Quality Assessment of Prune Jam with Different Concentration Methods Based on Physicochemical Properties, GC-IMS, and Intelligent Sensory Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Equipment
2.1.1. Materials
2.1.2. Experimental Equipment
2.2. Sample Preparation
2.3. Physicochemical Properties Analysis
2.3.1. Determination of Color
2.3.2. Determination of Browning Intensity (BI) and Soluble Solids (TSS)
2.3.3. Determination of Titratable Acid (TA) and L-Ascorbic Acid (AA) Assay
2.3.4. Determination of Dietary Fiber (DF) Assay
2.3.5. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. Determination of Texture Properties Analysis (TPA)
2.5. Sensory Evaluation
2.6. E-Nose Determination
2.7. E-Tongue Determination
2.8. GC-IMS Analysis of Volatile Compounds
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Concentration Methods on Color and Quality of Prune Jam
3.1.1. Changes of CPs
3.1.2. Changes in BI and TSS
3.1.3. Changes in TA and AA Content
3.1.4. Changes in DF Content
3.1.5. Changes in TPC and TFC
3.2. Texture Profile Analysis
3.3. E-Tongue Analysis

3.4. E-Nose Analysis
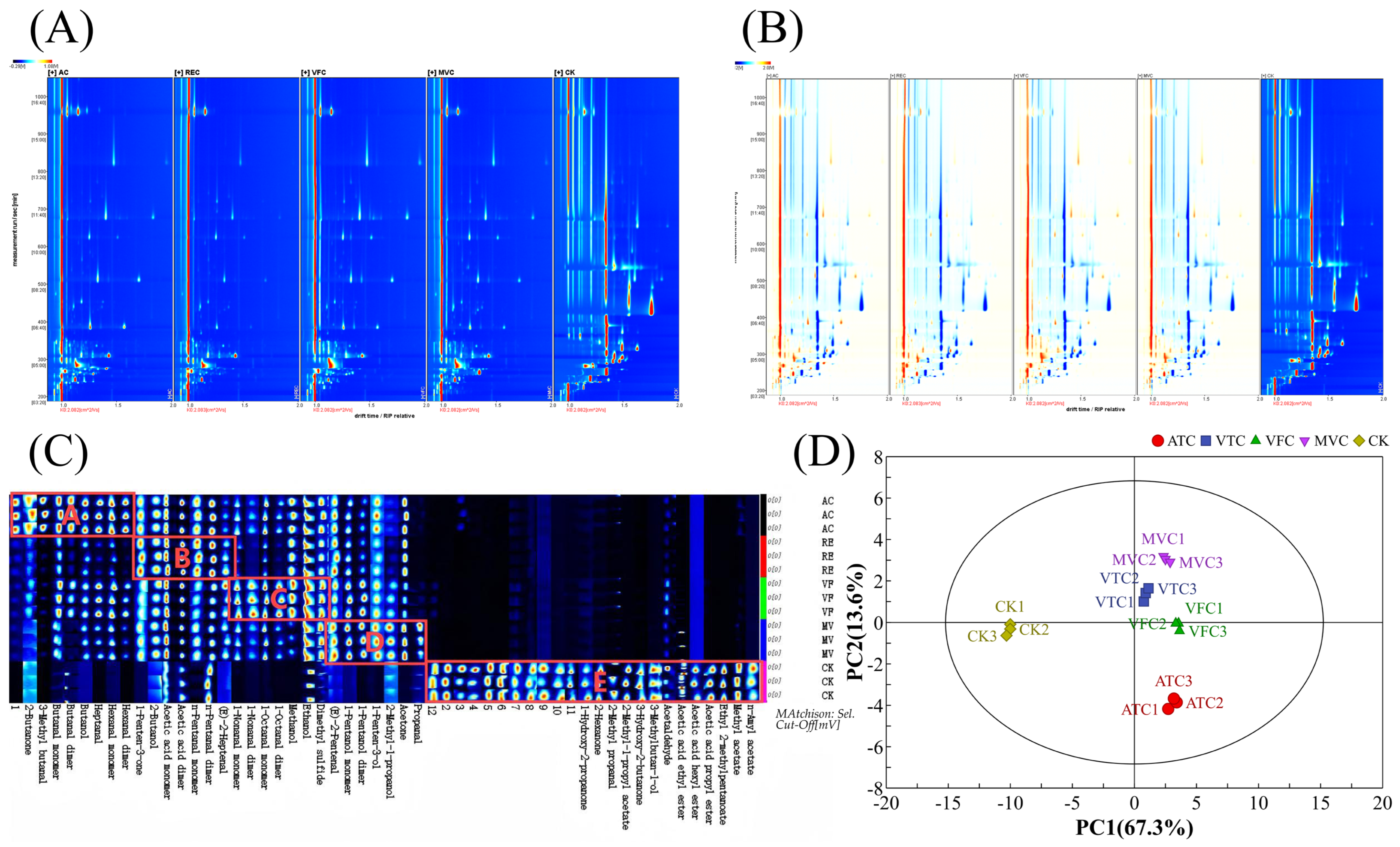
3.5. Analysis of Volatile Component Changes in Prune Jam After Concentration Treatment Based on GC-IMS


| No | Compound | VIP | Content (ug/kg) | Odor Description | ||||
|---|---|---|---|---|---|---|---|---|
| ATC | VTC | VFC | MVC | CK | ||||
| 1 | ATCetic acid ethyl ester | 2.0876 | 6.14 ± 0.23 c | 5.3 ± 0.28 c | 8.47 ± 0.46 c | 32.24 ± 3.15 b | 196.05 ± 10.52 a | fresh, fruity, sweet, grassy |
| 2 | Methanol | 2.04638 | 10.93 ± 0.43 c | 12.37 ± 0.15 b | 17.14 ± 0.85 a | 4.06 ± 0.96 d | 0.31 ± 0.03 e | alcohol, pungent |
| 3 | Ethanol | 1.79529 | 28.57 ± 0.49 d | 31.29 ± 0.42 c | 48.31 ± 0.23 a | 40.51 ± 0.84 b | 20.44 ± 1.03 e | aromaticity |
| 4 | Acetone | 1.65265 | 15.57 ± 0.94 b | 14.77 ± 0.24 b | 8.99 ± 0.13 c | 17.56 ± 1.98 a | 1.18 ± 0.11 d | fresh, apple, pear |
| 5 | 3-Hydroxy-2-butanone | 1.60444 | 2.88 ± 0.10 b | 2.36 ± 0.11 b | 4.23 ± 0.20 b | 3.53 ± 0.31 b | 132.86 ± 15.47 a | butter, cream |
| 6 | 3-Methyl butanal | 1.53947 | 4.75 ± 0.17 a | 0.53 ± 0.01 b | 0.58 ± 0.03 b | 0.53 ± 0.12 b | 0.26 ± 0.01 c | chocolate, fat |
| 7 | Propanal | 1.52113 | 0.59 ± 0.19 b | 0.52 ± 0.08 b | 0.84 ± 0.07 b | 5.13 ± 1.26 a | 1.27 ± 0.03 b | pungent, green grassy |
| 8 | Hexanal monomer | 1.38859 | 9.35 ± 0.23 a | 3.42 ± 0.15 d | 8.52 ± 0.31 b | 6.36 ± 0.29 c | 1.31 ± 0.07 e | fresh, green, fat, fruity |
| 9 | Hexanal dimer | 1.34278 | 5.27 ± 0.09 a | 0.65 ± 0.08 d | 3.75 ± 0.54 b | 1.8 ± 0.13 c | 0.4 ± 0.01 d | fresh, green, fat, fruity |
| 10 | 1-Nonanal monomer | 1.34278 | 8.55 ± 0.90 c | 4.44 ± 0.62 d | 14.30 ± 0.69 a | 9.97 ± 0.24 b | 1.46 ± 0.08 e | rose, citrus, strong oily |
