen, G.-H. Quantum Dots Applied to Methodology on Detection of Pesticide and Veterinary Drug Residues. J. Agric. Food Chem. 2018, 66, 1307–1319. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.; Zhu, J.; Ahmad, W.; Li, H.; Chen, Q. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020, 315, 126300. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Zareef, M.; Jiao, T.; Liu, S.; Xu, Y.; Viswadevarayalu, A.; Li, H.; Chen, Q. Signal optimized rough silver nanoparticle for rapid SERS sensing of pesticide residues in tea. Food Chem. 2021, 338, 127796. [Google Scholar] [CrossRef]
- Deng, J.; Ni, L.; Bai, X.; Jiang, H.; Xu, L. Simultaneous analysis of mildew degree and aflatoxin B1 of wheat by a multi-task deep learning strategy based on microwave detection technology. LWT 2023, 184, 115047. [Google Scholar] [CrossRef]
- Raveendran, J.; Docoslis, A. Detection and quantification of toxicants in food and water using Ag–Au core-shell fractal SERS nanostructures and multivariate analysis. Talanta 2021, 231, 122383. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Wang, C.; Liu, X.; El-Seedi, H.R.; Gómez, P.L.; Alzamora, S.M.; Zou, X.; Guo, Z. Enhanced composite Co-MOF-derived sodium carboxymethyl cellulose visual films for real-time and in situ monitoring fresh-cut apple freshness. Food Hydrocoll. 2024, 157, 110475. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci. Technol. 2021, 116, 1002–1028. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, H.; Lin, J.; Yao-Say Solomon Adade, S.; Chen, Q.; Xue, Z.; Chan, C. Non-destructive detection of multi-component heavy metals in corn oil using nano-modified colorimetric sensor combined with near-infrared spectroscopy. Food Control 2022, 133, 108640. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of Heavy Metals in Food and Agricultural Products by Surface-enhanced Raman Spectroscopy. Food Rev. Int. 2023, 39, 1440–1461. [Google Scholar] [CrossRef]
- Zhang, K.; Kwadzokpui, B.A.; Adade, S.Y.-S.S.; Lin, H.; Chen, Q. Quantitative and qualitative detection of target heavy metals using anti-interference colorimetric sensor Array combined with near-infrared spectroscopy. Food Chem. 2024, 459, 140305. [Google Scholar] [CrossRef]
- Haidar, I.; Day, A.; Martino, U.; Chevillot-Biraud, A.; Félidj, N.; Boubekeur-Lecaque, L. Bottom-up assembly of Au@Ag plasmonic nanocrystals: Issues to be addressed to achieve a good SERS substrate. Appl. Mater. Today 2019, 15, 462–471. [Google Scholar] [CrossRef]
- Sun, M.; Li, B.; Liu, X.; Chen, J.; Mu, T.; Zhu, L.; Guo, J.; Ma, X. Performance enhancement of paper-based SERS chips by shell-isolated nanoparticle-enhanced Raman spectroscopy. J. Mater. Sci. Technol. 2019, 35, 2207–2212. [Google Scholar] [CrossRef]