| 11 | Ethyl 2-methylpentanoate | 1.31415 | 1.72 ± 0.17 b | 1.89 ± 0.20 b | 1.85 ± 0.14 b | 1.98± 0.25 b | 89.93 ± 1.31 a | fresh fruit flavor, cucumber, apple peel, pineapple |
| 12 | Acetic acid dimer | 1.274 | 18.7 ± 1.23 b | 18.8 ± 0.52 b | 18.08 ± 0.88 b | 15.68 ± 0.61 b | 24.59 ± 5.14 a | spicy |
| 13 | 1-Octanal monomer | 1.22022 | 9.79 ± 0.48 b | 5.28 ± 0.43 c | 13.71 ± 0.05 a | 10.04 ± 0.53 b | 2.06 ± 0.06 d | aldehyde, waxy, citrus, orange, fruity, fatty |
| 14 | Butanal dimer | 1.12242 | 3.37 ± 0.10 a | 0.65 ± 0.03 e | 2.37 ± 0.12 b | 0.91 ± 0.02 d | 1.59 ± 0.07 c | pungent, fruity, green leaf |
| 15 | Heptanal | 1.02522 | 4.04 ± 0.16 b | 0.93 ± 0.06 d | 4.31 ± 0.18 a | 2.52 ± 0.19 c | 0.51 ± 0.05 e | fresh, aldehyde, fatty, green herbs, wine, fruity |
| 16 | Acetaldehyde | 1.4 ± 0.10 c | 0.82 ± 0.01 c | 2.44 ± 0.04 b | 1.59 ± 0.09 bc | 3.78 ± 1.05 a | green, slight fruity | |
| 17 | 2-Butanone | 0.48 ± 0.02 a | 0.21 ± 0.00 c | 0.3 ± 0.02 b | 0.15 ± 0.01 d | 0.11 ± 0.02 e | fruity, camphor | |
| 18 | 1-Penten-3-ol | 0.55 ± 0.05 b | 0.38 ± 0.05 c | 0.53 ± 0.03 b | 0.63 ± 0.04 a | 0.11 ± 0.02 d | ethereal, green, tropical fruity | |
| 19 | 3-Methylbutan-1-ol | 0.83 ± 0.07 b | 0.92 ± 0.06 b | 0.91 ± 0.09 b | 0.96 ± 0.03 b | 17.35 ± 0.93 a | whiskey, banana, fruity | |
| 20 | 1-Pentanol monomer | 3.69 ± 0.29 b | 3.88 ± 0.18 b | 6.02 ± 0.10 a | 6.28 ± 0.13 a | 1.05 ± 0.22 c | balsamic | |
| 21 | 1-Pentanol dimer | 0.80 ± 0.09 b | 0.78 ± 0.04 b | 2.06 ± 0.08 a | 2.02 ± 0.01 a | 0.37 ± 0.02 c | balsamic | |
| 22 | Acetic acid monomer | 16.64 ± 0.17 c | 18.01 ± 0.26 b | 18.92 ± 0.18 a | 18.53 ± 0.35 ab | 15.85 ± 0.83 c | spicy | |
| 23 | 2-Methyl-1-propanol | 0.18 ± 0.02 c | 0.16 ± 0.02 c | 0.42 ± 0.03 b | 0.59 ± 0.02 a | 0.19 ± 0.01 c | fresh, alcoholic, leather | |
| 24 | Butanol | 2.29 ± 0.04 a | 1.27 ± 0.09 d | 1.86 ± 0.05 b | 1.59 ± 0.04 c | 0.39 ± 0.04 e | wine | |
| 25 | Methyl acetate | 3.51 ± 0.09 b | 0.92 ± 0.04 c | 1.76 ± 0.03 c | 1.92 ± 0.49 c | 36.8 ± 1.36 a | ester, green | |
| 26 | Acetic acid propyl ester | 0.36 ± 0.04 b | 0.43 ± 0.04 b | 0.69 ± 0.11 b | 0.52 ± 0.03 b | 18.68 ± 0.54 a | fruity, pear | |
| 27 | 2-Butanol | 0.76 ± 0.06 b | 0.85 ± 0.06 a | 0.82 ± 0.03 a | 0.86 ± 0.06 a | 0.55 ± 0.24 b | fruity | |
| 28 | 2-Methyl-1-propyl acetate | 0.39 ± 0.05 b | 0.36 ± 0.01 b | 0.41 ± 0.03 b | 0.39 ± 0.03 b | 13.6 ± 0.65 a | fruity, raw pear and raspberry | |
| 29 | 1-Octanal dimer | 2.39 ± 0.23 b | 0.81 ± 0.08 c | 5.21 ± 0.29 a | 2.55 ± 0.19 b | 0.56 ± 0.15 c | aldehyde, waxy, citrus, orange, fruity, fatty | |
| 30 | Acetic acid hexyl ester | 0.2 ± 0.03 b | 0.24 ± 0.02 b | 0.24 ± 0.02 b | 0.23 ± 0.01 b | 0.94 ± 0.08 a | fruity, green, apple, banana, sweet | |
| 31 | 2-Hexanone | 0.76 ± 0.06 b | 0.5 ± 0.05 c | 0.7 ± 0.06 bc | 0.64 ± 0.07 bc | 6.29 ± 0.24 a | fruity, fungal, meaty, buttery | |
| 32 | Dimethyl sulfide | 4.26 ± 0.25 b | 4.03 ± 0.06 b | 5.15 ± 0.10 a | 4.95 ± 0.23 a | 1.98 ± 0.63 c | cabbage, sulfur, gasoline | |
| 33 | Butanal monomer | 2.12 ± 0.08 a | 1.28 ± 0.03 c | 2.25 ± 0.02 a | 1.94 ± 0.14 b | 0.34 ± 0.07 d | pungent, fruity, green leaf | |
| 34 | 2-Methyl propanal | 0.38 ± 0.11 b | 0.14 ± 0.01 b | 0.44 ± 0.08 b | 0.24 ± 0.02 b | 2.57 ± 1.32 a | banana, melon, slightly nutty | |
| 35 | n-Pentanal monomer | 4.76 ± 0.06 b | 5.74 ± 0.12 a | 3.63 ± 0.09 c | 4.59 ± 0.14 b | 0.81 ± 0.02 d | green grassy, faint banana, pungent | |
| 36 | n-Pentanal dimer | 11.32 ± 0.41 c | 12.54 ± 0.27 b | 10.27 ± 0.42 d | 10.35 ± 0.34 d | 17.78 ± 0.38 a | green grassy, faint banana, pungent | |
| 37 | 1-Penten-3-one | 1.43 ± 0.08 c | 1.68 ± 0.09 a | 1.52 ± 0.05 bc | 1.62 ± 0.13 ab | 0.12 ± 0.01 d | strong pungent odors | |
| 38 | n-Amyl acetate | 0.14 ± 0.03 b | 0.15 ± 0.02 b | 0.19 ± 0.00 b | 0.15 ± 0.02 b | 2.22 ± 0.39 a | bananas, apples, pears | |
| 39 | (E)-2-Pentenal | 1.47 ± 0.12 b | 1.82 ± 0.17 a | 1.37 ± 0.03 b | 2.12 ± 0.32 a | 0.42 ± 0.02 c | potato, peas | |
| 40 | 1-Nonanal dimer | 0.98 ± 0.17 b | 0.47 ± 0.01 c | 2.37 ± 0.24 a | 1.08 ± 0.09 b | 0.38 ± 0.03 c | rose, citrus, strong oily | |
| 41 | (E)-2-Heptenal | 0.63 ± 0.10 b | 1.13 ± 0.20 a | 1.45 ± 0.37 a | 1.30 ± 0.06 a | 0.50 ± 0.05 b | spicy, green vegetables, fresh, fatty | |
| 42 | 1-Hydroxy-2 propanone | 0.57 ± 0.09 bc | 0.42 ± 0.04 c | 0.85 ± 0.02 b | 0.66 ± 0.02 bc | 2.86 ± 0.41 a | pungent, caramel, fresh | |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATC | atmospheric thermal concentration |
| VFC | vacuum freezing concentration |
| MVC | microwave vacuum concentration |
| VTC | vacuum thermal concentration |
| CK | control group |
| GC-IMS | gas chromatography–ion mobility spectrometry |
| PCA | principal component analysis |
| BI | browning intensity |
| CPs | color parameters |
| TA | titratable acidity |
| AA | ascorbic acid |
| DF | dietary fiber |
| TPC | total phenolic content |
| TFC | total flavonoid content |
| TPA | texture properties analysis |
| PLS-DA | partial least squares discriminant analysis |
| VIP | variable importance in projection |
| QDA | quantitative descriptive analysis |
References
- Miao, H.; Yuqi, D.; Wenli, Y.; Yicong, L.; He, C.; Fu, C.; Peng, J.; Wang, P. Research progress on nutritional quality characteristics and processing of prunes. Process. Agric. Prod. 2025, 8, 97–101. [Google Scholar] [CrossRef]
- Chang, G.-H.; Chang, S.-W.; Kim, M.-E.; Chang, Y.-J.; Ko, S.-Y. N-Butanol Extracts of Panax Notoginseng Inhibit Osteoclatogenesis by Suppressing Mapk and Downregulating NFATC1 and CFOS in LPS-Activated Raw 264.7 Cells. Bone 2010, 47, S209. [Google Scholar] [CrossRef]