- Wang, H.; Levin, C.S.; Halas, N.J. Nanosphere Arrays with Controlled Sub-10-nm Gaps as Surface-Enhanced Raman Spectroscopy Substrates. J. Am. Chem. Soc. 2005, 127, 14992–14993. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, X.; Jayan, H.; Yin, L.; Xue, S.; El-Seedi, H.R.; Zou, X. Recent developments and applications of surface enhanced Raman scattering spectroscopy in safety detection of fruits and vegetables. Food Chem. 2024, 434, 137469. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Chen, H.; Fu, X.; Fu, J.; Huang, Y.; Zhang, Y. Tailoring SERS sensitivity in AgNWs-ZIF-67 substrates: Effects of MOF thickness and probe-pore size matching. Talanta 2025, 287, 127624. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Zhang, X.; Shen, Y.; Khalifa, S.A.M.; Huang, X.; Shi, J.; Li, Z.; Zou, X. Green and sustainable self-cleaning flexible SERS base: Utilized for cyclic-detection of residues on apple surface. Food Chem. 2024, 441, 138345. [Google Scholar] [CrossRef]
- Xu, N.; Sun, D.; Sun, H.; Ding, Q.; Zong, J.; Qu, Y.; Hong, M.; Tong, K. The applications of bio-orthogonal Raman labels for visualizing lipids in eukaryotic cells. TrAC Trends Anal. Chem. 2025, 183, 118099. [Google Scholar] [CrossRef]
- Al-Shaebi, Z.; Akdeniz, M.; Ahmed, A.O.; Altunbek, M.; Aydin, O. Breakthrough Solution for Antimicrobial Resistance Detection: Surface-Enhanced Raman Spectroscopy-based on Artificial Intelligence. Adv. Mater. Interfaces 2023, 2300664. [Google Scholar] [CrossRef]
| Contaminants | Plasmonic Nanostructures | Detection Method | Extra Technology or Functional Molecules | Limit of Detection | References |
|---|---|---|---|---|---|
| E. coli | Au@Ag NRs | Labeled | Antibody | 102 CFU/mL | [55] |
| E. piscicida, E. coli, V. anguillarum, V. harveyi and P. plecoglossicida | Au@Ag NPs | Label-free | Separation and enrichment of magnetic materials | Classification; 105 CFU/mL (E. piscicida) | [56] |
| S. aureus | Au-assisted magnetic nanoparticles; Au@Ag-ATP | Labeled | Hybridization chain reaction, aptamer | 0.25 CFU/mL | [57] |
| E. coli and S. aureus | AuAg@PB MOF | Label-free | 4-MPBA-functionalized substrate, dual-modal sensing | 42 CFU/mL (E. coli); 45 CFU/mL (S. aureus) | [58] |
| Listeria innocua | AuAg@pSiNWs | Label-free | / | 1.14 × 104 CFU/mL | [59] |
| Salmonella | Au-Ag NPs/Si | Label-free | / | 1 CFU/mL | [60] |
| Clostridium perfringens , Bacillus subtilis and S. aureus | Au@AgNPs/Van-PDMS | Label-free | Machine learning algorithms | 3 CFU/mL (S. aureus) | [61] |
| S. aureus | Au@AgPt nanozyme array | Label-free (indirect) | Dual mode | 38 CFU/mL (colorimetric); 6 CFU/mL (SERS) | [62] |
| P. aeruginosa, S. typhimurium and E. coli | MoDAu@Ag | Labeled | Antibody, LFIA | 29 cells/mL; 34 cells/mL; 40 cells/mL | [63] |
| E. coli, S. aureus and P. aeruginosa | Au-Ag-stuffed nanopancakes | Labeled (internal standard) | Triple-functional substrates | 7 CFU/mL | [64] |
| PAT | Au@AgMBA NPs; AuNSs-cDNA | Labeled | Aptamer | 0.0281 ng/mL | [65] |