- Graef, J.L.; Ouyang, P.; Wang, Y.; Rendina-Ruedy, E.; Lerner, M.R.; Marlow, D.; Lucas, E.A.; Smith, B.J. Dried Plum Polyphenolic Extract Combined with Vitamin K and Potassium Restores Trabecular and Cortical Bone in Osteopenic Model of Postmenopausal Bone Loss. J. Funct. Foods 2018, 42, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Zhipeng, W.; Ti, J.; Qingping, D.; Wei, L.; Wei, W.; Xuewen, L. Changes of reactive oxygen species metabolism and cell wall metabolism of prunes during storage at ice temperature. Mod. Food Technol. 2025. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Fan, L.; Yang, Y.; Yi, M.; Wang, W. Study on the characteristics and quality of prune hot air drying. Food Ind. Sci. Technol. 2023, 44, 103–110. [Google Scholar] [CrossRef]
- Igual, M.; Contreras, C.; Martínez-Navarrete, N. Colour and Rheological Properties of Non-Conventional Grapefruit Jams: Instrumental and Sensory Measurement. LWT-Food Sci. Technol. 2014, 56, 200–206. [Google Scholar] [CrossRef]
- Khan, M.U.; Hamid, K.; Tolstorebrov, I.; Eikevik, T.M. A Comprehensive Investigation of the Use of Freeze Concentration Appro Aches for the Concentration of Fish Protein Hydrolysates. Food Chem. 2024, 452, 139559. [Google Scholar] [CrossRef]
- Ai, Z.; Xiao, Z.; Liu, M.; Zhou, L.; Yang, L.; Huang, Y.; Xiong, Q.; Li, T.; Liu, Y.; Xiao, H.; et al. Evaluation of Innovative Drying Technologies in Gardenia Jasminoides Ellis Drying Considering Product Quality and Drying Efficiency. Food Chem. X 2024, 24, 102052. [Google Scholar] [CrossRef]
- Norouzi, S.; Fadavi, A.; Darvishi, H. The Ohmic and Conventional Heating Methods in Concentration of Sour Cherry Juice: Quality and Engineering Factors. J. Food Eng. 2021, 291, 110242. [Google Scholar] [CrossRef]
- Fadavi, A.; Yousefi, S.; Darvishi, H.; Mirsaeedghazi, H. Comparative Study of Ohmic Vacuum, Ohmic, and Conventional-Vacuum Heating Methods on the Quality of Tomato Concentrate. Innov. Food Sci. Emerg. Technol. 2018, 47, 225–230. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, Y.; Gao, Z.; Zhang, M.; Yang, X.; Mu, S.; Qu, K. Effects of Different Concentration Methods on the Quality and Volatile Components of Sea Buckthorn Clear Juice. LWT-Food Sci. Technol. 2024, 206, 116556. [Google Scholar] [CrossRef]
- Zhao, P.; Li, W.; Shang, J.; Jin, T.; Wang, Y.; Yan, L.; Liu, C. The effect of color protection and concentration process on the quality of pear paste. Food Res. Dev. 2019, 40, 138–144. [Google Scholar]