| ZEN | AuMBA@AgMBA NPs | Labeled | Antibody | 3 μg/kg | [23] |
| ZEN | AuDTNB@Ag NPs | Labeled | Aptamer | 0.001 ng/mL | [66] |
| OTA | AuMBA@AuAg NPs | Labeled | Aptamer | 0.004 ng/mL | [67] |
| AFB1 | Au-Ag Janus NPs | Labeled | Aptamer | 0.5 pg/mL | [37] |
| AFB1 | AuMBA@Ag NPs; ITO-Au-GO | Labeled | Aptamer | 0.1 pg/mL | [68] |
| AFB1 | Au@Ag bimetallic nanostars | Labeled | Aptamer | 58.9 pg/mL | [69] |
| AFB1 | Ag@Au IP6 bifunctional nanozymes | Labeled | Dual mode, aptamer | 0.58 pg/L | [70] |
| DON | AuNR@Ag@SiO2-AuNP | Labeled | Antibody, LFIA | 0.053 fg/mL | [71] |
| AFB1 and ZEN | Fe3O4@PEI/AuMBA@AgMBA | Labeled | Antibody, LFIA | 0.095 μg/kg (AFB1); 1.896 μg/kg (ZEN) | [72] |
| OTA, AFB1 and ZEN | AuNBA@Ag, Au4-MBA@Ag and AuDNTB@Ag | Labeled | Antibody, vertical flow immunoassay | 8.2 fg/mL (OTA), 13.7 fg/mL (AFB1), 47.6 fg/mL (ZEN) | [73] |
| PAT | Au@Ag NPs, MOF and AuNRs | Labeled | Aptamer | 0.0465 ng/mL | [74] |
| Thiabendazole | Au@Ag NRs | Label-free | / | 0.032 mg/kg | [75] |
| Thiram and acetamiprid | Au@Ag NPs | Label-free | Simultaneous detection | 0.076 μM (thiram); 1.22 μM (acetamiprid) | [11] |
| 2,4-D and imidacloprid | Au@Ag nanoflowers | Label-free | Chemometric algorithms | 2.98 μg/L (2,4-D); 5.5 μg/L (imidacloprid) | [76] |
| Thiram | PAN/Cu2O@Ag/Au@AgNPs | Label-free | Deep learning algorithm | 0.02 mg/L | [77] |
| Thiram and thiabendazole | Au@4-MBA@Ag array | Label-free | Ratio Raman | 0.38 μg/L (thiram); 25 μg/L (thiabendazole) | [78] |
| Acetamiprid and carbendazim | AuMBA@AgMBA NPs | Labeled | LFIA | 0.27 μg/kg (acetamiprid); 1.71 μg/kg (carbendazim) | [79] |
| Crystal violet, thiram and carbaryl | SiO2@AuAg | Label-free | / | 6.95 × 10−7 M (crystal violet); 5.56 × 10−7 M (thiram); 7.14 × 10−6 M (carbaryl) | [80] |
| Thiram and pymetrozine | Au-Ag octahedral hollow cages | Label-free | Machine learning algorithms | 0.286 μg/kg (thiram); 29 μg/kg (pymetrozine) | [34] |
| Thiram | Cellulose nanofiber/AuNRs@Ag | Label-free | / | 10−11 M | [81] |
| Malachite green | Chiral spiny L-Au@Ag@ZIF-8 | Label-free | / | 6.56 × 10−10 M | [82] |
| Acetamiprid | Ag@ZIF-8@Au nanoparticles | Label-free | / | 9.027 × 10−10 M | [83] |
| Carbaryl | ZIF-8@Ag/AAB/Au@Ag | Label-free | / | 5.72 × 10−3 µg/mL | [84] |
| Chloramphenicol | Ascorbate-functionalized Au@Ag NPs | Label-free | Chemometric algorithms | 2.73 × 10−5 μg/mL | [85] |
| Chloramphenicol | Au@Ag NBPs/SiO2 nanoarray | Labeled | DNA enzyme amplification strategy | 6.42 × 10−13 M | [86] |
| Amoxicillin and fenobucarb | Ag-Au alloy nanoparticles | Label-free | / | / | [87] |
| RhB | Ag@SiO2-Au NPs | Label-free | / | 5 × 10−9 M | [88] |
| Paracetamol and furazolidone | Ag/Au/AgCl heterostructure | Label-free | / | 2.8 × 10−12 M (paracetamol); 1.9 × 10−11 M (furazolidone) | [89] |
| Ciprofloxacin and chloramphenicol | TiO2/Au/Ag nanorod arrays | Label-free | / | 10−9 M (ciprofloxacin); 10−8 M (chloramphenicol) | [90] |