- Trishitman, D.; Negi, P.S.; Rastogi, N.K. Concentration of Pomegranate Juice by Forward Osmosis or Thermal Evaporation and Its Shelf-Life Kinetic Studies. Food Chem. 2023, 399, 133972. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhuang, S.; Gu, Y.; Shen, Y.; Zhang, W.; Ma, L.; Xiao, G.; Wang, Q.; Zhong, Y. Effect of Storage Time on the Volatile Compounds and Taste Quality of Meixian Green Tea. LWT-Food Sci. Technol. 2023, 173, 114320. [Google Scholar] [CrossRef]
- Lee, B.; Seo, J.D.; Rhee, J.-K.; Kim, C.Y. Heated Apple Juice Supplemented with Onion Has Greatly Improved Nutritional Quality and Browning Index. Food Chem. 2016, 201, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, H.; Xu, L.; Yu, C.; Hu, M.; Zhong, B.; Tu, Z.; Peng, B. Exploration of the Changes in Volatile Flavor Compounds of Low Salt Dry-Curing Grass Carp (Ctenopharyngodon Idella) Blocks during Cold Storage Detected by E-Nose, HS-SAFE-GC-MS and HS-GC-IMS. LWT-Food Sci. Technol. 2025, 218, 117470. [Google Scholar] [CrossRef]
- Opara, U.L.; Al-Ani, M.R.; Al-Rahbi, N.M. Effect of Fruit Ripening Stage on Physico-Chemical Properties, Nutritional Composition and Antioxidant Components of Tomato (Lycopersicum Esculentum) Cultivars. Food Bioprocess Technol. 2011, 5, 3236–3243. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Nadeem, M.; Khalid, W.; Ainee, A.; Roheen, T.; Javaria, S.; Ahmed, A.; Fatima, H.; Riaz, M.N.; Khalid, M.Z.; et al. Optimization of Ultrasound Assisted Extraction and Characterization of Functional Properties of Dietary Fiber from Oat Cultivar S2000. LWT 2024, 197, 115875. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Fischer, A.; Legler, A.D.S.; Oner, M.E.; Wolken, H.F.; Köpsel, M.; Ozogul, Y.; Özyurt, G.; Biase, D.D.; Ozogul, F. Physical, Chemical, and Sensory Properties of Water Kefir Produced from Aronia Melanocarpa Juice and Pomace. Food Chem. X 2023, 18, 100683. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Uslu, N.; Özcan, M.M.; Alkaltham, M.S.; Hayat, K. The Effect of Oven Drying on Bioactive Compounds, Antioxidant Activity, and Phenolic Compounds of White and Red-skinned Onion Slices. J. Food Process. Preserv. 2020, 45, e15173. [Google Scholar] [CrossRef]
- Chen, L.; Opara, U.L. Texture Measurement Approaches in Fresh and Processed Foods—A Review. Food Res. Int. 2013, 51, 823–835. [Google Scholar] [CrossRef]
- Zheng, F.; Gan, S.; Zhao, X.; Chen, Y.; Zhang, Y.; Qiu, T.; Zheng, P.; Zhai, X.; Dai, Q. Unraveling the Chemosensory Attributes of Chinese Black Teas from Different Regions Using GC-IMS Combined with Sensory Analysis. LWT-Food Sci. Technol. 2023, 184, 114988. [Google Scholar] [CrossRef]
- Bai, J.; Fan, Y.; Zhu, L.; Wang, Y.; Hou, H. Characteristic Flavor of Antarctic Krill (Euphausia Superba) and White Shrimp (Penaeus Vannamei) Induced by Thermal Treatment. Food Chem. 2022, 378, 132074. [Google Scholar] [CrossRef]
- He, Z.; Wang, H.; Wang, W.; Shen, X.; Yu, C.; Yue, H.; Wang, H.; Feng, T.; Yao, L. The Volatile and Sensorial Profiles of Processed Damask Rose (Rosa Damascena Mill.) Products Cultivated in China under Different Process Treatments. Ind. Crops Prod. 2024, 222, 119716. [Google Scholar] [CrossRef]
- Nistor, O.V.; Șeremet, L.; Mocanu, G.D.; Barbu, V.; Andronoiu, D.G.; Stănciuc, N. Three Types of Red Beetroot and Sour Cherry Based Marmalades with Enhanced Functional Properties. Molecules 2020, 25, 5090. [Google Scholar] [CrossRef] [PubMed]
- Korus, A.; Jaworska, G.; Bernaś, E.; Juszczak, L. Characteristics of Physico-Chemical Properties of Bilberry (Vaccinium Myrtillus L.) Jams with Added Herbs. J. Food Sci. Technol. 2014, 52, 2815–2823. [Google Scholar] [CrossRef]
- Wu, D.; Xia, Q.; Cheng, H.; Zhang, Q.; Wang, Y.; Ye, X. Changes of Volatile Flavor Compounds in Sea Buckthorn Juice during Fermentation Based on Gas Chromatography–Ion Mobility Spectrometry. Foods 2022, 11, 3471. [Google Scholar] [CrossRef]
- Han, S.-H.; Zhu, J.-K.; Shao, L.; Yue, C.-H.; Li, P.-Y.; Bai, Z.-Y.; Luo, D.-L. Effects of Ultrasonic Treatment on Physical Stability of Lily Juice: Rheological Behavior, Particle Size, and Microstructure. Foods 2024, 13, 1276. [Google Scholar] [CrossRef]
- Shen, C.; Cai, Y.; Wu, X.; Gai, S.; Wang, B.; Liu, D. Characterization of Selected Commercially Available Grilled Lamb Shashliks Based on Flavor Profiles Using GC-MS, GC × GC-TOF-MS, GC-IMS, E-Nose and E-Tongue Combined with Chemometrics. Food Chem. 2023, 423, 136257. [Google Scholar] [CrossRef]
- Jabbari, S.-S.; Jafari, S.M.; Dehnad, D.; Shahidi, S.-A. Changes in Lycopene Content and Quality of Tomato Juice during Thermal Processing by a Nanofluid Heating Medium. J. Food Eng. 2018, 230, 1–7. [Google Scholar] [CrossRef]
- Wu, S.; Xiong, S.; Pan, S. Effect of vacuum concentration and atmospheric boiling on the quality and rheological properties of citrus jam. J. Huazhong Agric. Univ. 2023, 42, 194–201. [Google Scholar] [CrossRef]
- Yang, Z.; Miao, N.; Zhang, X.; Li, Q.; Wang, Z.; Li, C.; Sun, X.; Lan, Y. Employment of an Electronic Tongue Combined with Deep Learning and Transfer Learning for Discriminating the Storage Time of Pu-Erh Tea. Food Control. 2021, 121, 107608. [Google Scholar] [CrossRef]
- Tkacz, K.; Chmielewska, J.; Turkiewicz, I.P.; Nowicka, P.; Wojdyło, A. Dynamics of Changes in Organic Acids, Sugars and Phenolic Compounds and Antioxidant Activity of Sea Buckthorn and Sea Buckthorn-Apple Juices during Malolactic Fermentation. Food Chem. 2020, 332, 127382. [Google Scholar] [CrossRef]
- Jia, X.; Ren, J.; Fan, G.; Reineccius, G.A.; Li, X.; Zhang, N.; An, Q.; Wang, Q.; Pan, S. Citrus Juice Off-Flavor during Different Processing and Storage: Review of Odorants, Formation Pathways, and Analytical Techniques. Crit. Rev. Food Sci. Nutr. 2022, 64, 3018–3043. [Google Scholar] [CrossRef]