| R6G | WS2/Au@Ag nanocomposites | Label-free | Deep learning algorithm | 10−14 M | [91] |
| Chloramphenicol | Au@Ag NPs | Label-free | chemometric algorithms | 1 × 10−5 μg/mL | [92] |
| Tetracycline | Fe3O4@h-TiO2/Au nanochains and Au@Ag NPs | Labeled | Aptamer, cascade amplification | 15.91 pg/mL | [93] |
| Cr (VI) | Au@Ag nano-sea urchins | Labeled | Methimazole-functionalized | 0.956 ng/L | [94] |
| Pb2+ | Au@Ag NRs | Labeled | Glutathione and 4-MBA-functionalized | 0.021 μg/L | [95] |
| Hg2+ | Au@Ag NPs | Labeled | Colorimetric/SERS dual-mode, etching Ag shell | 2 μM (naked eye); 0.2 nM(UV-vis); 0.1 nM (SERS) | [96] |
| Hg2+ | Au@Ag/graphene-upconversion nanohybrids | Labeled | Fluorescence/SERS dual-mode, aptamer | 0.33 ppb (SERS); 1 ppb (fluorescence) | [97] |
| Hg2+ | Au@Ag/COF | Labeled | Y-shaped DNA-functionalized | 5.0 × 10−16 M | [98] |
| R6G, thiram, melamine and piroxicam | Au@SiO2@Ag@SiO2 composites | Label-free | / | 10−9 M (R6G); 10−6 M (thiram); 10−3 M (melamine); 10−3 M (piroxicam); | [99] |
| Bacteria spores | Ag@AuNP array | Label-free | Chemometric algorithms | 10 CFU/mL | [100] |
| Malachite green | Au nanobipyramid@Ag | Label-free | / | 0.1 nM | [101] |
| Amaranth and allura red | PDDA/Ag/Au hybrid plasmonic optical cavity | Label-free | / | 0.3022 mg/L (amaranth); 0.2482 mg/L (allura red) | [102] |
| Six colorants | GO/Au@Ag nanobones | Label-free | Machine learning algorithms | / | [103] |
| Polystyrene nanoplastics | Ag@Au film | Label-free | / | 25 (310 nm), 50 (50, 70 nm) μg/mL | [104] |
| SEC | Au-Ag Janus NPs | Labeled | MBIA-functionalized, antibody | 0.55 pg/mL | [105] |
| SEC | Au-Ag Janus NPs | Labeled | MBIA-functionalized, aptamer | 0.83 pg/mL | [106] |
| β-lactoglobulin | Au-Ag nanourchins | Labeled | Aptamer | 0.07 ng/mL | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Shen, C.; Yin, X.; Cheng, J.; Liu, C.; Yu, Z. Au-Ag Bimetallic Nanoparticles for Surface-Enhanced Raman Scattering (SERS) Detection of Food Contaminants: A Review. Foods 2025, 14, 2109. https://doi.org/10.3390/foods14122109
Yu P, Shen C, Yin X, Cheng J, Liu C, Yu Z. Au-Ag Bimetallic Nanoparticles for Surface-Enhanced Raman Scattering (SERS) Detection of Food Contaminants: A Review. Foods. 2025; 14(12):2109. https://doi.org/10.3390/foods14122109
Chicago/Turabian StyleYu, Pengpeng, Chaoping Shen, Xifeng Yin, Junhui Cheng, Chao Liu, and Ziting Yu. 2025. "Au-Ag Bimetallic Nanoparticles for Surface-Enhanced Raman Scattering (SERS) Detection of Food Contaminants: A Review" Foods 14, no. 12: 2109. https://doi.org/10.3390/foods14122109
APA StyleYu, P., Shen, C., Yin, X., Cheng, J., Liu, C., & Yu, Z. (2025). Au-Ag Bimetallic Nanoparticles for Surface-Enhanced Raman Scattering (SERS) Detection of Food Contaminants: A Review. Foods, 14(12), 2109. https://doi.org/10.3390/foods14122109