- Velotto, S.; Palmeri, R.; Alfeo, V.; Gugino, I.M.; Fallico, B.; Spagna, G.; Todaro, A. The Effect of Different Technologies in Pomegranate Jam Preparation on the Phenolic Compounds, Vitamin C and Antioxidant Activity. Food Biosci. 2023, 53, 102525. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Yang, G.; Zhang, W.-W.; Thakur, K.; Hu, F.; Khan, M.R.; Ni, Z.-J.; Wei, Z.-J. Physicochemical and Functional Properties of Carboxymethylated Insoluble Dietary Fiber of Lycium Barbarum Seed Dreg. Food Chem. X 2024, 22, 101270. [Google Scholar] [CrossRef]
- Vaz, A.A.; Bellí, G.; Oms-Oliu, G.; Martín-Belloso, O.; Odriozola-Serrano, I. Exploring the Prebiotic Potential of Unpurified Apple Dietary Fibre Concentrate. LWT-Food Sci. Technol. 2025, 222, 117608. [Google Scholar] [CrossRef]
- Scaglioni, P.T.; de Souza, T.D.; Schmidt, C.G.; Badiale-Furlong, E. Availability of Free and Bound Phenolic Compounds in Rice after Hydrothermal Treatment. J. Cereal Sci. 2014, 60, 526–532. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Guerra-Valle, M.; Astudillo-Lagos, M. Impact of Block Cryoconcentration on Polyphenol Retention in Blueberry Juice. Food Biosci. 2017, 20, 149–158. [Google Scholar] [CrossRef]
- da Silva Haas, I.C.; de Espindola, J.S.; de Liz, G.R.; Luna, A.S.; Bordignon-Luiz, M.T.; Prudêncio, E.S.; de Gois, J.S.; Fedrigo, I.M.T. Gravitational Assisted Three-Stage Block Freeze Concentration Process for Producing Enriched Concentrated Orange Juice (Citrus Sinensis L.): Multi-Elemental Profiling and Polyphenolic Bioactives. J. Food Eng. 2022, 315, 110802. [Google Scholar] [CrossRef]
- Kh, A.A.; Mi, S.; Tian, H.; Xu, X.; Abdo, A.A.A.; Aleryani, H.; Wang, X. Evaluation of Flavor Characteristics in Chinese Wheat Flour Paste Using Electronic-nose, Electronic-tongue, and Headspace-gas Chromatography-ion Mobility Spectrometry at Different Fermentation Stages. J. Sci. Food Agric. 2024, 105, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.I.; Lozano, J.E.; Genovese, D.B. Effect of Formulation Variables on Rheology, Texture, Colour, and Acceptability of Apple Jelly: Modelling and Optimization. LWT-Food Sci. Technol. 2015, 62, 325–332. [Google Scholar] [CrossRef]
- Chen, L.; Ning, F.; Zhao, L.; Ming, H.; Zhang, J.; Yu, W.; Yi, S.; Luo, L. Quality Assessment of Royal Jelly Based on Physicochemical Properties and Flavor Profiles Using HS-SPME-GC/MS Combined with Electronic Nose and Electronic Tongue Analyses. Food Chem. 2023, 403, 134392. [Google Scholar] [CrossRef] [PubMed]
- Kan, Q.; Cao, L.; He, L.; Wang, P.; Deng, G.; Li, J.; Fu, J.; Huang, Q.; Ho, C.-T.; Li, Y.; et al. Tracing the Change of the Volatile Compounds of Soy Sauce at Different Fermentation Times by PTR-TOF-MS, E-Nose and GC–MS. Food Chem. X 2025, 25, 102002. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, W.; Rong, Y.; Lou, B. Characterisation of Taste-active Compositions, Umami Attributes and Aroma Compounds in Chinese Shrimp. Int. J. Food Sci. Technol. 2021, 56, 6311–6321. [Google Scholar] [CrossRef]
- Li, Y.; Lu, K.; Wang, M.; Wang, X.; Li, H.; Xie, W. Aroma Composition and Sensory Correlation in Jiaodong Shrimp Paste: A Comparative Analysis of Solid, Liquid, and Oil Phases. Food Res. Int. 2025, 209, 116265. [Google Scholar] [CrossRef]
- Jia, X.; Cui, H.; Qin, S.; Ren, J.; Zhang, Z.; An, Q.; Zhang, N.; Yang, J.; Yang, Y.; Fan, G.; et al. Characterizing and Decoding the Key Odor Compounds of Spirulina Platensis at Different Processing Stages by Sensomics. Food Chem. 2024, 461, 140944. [Google Scholar] [CrossRef]

| Sample | CK | ATC | VTC | VFC | MVC |
|---|---|---|---|---|---|
| TSS/% | 9.34 ± 0.45 b | 44.78 ± 0.36 a | 43.76 ± 0.22 a | 41.91 ± 0.11 a | 42.77 ± 0.37 a |
| Sample | Hardness/g | Adhesiveness/(g.s) | Cohesiveness/g | Gumminess/g | Chewiness/g |
|---|---|---|---|---|---|
| CK | 59.53 ± 3.24 e | −8.60 ± 0.45 a | 0.68 ± 0.02 c | 49.60 ± 1.85 d | 51.61 ± 4.32 e |
| ATC | 183.92 ± 5.94 a | −155.89 ± 2.73 e | 0.96 ± 0.05 a | 183.45 ± 5.53 a | 190.17 ± 5.13 a |
| VTC | 144.47 ± 5.09 c | −112.57 ± 2.44 c | 0.85 ± 0.03 b | 153.93 ± 12.80 b | 136.94 ± 2.07 c |
| VFC | 100.91 ± 2.03 d | −62.20 ± 5.17 b | 0.73 ± 0.03 c | 120.78 ± 4.10 c | 97.73 ± 5.31 d |
| MVC | 167.40 ± 1.49 b | −140.76 ± 8.83 d | 0.87 ± 0.01 b | 161.04 ± 7.23 b | 162.03 ± 5.61 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Zhao, L.; Wang, W.; Du, Q.; Li, W.; Sun, T.; Huang, S. Quality Assessment of Prune Jam with Different Concentration Methods Based on Physicochemical Properties, GC-IMS, and Intelligent Sensory Analysis. Foods 2025, 14, 2084. https://doi.org/10.3390/foods14122084
Yang R, Zhao L, Wang W, Du Q, Li W, Sun T, Huang S. Quality Assessment of Prune Jam with Different Concentration Methods Based on Physicochemical Properties, GC-IMS, and Intelligent Sensory Analysis. Foods. 2025; 14(12):2084. https://doi.org/10.3390/foods14122084
Chicago/Turabian StyleYang, Rui, Langhan Zhao, Wei Wang, Qingping Du, Wei Li, Tongle Sun, and Shihao Huang. 2025. "Quality Assessment of Prune Jam with Different Concentration Methods Based on Physicochemical Properties, GC-IMS, and Intelligent Sensory Analysis" Foods 14, no. 12: 2084. https://doi.org/10.3390/foods14122084
APA StyleYang, R., Zhao, L., Wang, W., Du, Q., Li, W., Sun, T., & Huang, S. (2025). Quality Assessment of Prune Jam with Different Concentration Methods Based on Physicochemical Properties, GC-IMS, and Intelligent Sensory Analysis. Foods, 14(12), 2084. https://doi.org/10.3390/foods14122084







